Abstract
Nuts are dry, single-seeded fruits, with a combination of beneficial compounds that aid in disease prevention and treatment. This review aims to summarize the antioxidant components and the nutraceutical properties and applications of hazelnut, almond, and pistachio skins, as well as discuss their ability to prevent and treat specific diseases based on in vitro and in vivo studies. The search strategy included searching PubMed database and Google Scholar for relevant articles published in English. Research articles focusing on hazelnut, pistachio, and almond were included. The nut skin extracts were considered and other by-products were excluded from this search. Pistachio and almond skin hydroalcoholic extracts have antibacterial effects and decrease the risk of liver cancer by eliminating reactive oxygen species. Moreover, hazelnut skin can lower plasma against low-density lipoprotein-cholesterol, thus reducing the risk of colon cancer, and its polyphenolic extract can also decrease the formation of advanced glycation end products in vitro with multidimensional effects. Overall, hazelnut, pistachio, and almond skins are a great source of antioxidants, making them suitable for nutraceuticals’ development.
Keywords:
anti-inflammation; antioxidant activity; chronic disease; diabetes; fruit; oxidative stress; phenols; testa 1. Introduction
Nuts are used to describe dry, single-seeded fruits growing on trees. They are generally composed of leaf, green leafy cover, hull, hard shell, and skin. They are nutrient-dense foods, widely consumed as components of healthy diets worldwide and in both raw and processed forms [1].
There are many types of edible tree nuts such as almonds (Prunus amigdalis), hazelnuts (Corylus avellana), walnuts (Juglans regia), pistachios (Pistachia vera), pine nuts (Pinus pinea), cashews (Anacardium occidentale), pecans (Carya illinoiensis), macadamias (Macadamia integrifolia), and Brazil nuts (Bertholletia excelsa). Peanuts (Arachis hypogea) are botanically groundnuts or legumes, but are identified as part of the nut food group from a consumer’s perspective. Moreover, chestnuts (Castanea sativa) are considered nuts, although they differ from common nuts in terms of nutrient profile, having a higher starch content than others [2].
Tree nuts’ production worldwide varies according to a recent statistic from the International Nut and Dried Fruits Council (INC) showing the evolution of the world’s production of different nuts. For instance, the United States (US) is the leading country for almond production (79%), followed by both Australia and Spain (7%). Moreover, China is the world’s biggest walnut producer (47%) followed by the United States and Chile, at 31% and 7%, respectively. Australia and Western Africa are considered the leading countries for producing macadamias (25%), followed by China (14%) [3].
In Western countries, individuals consume whole nuts (fresh or roasted) as a snack, in desserts, or as part of a meal. Additionally, nuts oils are added as a versatile food ingredient to commercial products including sauces, pastries, ice creams, and baked goods. The consumption of nuts has increased in recent years because they are considered as a good energy source and beneficial to include in the daily diet. Nuts have an optimal nutritional density regarding healthy minerals and contain high amounts of vegetable protein and fat, especially unsaturated fatty acids (UFAs) [4]. Nuts also contain various nutrients and provide dietary fibers, vitamins, minerals, and other bioactive compounds [5].
Nuts’ skins are a good source of antioxidants thanks to the presence of polyphenolic compounds and other phytochemicals that can delay or inhibit lipid oxidation and neutralization of free radicals, contributing to disease prevention and treatments [1]. Antioxidant activity (AO) can be detected by several chemical or biological methods such as ferric reducing antioxidant power (FRAP) assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, and 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulphonic acid (ABTS) [1]. Naturally, antioxidants are nutrient and non-nutrient (phytochemicals) compounds with various types and properties. Nutrient antioxidants possess a weak AO (e.g., vitamin A, vitamin C, and selenium). Regarding non-nutrient antioxidants, they are considered much stronger in their activity, especially phenolic compounds [5].
1.1. Characteristics and Compositions of Pistachio Nut and Skin
Pistachio nut (Pistacia vera) is one of the world’s most popular tree nuts, belonging to the Anacardiaceae family, which comprises about 70 genera and over 600 species. P. vera is considered the only species of the genus commercially cultivated, while the other species are mostly used as rootstocks [6]. The pistachio fruits are drupe and grape-like clusters, containing a single seed covered with a soft and thin seed coat (skin/testa), the edible part (Figure 1a). The seed is covered with an inedible, smooth, hard, and cream-colored shell, which is alternatively covered by a thin and pale green colored hull, forming a blush of red color during maturity (Figure 1b) [7]. Production of pistachios is highly concentrated in the United States, with a percentage of 47% in comparison with other countries such as Turkey (30%) and Iran (19%) according to the INC 2021 statistics [3].

Figure 1.
Pistacia vera L. open hard shell showing the red-purple skin (a); pistachio ripe fruit (hull) (b) [7].
Pistachio skin (testa) is the soft, thin, and edible seed coat that appears in red-purple. It is the main by-product, reaching more than 60%, with Iran producing around 400,000 tons of pistachio skin annually. Furthermore, various processing methods such as roasting and bleaching can cause destruction of bioactive compounds in pistachio skins [8].
Pistachio skin has value; although limited, it can be used in appropriate methods by different industries and fields. Testa is a rich source of phenolic or antioxidant compounds such as flavonoids (e.g., myricetin), gallotannins, and other phenolic compounds. In addition, the skin is used to prepare natural polymers thanks to its cellulose content [8]. Furthermore, it can be used in food and food processing, cosmetics, and pharmaceutical industries [7,8]. Moreover, the presence of various beneficial bioactive compounds in pistachio skin makes them valuable to add to an individual’s daily diet, which can contribute to disease prevention, as many studies have reported [5,7].
1.2. Characteristics and Compositions of Almond Nut and Skin
Prunus dulcis or almonds are a stone fruit under the Rosaceae family, and their trees require sustainable irrigation practices for cultivation and development, specifically in arid and dry regions [9]. As previously stated, the United States is the world’s largest producer of almonds, surpassing 1.3 million metric tons (MT) according to the INC statistical yearbook 2020/21 [3]. Almond nut consists of five main parts including the outer cover or hull with a greenish appearance, the intermediate shell, the edible brown skin coating, and the seed or kernel produced after the full ripening cycle of the fruit (Figure 2) [9]. The almond’s hull is the heaviest part of the kernel with a total fresh weight of around 52%, affected by the variable ripening cycle that changes according to cultivation conditions [10].
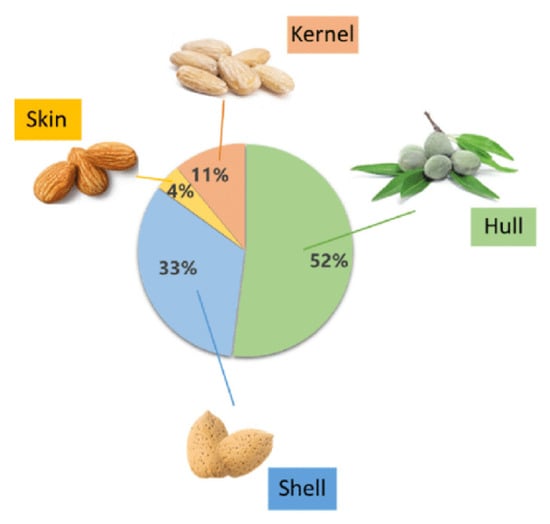
Figure 2.
Weight proportion of the main structural parts of almond fruit [9].
Almond skin serves as a protective layer for the kernel against microbial contamination and oxidation. It appears as a brown leathery coating and represents 4% of the total weight (Figure 2). It contains many beneficial phytochemicals properties, comprising between 70% and 100% of the total phenols in the whole almond fruit [9].
Moreover, compounds have synergistic effects when linked with vitamins C and E, protecting against low-density lipoprotein-cholesterol (LDL-C) oxidation and improving the AO. Furthermore, the skin contains different quantities of triterpenoids, especially betulinic acid, oleanoic acid, and ursolic acid, which have anti-inflammatory, anticancer, and antiviral activities against human immunodeficiency virus (HIV) [9].
Almond skins are used in various products, including as a base component in wheat flour and as coloring for altering biscuits color [11]. A recent study proposed using almond skin extract for patients with intestinal inflammatory diseases [12]. In addition, the skin is a great source of fiber that serves as a prebiotic, affecting the gut microbiome [13].
1.3. Characteristics and Compositions of Hazelnut and Its Skin
Hazelnuts (Corylus avellana L.) belong to the Betulaceae family and are one of the most famous types of nuts worldwide. They are ranked third in the global nut market, as its production reaches over 863,000 MT per year. The largest producer of hazelnuts is Turkey (60%), estimated at 320,000 MT in 2020/21 statistics (Figure 3) [3]. The hazelnut consists of the green shell, the hard shell, leaves, skin, and the edible kernel [14].
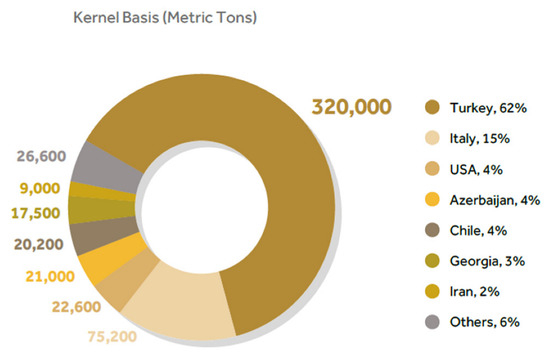
Figure 3.
INC 2020/21 statistics for hazelnut production in kernel bases (metric tons) [3].
Hazelnuts are used in a wide range of applications with increased demands for them in the global markets. They are used mainly as a feedstock in the food industry, especially in the sweet sector such as chocolate bars, ice creams, dairy products, and coffee. Furthermore, it can be added to many other products, from cereals and bread to yogurts and salads [14]. Moreover, hazelnut oil is used in cosmetic, medicinal, massaging, and cooking products. Hazelnut also contains filbert, which is a flavoring compound added in per-fumes [15]. Recently, the use of hazelnuts in other fields has increased, where hazelnut oil is considered a source of biodiesel fuel, as its chemical content is approximately similar to natural oil [16].
Hazelnut skin is a by-product of the roasting process and contains 7.5% moisture, 8% protein, 14.5% fat, and 1.7% ash. Dietary fiber represents the most abundant constituent of hazelnut skin (67.7%), of which 57.7% is insoluble fiber [17]. Moreover, the skin has a good AO compared with other food items [18]. According to Özdemir et al., the total phenolic content expressed as mg gallic acid equivalents per gram (mg GAE/g) is 233 mg GAE/g in skin. The skin of hazelnuts is used as a coloring agent in foods because of its natural brown color [17]. Similarly, it is an ingredient in different processed foods, especially bakery and confectionery products [18]. Skins can be used as an enrichment ingredient to produce functional yogurt [19] and can be added in freshly made pasta, increasing the total fiber content and AO [20]. The current review aims to explore the benefits of hazelnut, almond, and pistachio skins and their ability to prevent and treat diseases.
2. Materials and Methods
2.1. Search Strategy
Studies published from 1 January 2010 through 31 March 2022 were involved in the research based on the most related papers and subjects published within this period. Articles published in English were used by searching the PubMed database and manual search using Google Scholar. The search strategy was based on the following items: “hazelnut”, “almond” AND “pistachio” OR “skin” OR “disease prevention” OR “antioxidant activity”.
2.2. Inclusion and Exclusion Criteria
For all of the relevant abstracts, full publications were retrieved for evaluation based on criteria established a priori. In vitro and in vivo studies were included, while in-silico-based research articles were excluded. Research articles focusing on hazelnut, pistachio, and almond were included; however, the whole nuts and their skin extract were considered and other by-products were excluded.
3. Results and Discussion
Owing to all of the beneficial compounds found in the skins of pistachios, almonds, and hazelnuts, they are proved to have potential roles in the prevention and treatment of diseases by various studies shown in Table 1.

Table 1.
Studies on the effect of pistachio, almond, and hazelnut skin in health treatment.
3.1. Antioxidant Effects of Pistachio and Its Skin
Pistachios contain many phenolic compounds such as flavonoids and its subclasses (flavan-3-ols, flavanones, isoflavones, and flavones), which can reach up to 16–70 mg/100 g depending on the type [7,27]. A study performed by Moreno-Rojas et al. analyzed the antioxidant potential of 11 pistachio cultivars by different methods with significant differences (p < 0.01) in the AO values measured by ABTS (2.51 mmol/100 g) and DPPH (1.97 mmol/100 g) on average in 2020 [28]. Variations seen in AO between cultivars are due to different climatic conditions between years [28].
A study by performed by Nuzzo et al. shows the importance of AO in pistachio, indicating that the regular intake of pistachio can decrease the deleterious effects of a high-fat diet in the brain of obese mice [29]. Phenolic compounds in pistachios can attenuate the reactive oxygen species (ROS) generated in the mitochondria and induced by redox stress in the mouse brain, leading to neuroprotective effects (e.g., decreased brain apoptosis, decreased brain lipid, and improvement in mitochondrial function) (Figure 4) [29].
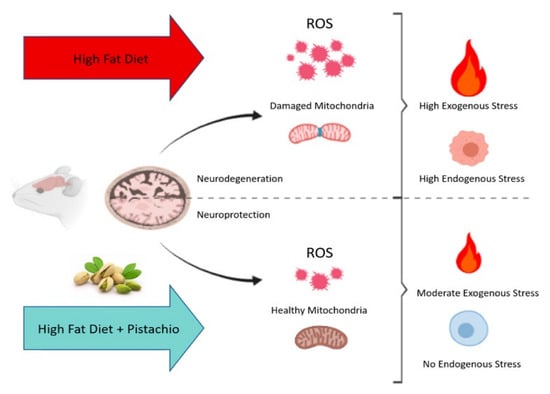
Figure 4.
The neurodegenerated effects of a high-fat diet in comparison with the neuroprotective effects of regular consumption of pistachio [29].
Pistachios can contribute to disease prevention, as many studies have reported. For instance, it has been found in a randomized trial that adults with type 2 diabetes on a pistachio diet had a significant reduction in systolic ambulatory blood pressure by 3.5 ± 2.2 mmHg. These effects may be mediated by their high concentrations of magnesium and low concentrations of sodium [30]. Additionally, pistachios are a rich source of dietary fiber, which promotes satiety and likely favors adherence in weight reduction programs. This is supported by clinical data by Fantino et al., who demonstrated that daily intake of 44 g of pistachios during a 12-week period did not adversely affect body weight control in healthy French women [31]. Furthermore, Sari et al. demonstrated that consuming (30–80 g/day) pistachios can lower the total cholesterol to 10.1% and LDL-C to 8.6% [32]. London et al. also reported improvements in blood glucose levels, in endothelial function, and in some indices of inflammation and oxidative status among healthy young men following increased intake of pistachios [33].
Meanwhile, another study indicated that the consumption of high-carbohydrate foods in addition to pistachios reduces the absorption of carbohydrate as well as postprandial glucose levels [34]. On the other hand, there is epidemiological evidence showing pistachios’ biological mechanisms and potential in reducing the risk of cancer and the effects of anticancer drugs, including that pistachios incorporated into the diet with anticancer drugs will lessen the disruptions in motor and cognitive functions [27]. Moreover, the study of Sadeghpour and Noorbakhsh reported the therapeutic potential of pistachio skin extract and its antibacterial effect on gram-positive bacteria [21].
In particular, positive effects were observed by Harandi et al. using the skin of pistachios’ hydroalcoholic extract and its liposomal form on human liver cancer cells (HepG2) cell line, as their viability decreased [22]. This reduction occurred as phenolic compounds found in pistachio and their high AO can eliminate ROS, which promote cancer cell proliferation. Nonetheless further research and clinical applications need to be conducted to examine the role of pistachio skin in the treatment and prevention of cancer.
3.2. Antioxidant, Antiviral, and Antibacterial Effects of Almond and Its Skin
Almonds contain high amounts of phenolic compounds, but the total content detected by studies varies owing to the cultivars selected, protocol of extraction, and detection method used for analyzing these compounds [9]. For example, Čolić et al. confirmed the presence of 28 polyphenols in almonds grown in Serbia, with catechin predominating up to 46.3% of total phenols in addition to chlorogenic acid, naringenin, rutin, apigenin, and astragalin [35]. Another study identified several other polyphenolic compounds including stilbenes (the least polyphenol found), polydatin in kernels (0.7 ng/g), skins (1.8 ng/g), blanch water (72 ng/g), and oxyresveratrol with piceatannol in blanch water (17 ng/g) [36]. AO was detected by several methods in almonds and their by-products. Various almond cultivars displayed high radical scavenging activity (SA) rates with high DPPH-SA reaching up to 90%. Similarly, the skin showed variations regarding DPPH-SA due to processing, as dehydration has the ability to promote higher activities in dried almond skins (40.4 µmol of Trolox equivalents). Similarly, the skin showed variations regarding DPPH-SA due to processing, as dehydration has the ability to promote higher activities in dried almond skins (40.4 µmol of Trolox equivalents (TE)/g almond skin) in comparison with non-dried skins [11].
Almond nuts and their skins have been used for disease treatment since ancient times, and studies have reported their beneficial effects [9]. A study has recently proposed using almond skin extract for patients with intestinal inflammatory diseases [12]. In addition, the skin is a great source of fiber that serves as a prebiotic, affecting the gut microbiome [13]. Regular consumption of almonds can reduce cardiovascular disease (CVD) risk factors. A randomized controlled trial carried out by Jamshed et al. concluded that eating 10 g/day of almonds before breakfast can reduce serum uric acid in coronary artery disease (CAD) patients, thus protecting against vascular and kidney damage [37]. Concerning the anti-inflammation feature of almonds, a crossover study reported that high monounsaturated fatty acids (MUFAs) and other components present in almonds were responsible for reducing E-selectin and C-reactive protein levels, which mediate inflammation [38]. Almond-derived compounds are also reported to possess other activities including anticancer (e.g., preventing the development of breast cancer), antimicrobial, and antiviral activities with polyphenolic compounds tested in vitro [23,39]. A study tested the antibacterial and antiviral effect of a mix of polyphenols present in natural almond skin (NS MIX) on different clinical strains of Staphylococcus aureus and Herpes simplex virus type I (HSV-1). Upon evaluation, almond skin polyphenolic extracts exhibited antimicrobial activity against S. aureus and antiviral activity observed by a decrease in the viral titer and viral DNA accumulation in the cells of HSV-1. However, further studies are required to establish possible synergistic effects with antibiotics or antivirals [23].
A randomized, double-blind, placebo-controlled study by Fonollá et al. on adults with moderate hypercholesterolemia, aged from 18 to 65 years old, was carried out to evaluate the effect of supplementation with a combination of almond and olive extracts [24]. It concluded that daily consumption of both extracts has a protective ability against LDL-C oxidation and prevents inflammatory status in subjects with moderate hypercholesterolemia [24].
3.3. Antioxidant Effects of Hazelnut and Its Skin
Like all nuts, hazelnuts have high AO, containing 291–875 mg/100 g of polyphenols. The main phenolic compounds present in hazelnut skin are phenolic acids (e.g., hydroxybenzoic acids, gallic acid, protocatechuic acid, salicylic acid, vanillic acid, and many others), flavonoids (e.g., quercetin, quercetin glucuronide, quercetin hexoside isomer, quercetin-3-O-glucoside, and others), hydrolysable tannins (e.g., ellagic acid hexoside isomer, ellagic acid pentoside isomer, flavogallonic acid dilactone isomer, bis (hexahydroxydiphenoyl)-glucose (HHDP-glucose) isomer, and valoneic acid dilactone/sanguisorbic acid dilactone), and other phenolics (e.g., dihydroxycoumarin and procyanidin dimer) [40].
A study performed by Pycia et al. analyzed the antioxidant potential of hazelnut fruit with various cultivars during its progress through maturation [41]. They reported that, as maturation progresses, the antioxidant potential of nuts harvested was lower than the previously harvested batch, reaching an average of 95% for ABTS, 86% for DPPH, and 89% for FRAP [41]. In another study, a total of 31 polyphenolic subclass flavan-3-ols were identified and quantified with an average content of total polyphenols of about 675 mg/100 g. Additionally, 3.5% of flavonols and dihydrochalcones were represented in the different hazelnut samples, with phenolic acids being the least amount of total polyphenolics found in the skin (<1%) [18].
In a randomized controlled trial by Guaraldi et al. that evaluated the effects of short-term consumption of hazelnuts on oxidative stress and DNA damage in children and adolescents with primary hyperlipidemia, an improvement was detected in cell DNA protection and resistance against oxidative stress [42]. The authors concluded that the consumption of hazelnut is associated with reduced levels of DNA strand breaks, formamidopyrimidine DNA glycosylase sensitive site, and hydrogen peroxide-induced DNA strand breaks [42].
Hazelnuts can prevent many chronic diseases such as CAD, strokes, Alzheimer’s (reducing its symptoms), cancer, and atherosclerosis [43]. Generally, a diet containing a high dose of nuts lowers cholesterol. For example, a previous study showed that ingesting three various forms of hazelnuts leads to an equally enhanced lipoprotein profile and α-tocopherol concentrations in mildly hypercholesterolemic individuals, reducing the risk for CVDs [44]. Moreover, as hazelnuts are high in polyphenols, they contribute to high antioxidant potential, antimicrobial activities, and decrease the risk of inflammatory diseases when they act synergistically with phytochemicals present in nuts [41].
A systematic review by Perna et al. showed an analysis of nine studies about hazelnut addition in diets and evaluated its effect on blood lipids and body weight [45]. Subsequently, the analysis indicated that hazelnut intake reduced LDL-C, while the high-density lipoprotein-cholesterol, triglycerides, and body mass index remained unchanged. These findings support the fact that hazelnut consumption may prevent the risk of suffering from a CVD [45].
The important action of hazelnut skin is demonstrated in an in vivo animal study in improving the plasma profile of the lithocholic/deoxycholic bile acid fecal ratio in hamsters fed a high-fat diet, which included hazelnut skin. The results found that hazelnut skin has lipid-lowering blood effects, specifically LDL-C, triglycerides, and non-esterified free fatty acids (FAs), thus reducing the risk factor of colon cancer [25].
Non-enzymatic reactions of sugar with protein side chains in cells can produce advanced glycation end-products (AGEs), some of which are oxidoreductive in nature. AGEs at higher amounts can cause diabetic complications such as renal failure, oxidative stress, and chronic inflammation. Thus, a study was performed to evaluate the antiglycation effects of polyphenol compounds extracted from hazelnut skin, resulting in reduced AGEs’ formation [26].
4. Future Directions
As shown in Table 1 and Figure 5, pistachio skin properties are based on a strong antibacterial effect and decreased variability of cancer cells, as ROS are eliminated by phenolic compounds [38,39]. This is a very interesting study because, in 2018, Seffadinipour et al. demonstrated the potential effects of pistachios on the inhibition of angiogenesis [46]. Although the almond skin did not show high AO, clear effects on antimicrobial and antiviral activity against S. aureus and HSV-1, respectively, have been demonstrated [23]. Hazelnuts provide potential protective effects against LDL oxidation and prevent inflammatory status in subjects with moderate hypercholesterolemia, as concluded by a recent meta-analysis [45]. Moreover, hazelnuts showed an effect on triglycerides and non-esterified free FAs, thus reducing the risk factor of colon cancer [25]. The potential effects of the polyphenolic extract of hazelnut skins can reduce the formation of AGEs in vitro [26]. It is worth mentioning that AGEs contribute to the pathogenesis of several chronic diseases, like cancer and diabetes. For this reason, natural bioactive molecules present in hazelnut skin that are able to inhibit their production could be an interesting new strategy for supporting therapeutic approaches with a positive effect on human health.
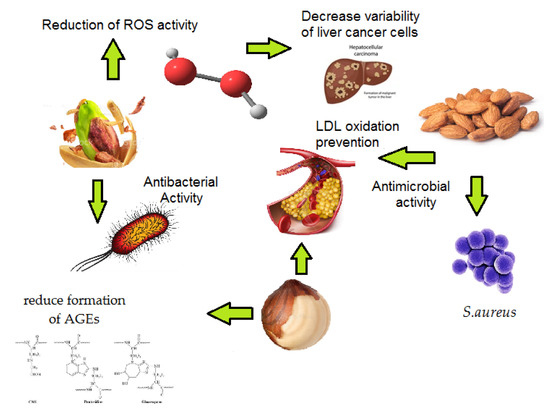
Figure 5.
Mechanisms of action of almond, hazelnut, and pistachio skin on cancer cells, LDL oxidation, and antimicrobial activity.
5. Conclusions
To conclude, nuts are nutrient-dense foods that are widely consumed as components of healthy diets globally, in both raw and roasted forms. These nuts may have the potential to be developed as nutraceuticals or dietary supplements in the foreseeable future, as research has shown nut skins to possess high AOs owing to the high amounts of phenolic compounds present in them. Ultimately, the bioactive compounds in nut skins could be optimized and validated for the prevention and treatment of many pathological conditions, including mental disorders, CVDs, cancer, and diabetes.
Author Contributions
Conceptualization, S.P., C.F. and T.A.A.; methodology, D.M., M.R. and M.H.; validation, S.P., D.M. and C.G.; data curation, M.H., D.M. and M.R.; writing—original draft preparation, S.P., D.M., T.A.A. and M.H.; writing—review and editing, D.M. and T.A.A.; visualization, S.P. and M.H.; supervision, S.P. and D.S.; project administration, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, S.K.; Alasalvar, C.; Bolling, B.W.; Shahidi, F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits—A comprehensive review. J. Funct. Foods 2016, 26, 88–122. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- The International Nut and Dried Fruits Council (INC). Available online: https://www.nutfruit.org/files/tech/1621253983_INC_Statistical_Yearbook_2020-_2021.pdf (accessed on 12 March 2022).
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Artiaga, L.; García-Romo, S.J.; Rosas-Burgos, C.E.; Cinco-Moroyoqui, J.F.; Vidal-Quintanar, L.R.; Carbonell-Barrachina, A.A.; Burgos-Hernández, A. Antioxidant, Antimutagenic and Cytoprotective Properties of Hydrosos Pistachio Nuts. Molecules 2019, 24, 4362. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plant 2021, 11, 18. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Ros, E.; Singh, A.; O’Keefe, J.H. Nuts: Natural Pleiotropic Nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Santos, R.; Saavedra, M.J.; Aires, A.; Pascual-Seva, N.; Barros, A. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly)phenols. Ind. Crops Prod. 2019, 132, 186–196. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT- Food Sci. Technol. 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Lauro, M.R.; Marzocco, S.; Rapa, S.F.; Musumeci, T.; Giannone, V.; Picerno, P.; Aquino, R.P.; Puglisi, G. Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract. Pharmaceutics 2020, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Ra, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Król, K.; Gantner, M. Morphological Traits and Chemical Composition of Hazelnut from Different Geographical Origins: A Review. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Kornsteiner, K.M.; Wagner, K.H.; Elmadfa, I. Phytosterol content and fatty acid pattern of ten different nut types. Int. J. Vitam. Nutr. Res. 2013, 83, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Saydut, A.; Erdogan, S.; Kafadar, A.B.; Kaya, C.; Aydin, F.; Hamamci, C. Process optimization for production of biodiesel from hazelnut oil, sunflower oil, and their hybrid feedstock. Fuel 2016, 183, 512–517. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Yılmaz, C.; Durmaz, G.; Gökmen, V. Hazelnut skin powder: A new brown colored functional ingredient. Food Res. Int. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic Composition of Hazelnut Skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef]
- Dinkçi, N.; Aktaş, M.; Akdeniz, V.; Sirbu, A. The Influence of Hazelnut Skin Addition on Quality Properties and Antioxidant Activity of Functional Yogurt. Foods 2021, 10, 2855. [Google Scholar] [CrossRef]
- Zeppa, G.; Belviso, S.; Bertolino, M.; Cavallero, M.C.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Giorgis, M.; Grosso, A.; Rolle, L.; et al. The effect of hazelnut roasted skin from different cultivars on the quality attributes, polyphenol content and texture of fresh egg pasta. J. Sci. Food Agric. 2014, 95, 1678–1688. [Google Scholar] [CrossRef]
- Sadeghpour, M.; Noorbakhsh, F. The effect of the fresh peel extract pistachio (Pistacia Atlantica) on the growth of staphylococcus aureus, streptococcus pyogenes and bacillus cereus isolated from clinical specimens in vitro. Stud. Med. Sci. 2015, 26, 813–823. [Google Scholar]
- Harandi, H.; Majd, A.; Khanamani Falahati-Pour, S.; Mahmoodi, M. Toxicity Effect of Hydro-Alcoholic Extract of Pistachio Hull and Its Liposomal Form on Liver Cancer Cells (HepG2). J. Rafsanjan UMS 2020, 18, 1035–1048. [Google Scholar]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, T.M.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Fonollá, J.; Maldonado-Lobón, J.A.; Luque, R.; Rodríguez, C.; Bañuelos, Ó.; López-Larramendi, J.L.; Olivares, M.; Blanco-Rojo, R. Effects of a Combination of Extracts from Olive Fruit and Almonds Skin on Oxidative and Inflammation Markers in Hypercholesterolemic Subjects: A Randomized Controlled Trial. J. Med. Food 2021, 22, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; Puiggròs, F.; Suárez, M.; Crescenti, A.; Laos, S.; Ruiz, J.A.; Alonso, V.; Moragas, J.; del Bas, J.M.; Arola, L. The intake of a hazelnut skin extract improves the plasma lipid profile and reduces the lithocholic/deoxycholic bile acid faecal ratio, a risk factor for colon cancer, in hamsters fed a high-fat diet. Food Chem. 2015, 167, 138–144. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef]
- Ghasemynasabparizi, M.; Ahmadi, A.; Mazloomi, S.M. A review on pistachio: Its composition and benefits regarding the prevention or treatment of diseases. J. Occup. Health Epidemiol. 2015, 4, 57–69. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Velasco-Ruiz, I.; Lovera, M.; Ordoñez-Díaz, J.L.; Ortiz-Somovilla, V.; De Santiago, E.; Arquero, O.; Pereira-Caro, G. Valuation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxidants 2022, 11, 609. [Google Scholar] [CrossRef]
- Nuzzo, D.; Galizzi, G.; Amato, A.; Terzo, S.; Picone, P.; Cristaldi, L.; Mul, F.; Di Carlo, M. Regular Intake of Pistachio Mitigates the Deleterious Effects of a High Fat-Diet in the Brain of Obese Mice. Antioxidants 2020, 9, 317. [Google Scholar] [CrossRef]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: A randomized trial. J. Am. Heart Assoc. 2014, 3, e000873. [Google Scholar] [CrossRef]
- Fantino, M.; Bichard, C.; Mistretta, F.; Bellisle, F. Daily consumption of pistachios over 12 weeks improves dietary profile without increasing body weight in healthy women: A randomized controlled intervention. Appetite 2020, 144, 104483. [Google Scholar] [CrossRef]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- London, H.A.; Pawlak, R.; Colby, S.E.; Wall-Bassett, E.; Sira, N. The Impact of Pistachio Consumption on Blood Lipid Profile. Am. J. Lifestyle Med. 2013, 7, 274–277. [Google Scholar] [CrossRef]
- Holligan, S.D.; West, S.G.; Gebauer, S.K.; Kay, C.D.; Kris-Etherton, P.M. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br. J. Nutr. 2014, 112, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.C.; Natić, M.M. Fatty acid and phenolic profiles of almond grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Bolling, B.W. Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC-MS. Food Chem. 2014, 148, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Gilani, A.U.; Sultan, A.T.; Amin, F.; Arslan, J.; Ghani, S.; Masroor, M. Almond supplementation reduces serum uric acid in coronary artery disease patients: A randomized controlled trial. Nutr. J. 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Connell, K.M.; Sabaté, J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: A randomised, controlled, crossover study. Br. J. Nutr. 2010, 10, 907–912. [Google Scholar] [CrossRef]
- Soriano-Hernandez, A.D.; Madrigal-Perez, D.G.; Galvan-Salazar, H.R.; Arreola-Cruz, A.; Briseño-Gomez, L.; Guzmán-Esquivel, J.; Dobrovinskaya, O.; Lara-Esqueda, A.; Rodríguez-Sanchez, I.P.; Baltazar-Rodriguez, L.M.; et al. The protective effect of peanut, walnut, and almond consumption on the development of breast cancer. Gynecol. Obstet. Investig. 2015, 80, 89–92. [Google Scholar] [CrossRef]
- Pelvan, E.; Olgun, E.Ö.; Karadağ, A.; Alasalvar, C. Phenolic profiles and antioxidant activity of Turkish Tombul hazelnut samples (natural, roasted, and roasted hazelnut skin). Food Chem. 2018, 244, 102–108. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G. Changes in Antioxidant Activity, Profile, and Content of Polyphenols and Tocopherols in Common Hazel Seed (Corylus avellana L.) Depending on Variety and Harvest Date. Molecules 2019, 25, 43. [Google Scholar] [CrossRef]
- Guaraldi, F.; Deon, V.; Del Bo’, C.; Vendrame, S.; Porrini, M.; Riso, P.; Guardamagna, O. Effect of short-term hazelnut consumption on DNA damage and oxidized LDL in children and adolescents with primary hyperlipidemia: A randomized controlled trial. J. Nutr. Biochem. 2018, 57, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Guiné, P.F.; Correia, M.R. Hazelnut: A Valuable Resource. ETP Int. J. Food Eng. 2020, 6, 67–72. [Google Scholar] [CrossRef]
- Tey, L.S.; Brown, C.R.; Chisholm, W.A.; Delahunty, C.M.; Gray, R.A.; Williams, M.S. Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur. J. Clin. Nutr. 2011, 65, 117–124. [Google Scholar] [CrossRef]
- Perna, S.; Giacosa, A.; Bonitta, G.; Bologna, C.; Isu, A.; Guido, D.; Rondanelli, M. Effects of hazelnut consumption on blood lipids and body weight: A systematic review and Bayesian meta-analysis. Nutrients 2016, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).