Abstract
Gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system (CNS), plays a pivotal role in maintaining the delicate balance between inhibitory and excitatory neurotransmission. Dysregulation of the excitatory/inhibitory balance is implicated in various neurological and psychiatric disorders, emphasizing the critical role of GABA in disease-free brain function. The review examines the intricate interplay between the gut–brain axis and CNS function. The potential impact of dietary GABA on the brain, either by traversing the blood–brain barrier (BBB) or indirectly through the gut–brain axis, is explored. While traditional beliefs questioned GABA’s ability to cross the BBB, recent research challenges this notion, proposing specific transporter systems facilitating GABA passage. Animal studies provide some evidence that small amounts of GABA can cross the BBB but there is a lack of human data to support the role of transporter-mediated GABA entry into the brain. This review also explores GABA-containing food supplements, investigating their impact on brain activity and functions. The potential benefits of GABA supplementation on pain management and sleep quality are highlighted, supported by alterations in electroencephalography (EEG) brain responses following oral GABA intake. The comprehensive overview encompasses GABA’s sources in the diet, including brown rice, soy, adzuki beans, and fermented foods. GABA’s presence in various foods and supplements, its association with gut microbiota, and its potential as a therapeutic strategy for neurological disorders are thoroughly examined. The articles were retrieved through a systematic review of the databases: OVID, SCOPUS, and PubMed (keywords “GABA”, “oral GABA“, “sleep”, “cognition”, “neurodegenerative”, “blood-brain barrier”, “gut microbiota”, “supplements” and “therapeutic”, and by searching reference sections from identified studies and review articles). This review presents the relevant literature available on the topic and discusses the mechanisms, effects, and hypotheses that suggest oral GABA benefits range from neuroprotection to blood pressure control. The literature suggests that oral intake of GABA affects the brain illustrated by changes in EEG scans and cognitive performance, with evidence showing that GABA can have beneficial effects for multiple age groups and conditions. The potential clinical and research implications of utilizing GABA supplementation are vast, spanning a spectrum of diseases ranging from neurodegeneration to blood pressure regulation. Importantly, recommendations for the use of oral GABA should consider the dosage, formulation, and duration of treatment as well as potential side effects. Effects of GABA need to be more thoroughly investigated in robust clinical trials to validate efficacy to progress the development of alternative treatments for a variety of disorders.
Keywords:
GABA; oral GABA; gut microbiota; neurodegenerative; cognition; blood–brain barrier; supplements; therapeutic 1. Introduction
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter. With widespread distribution and abundant presence in the brain and spinal cord, GABA plays a pivotal role in mediating and modulating a diverse array of central nervous system (CNS) functions [1,2]. In the mature brain, GABA and glutamate are balanced, exerting inhibitory and excitatory effects, respectively, and this homeostasis is vital in disease-free brain function [3,4]. Substantiating this crucial role, a wealth of evidence points to the diverse pharmacological effects exhibited by GABA receptor agonists and antagonists, ranging from anxiolysis and hypnosis to muscle relaxation, amnesia, cognitive enhancement, stimulant, and anticonvulsant activities [5].
The GABAergic system operates through two key receptors in the CNS, namely GABAA and GABAB receptors, each contributing to synaptic inhibition. GABAA receptors are heteropentamers, comprising more than 20 subunits [4,5,6]. As chloride ion channels, these receptors are distributed throughout the CNS. In contrast, GABAB receptors utilize G-protein-coupled mechanisms, primarily belonging to the pertussis toxin-sensitive Gi/o family, which regulates specific ion channels and cAMP cascades. GABAB receptors mediate slow synaptic inhibition and are critical regulators of neuronal excitability. The varied subunit compositions of the receptor subtypes contribute to the functional versatility of the GABAergic system, enabling fine modulation of neuronal activity [6].
GABA is synthesized through the conversion of glutamate via the action of glutamate decarboxylase and vitamin B6. Synthesized GABA is released onto the post-synaptic terminals of neurons. Despite glutamate being the precursor for GABA, in the CNS it is an excitatory neurotransmitter, and GABA functions as an inhibitory neurotransmitter. Dysregulation of the glutamate–GABA balance, or the excitatory/inhibitory (E/I) balance, has been implicated in various pathologies, including psychiatric disorders such as generalized anxiety, schizophrenia, autism spectrum disorder, major depressive disorder, Alzheimer’s disease, and other dementias [4,7,8].
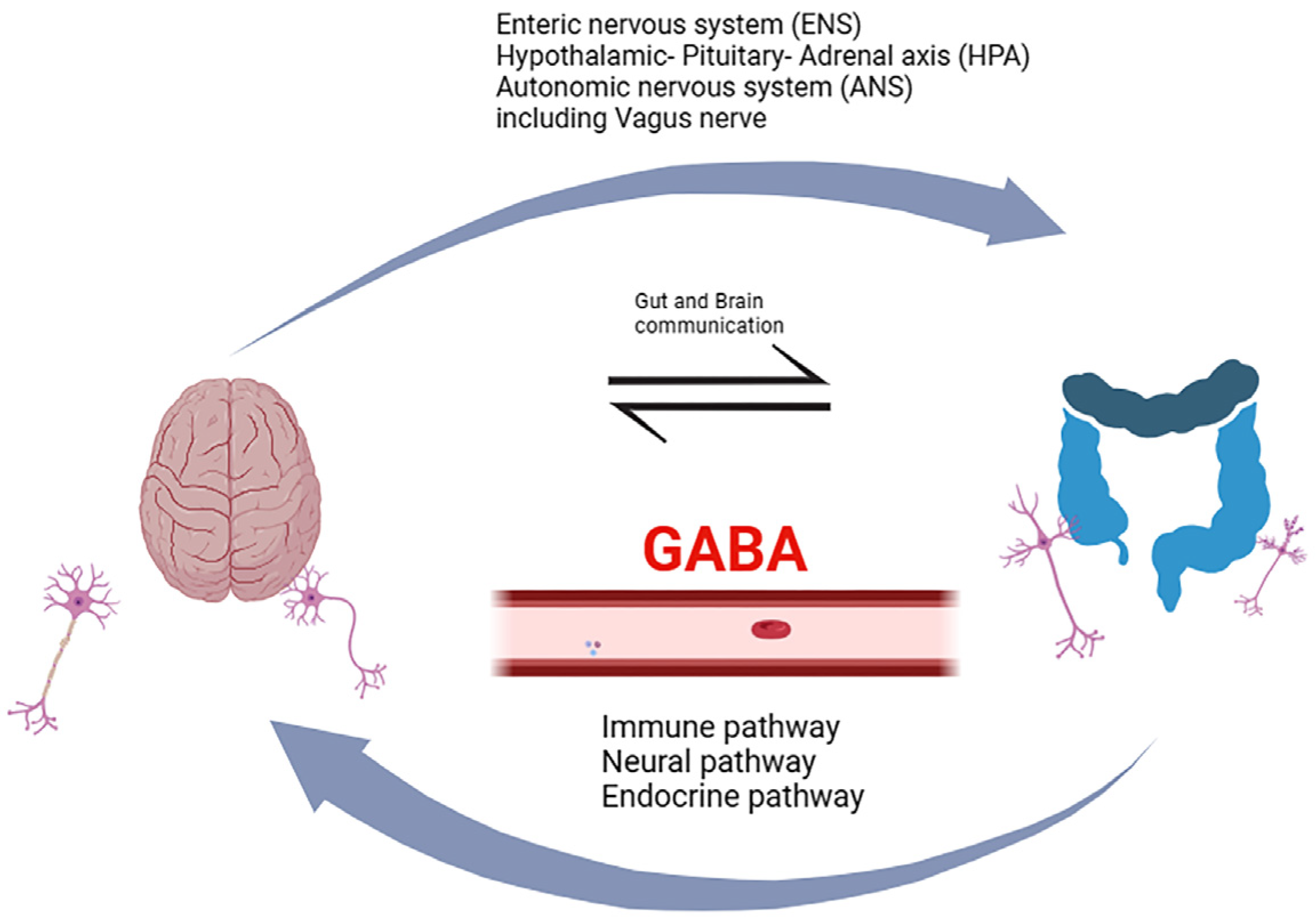
Decades of research bring the gut–brain axis to the forefront, an intricate and bidirectional communication system integrating peripheral intestinal function with emotional and cognitive brain centers through neuro-immuno-endocrine mediators (Figure 1). Within this context, gut microbiota has been associated with various CNS disorders, including dementia [9]. Dysbiosis of gut microbiota has been linked to the secretion of amyloid and lipopolysaccharides (LPS), disrupting gastrointestinal permeability and the blood–brain barrier (BBB). This disruption sets in motion inflammatory signaling pathways, leading to neuroinflammation, neuronal injury, and ultimately neuronal death [9,10]. The gut microbiota is a source of GABA present in the gut, but oral GABA intake needs to be considered as well. It is vital to investigate how GABA present in the gut may affect the brain and whether it has a direct effect by crossing the BBB or works indirectly by acting on the enteric nervous system (ENS) or the vagus nerve [11].

Figure 1.
A schematic diagram showing the proposed mechanisms contributing to the functioning of the gut–brain axis. The mechanisms involve bidirectional communication between the gastrointestinal (GI) tract and the central nervous system (CNS), integrating neural pathways, immune pathways, as well as the endocrine pathway, and the involvement of the enteric (ENS) and autonomic nervous (ANS) systems, including the vagus nerve. This intricate network relies on a dynamic interplay of signals between gut microbiota, intestinal epithelial cells, and various components of the nervous and immune systems. The gut–brain axis facilitates this communication and dietary GABA might help to maintain this. Importantly, the gut microbiota, comprising trillions of microorganisms, also influences this axis by producing GABA, other metabolites, and bioactive compounds. Created in BioRender.com (https://www.biorender.com, accessed on 5 April 2024).
Exploring the impact of dietary GABA on the brain via gut interactions and its potential to influence the BBB directly or indirectly raises questions about the underlying mechanisms. Nonetheless, the extent to which GABA can effectively traverse the BBB remains a subject of ongoing debate. In a study involving rat brain grafts, fetal neocortex, or substantia nigra was transplanted into a young adult rat cortex or striatum. This allowed examine how the BBB lets [3H] GABA pass. Initially, [3H] GABA could cross the BBB and enter transplanted areas, particularly the neocortex, for up to 4 weeks. In substantia nigra grafts, the BBB became better at stopping GABA passage after 4 weeks. This suggests that the BBB gradually forms and restricts GABA movement. In the early stages, GABA was taken up by neurons or glia in the grafts. Ultimately, the BBB’s barrier to GABA was established beyond four weeks. This study highlights how the BBB evolves and controls GABA access to the brain [12]. On the other hand, another study conducted on rats tested the BBB’s permeability to GABA following intraperitoneal injection and intraperitoneal GABA plus L-Arginine (L-Arg) administration. GABA and L-Arg injected together showed a fourfold increase in brain GABA level (383.3%) compared to untreated rats. Intraperitoneal GABA alone showed an increased brain GABA concentration of 33%, while L-Arg alone increased GABA concentration by 65%, compared to untreated rats. The study also observed dose-dependent nitric oxide (NO) production in the brain when rats were injected with L-Arg, and the peak concentration of NO production was observed at 2000 mg of L-Arg. This suggests that high NO concentrations in the brain following L-Arg administration may increase the permeability of the BBB to peripheral GABA due to NO’s role in vasodilation. The authors conclude that NO contributes to increased BBB permeability to GABA [13]. Nevertheless, while traditional beliefs suggest that GABA may not traverse the BBB, recent research challenges this notion. Some studies propose that GABA can indeed cross the BBB facilitated by specific GABA-transporter systems in the brain [13,14]. These pathways could offer a means for oral absorption of GABA and its analogs. However, while these animal studies provide some evidence that small amounts of GABA can cross the BBB there is a lack of human data to support the role of transporter-mediated GABA entry into the brain. Moreover, oral administration of GABA might influence the brain via the gut–brain axis (Figure 1). Notably, studies have shown alterations in electroencephalography (EEG) brain responses following oral GABA intake in comparison to controls like water/L-theanine or dextrin placebo [15]. However, it is important to note that the mechanism by which oral GABA affects the brain is unclear and further research needs to be performed to provide clarity. It is crucial to consider that experiments testing GABA’s ability to cross the BBB employed different methodologies; this is likely one reason for the difference in outcomes and the conflicting viewpoints. Also important to factor in possible species-specific differences in GABA transport mechanisms and the age and disease model used in these studies. GABA transport seems to be limited under normal physiological conditions in the adult brain [16,17,18,19,20,21,22] but can increase during development [12,14] and transport can be altered in disease conditions [19].
In Europe, GABA is utilized as an ingredient in food supplements. In 2009, the European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition, and Allergies assessed health claims regarding GABA’s impact on cognitive function. They concluded that a definitive cause-and-effect relationship between GABA intake and the asserted cognitive functions had not been firmly established [23]. In recent times, interest has surged in GABA-containing food supplements and their hypothetical impact on brain activity and functions. A study found that consistent administration of GABA to rats and dogs, even at doses up to 1 g/kg/day, did not exhibit any indications of toxicity [24]. Notably, some research has linked GABA to reductions in blood pressure, implying a potential risk of hypotension when used alongside antihypertensive drugs [24]. Oral GABA’s potential to affect stress and sleep was examined through various studies. One of which indicated an increased heart rate variability (HRV) and parasympathetic activity, suggesting stress reduction [25]. Another study looked at GABA’s ability to affect brain wave patterns and reported increasing alpha and decreasing beta waves suggesting relaxation [26]. In addition, cortisol, and chromogranin A (CgA) level reduction in GABA consumers indicates potential stress reduction, and reduced sleep latency. It is important to note that evidence for GABA’s effect on improved sleep maintenance is limited [27]. Further research is warranted to establish optimal doses of GABA and its long-term effects on stress and sleep. GABA-enriched products as a potential food source to mitigate inflammatory responses were also proposed, offering practical applications in health and wellness. The anti-inflammatory activity of GABA-enriched products derived from Lactobacillus fermented rice bran solution exhibited inhibitory effects on the expression of inflammatory enzymes, including inducible nitric oxide synthase and cyclooxygenase-2, and reduced the generation of pro-inflammatory cytokines like interleukin (IL)-6, IL-1β, tumor necrosis factor α, and monocyte chemoattractant protein-1 [28].
Dietary GABA encompasses foods like brown rice, soy and adzuki beans, chestnuts, and mushrooms [29]. It can also be found in foods, such as tea, tomato, soybean, germinated rice, and some fermented foods [29]. Additionally, some foods are GABA-enriched such as cereals, sourdough breads, cheeses, and fermented sausages, which also contribute to GABA intake [30,31]. GABA supplements, like rice bran and Sarcodon aspratus extracts, offer additional GABA sources. As mentioned earlier, gut microbiota also plays a role in gut GABA production and maintenance of normal gut GABA levels. Nutritional support of the GABAergic system with GABA or GABA receptor ligands, food, and vitamins has been suggested as treatment options for neurodevelopmental and aging-related disorders associated with GABAergic dysfunction [32,33,34]. Vitamin D deficiency is known to induce an E/I imbalance and supplementation has been reported to recover impairments of the GABAergic system [33,35]. These findings highlight the importance of diet in maintaining healthy brain function and the opportunity for nutritional support to correct the E/I imbalance.
This review aims to provide a comprehensive overview of the effect of dietary GABA and GABA supplements on the brain in both health and disease. We will explore the potential impact of GABA-containing food and GABA supplements on brain function, delving into the proposed mechanisms of action. Additionally, we will address the intriguing controversy surrounding GABA’s direct versus indirect influence on the brain through the gut–brain axis. The understanding of the intricate interplay between dietary GABA, gut microbiota, and CNS function holds great promise for unveiling novel therapeutic strategies, including probiotic-based interventions, to address neurological disorders and promote brain health. Through an in-depth exploration of these mechanisms, we aspire to contribute to the advancement of the field and deepen our comprehension of GABA’s multifaceted role in regulating brain function. Here we review articles that propose health benefits to oral GABA in humans and animal models that can be linked to the involvement of the nervous system.
2. The Effect of Dietary GABA on Sleep
In recent years, dietary GABA has garnered interest in sleep aids. Multiple studies have explored its potential to enhance sleep quality and duration, shedding light on promising approaches for better sleep. One study examined the effect of consuming GABA-enriched rice in two groups (containing 16.8 mg GABA in a daily portion of 150 g GABA rice vs. 4.1 mg GABA in 150 g white rice per day) of healthy middle-aged patients with poor sleep. Participants were given the rice for 8 weeks. Enhanced sensations upon awakening were discovered in the GABA-enriched rice-consuming group during both the fourth week and the tenth week of the study, in comparison to the white rice group. However, no significant impact of GABA rice on the Visual Analog Scale (VAS) sleepiness score was observed [36]. Another study using GABA-enriched rice documented that among post-menopausal women, the ingestion of GABA rice containing 26.4 mg of GABA three times daily resulted in the improvement of insomnia scores as assessed by the Kupperman Menopause Index. This enhancement was notable during the fourth week of the treatment period when compared to the control rice group [37]. These two studies illustrated the positive outcome resulting from the consumption of GABA-enriched rice among participants with poor sleep and insomnia.
Other studies took a different approach and looked at the effect biosynthetic rice might have on sleep in disease-free elderly patients. GABA consumption improved the maintenance and onset of sleep, and drowsiness in the morning. Recovery from fatigue in the GABA group was seen after 4 weeks although the placebo group showed the same result [38]. However, other studies illustrate the effectiveness of biosynthetic GABA. One such investigation includes three intervention studies, conducted with notably small sample sizes each spanning 1 to 3 weeks, delving into the impact of biosynthetic GABA consumption on sleep quality among individuals experiencing suboptimal sleep. One study enrolled participants scoring above 5 on the Pittsburgh Sleep Quality Index (PSQI) and the other two involved individuals with PSQI scores exceeding 6. In the initial 1-week intervention study, the ingestion of 100 mg GABA was delivered in the form of GABA powder, contained in gelatin capsules. Each capsule contained 100 mg of GABA powder, along with other components like glutamic acid, other amino acids, minerals, and water. As opposed to a control GABA consumption led to an improvement in sensations upon waking scores. Additionally, the results indicated a decrease in sleep onset latency and an increase in the total duration of non-REM sleep stages (N1, N2, and N3/SWS) following the intervention. The observed trends suggest enhancements in PSQI scores, sleep satisfaction, and ease of falling asleep ratings, alongside the augmented duration of light non-REM sleep and sleep efficiency within the GABA group compared to the control after the treatment period [37]. This highlights GABA’s potential to positively influence sleep parameters, making it a promising avenue for further research.
More evidence of oral GABA’s effect on sleep is exemplified in a week-long intervention study focused on middle-aged individuals grappling with sleep disturbances. The investigation unveiled a noticeable trend toward decreased sleep onset latency, specifically in the context of the 100 mg GABA capsule intake as compared to the control group. However, when examining various parameters including PSQI total score, sleep satisfaction, feelings upon awakening, ease of falling asleep ratings, along with measurements of non-REM sleep latency, REM sleep duration, non-REM sleep duration, frequency of awakenings, and delta wave power, no statistically significant differences emerged between the GABA-only group and the control group [39]. Another study was conducted in 4 weeks and focused on middle-aged individuals who reported struggling with poor sleep quality. The study revealed that the intake of a 300 mg GABA tablet, as compared to a control tablet, led to a reduction in sleep onset latency following the intervention. The researchers also noted a decrease in N2 sleep duration as a percentage and a decline in the severity index of insomnia (ISI). Furthermore, improvements were observed in various PSQI parameters, including the total score, sleep quality, sleep latency, and total sleep time within the GABA group when comparing pre-treatment and post-treatment scores. However, the study did not state any notable differences between the GABA group and the placebo/control group. Notably no statistically significant effects were found concerning PSQI sleep efficiency scores, total sleep time, the percentages of stage 1 and 3 non-REM sleep, REM sleep percentage, wake after sleep onset (WASO) in minutes, REM sleep latency, sleep efficacy, arousal index, apnea-hypopnea index (AHI), or respiratory distress index (RDI) [40].
Another study conducted on human subjects utilized sake yeast which has been observed to take up GABA during the primary stage of sake brewing [41]. For the clinical trial, tablets were prepared, each containing 125 mg of sake yeast powder, approximately equivalent to 75 mg of dry sake yeast. Participants were instructed to ingest four tablets per day, with each tablet containing 125 mg of sake yeast powder. The tablets were taken 1 h before bedtime, over a period of 4 days for each test condition. A placebo group was also used for comparison. Sake yeast supplementation significantly augmented sleep quality, evident in a robust 110% amplification of EEG delta power slow-wave sleep (SWS) is a phase of deep sleep crucial for physical restoration and cognitive functions. The amplification of EEG delta power in the context of sleep quality refers to an increase in the intensity or magnitude of the brain’s electrical activity in the delta frequency range (0.5–4 Hz) during the SWS stage. SWS is a phase of deep sleep crucial for physical restoration and cognitive functions. Higher delta power indicates a greater predominance of slow-wave activity during sleep, suggesting a deeper and more restorative quality of sleep. Moreover, the subjective perception of sleep quality, especially in terms of ‘sleepiness upon waking’, exhibited a notable enhancement, showcasing a significant difference compared to the placebo (p = 0.02). These quantitative outcomes highlight the potential of sake yeast supplementation to positively influence sleep patterns and the subjective perception of sleep, ultimately fostering an improved overall sleep quality [42].
Other studies examined how GABA-rich foods such as Passiflora incarnata L. (Passionflower (PI)), which contains high levels of GABA, may influence behaviors and brain function [43,44]. One study investigated the sleep-inducing effects of PI extract on rodents; this included both mice and rats. The study used brain tissue samples from the animals treated with the PI extract. They performed immunohistochemistry staining with a c-Fos-specific antibody on the brain tissue samples of rodents and observed a notable affected on brain activity, specifically the mammillary body region, as evidenced by increased c-Fos-positive cells. Moreover, the extract influenced intracellular ROS levels and upregulated GABA receptors and GAD1 mRNA expression, suggesting a potential mechanism involving GABAergic signaling. Notably, the PI extract significantly increased serum melatonin levels, a critical marker for potential sleep-inducing effects due to its fundamental role in regulating the sleep-wake cycle, also known as the circadian rhythm. Additionally, the extract led to prolonged immobility time and increased palpebral closing time, further indicating its sedative or sleep-inducing properties. In conclusion, PI extract demonstrates promising sleep-inducing effects in animal models suggesting its potential as a natural alternative for managing sleep disorders [44]. Another study was conducted to investigate the sleep-promoting effects of a mixture of GABA and 5-hydroxytryptophan (5-HTP) specifically using ICR mice and Sprague-Dawley rats. The GABA/5-HTP mixture was administered orally to the study subjects at specific doses, GABA at 60 mg/kg and 5-HTP at 6 mg/kg dissolved in 0.9% physiological saline. The drugs were given to the subjects one hour before conducting the experiments related to sleep analysis using the pentobarbital-induced sleep test. The administration of the GABA/5-HTP mixture significantly increased the sleep onset ratio by more than 65%. Additionally, in mice treated with a hypnotic dose of pentobarbital (42 mg/kg), the GABA/5-HTP mixture significantly reduced sleep latency and significantly prolonged the duration of sleep. These results highlight the sleep-promoting potential of the GABA/5-HTP mixture, showcasing its ability to enhance sleep onset and duration effectively in the study subjects [45].
This set of studies delved into exploring the potential of dietary GABA as a sleep aid, encompassing both human and animal research. One study primarily targeted middle-aged individuals grappling with poor sleep, comparing the consumption of GABA-enriched rice to white rice. The results showed an improvement in awakening sensations for the GABA group during specific weeks, although no significant changes in sleepiness scores were observed. Another study involving post-menopausal women revealed that GABA rice consumption led to better insomnia scores compared to a control group. The effects of biosynthetic GABA on sleep were also investigated in elderly patients, showcasing improved sleep maintenance and onset as well as increased morning alertness. However, it is important to note that placebo effects were also evident in these cases. Differing results emerged from smaller studies focusing on biosynthetic GABA, some demonstrating improved wakefulness scores and non-REM sleep stages, but not consistently across all sleep parameters. Studies involving middle-aged individuals indicated trends toward reduced sleep onset latency with GABA capsules, although not consistently significant effects on various sleep metrics were observed.
In summary, the potential impact of dietary GABA on sleep quality appears promising, showing potential benefits in terms of awakening sensations and specific aspects of sleep in both human and animal studies. However, the results varied across studies and parameters, possibly influenced by differences in the source and administration methods of GABA and the specific groups (e.g., health status, age, sex, ethnicity) on which the studies were conducted. Factors such as the short duration of the studies and variations in participant numbers may have contributed to the diverse outcomes observed. Further research and standardized methodologies are essential to better understand the potential of GABA as a sleep aid.
3. Effects of Dietary GABA on Anxiety
A study on the oral effect of GABA in rats explored alterations in anxiety-related behavior using stress models and behavioral tests. In the open field test, emotional stress led to a notable 84% reduction in center time compared to controls. However, GABA at 1 mg/kg and 2 mg/kg increased center time significantly (t = −3.384, p = 0.002 and t = −5.256, p < 0.001, respectively). Notably, GABA at 2 mg/kg also increased the distance traveled in the center (t = −3.342, p = 0.002). Locomotor and exploratory behaviors showed no significant differences among groups. In the elevated plus maze test, stressed rats spent less time in open arms, while GABA at 2 mg/kg markedly increased this duration (t = −4.225, p < 0.001). Entries into open arms decreased in stressed rats but were improved by GABA at 2 mg/kg. Regarding blood composition, it was noted that plasma NO metabolites nitrate and nitrite (NOx) levels were higher in stressed rats and the 0.5 mg/kg GABA group compared to controls (t = −4.828, p < 0.001 and t = −3.219, p = 0.003, respectively). However, GABA at 1 mg/kg and 2 mg/kg reduced plasma NOx levels significantly (t = 2.349, p = 0.025 and t = 3.219, p = 0.003). In the frontal cortex, NOx levels were lower in stress-exposed rats (t = 2.300, p = 0.028), but GABA doses showed a dose-dependent increase. Correlation analysis revealed grooming in the open field negatively correlated with NOx levels (p < 0.01), as did open field movement time (p < 0.01). In the elevated plus maze, the anxiety index negatively correlated with NOx levels (p < 0.01). In conclusion, oral GABA supplementation exhibited anxiolytic potential. GABA’s influence on NOx levels correlated with behavioral changes, suggesting complex interactions that need to be further studied to be understood [46].
Another study explored the effect of GABA-enriched chocolate on humans to assess stress using heart rate variability and salivary CgA. The first experiment examined the heart rate variability (HRV) using two measures of heart rate variability (HRV): LF/HF and HFnu, after people had 10 g of GABA-enriched chocolate (28 mg GABA) or placebo. After 14 min of a task (about 31–34 min after eating), both the GABA chocolate and placebo groups had higher LF/HF values compared to before eating (p < 0.05). Around 6.5–9.5 min after the task (about 36.5–39.5 min after eating), the LF/HF value was significantly lower in the GABA chocolate group compared to placebo (p < 0.05). At ECG-1 (14 min post-task), HFnu values in both groups were lower compared to before eating (p < 0.05). At 6.5–9.5 min after the task and at 12–15 min after the task (about 36.5–39.5 min and 42–45 min after eating), the GABA chocolate group had higher HFnu values than placebo (p < 0.05). The other experiment tested for changes in salivary CgA after participants had GABA-enriched chocolate (28 mg GABA) or placebo. In the placebo group, the levels of salivary CgA were significantly higher at 30 min and 50 min after eating compared to before eating (p < 0.05). In the GABA chocolate group, there were no significant differences between these times. While the 30-min salivary CgA level in the GABA chocolate group was a bit lower than in the placebo group (p = 0.060) [47].
4. Effects of Dietary GABA on Pain Modulation
The GABAergic system in the CNS plays a significant role in pain modulation. Over nearly three decades, extensive research has delved into the intricate interplay of GABAergic transmission in pain perception and mediation, covering acute, inflammatory, neuropathic, and chronic pain [1]. This understanding has propelled the exploration of oral GABA supplementation as a potential therapeutic approach for pain management. Numerous studies have highlighted the critical association between reduced GABAergic tone, characterized by diminished GABA activity within the spinal cord, and heightened pain sensitivity, particularly in neuropathic pain models [48]. This reduction stems from disrupted GABA synthesis, loss of GABA-producing spinal neurons, and alterations in GABA transport mechanisms, culminating in compromised inhibitory effects and heightened neural excitability within the CNS, ultimately amplifying pain perception. Pharmaceuticals structurally resembling GABA, such as gabapentin and pregabalin, have demonstrated efficacy in pain management by modulating specific central nervous system channels, mitigating neural excitation, and alleviating pain [1,48]. Although they do not directly bind to GABA receptors, they influence GABA-associated pathways, presenting promising options for pain relief [49,50]. In parallel, a burgeoning area of research explores the neuromodulatory potential of microbial-derived GABA, particularly in the context of visceral pain [51]. Certain commensal bacterial species, including GABA-producing Bifidobacterium dentium, have been investigated for their ability to modulate colonic sensory afferent excitability and influence pain perception within the gastrointestinal tract [52]. Further research in this domain holds promise for innovative nutritional interventions and microbiome-based therapeutics targeting abdominal pain and related conditions. Additionally, studies have shed light on the pivotal role of GABAergic neurons, especially parvalbumin (PV) interneurons, in mitigating anxiety induced by chronic inflammatory pain [53]. GABAergic activity within the anterior cingulate cortex (ACC) has been shown to alleviate anxiety-like behaviors associated with chronic inflammatory pain, underscoring the potential of GABAergic signaling as a therapeutic modality for managing both chronic pain and its consequential anxiety [53]. Furthermore, intriguing findings have revealed a complex interplay between thalamic GABA levels and altered cortical brain rhythms in individuals experiencing chronic neuropathic pain [54]. Although conventional expectations of reduced GABAergic activity in chronic pain were not fully confirmed, the correlation observed between thalamic GABA and modified cortical rhythms provides valuable insights into the neural mechanisms contributing to chronic pain perception. In conclusion, the collective research underscores the potential of GABAergic modulation, whether through oral GABA supplementation or microbial-derived GABA, as a promising avenue for pain management. Understanding GABA’s role in pain perception and exploring therapeutic interventions targeting the GABAergic system offer exciting prospects for alleviating a spectrum of pain disorders. Continued research endeavors are imperative to fully unlock the therapeutic potential of GABAergic interventions in the field of pain management.
5. Neuroprotective and Cognition-Enhancing Effects of Dietary GABA
GABA has calming and inhibitory properties, preventing excessive neuronal activity and playing a critical role in the regulation of neural signaling. The potential neuroprotective properties of GABA have garnered significant interest, suggesting its ability to reduce neuronal damage and cell death by counteracting excitotoxicity, a process wherein excessive glutamate activity leads to cellular harm [55,56]. GABA achieves this by promoting inhibitory actions and mitigating overstimulation of neurons, thus potentially preserving neuronal integrity and function, providing a potential avenue for neuroprotection in various neurological conditions, such as traumatic brain injury (TBI) and neurodegenerative diseases [55,56]. However, further research is imperative to fully comprehend and harness the neuroprotective potential of GABA. In the context of TBI, neurotransmitter dynamics significantly contribute to the injury’s pathophysiological cascades [55]. Following TBI and in neurodegenerative diseases, disruptions in glutamate and GABA levels, along with alterations in the expression of specific receptors and transporters, perturb the intricate balance between neuronal excitation and inhibition [55]. GABA critically modulates excitatory pathways in the brain. However, the loss of GABA-producing cells post-TBI and in neurodegenerative diseases, and the remodeling of the glutamate and GABA receptor and transporter systems in neurodegenerative diseases disrupt this equilibrium, resulting in heightened cell injury and apoptotic processes [4,55,57,58]. Excitotoxicity—characterized by excessive glutamate activity—is a pivotal contributor to cellular damage in these neurological disorders [57,58,59] (Figure 2). Furthermore, several studies suggest that targeted interventions focusing on GABAergic pathways and comprehending the nuanced shifts in GABA receptor function hold promise for neuroprotective strategies against TBI and neurodegenerative diseases [32,55,56]. High levels of glutamate can induce damage to the GABA system, including perturbed receptor subunit expression, clustering, and malfunctioning of the GABARs, contributing to the pathogenesis of various neurological disorders, such as autism spectrum disorder, AD, epilepsy, ischemic conditions, and Huntington’s disease [4,60,61,62]. The synaptic pool of GABAARs is finely controlled by the regulation of receptor internalization, recycling, and lateral diffusion. In disease conditions, these mechanisms are disrupted leading to the weakening of synapses and malfunctioning of GABAergic neuronal transmission [61] (Figure 2). Oligodendrocytes also express GABA receptors, which play a crucial role in their survival and differentiation. These cells are exceptionally vulnerable to excitotoxicity triggered by glutamate, leading to their dysfunction and cell death. Glutamate overactivation primarily targets ionotropic glutamate receptors, such as AMPA and kainate receptors, in oligodendrocytes, resulting in sustained calcium influx that disrupts mitochondrial function, triggering the generation of reactive oxygen species (ROS) and initiating apoptotic pathways ultimately leading to oligodendroglia and neuronal death [63] (Figure 2).
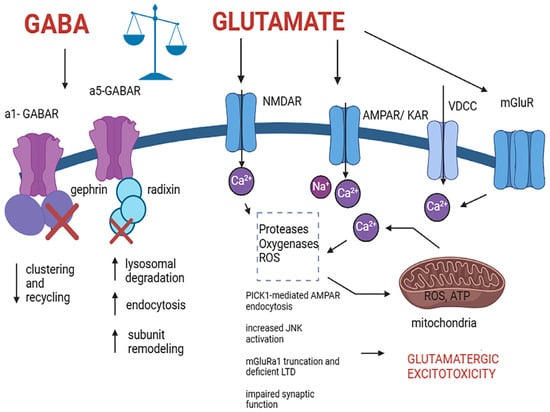
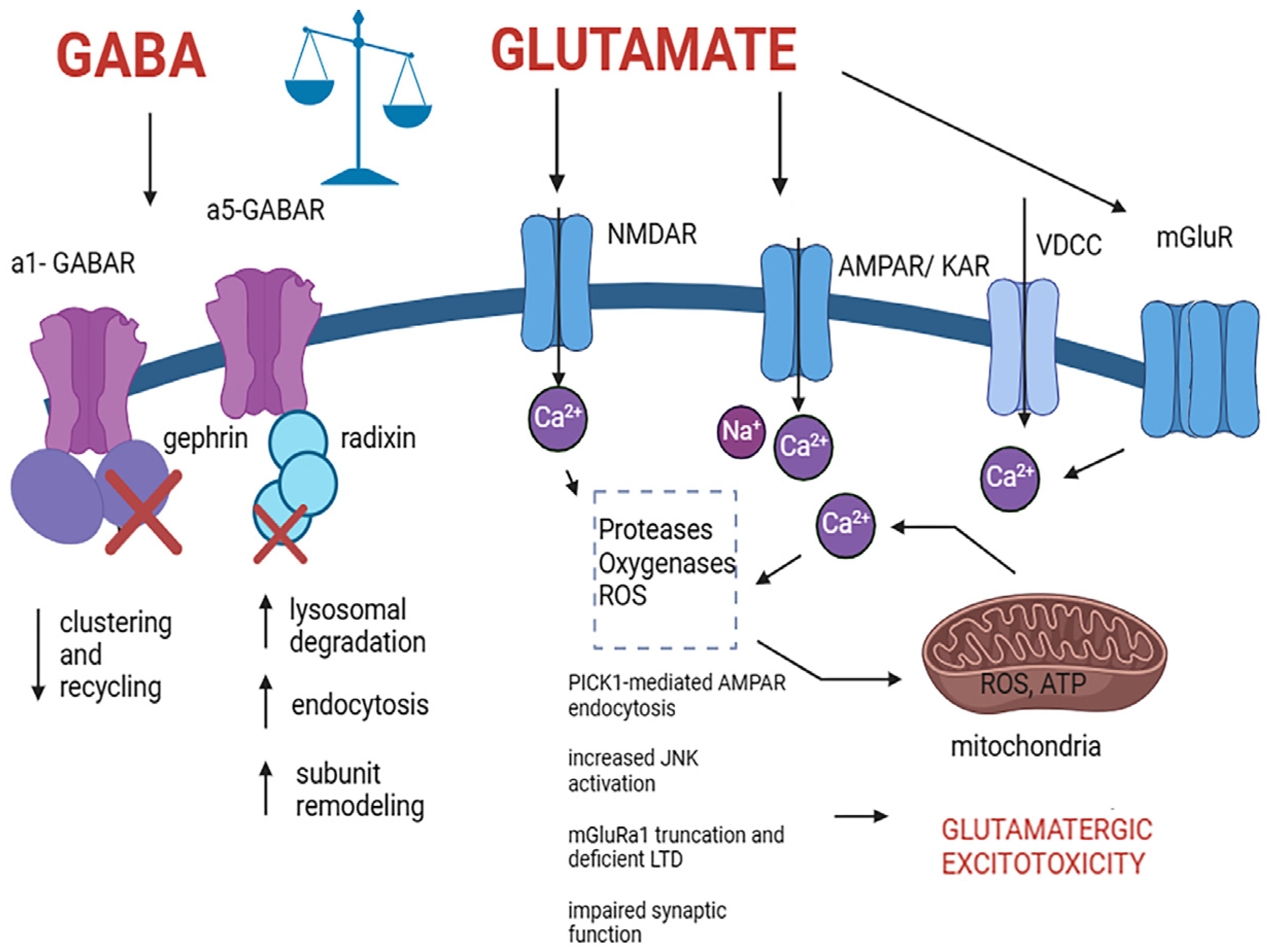
Figure 2.
Pathological alteration in cells expressing GABA and glutamate receptors induced by excitotoxic mechanisms that lead to cell death. The process involves excessive glutamate release and activation of NMDA, AMPA, and kainate receptors, leading to an excessive influx of Ca2+ which induces a variety of pathological mechanisms including PICK1-mediated AMPAR endocytosis, increased JNK activation, mGluRa1 truncation and deficient LTD, impaired synaptic function and subsequently resulting in neuronal death. Elevated cytoplasmic Ca2+ prompts mitochondrial Ca2+ uptake, which potentially leads to ROS production, ATP inhibition, GABA receptor clustering and recycling deficit, remodeling, and malfunctioning, and excitotoxic cell death. Abbreviations: GABA type A alpha 1 subunit containing receptor (a1-GABAR), GABA type A alpha 5 subunit containing receptor (a5-GABAR), N-methyl-D-aspartate receptor (NMDAR), alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR), kainic acid receptor (KAR), voltage-dependent calcium channel (VDCC), metabotropic glutamate receptor (mGluR), protein interacting with C kinase 1 (PICK1), reactive oxygen species (ROS), adenine triphosphate (ATP). Created with BioRender.com (https://www.biorender.com, accessed on 21 March 2024).
A study performed on rats aimed to explore the effects of orally supplemented glutamate and GABA on learning and memory performance, as well as to examine their influence on the levels of these amino acids in the brain. Three groups of rats were subjected to oral supplementation with either drinking water (control group) or a suspension of tablets containing GABA or glutamate for a duration of four weeks. Cognitive performance was assessed through behavioral tests, including the Novel Object Recognition test, Morris Water Maze, and Passive Avoidance test, measuring recognition, spatial reference, and aversive memory, respectively. Additionally, the levels of GABA, glutamate, and acetylcholine (ACh) were quantified in the rat hippocampus. The results indicated that chronic oral administration of GABA or glutamate tablets significantly impacted brain function, leading to alterations in GABA and glutamate content in the rat hippocampus. Specifically, glutamate supplementation was found to enhance memory performance by increasing acetylcholine (ACh) levels, distinguishing its effects from those of GABA [64]. A different study looking at mice with memory impairment induced by scopolamine (Sco) and ethanol (EtOH) examined the effect of fermented Laminaria japonica (FL), a type of sea tangle used in food, which is rich in GABA and is believed to boost cognitive function and potentially help treat common neurodegenerative disorders. The study was conducted on mice using the passive avoidance (PA) and Morris water maze (MWM) tests to assess memory impairment induced by Sco and EtOH. The study also analyzed ACh and acetylcholinesterase (AChE) activity, as well as the expression of muscarinic acetylcholine receptor (mAChR), cAMP response element binding protein (CREB), and extracellular signal-regulated kinases 1/2 (ERK1/2) in the hippocampus. Immunohistochemical analysis was performed, and biochemical blood analysis measured alanine transaminase (ALT), aspartate transaminase (AST), triglyceride (TG), and total cholesterol (TC) levels. The study included seven groups: a control group, three Sco-induced dementia groups, three EtOH-induced dementia groups, a positive control group administered donepezil (Dpz), and an FL (50 mg/kg) treatment group. In support of the impact of dietary GABA on the CNS, FL50 significantly reduced AST and ALT levels induced by EtOH. FL50 treatment improved step-through latency time in the PA test and reduced escape latency times in the MWM test for Sco- and EtOH-induced dementia. FL50 reversed the anticholinergic effects of Sco and EtOH by decreasing AChE activity and increasing ACh concentration. FL50 also increased ERK1/2 protein expression and p-CREB (ser133) in hippocampal tissue. In conclusion, these findings suggest that FL may be an effective intervention for Sco- and EtOH-induced dementia, demonstrating the potential to reverse cognitive impairment and neuroplastic dysfunction [65].
Another study conducted on memory-impaired mice examined the impact of fermented foods high in GABA. The study was initiated by employing a 12% (w/v) concentration of monosodium glutamate (MSG) for the fermentation process to produce GABA using the lactic acid bacteria Lactobacillus sakei B2-16, which was originally isolated from kimchi. The conversion yield of GABA from MSG was determined, and the highest yield was observed with this bacterial strain. For the in vivo experiments, mice were administered Sco to induce memory impairment. The memory recovery effects of GABA were assessed through PA tests. Various concentrations of GABA were tested, and a significant improvement in long-term memory recovery was noted at a concentration of 46.69 mg/mL, reducing the recovery time from 132 to 48 s. Subsequently, a fermentation broth containing 46.69 mg/mL of GABA was used to assess the dose-dependent memory enhancement, with a notable 85% improvement observed at a concentration of 667 mg/mL. In summary, the study demonstrated that GABA obtained from MSG fermentation, particularly with Lactobacillus sakei B2-16, could enhance memory recovery in mice subjected to Sco-induced memory impairment. The findings suggest the potential use of GABA as a natural and functional substance obtained from fermentation processes for various purposes, including cognitive enhancement [66].
A study conducted on mice aimed to investigate the protective effects of a freeze-dried powder derived from a fermentation milk whey containing a high-yield GABA strain (FDH-GABA) against D-galactose-induced brain injury and gut microbiota imbalances in a prematurely aged mouse model. The mice received subcutaneous injections of D-galactose to simulate premature aging. The effects of FDH-GABA were assessed by measuring antioxidant activities, anti-inflammatory markers, autophagy, PI3K/AKT/mTOR signaling pathway alterations, neurotransmitter levels, and changes in intestinal microorganisms. Compared to the control group, FDH-GABA-treated mice exhibited improved antioxidant stress, reduced inflammation, enhanced autophagy, inhibition of the PI3K/AKT/mTOR signaling pathway, restored neurotransmitter levels, and recovery of intestinal flora diversity. Pathological observations confirmed the protective effects of FDH-GABA against damage to the brain and intestine in D-galactose-induced aging mice. These findings suggest that FDH-GABA intervention provides a means to alleviate neurodegenerative changes associated with aging in this mouse model [67].
Evidence of oral GABA delivery on cognitive performance in humans is limited. Administration of an oral dose of 800 mg GABA to participants performing a stop-change task led to an enhanced action selection [68]. Oral administration of the same dose of GABA also enhanced attentional processing. While spatial attention was unaffected by GABA, performance in the temporal attention task was significantly improved [69]. The acute effects of an 800 mg dose of GABA on cognitive flexibility in healthy young adults were also explored. GABA intake decreased cognitive flexibility during task switching, but there was no effect on Stroop task accuracy suggesting a nonlinear or U-shaped relationship between GABA intake and cognitive performance [70]. In a randomized, double-blinded, placebo-controlled, crossover trial the same GABA dose increased visual search time compared to the placebo but did not affect visual search accuracy, temporal attention, or visual working memory precision [71]. Furthermore, the GABA-enriched fermented Laminaria japonica intake provided a protective effect against cognitive impairment associated with dementia [72]. The treatment significantly improved scores in the K-MMSE, numerical memory test, Raven test, and iconic memory, compared to the placebo group. These studies discussed highlighted oral GABA as a potential neuroprotective and cognitive-enhancing pharmacological intervention. However, further research is required to provide convincing evidence. All studies have several limitations, including small sample size, administration of a single dose of GABA, examination of the effect at a single time point, assessment of a specific age group of participants, and a limited number of indicators of cognitive function.
6. Other Effects of Oral GABA
Several articles discussed a variety of oral GABA-induced effects on CNS and their potential to treat disease conditions. For example, multiple studies conducted in both animals and humans examined the effect of oral GABA on blood pressure. Regarding animal models, one study focused on assessing the blood-pressure-lowering effects of GABA and GABA-enriched fermented milk product (FMG) through low-dose oral administration in spontaneously hypertensive (SHR/Izm) and normotensive Wistar–Kyoto (WKY/Izm) rats. The FMG, a non-fat fermented milk product produced by lactic acid bacteria, contained GABA derived from milk protein during fermentation. A single oral dose of GABA or FMG (5 mL/kg; 0.5 mg GABA/kg) resulted in a significant (p > 0.05) reduction in blood pressure in SHR/Izm rats from 4 to 8 h post-administration, without affecting WKY/Izm rats. The hypotensive activity of GABA exhibited dose dependency, ranging from 0.05 to 5.00 mg/kg in SHR/Izm. Chronic administration of experimental diets to SHR/Izm revealed a significantly slower increase in blood pressure compared to the control group at 1 or 2 weeks after initiating the GABA or FMG diet, respectively (p > 0.05), maintaining this difference throughout the feeding period. The time profile of blood pressure changes due to FMG administration mirrored that of GABA. FMG did not inhibit the angiotensin 1-converting enzyme, and a peptide-containing fraction from reverse-phase chromatography in FMG lacked a hypotensive effect in SHR/Izm rats. The findings indicate that low-dose oral GABA exerts a hypotensive effect in SHR/Izm, and the hypotensive action of FMG is attributed to GABA [73].
Another study conducted on rats employed an experimental approach to investigate the potential antihypertensive effects of GABA in spontaneously hypertensive rats (SHR), utilizing acute and chronic administration studies. The GABA-rich tomato cultivar ‘DG03-9’ and purified GABA were administered orally to SHR, and blood pressure measurements were conducted using the tail-cuff method. In the acute administration study, ‘DG03-9’ exhibited a substantial and dose-dependent reduction in systolic blood pressure (SBP) in SHR. Notably, the 10 g/kg dose of ‘DG03-9’ demonstrated a significant antihypertensive effect at 4 h, 6 h, and 8 h post-administration, compared to the control group (p < 0.01). Similarly, the 2 g/kg ‘DG03-9’ dose showed a significant antihypertensive effect at 6 h and 8 h after administration (p < 0.01). These findings were supported by the observation that the maximal decrease in SBP occurred at 8 h after administration, with SBP values of 159.5 ± 1.7 mmHg for the 10 g/kg ‘DG03-9’ group (p < 0.01). To discern the specific contribution of GABA to the antihypertensive effects, a comparative analysis was conducted between ‘DG03-9’ and an equivalent amount of purified GABA administered alone. The results revealed that both the GABA-rich tomato cultivar and purified GABA exerted similar and significant antihypertensive effects at 4 h, 6 h, 8 h, and 24 h after administration (p < 0.05). The similarity in the antihypertensive responses between ‘DG03-9’ and GABA alone underscores the pivotal role of GABA in mediating the observed blood pressure reduction. Furthermore, the chronic administration study investigated the sustained antihypertensive effects of ‘DG03-9’ over 4 weeks. The results indicated that the GABA-rich tomato cultivar, when included in the diet, did not significantly affect body weight, food intake, or water consumption. However, chronic administration of ‘DG03-9’ demonstrated a sustained antihypertensive effect, highlighting its potential as a long-term intervention for hypertension. In conclusion, this study provides compelling evidence that GABA, particularly derived from the GABA-rich tomato cultivar ‘DG03-9’, exerts significant and dose-dependent antihypertensive effects in SHR. The acute and chronic administration studies underscore the potential therapeutic implications of GABA in mitigating hypertension, suggesting avenues for further exploration in the context of dietary interventions and functional foods [74]. The aforementioned studies provide insight into the possible use of oral GABA for blood pressure modulation.
Importantly, the antihypertensive effects of dietary GABA have also been demonstrated in human intervention trials. In a randomized, placebo-controlled, single-blind trial conducted at the Cardiovascular Disease Center, Tokyo Metropolitan Police Hospital in Japan, the study aimed to investigate the potential effects of a newly developed fermented milk product containing GABA, denoted as FMG, on the blood pressure of individuals with mild hypertension. The study enrolled a total of 39 participants diagnosed with mild hypertension, comprising 16 women and 23 men, with an age range of 28–81 years and a mean age of 54.2 years. The intervention involved a 12-week period during which participants were randomly assigned to daily intake of either FMG or a placebo (weeks 1–12), followed by 2 weeks of no intake (weeks 13 and 14). Throughout the trial, peripheral blood pressure and heart rate measurements were systematically collected at weeks 0, 2, 4, 8, 12, and 14. The study findings revealed a significant reduction in blood pressure within 2 to 4 weeks of FMG intake, and this reduction was sustained throughout the 12-week intervention period. Specifically, the FMG group exhibited a mean decrease of 17.4 ± 4.3 mmHg in systolic BP and 7.2 ± 5.7 mmHg in diastolic blood pressure after 12 weeks, with both values differing significantly from baseline levels (p < 0.01). Furthermore, the systolic blood pressure of the FMG group differed significantly from the placebo group (p < 0.05). Other parameters, including heart rate, body weight, and various hematological and blood chemistry variables, showed no significant variations between the FMG and placebo groups throughout the study. The results suggest that FMG may contribute to lowering blood pressure in individuals with mild hypertension [75].
Another study explores the potential blood pressure-lowering effects of GABA, through the consumption of a cheese naturally enriched in GABA. Two GABA-producing strains, Lc. lactis ssp. lactis ULAAC-A23 and ULAAC-H13 were used in the study. Cheese manufacturing involved the inoculation of raw milk with these strains along with Lactococcus lactis ssp. cremoris W62 and standard Cheddar cheese production procedures were followed with modifications for enhanced GABA production. The experimental Cheddar cheeses containing GABA were produced in two batches and compared with placebo cheeses. Composition analyses, including fat, protein, sodium, energy, moisture, and pH, were conducted for both types of cheese. In the cheeses used for the clinical study, the GABA concentration in those containing strain Lc. lactis ssp. lactis ULAAC-H13 was 16 mg of GABA per 50 g compared to 0.12 mg in placebo cheeses. The clinical study involved 23 men with slightly elevated blood pressure. A randomized, placebo-controlled, double-blind, parallel design was employed, where participants were assigned to consume either GABA-enriched cheese or placebo cheese daily for 12 weeks. Blood pressure, heart rate, body weight, and waist circumference were recorded at regular intervals. The cheese characteristics, microbiological changes, proteolysis, and hardness were assessed to ensure the quality and consistency of the GABA-containing cheeses. In the clinical study, GABA-enriched cheese consumption led to a significant decrease in blood pressure within 2 to 4 weeks, with a sustained reduction throughout the 12-week intervention. The mean decrease after 12 weeks was 17.4 ± 4.3 mmHg in systolic blood pressure and 7.2 ± 5.7 mmHg in diastolic blood pressure. No significant changes were observed in heart rate, body weight, or other metabolic parameters. The GABA group showed a statistically significant difference in systolic blood pressure compared to the placebo group. The study demonstrates the successful production of Cheddar cheese enriched with GABA-producing strains, resulting in a significant increase in GABA concentrations. Consumption of this GABA-enriched cheese by individuals with slightly elevated blood pressure led to a notable reduction in blood pressure. The findings suggest that GABA-enriched cheese may be a promising non-pharmacological intervention for managing hypertension [76]. Hypertension is a disease that ranges in gravity and can be complicated by further cardiovascular issues. There might be a potential avenue for oral GABA in combination with other antihypertensive drugs however this needs to be researched to determine effectiveness and safety as well as dosage and possible side effects.
A study explored the potential of dietary GABA in managing depression [77]. The influence of GABA-enriched fermented milk was examined on crucial neurotransmitters 5-hydroxytryptamine, norepinephrine, and dopamine in the mouse hippocampus essential for mood regulation using enzyme-linked immunosorbent assay (ELISA). The results indicated a positive impact, suggesting potential antidepressant effects of GABA-enriched food by enhancing these neurotransmitter levels. This finding highlights that GABA-enriched fermented milk is a promising avenue for mitigating depressive symptoms by targeting key components of mood regulation [77].
A study examined the potential of dietary GABA in obesity prevention [78]. Two hundred male C57BL/6 mice were divided into two study groups. In Study I, mice were categorized into a control group, a diet-induced obesity (DIO) group, and a diet-induced obesity-resistant (DIO-R) group based on body weights after a 20-week high-fat diet (HFD). The method of GABA administration in the study involved incorporating GABA into the drinking water of mice. In Study II, three groups of mice on an HFD received different doses of GABA through their drinking water (0.2%, 0.12%, and 0.06% g/mL). The administration of GABA via drinking water allowed for convenient and controlled delivery of the compound to the mice throughout the 20-week experiment. In Study II, mice were assigned to a control group, an HFD group, and three HFD groups receiving different doses of GABA. Body weights, water intake, fasting blood glucose (FBG), plasma lipid profiles, oxidative stress markers, and thyroid-related parameters were assessed. DIO mice exhibited a significant increase in body weight compared to the control group (p < 0.05). GABA administration in HFD mice led to a dose-dependent reduction in body weight gain. FBG levels were elevated in DIO mice, while GABA-treated groups showed improved blood glucose levels. In terms of plasma lipid status, DIO mice displayed elevated levels of total cholesterol, LDL-C, and triacylglycerol (TG) compared to controls. GABA administration attenuated these lipid abnormalities, with a significant reduction in TC, LDL-C, and TG levels. Oxidative stress assessment revealed that DIO mice displayed increased ROS production and decreased antioxidant enzyme activities. GABA administration led to a dose-dependent reduction in ROS levels and restoration of antioxidant enzyme activities. Additionally, DIO mice exhibited elevated plasma thyroid-stimulating hormone (TSH) levels, suggesting HPT axis dysfunction. GABA administration showed a trend toward normalization of TSH levels, indicating a potential role in mitigating thyroid dysfunction associated with obesity. This study highlights the beneficial effects of GABA administration in mitigating obesity, improving glucose homeostasis, restoring lipid profiles, and modulating oxidative stress and thyroid function in HFD-induced obese mice. Importantly, there is a growing number of studies supporting the anti-obesity effects of GABA in HFD mice [79,80,81]. These findings provide valuable insights into the complex interactions between oral GABA, obesity, and associated metabolic dysregulations [78,79,80,81].
7. The Effect of GABA Produced by the Gut Microbiota
Dysbiosis of the gut microbiota has been linked to various neurological disorders, including anxiety, depression, autism spectrum disorders, and neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease [82,83]. The potential of targeting the gut microbiota through personalized dietary interventions, probiotics, prebiotics, and fecal microbiota transplantation (FMT) was suggested as a promising approach to managing and treating neurological disorders [84,85]. In several studies the biosynthetic potential of specific gut-resident bacteria to produce GABA was investigated, offering potential avenues for modulating gut microbiota to support health. Bacterial strains isolated from the human gastrointestinal tract revealed that certain strains of Lactobacillus and Bifidobacterium exhibited the enzymatic capability to convert monosodium glutamate (MSG) into GABA [82]. Lactobacillus brevis strain DPC6108 demonstrated the highest efficiency in GABA production, achieving a complete conversion of MSG to GABA [82]. These findings shed light on the biosynthetic potential of specific gut-resident bacteria to produce GABA, presenting promising avenues for modulating the gut microbiota to support health and enhance overall well-being [82]. In previous studies, the gut–brain axis and its role in influencing neurological disorders were explored, emphasizing the potential of the gut microbiota as a therapeutic target in neurology [11]. Studies highlighted the intricate bidirectional communication between the gut and the brain, mediated by neural, endocrine, immune, and metabolic pathways, underscoring the potential of the gut microbiota in modulating this axis.
GABA is known to play a pivotal role in regulating mood and emotional responses. A study examined the gut microbiota and its correlation to GABA in patients with major depressive disorder (MDD). Quantitative analysis of the gut microbiota composition revealed a positive correlation between the abundance of Bacteroides and GABA levels. Individuals with higher concentrations of Bacteroides exhibited elevated GABA levels in the fecal samples, suggesting a potential modulatory effect of this gut bacterium on GABA production. To further elucidate the relationship between Bacteroides and GABA, additional in vitro experiments were conducted. Fecal samples with varying Bacteroides concentrations were incubated, and subsequent analyses demonstrated a dose-dependent increase in GABA production. This experiment provided mechanistic insights into the observed correlation, suggesting that Bacteroides might influence GABA levels, and this can occur through direct or indirect pathways in the gut. The neurobiological implications of these findings were underscored by correlating GABA levels with functional magnetic resonance imaging (fMRI) data. Individuals with higher GABA levels exhibited distinct patterns of functional connectivity within the neural circuits associated with depression, including alterations in the left dorsolateral prefrontal cortex (DLPFC) and the default mode network (DMN). In summary, the study not only identified a potential link between Bacteroides abundance and MDD but also shed light on the intricate relationship between gut microbiota, particularly Bacteroides, and GABA. These findings contribute to a deeper understanding of the complex interactions within the gut–brain axis and provide a foundation for future research exploring microbiota-mediated effects on neurotransmitter systems in the context of mental health [37]. Another study looked at the in vitro and in vivo effects of certain GABA-producing bacteria strains. Quinoa yogurt beverages (B-SP1, B-20194, and B-T6B10) were subjected to fermentation using Lactobacillus rhamnosus SP1, Weissella confusa DSM 20194. A two-year double-blind randomized placebo-controlled trial involved 423 pregnant women, receiving either a placebo or daily supplementation of Lactobacillus rhamnosus HN001 from 14–16 weeks of gestation to 6 months postpartum. Modified versions of the Edinburgh Postnatal Depression Scale and State-Trait Anxiety Inventory were employed to measure anxio-depressive states. The trial assessed the impact of Lactobacillus rhamnosus HN001 on depression and anxiety symptoms, maintaining a rigorous methodology. Depression scores were monitored even after controlling for potential confounding factors. The clinical trial demonstrated the significant impact of Lactobacillus rhamnosus HN001 supplementation on depression and anxiety symptoms in postpartum women. The probiotic group exhibited a markedly lower prevalence of depression and anxiety symptoms compared to the placebo group. Importantly, depression scores remained consistently lower, even after adjusting for potential confounding variables, providing robust evidence of the enduring effects of probiotic supplementation. The substantial GABA production observed in quinoa yogurt beverages, coupled with the persistent attenuation of depression and anxiety symptoms in the clinical trial, underscores the therapeutic promise of these probiotics. The findings provide a solid foundation for further targeted studies, emphasizing the need for exploration in individuals with diagnosed mental health disorders [86].
A study examined the impact of two Bifidobacterium adolescentis strains—namely, IPLA60004 (GABA-producing) and LMG10502T (non-GABA-producing)—cultured daily in an MRSc medium. C3H/HeJ mice were divided into vehicle, probiotic (IPLA60004), and control (LMG10502T) groups, subjected to a 14-day oral gavage regimen. Fecal samples, blood, and colonic contents were collected at intervals for analysis. IPLA60004 administration induced a distinctive gut microbiota modulation, marked by increased representation of beneficial genera such as Lactobacillus and Roseburia, contrasting with the effects observed with LMG10502T. Notably, IPLA60004 treatment led to a reduction in serum glutamate levels. Bacterial suspensions, containing approximately 108 CFU per day, were administered in sterilized milk. High-performance liquid chromatography was employed to determine glutamate and GABA concentrations in feces and colonic contents. Additionally, 16S rDNA sequencing revealed specific microbiome changes associated with IPLA60004 administration. Bifidobacterial administration did not affect animal growth, and both strains demonstrated similar recoverable bifidobacterial levels. Metabolite analysis in colonic contents showed no significant differences in GABA and MSG concentrations among treatment groups. However, the probiotic group exhibited lower total short-chain fatty acids, attributed to reduced acetic and butyric acids. Fecal and colonic microbiota profiling revealed dynamic changes over the intervention period, with IPLA60004 inducing higher abundances of Lactobacillus and Roseburia at late stages. Notably, Bifidobacterium representation was elevated in groups receiving IPLA60004 or LMG10502T compared to the vehicle group. Colonic content analysis at the end of the intervention showed no significant differences in alpha diversity but revealed distinctive beta diversity profiles, indicating unique microbiota compositions for control and probiotic groups. These findings highlight the potential modulatory effects of GABA-producing probiotics on gut microbiota and metabolic profiles, providing insights into their therapeutic implications [87]. Further research in this burgeoning field is essential to unravel the intricate mechanisms underlying the gut–brain axis and harness its potential for therapeutic interventions in neurological disorders [11].
8. Conclusions
The increasing interest in dietary GABA as a treatment avenue for neurological disorders stems from its role as a neurotransmitter regulating brain activity and its natural occurrence in certain foods. Recent research indicates therapeutic potential for neurological conditions through the consumption of GABA-rich foods and supplements, with studies delving into their impact on depression, anxiety, blood pressure, sleep, and cognitive disorders. Notably, certain strains of Lactobacillus rhamnosus bacteria, renowned for GABA production, exhibit antidepressant effects in animal and human studies. While promising in animal models, translating these findings to humans necessitates robust clinical trials to validate efficacy and safety across diverse populations. Investigating strain specificity, individual variability, mechanisms of action, optimal dosage, and treatment duration are critical aspects requiring exploration. Additionally, specificity to various neurological disorders, including Alzheimer’s and Parkinson’s diseases, warrants targeted studies to elucidate the broader applicability of GABA-based interventions. Further understanding of the microbiota–gut–brain axis and human metagenomic studies focusing on GABA-related genetic factors will collectively contribute to evidence-based dietary interventions in clinical settings. Collaborative efforts addressing these challenges are pivotal for advancing our comprehension of dietary GABA’s therapeutic role in neurological disorders.
Author Contributions
Conceptualization, S.A., A.S. and A.K.; methodology, S.A., A.S. and A.K.; writing—original draft preparation, S.A., A.S. and A.K.; writing—review and editing, A.S. and A.K.; supervision, A.K.; project administration, A.K.; funding acquisition, S.A. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
Health Research Board Summer Scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Enna, S.J.; McCarson, K.E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 2006, 54, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 11677. [Google Scholar] [CrossRef] [PubMed]
- Govindpani, K.; Calvo-Flores Guzman, B.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABA(A) receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006, 54, 231–263. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Sabir, S.; Sharma, S. GABA Receptor. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lam, P.; Newland, J.; Faull, R.L.M.; Kwakowsky, A. Cation-Chloride Cotransporters KCC2 and NKCC1 as Therapeutic Targets in Neurological and Neuropsychiatric Disorders. Molecules 2023, 28, 1344. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between gut and brain in Alzheimer’s disease: The role of gut microbiota modulation strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Rosenstein, J.M. Permeability of the blood–brain barrier to protein and [3H] GABA in intraparenchymal fetal CNS tissue grafts. Exp. Neurol. 1996, 142, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Shyamaladevi, N.; Jayakumar, A.R.; Sujatha, R.; Paul, V.; Subramanian, E.H. Evidence that nitric oxide production increases γ-amino butyric acid permeability of blood-brain barrier. Brain Res. Bull. 2002, 57, 231–236. [Google Scholar] [CrossRef]
- Al-Sarraf, H. Transport of 14C-γ-aminobutyric acid into brain, cerebrospinal fluid and choroid plexus in neonatal and adult rats. Brain Res. Dev. Brain Res. 2002, 139, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yoto, A.; Murao, S.; Motoki, M.; Yokoyama, Y.; Horie, N.; Takeshima, K.; Masuda, K.; Kim, M.; Yokogoshi, H. Oral intake of γ-aminobutyric acid affects mood and activities of central nervous system during stressed condition induced by mental tasks. Amino Acids 2012, 43, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.H.; Loscher, W. Cetyl GABA: Effect on convulsant thresholds in mice and acute toxicity. Neuropharmacology 1980, 19, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, K.; Sze, P.Y. Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.M.; Poulsen, H.E.; Paulson, O.B. Blood-brain barrier permeability in galactosamine-induced hepatic encephalopathy. No evidence for increased GABA-transport. J. Hepatol. 1988, 6, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bassett, M.L.; Mullen, K.D.; Scholz, B.; Fenstermacher, J.D.; Jones, E.A. Increased brain uptake of gamma-aminobutyric acid in a rabbit model of hepatic encephalopathy. Gastroenterology 1990, 98, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, N.M.; Elliott, K.A. Disposition of gamma-aminobutyric acid administered to mammals. J. Neurochem. 1958, 3, 139–143. [Google Scholar] [CrossRef]
- Oldendorf, W.H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol. 1971, 221, 1629–1639. [Google Scholar] [CrossRef]
- Loscher, W.; Frey, H.H. Transport of GABA at the blood-CSF interface. J. Neurochem. 1982, 38, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the Substantiation of Health Claims Related to Gamma-Aminobutyric Acid and Cognitive Function, No 1924/2006. EFSA J. 2009, 7, 1274. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.; Jelinek, H.F.; Viengkhou, V.; Johnston, G.A.; Matthews, S. Effect of GABA-fortified oolong tea on reducing stress in a university student cohort. Front. Nutr. 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lu, X.; Yang, S.; Zou, Y.; Zeng, F.; Xiong, S.; Cao, Y.; Zhou, W. The anti-inflammatory activity of GABA-enriched Moringa oleifera leaves produced by fermentation with Lactobacillus plantarum LK-1. Front. Nutr. 2023, 10, 1093036. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-Aminobutyric Acid (GABA): Biosynthesis, Role, Commercial Production, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 413–452. [Google Scholar]
- Zhao, S.; Ding, J.; Xiong, S. Development of Gamma-Aminobutyric Acid-Enriched Germinated Rice Products; AACC International Press: St. Paul, MN, USA, 2018; pp. 175–190. [Google Scholar]
- Mills, D.J. The Aging GABAergic System and Its Nutritional Support. J. Nutr. Metab. 2021, 2021, 6655064. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, Q.; Ren, P.; Xiang, W.; Xiao, L. The Synaptic and Circuit Functions of Vitamin D in Neurodevelopment Disorders. Neuropsychiatr. Dis. Treat. 2023, 19, 1515–1530. [Google Scholar] [CrossRef]
- El-Ansary, A. GABA and glutamate imbalance in Autism and their reversal as novel hypothesis for effective treatment strategy. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Tarasenko, A.S.; Krupko, O.O.; Kuchmerovska, T.M.; Lisakovska, O.O.; Trikash, I.O. Vitamin D deficiency induces the excitation/inhibition brain imbalance and the proinflammatory shift. Int. J. Biochem. Cell Biol. 2020, 119, 105665. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Patel, D.K.; Ganguly, K.; Lim, K.-T. Effects of GABA/β-glucan supplements on melatonin and serotonin content extracted from natural resources. PLoS ONE 2021, 16, e0247890. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. Nihon Shokuhin Kagaku Kōgaku Kaishi 2000, 47, 596–603. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Pandharipande, T.; Maru, I.; Kim, M. Effect of oral γ-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci. Biotechnol. 2016, 25, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Yamatsu, A.; Yamashita, Y.; Maru, I.; Yang, J.; Tatsuzaki, J.; Kim, M. The Improvement of Sleep by Oral Intake of GABA and Apocynum venetum Leaf Extract. J. Nutr. Sci. Vitaminol. 2015, 61, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.I.; Shin, Y.Y.; Chung, S.E.; Shin, W.C. Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: A randomized, double-blind trial. J. Clin. Neurol. 2018, 14, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Furukawa, A.; Hara, S.; Mizoguchi, H. Isolation and characterization of sake yeast mutants deficient in γ-aminobutyric acid utilization in sake brewing. J. Biosci. Bioeng. 2004, 97, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Monoi, N.; Matsuno, A.; Nagamori, Y.; Kimura, E.; Nakamura, Y.; Oka, K.; Sano, T.; Midorikawa, T.; Sugafuji, T.; Murakoshi, M.; et al. Japanese sake yeast supplementation improves the quality of sleep: A double-blind randomised controlled clinical trial. J. Sleep Res. 2016, 25, 116–123. [Google Scholar] [CrossRef]
- Elsas, S.M.; Rossi, D.J.; Raber, J.; White, G.; Seeley, C.A.; Gregory, W.L.; Mohr, C.; Pfankuch, T.; Soumyanath, A. Passiflora incarnata L. (Passionflower) extracts elicit GABA currents in hippocampal neurons in vitro, and show anxiogenic and anticonvulsant effects in vivo, varying with extraction method. Phytomedicine 2010, 17, 940–949. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, Y.; Yoon, S.; Kim, S.J.; Yi, S.S. Sleep-inducing effect of Passiflora incarnata L. extract by single and repeated oral administration in rodent animals. Food Sci. Nutr. 2020, 8, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-B.; Park, Y.; Suh, H.J. Sleep-promoting effects of the GABA/5-HTP mixture in vertebrate models. Behav. Brain Res. 2016, 310, 36–41. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ouyang, J.; Hu, Z.; Yang, J.; Chu, Y.; Huang, S.; Yang, Y.; Liu, C. Intervention mechanism of repeated oral GABA administration on anxiety-like behaviors induced by emotional stress in rats. Psychiatry Res. 2019, 271, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takishima, T.; Kometani, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with γ-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60, 106–113. [Google Scholar] [CrossRef] [PubMed]
- McCarson, K.E.; Enna, S.J. GABA pharmacology: The search for analgesics. Neurochem. Res. 2014, 39, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, Ł.; Sałat, K.; Podkowa, A.; Zaręba, P.; Nowaczyk, A. Potential role of selected antiepileptics used in neuropathic pain as human GABA transporter isoform 1 (GAT1) inhibitors—Molecular docking and pharmacodynamic studies. Eur. J. Pharm. Sci. 2017, 96, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Alles, S.R.A.; Cain, S.M.; Snutch, T.P. Pregabalin as a Pain Therapeutic: Beyond Calcium Channels. Front. Cell Neurosci. 2020, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, W.; Lu, Y.G.; Pan, Z.Z. Brain-derived neurotrophic factor-mediated downregulation of brainstem K-Cl cotransporter and cell-type-specific GABA impairment for activation of descending pain facilitation. Mol. Pharmacol. 2013, 84, 511–520. [Google Scholar] [CrossRef]
- Shao, F.-B.; Fang, J.-F.; Wang, S.-S.; Qiu, M.-T.; Xi, D.-N.; Jin, X.-M.; Liu, J.-G.; Shao, X.-M.; Shen, Z.; Liang, Y.; et al. Anxiolytic effect of GABAergic neurons in the anterior cingulate cortex in a rat model of chronic inflammatory pain. Mol. Brain 2021, 14, 139. [Google Scholar] [CrossRef]
- Di Pietro, F.; Macey, P.M.; Rae, C.D.; Alshelh, Z.; Macefield, V.G.; Vickers, E.R.; Henderson, L.A. The relationship between thalamic GABA content and resting cortical rhythm in neuropathic pain. Hum. Brain Mapp. 2018, 39, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores Guzman, B.; Vinnakota, C.; Govindpani, K.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The GABAergic system as a therapeutic target for Alzheimer’s disease. J. Neurochem. 2018, 146, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.; Kwakowsky, A. Metabotropic glutamate receptor 1 alpha: A unique receptor variant with variable implications for Alzheimer’s disease pathogenesis. Neural Regen. Res. 2023, 18, 2196–2197. [Google Scholar] [CrossRef] [PubMed]
- JH, Y.; Waldvogel, H.J.; RL, M.F.; Kwakowsky, A. iGluR expression in the hippocampal formation, entorhinal cortex, and superior temporal gyrus in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2197–2199. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Prabhakar, M.; Kumar, P.; Deshmukh, R.; Sharma, P.L. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013, 698, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Calvo-Flores Guzman, B.; Govindpani, K.; Waldvogel, H.J.; Faull, R.L. Gamma-aminobutyric acid A receptors in Alzheimer’s disease: Highly localized remodeling of a complex and diverse signaling pathway. Neural Regen. Res. 2018, 13, 1362–1363. [Google Scholar] [CrossRef]
- Mele, M.; Costa, R.O.; Duarte, C.B. Alterations in GABA(A)-Receptor Trafficking and Synaptic Dysfunction in Brain Disorders. Front. Cell Neurosci. 2019, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.D.; Gookin, S.E.; Crosby, K.C.; Schwartz, S.L.; Tiemeier, E.; Kennedy, M.J.; Dell’Acqua, M.L.; Herson, P.S.; Quillinan, N.; Smith, K.R. Stepwise disassembly of GABAergic synapses during pathogenic excitotoxicity. Cell Rep. 2021, 37, 110142. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Ochoa-Bueno, B.I.; Ruiz, A.; Ozalla, M.; Matute, C.; Sánchez-Gómez, M.V. GABA Receptor Agonists Protect from Excitotoxic Damage Induced by AMPA in Oligodendrocytes. Front. Pharmacol. 2022, 13, 897056. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, S.; Madiha, S.; Khaliq, S.; Shahzad, S.; Batool, Z.; Haider, S. Impact of oral supplementation of Glutamate and GABA on memory performance and neurochemical profile in hippocampus of rats. Pak. J. Pharm. Sci. 2017, 30, 1013–1021. [Google Scholar] [PubMed]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. GABA-enriched fermented Laminaria japonica improves cognitive impairment and neuroplasticity in scopolamine- and ethanol-induced dementia model mice. Nutr. Res. Pract. 2018, 12, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.C.; Choi, W.Y.; Kim, J.S.; Lee, C.G.; Ahn, J.H.; Cho, H.Y.; Lee, S.H.; Cho, J.S.; Joo, S.J.; Lee, H.Y. Enhancement of the Cognitive Effects of γ-Aminobutyric Acid from Monosodium Glutamate Fermentation by Lactobacillus sakei B2-16. Food Biotechnol. 2012, 26, 29–44. [Google Scholar] [CrossRef]
- He, W.; Song, H.; Yang, Z.; Zhao, S.; Min, J.; Jiang, Y. Beneficial effect of GABA-rich fermented milk whey on nervous system and intestinal microenvironment of aging mice induced by D-galactose. Microbiol. Res. 2024, 278, 127547. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; Stock, A.K.; Beste, C.; Colzato, L.S. gamma-Aminobutyric acid (GABA) administration improves action selection processes: A randomised controlled trial (retracted). Sci. Rep. 2015, 5, 12770. [Google Scholar] [CrossRef] [PubMed]
- Leonte, A.; Colzato, L.S.; Steenbergen, L.; Hommel, B.; Akyurek, E.G. Supplementation of gamma-aminobutyric acid (GABA) affects temporal, but not spatial visual attention. Brain Cogn. 2018, 120, 8–16. [Google Scholar] [CrossRef]
- Lim, L.W.; Aquili, L. GABA Supplementation Negatively Affects Cognitive Flexibility Independent of Tyrosine. J. Clin. Med. 2021, 10, 1807. [Google Scholar] [CrossRef]
- Tinok, A.A.; Karabay, A.; Jong, J.; Balta, G.; Akyurek, E.G. Effects of gamma-aminobutyric acid on working memory and attention: A randomized, double-blinded, placebo-controlled, crossover trial. J. Psychopharmacol. 2023, 37, 554–565. [Google Scholar] [CrossRef]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. The Effects of Fermented Laminaria japonica on Short-Term Working Memory and Physical Fitness in the Elderly. Evid. Based Complement. Altern. Med. 2018, 2018, 8109621. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kimura, M.; Kasaha, K.; Matsumoto, K.; Sansawa, H.; Yamori, Y. Effect of a γ-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar–Kyoto rats. Br. J. Nutr. 2004, 92, 411–417. [Google Scholar] [CrossRef]
- Yoshimura, M.; Toyoshi, T.; Sano, A.; Izumi, T.; Fujii, T.; Konishi, C.; Inai, S.; Matsukura, C.; Fukuda, N.; Ezura, H.; et al. Antihypertensive effect of a gamma-aminobutyric acid rich tomato cultivar ‘DG03-9’ in spontaneously hypertensive rats. J. Agric. Food Chem. 2010, 58, 615–619. [Google Scholar] [CrossRef]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Pouliot-Mathieu, K.; Gardner-Fortier, C.; Lemieux, S.; St-Gelais, D.; Champagne, C.P.; Vuillemard, J.C. Effect of cheese containing gamma-aminobutyric acid-producing lactic acid bacteria on blood pressure in men. PharmaNutrition 2013, 1, 141–148. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef]
- Xie, Z.; Xia, S.; Le, G.-W. Gamma-aminobutyric acid improves oxidative stress and function of the thyroid in high-fat diet fed mice. J. Funct. Foods 2014, 8, 76–86. [Google Scholar] [CrossRef]
- Jin, H.; Han, H.; Song, G.; Oh, H.J.; Lee, B.Y. Anti-Obesity Effects of GABA in C57BL/6J Mice with High-Fat Diet-Induced Obesity and 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2024, 25, 995. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.S.; Dizon, M.P.; Yaw, C.K.; Kaufman, D.L. Oral treatment with gamma-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Hoang, T.H.; Kim, Y.M.; Jang, G.H.; Seok, C.H.; Gwak, Y.G.; Lim, J.; Kim, J.; Chae, H.J. GABA and Fermented Curcuma longa L. Extract Enriched with GABA Ameliorate Obesity through Nox4-IRE1alpha Sulfonation-RIDD-SIRT1 Decay Axis in High-Fat Diet-Induced Obese Mice. Nutrients 2022, 14, 1680. [Google Scholar] [CrossRef]
- Tette, F.-M.; Kwofie, S.K.; Wilson, M.D. Therapeutic Anti-Depressant Potential of Microbial GABA Produced by Lactobacillus rhamnosus Strains for GABAergic Signaling Restoration and Inhibition of Addiction-Induced HPA Axis Hyperactivity. Curr. Issues Mol. Biol. 2022, 44, 1434–1451. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Moos, W.H.; Faller, D.V.; Harpp, D.N.; Kanara, I.; Pernokas, J.; Powers, W.R.; Steliou, K. Microbiota and Neurological Disorders: A Gut Feeling. BioResearch Open Access 2016, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kviatcovsky, D.; Zheng, D.; Elinav, E. Gut microbiome and its potential link to personalized nutrition. Curr. Opin. Physiol. 2021, 22, 100439. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Tamés, H.; Sabater, C.; Royo, F.; Margolles, A.; Falcón, J.M.; Ruas-Madiedo, P.; Ruiz, L. Mouse intestinal microbiome modulation by oral administration of a GABA-producing Bifidobacterium adolescentis strain. Microbiol. Spectr. 2024, 12, e0258023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).