Abstract
Low physical activity of patients is a global problem and associated with loss of strength and independent mobility. This study analyzes the effect of general physical activity promoting interventions on functional and hospital outcomes in patients hospitalized over 48 h. Five electronic databases were searched for randomized controlled trials. For outcomes reported in two studies or more, a meta-analysis was performed to test between-group differences (intervention versus control) using a random-effects model. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to rate the certainty of evidence for each outcome. Out of 23,302 identified studies, we included four studies (in total n = 368 participants). We found with moderate certainty of evidence 0 reported falls in the intervention (n = 126) versus five reported falls in the control (n = 122), a non-statistically significant difference between intervention and control groups (p = 0.06). In addition, we found with (very) low certainty of evidence no statistically significant differences between groups on activities of daily living (ADL-activity) and time spent standing and walking. Overall, we found no conclusive evidence on the effect of general physical activity promoting interventions on functional outcomes. More research is needed to understand and improve the effect of general physical activity promoting interventions for patients during the hospital stay.
1. Introduction
Sedentary behavior during the hospital stay is common in patients admitted to the hospital [1]. Several studies estimate that hospitalized patients who can walk spend approximately 70–82% of their time during daytime lying in bed [1,2,3]. Excessive bed rest can lead to functional decline and deconditioning [4,5], which may result in complications, increased hospital readmissions and health problems that are not directly related to the primary cause for hospitalization [6,7,8]. This phenomenon is better known as hospitalization-associated disability: an avoidable and unnecessary increasing functional decline in patients that occurs during care [9].
On the positive side, increasing physical activity in patients during their hospital stay shows beneficial effects on their health status [10]. Even small amounts of physical activity are known to reduce the risk of disease and disability in public health [11,12,13]. Increasing inpatient physical activity might, therefore, counteract the negative consequences of hospitalization-associated disability. Higher levels of physical activity are related to better functional outcomes [14], reduced length of hospital stay [15,16], and diminished readmission [17]. It is our hypothesis that the negative consequences of sedentary behavior reduce when inpatient physical activity levels increase with general physical activity promoting interventions. Therefore, the current study aims to estimate the effect of general physical activity promoting interventions on functional and hospital outcomes in patients hospitalized over 48 h.
2. Materials and Methods
This systematic review and meta-analysis of randomized controlled trials was conducted following the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [18], and the Cochrane handbook [19]. This review was a priori registered in the International Prospective Register of Systematic Reviews (PROSPERO) database: CRD42017059178. We used the definition of physical activity as provided by Caspersen et al.: ‘physical activity is any bodily movement produced by skeletal muscles that requires energy expenditure’ [20]. We defined general physical activity promoting interventions as non-disease-specific interventions aiming to promote the physical activity of patients during their hospital stay, which could be administered to patients with different medical indications without the need for supervision of specialized staff (unsupervised). General physical activity promoting interventions should be applicable to a broad range of patients; interventions used in specific patient populations were considered specific interventions. In addition, general physical activity promoting interventions should not consist solely of supervised exercises, as these interventions were considered (physical) exercise therapy rather than general physical activity promoting interventions [21].
We searched five electronic databases for published studies up to January 2020: MEDLINE, Cochrane Central Register of Controlled Trials, EMBASE, CINAHL, and PEDro. The search comprised studies that included patients, hospitalized over 48 h. No selection was made based on the reason for hospital admission (condition), age or other patient characteristics. Types of interventions that were considered relevant were: physical activity promoting interventions, early ambulation, mobility programs, exercise, fitness, locomotion, stepping, and self-care. Ineligible interventions were supervised training programs, high-intensity interval training, and disease-specific exercise programs, because these interventions were not considered as generic but as specific physical activity promoting interventions. No selection was made on the minimum duration or intensity of physical activity stimulation. Outcomes of interest in the search were: activities of daily living, muscle strength, quality of life, functional recovery, functional impairment, functional decline, disability, and inability (functional outcomes); length of hospital stay, patient readmission, and patient discharge (hospital outcomes). The search was limited to studies published in the English language. The complete search strategies were constructed with the support of an experienced librarian (OYC), see Supplementary Table S1.
We included randomized controlled trials that compared usual hospital care with usual care and the addition of general physical activity promoting interventions in hospitalized patients for at least 48 h. Studies were eligible if the intervention was (1) studied according to a randomized controlled trial design; (2) non-disease-specific; (3) not individually tailored; (4) conducted in a hospital; (5) without specialized staff supervising the intervention (unsupervised); and (6) was evaluated on functional or hospital outcomes. Studies were excluded if the intervention was conducted as part of a (home-based) exercise program.
Titles and abstracts of retrieved studies were independently screened by two reviewers (JS and NK screened studies published until May 2018, and EK and RB screened studies published between June 2018 and January 2020). Studies that provided insufficient information in the abstract regarding the eligibility criteria were retrieved for full-text evaluation (NK). Two reviewers independently evaluated full-text studies and determined their eligibility for inclusion in our review. Disagreements were resolved by consensus and, if disagreement persisted, by another review author (TH).
Functional outcomes were used to assess the effect of physical activity promotion on (instrumental) activities of daily living. The studies included information on: reported falls, activities of daily living, time spent in different levels of physical activity (time spent lying, sitting, standing, cycling, or walking), community mobility, hospital mobility, and revolutions cycled [22]. In this review and meta-analysis, community mobility was defined as ‘the ability of a person to purposefully move out of the room in which the person sleeps to another area in a specific time period’ [23].
Two reviewers (JS and NK) used standardized forms to independently extract the following data from each eligible study: study characteristics such as authors, year of publication, setting, type of intervention, and follow-up duration; study population characteristics such as age and gender; and study outcomes. Details on the type of intervention and usual care were extracted, and, if available, information about the frequency, intensity, and time. Details on the intervention were extracted and reported using the Template for Intervention Description and Replication (TIDieR) checklist, see Supplementary Tables S2–S5 [24].
Two reviewers (JS and NK) independently assessed the risk of bias in each included study using the Cochrane ‘Risk of bias-2’ tool [25,26]. Tables with the completed Risk of Bias-2 assessment are provided in Supplementary Tables S6–S9. Any disagreement was resolved by consensus between the two reviewers, and in cases where no consensus was achieved, another review author (TH) acted as an arbitrator. We assessed the risk of bias for the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, selection of reported results. Assessors rated the risk of bias low, unclear, or high for all domains and overall [27].
In this study, we compared general physical activity promoting interventions with usual care. A meta-analysis was conducted for outcomes reported in two studies or more using a random-effects model, with a p-value of <0.05 considered as statistically significant. We calculated standardized outcomes (e.g., percentage time per day) if two studies reported outcomes of the same construct that were expressed in different units of measurement (e.g., minutes per day and percentage per day) or with different measurement instruments (e.g., performance tests and questionnaires). Standardized mean differences and weighted mean differences were calculated as part to compare outcomes in the meta-analysis. The statistical heterogeneity of the treatment effect among studies was assessed using the inconsistency I2 test, in which values greater than 50% were considered indicative of high heterogeneity [19]. Fisher’s exact test was used to calculate the relative effect of physical activity promoting interventions on reported falls. Analyses were performed using Stata software, version 15.0 (Stata Inc., College Station, TX, USA).
To assess the certainty of evidence, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to rate the certainty of evidence for each outcome [28,29]. The GRADE-methodology is constructed upon five different items (study limitations; inconsistency of results; indirectness of evidence; imprecision of outcome estimates, and publication bias), which were independently assessed by two reviewers (JS and NK). Possible outcomes for each measure ranged from ‘very low certainty of evidence’ (we are very uncertain about the estimate), to ‘high certainty of evidence’ (further research is unlikely to change our confidence in the estimate of the effect) [28]. Any discrepancy in judgment was solved by consensus between the two reviewers, and in cases where no consensus was achieved, another review author (TH) acted as an arbitrator. Using the GRADEpro Guideline Development Tool (GRADEpro Guideline Development Tool (software), McMaster University, 2015, developed by Evidence Prime Inc., Hamilton, ON, Canada), a ‘summary of findings table’ was generated for all outcomes.
3. Results
Figure 1 shows a flow chart of the included studies.
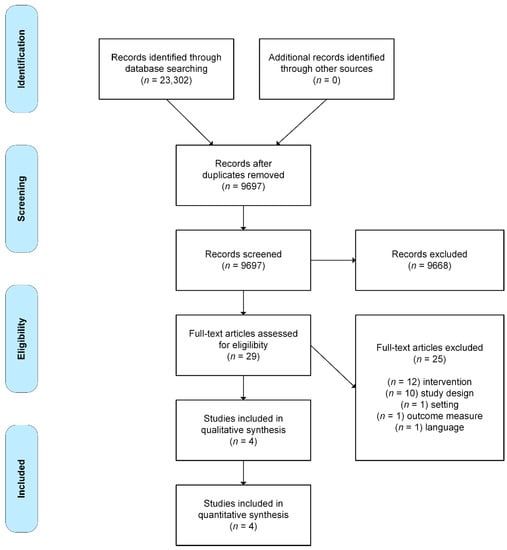
Figure 1.
Flow chart of the study selection process, adapted from Moher et al. [18].
Study characteristics of the four included trials are presented in Table 1. Details on the interventions are provided in Supplementary Tables S2–S5.

Table 1.
Study characteristics, population, intervention versus control, and outcomes.
Brown et al. [30] included 100 patients (n = 8 loss to follow-up), aged 65 years or older who had a medical reason for admission. The mean age of the study population was 74 years (standard deviation (SD): 7 years) and included three females (3%). Common diagnoses included pneumonia, heart failure, and chronic obstructive pulmonary disease exacerbations. A standardized ambulation protocol was used for the intervention group, in which patients were assisted with ambulation up to twice daily. Besides, a behavioral strategy was used to encourage ambulation of patients by focusing on goal setting and addressing mobility barriers. Patients reported falls with a daily 24 h diary during the hospital stay. Activities of daily living were examined with a questionnaire on independent activities of daily living performance (ADL-activity) from 7 (independent) to 21 (total assistance). The community mobility was assessed with the Life-Space Assessment (0–120, higher scores representing greater mobility). Data on the time spent out of bed were not collected as a result of a technical failure of the accelerometry (brand: not reported).
Dall et al. [31] included 141 patients with a pulmonary diagnosis (n = 48 loss to follow-up). The mean age of the study participants was 73 years (SD: 13 years). The intervention consisted of visual feedback about daily time spent lying in bed, sitting, standing, and walking with a tablet. Methods for data collection on reported falls were not provided. Data on the time spent lying in bed, sitting, standing, and walking were collected in minutes per day with a tri-axial accelerometer (brand: not reported) supported with medical Band-aids.
Killey and Watt [32] included 77 patients with a minimum age of 70 years with provisional diagnoses including heart-, lung-, and diabetes-related morbidities (n = 29 loss to follow-up). The mean age of the study population was 83 years (SD: 7 years). The intervention was extra walking twice-daily, patients were instructed to walk the maximum distance they were able to comfortably cover. Methods for data collection on reported falls were not provided. ADL-activity performance was measured with the Barthel Index (range: 0–100, 100 as highest possible independence). Data on hospital mobility were collected as maximum distance walked in meters.
McGowan et al. [33] included 50 patients over the age of 65 years with an acute medical diagnosis (n = 2 loss to follow-up). The study participants had a mean age of 85 years (SD: 7 years). The pedal exercises intervention consisted of ‘5 min of chair-based pedal exercises three times a day with no specified targets on number of revolutions’ on an Able 2-pedal exerciser. ADL-activity was scored with the Elderly Mobility Scale, a 20-point ordinal scale from 0 ‘full dependent in mobility’ to 20 ‘independent’. Data on the number of revolutions were collected with a build-in pedometer and data on the time spent on the pedal exerciser with an accelerometer (ActivPal®, developed by PAL Technologies, Glasgow, United Kingdom).
Risk of bias-2 scores are presented in Table 2.

Table 2.
Risk of bias-2 scores of the included studies.
The overall risk of bias score was ‘high’ for three studies [31,32,33]; one study scored ‘some concerns’ [30]. The randomization process was judged to be of high risk of bias in two studies as a result of no random allocation sequence and no concealed allocation [31,32]. None of the studies provided information on deviations from intended interventions, resulting in some concerns in all included studies [30,31,32,33]. Two studies were judged to be of high risk of bias due to missing outcome data, because both studies had a high loss to follow-up and did not perform an intention-to-treat analysis or missing data analysis [32]. The study of Dall et al. [31] showed a high risk of bias regarding measurement of outcomes, as they provided no details on the methods for reported falls and no information on the psychometric quality of the accelerometry used. Selection of reported results raised some concerns in three studies, as these studies provided no study protocol [32], data on within-group differences [30], and data on between-group differences [33].
Table 3 shows a summary of findings for each outcome.

Table 3.
Summary of findings according to Grading of Recommendations Assessment, Development, and Evaluation (GRADE) certainty of evidence.
The reported falls were assessed in three studies [30,31,32]. Patients in the control groups reported 5 falls per 126 patients in contrast to 0 falls per 122 patients with the general physical activity promoting interventions, showing no statistically significant difference (p: 0.06). The certainty of evidence for reported falls was ‘moderate’ as a result of the indirectness of outcomes (reported falls are a surrogate outcome for actual falls incidents). The ADL-activity performance was examined in three studies, comprising data of 203 participants [30,32,33]. The standardized mean difference ADL-activity performance was −0.07 (95% CI: −0.64 to 0.51), showing no statistically significant difference between groups (Figure 2). The certainty of evidence for ADL-activity was ‘low’ due to study limitations (attrition of participants) and imprecision (large confidence intervals around the estimated mean).Two studies analyzed the time spent standing and walking during the hospital stay, comprising data of 141 participants [31,33]. The weighted mean difference time spent standing and walking was 2.0% per day (95% CI: −2.8% to 6.9%) showing no statistically significant difference between groups (Figure 3). The certainty of evidence for time spent standing and walking was ‘very low’ as a result of study limitations (unknown psychometric quality of accelerometry), imprecision (high standard deviations from the estimated mean), and inconsistent outcomes (results in two studies showed opposite standardized mean differences). None of the included studies reported hospital outcomes.
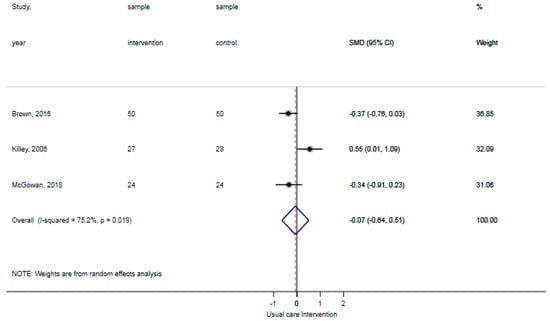
Figure 2.
Forst plot activities of daily living (ADL)-activity performance showing no statistically significant difference between usual care and intervention.
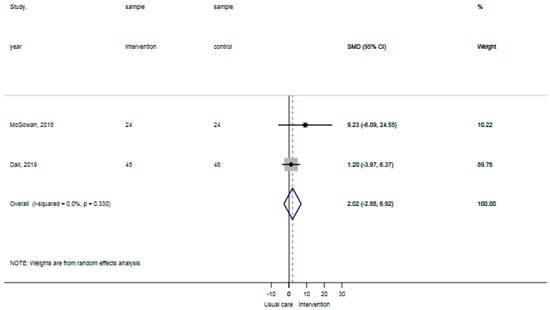
Figure 3.
Forst plot time spent standing and walking showing no statistically significant difference between usual care and intervention.
4. Discussion
In this systematic review and meta-analysis, we specifically studied the outcomes of general physical activity promoting interventions in patients hospitalized over 48 h. After an extensive literature search, we identified just four studies that met our eligibility criteria. We found no statistically significant effect (moderate certainty of evidence) of general physical activity promoting interventions on reported falls during the hospital stay. Besides, we found no statistically significant effect (very low certainty of evidence) of interventions on physical activity in patients enrolled in general physical activity promoting interventions compared to usual care. Finally, we found no statistically significant effect (low certainty of evidence) for physical activity promoting interventions on ADL-activity in patients during their hospital stay.
To our knowledge, this is the first systematic review of randomized controlled trials to study the effectiveness of interventions aiming to promote physical activity in the entire hospital population without additional supervision of specialized staff. We specifically looked at activity promoting interventions that could be employed in the whole hospital for all patients without additional specialized supervision, as the workload of hospital personnel is already perceived as high [34]. The most similar systematic review studied the effects of supervised activity interventions in older hospitalized patients [35]. They concluded, without a GRADE analysis, that evidence for the effect of physical interventions on physical performance in older patients during hospitalization was uncertain, which is in line with our findings. The use of general physical activity promoting interventions might be promising looking at the direction of reported falls; however, there is little evidence available. Hypothetically, this could mean that people admitted to the hospital do not necessarily need supervised general interventions; they have more to gain from interventions that focus on the context in which physical activity care is provided [36].
In our review and meta-analysis, we aimed to include hospitalized patients of all ages; however, the subjects included in our review are all of older age. This shift towards older patients is likely caused by the fact that we did not include disease-specific interventions, in which more patients of varying age are present. We hypothesize that this might have overestimated the findings of our review to some extent. After all, hospitalized older patients have an increased risk for developing hospital-associated disability [37], and ADL dependence compared to younger patients [38]. On the one hand, it is thought that promoting physical activity in this population with older patients has the potential to elicit greater effects, as the consequences of physical activity are more pronounced. On the other hand, the physical inactivity epidemic targets all patients in the hospital and does not discriminate for age [1]. Although frail older patients are more prone to the negative consequences of inactivity during hospitalization than the relatively younger patients, there is evidence suggesting that almost half of the relatively younger patients were significantly affected by sedentary behavior during hospitalization [14]. A recent systematic review and meta-analysis by Fazio et al. [39] confirms that a broad spectrum of inpatient populations is physically inactive with a point estimate of 70 min walking a day (interquartile range: 58–83 min). Physical activity levels of patients during their hospital stay are low and do not seem to be associated with their level of illness [2,40,41]; however, it seems reasonable that the level of illness is somehow related to patients’ ability and willingness to be physically active [42,43]. In other words, interventions targeting the entire hospital population might have a greater impact as a whole.
There are several explanations for the limited effectiveness of the interventions in terms of reported falls, ADL-activity, and time spent standing and walking. First, we included few studies that might compromise the validity of meta-analysis [44]. Second, interventions were not supervised, which means that activities and outcomes might be under- or overestimated. Third, the practice of any physical activity requires a minimum of time and exposure to take advantage of it [11]. It is not known how often the patients in the included studies exceeded a minimum of time. A fourth explanation is that the interventions might have been too simplistic. All included interventions have mono-faceted treatment approaches [30,32], while the underlying mechanism that triggers physically inactive behavior in the hospital is multi-faceted. Previous research demonstrates that physical inactivity is more than just patient-related characteristics such as functional status, pain, and shortness of breath [9,45]. Inactivity can be triggered by the built environment of the hospital (e.g., the inactivating hospital bed centric approach to care, the lack of privacy and shelter in a hospital room), the (lack of) materials to mobilize patients (e.g., being connected to drains or catheters, lack of chairs to sit, lack of rollers to walk), and the mindset of both patients (e.g., beliefs that to be in the hospital is to be in bed or that patients are not welcome outside of their rooms) and healthcare professionals (e.g., dedication to engage patients in physical activity or thoughts that patients are better off lying in bed) [46,47,48]. Given this plethora of related variables, one could expect a multi-faceted approach to tackle physical inactivity in the hospital setting. Future researchers might need to view physical activity promoting interventions in the hospital as complex interventions, which need to be developed and evaluated accordingly [49,50].
Our systematic review and meta-analysis have an important limitation that needs to be addressed, namely, that only four studies were eligible for inclusion. One of the reasons was the inclusion of randomized controlled trials only. We have excluded 10 full-text studies based on study design, because these studies did not have a randomized controlled trial design to study intervention effect estimates. However, the excluded studies may contain useful information and important outcomes related to general physical activity promoting interventions. Nevertheless, to our knowledge, even if we had broadened our inclusion criteria in terms of the study design with, for example, before–after studies, no additional studies in hospitalized patients have been identified.
Even though the effects of physical activity promoting interventions remain largely uncertain, healthcare professionals should still take the detrimental consequences of physical inactivity during the hospital stay seriously [7,8,9,51]. The moderate certainty of evidence that these general, unsupervised interventions reduce the number of falls, should promote healthcare professionals to explore the possibilities of promoting physical activity. It is important that healthcare professionals appreciated the complex nature of physical inactivity in the hospital and understand that this behavior is ingrained in both patients’ expectations as well as the build hospital environment [46,52]. Researchers might consider the use of cluster randomized controlled trial designs or pragmatic, quasi-interrupted time series to further study the effect of general physical activity promoting interventions in the complex hospital context [53].
5. Conclusions
In conclusion, we found no statistically significant effect of general physical activity promoting interventions on functional outcomes. The meta-analysis showed no statistically significant difference of reported falls between participants in the intervention and control groups with a moderate certainty of evidence. No statistically significant difference between intervention and control groups was found for ADL-activity and time spent standing and walking with a (very) low certainty of evidence. Although general physical activity promoting interventions might have positive effects on functional outcomes in patients hospitalized over 48 h according to observational studies, currently, there is a lack of well-designed experimental studies to make recommendations with a high degree of certainty of evidence.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/3/1233/s1. Table S1: Complete search strategy for MEDLINE, searched from inception till January 2020 Table S2: TiDieR checklist Brown et al. Table S3: TiDieR checklist Dall et al. Table S4: TiDieR checklist Killey and Watt. Table S5: TiDieR checklist McGowan et al. Table S6–S9: Completed Risk of Bias-2 templates.
Author Contributions
Conceptualization: J.P.H.S., N.K., J.B.S., T.J.H. Data curation: J.P.H.S., N.K. Data analysis: J.P.H.S., N.K., J.B.S., T.J.H. Methodology: J.P.H.S., N.K., J.B.S., T.J.H. Project administration: N.K. Supervision: J.B.S., T.J.H. Writing—original draft: J.P.H.S., N.K., T.J.H. Writing—review and editing J.P.H.S., N.K., J.B.S., T.J.H. Guarantor: T.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any funding from the public, commercial or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, N.K., upon reasonable request.
Acknowledgments
We thank On-Ying Chan (O.-Y.C.), information specialist at the Radboud university medical center, for her support with the search strategy. Emily Klooster (E.K.) and Rafael Brouwer (R.B.) are thanked for their help in the update of the search (June 2018–January 2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mudge, A.M.; McRae, P.; McHugh, K.; Griffin, L.; Hitchen, A.; Walker, J.; Cruickshank, M.; Morris, N.R.; Kuys, S. Poor mobility in hospitalized adults of all ages. J. Hosp. Med. 2016, 11, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.M.; Bodilsen, A.C.; Petersen, J.; Beyer, N.; Andersen, O.; Lawson-Smith, L.; Kehlet, H.; Bandholm, T. Twenty-four-hour mobility during acute hospitalization in older medical patients. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Redden, D.T.; Flood, K.L.; Allman, R.M. The underrecognized epidemic of low mobility during hospitalization of older adults. J. Am. Geriatr. Soc. 2009, 57, 1660–1665. [Google Scholar] [CrossRef]
- Peel, N.M.; Paul, S.K.; Cameron, I.D.; Crotty, M.; Kurrle, S.E.; Gray, L.C. Promoting Activity in Geriatric Rehabilitation: A Randomized Controlled Trial of Accelerometry. PLoS ONE 2016, 11, e0160906. [Google Scholar] [CrossRef] [PubMed]
- Surkan, M.J.; Gibson, W. Interventions to Mobilize Elderly Patients and Reduce Length of Hospital Stay. Can. J. Cardiol. 2018, 34, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Falvey, J.R.; Mangione, K.K.; Stevens-Lapsley, J.E. Rethinking Hospital-Associated Deconditioning: Proposed Paradigm Shift. Phys. Ther. 2015, 95, 1307–1315. [Google Scholar] [CrossRef]
- Allen, C.; Glasziou, P.; Del Mar, C. Bed rest: A potentially harmful treatment needing more careful evaluation. Lancet 1999, 354, 1229–1233. [Google Scholar] [CrossRef]
- Krumholz, H.M. Post-Hospital Syndrome—An Acquired, Transient Condition of Generalized Risk. N. Engl. J. Med. 2013, 368, 100–102. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Thompson, P.D.; Eijsvogels, T.M.H. New Physical Activity Guidelines: A Call to Activity for Clinicians and Patients. JAMA 2018, 320, 1983–1984. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.A.; Benatar, J.; Maddison, R. Living longer by sitting less and moving more. Curr. Opin. Cardiol. 2015, 30, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Zisberg, A.; Shadmi, E.; Sinoff, G.; Gur-Yaish, N.; Srulovici, E.; Admi, H. Low mobility during hospitalization and functional decline in older adults. J. Am. Geriatr. Soc. 2011, 59, 266–273. [Google Scholar] [CrossRef]
- Agmon, M.; Zisberg, A.; Gil, E.; Rand, D.; Gur-Yaish, N.; Azriel, M. Association between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern. Med. 2017, 177, 272–274. [Google Scholar] [CrossRef]
- McCullagh, R.; Dillon, C.; Dahly, D.; Horgan, N.F.; Timmons, S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol. Meas. 2016, 37, 1872–1884. [Google Scholar] [CrossRef]
- Fisher, S.R.; Graham, J.E.; Ottenbacher, K.J.; Deer, R.; Ostir, G.V. Inpatient Walking Activity to Predict Readmission in Older Adults. Arch. Phys. Med. Rehabil. 2016, 97, S226–S231. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.1; Updated September 2020; Cochrane: Oxford, UK, 2020; Available online: www.training.cochrane.org/handbook (accessed on 25 September 2020).
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Dasso, N.A. How is exercise different from physical activity? A concept analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef]
- Jobe, J.B.; Smith, D.M.; Ball, K.; Tennstedt, S.L.; Marsiske, M.; Willis, S.L.; Rebok, G.W.; Morris, J.N.; Helmers, K.F.; Leveck, M.D.; et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Control. Clin. Trials 2001, 22, 453–479. [Google Scholar] [CrossRef]
- McCrone, A.; Smith, A.; Hooper, J.; Parker, R.; Peters, A. The Life-Space Assessment Measure of Functional Mobility Has Utility in Community-Based Physical Therapist Practice in the United Kingdom. Phys. Ther. 2019, 99, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Sterne, J.A.C.; Savović, J.; Page, M.J.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. A revised tool for assessing risk of bias in randomized trials. In Cochrane Methods. Cochrane Database of Systematic Reviews; Chandler, J., McKenzie, J., Boutron, I., Welch, V., Eds.; Cochrane: London, UK, 2016; Volume 10. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Schünemann, H.B.J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; GRADE Working Group: Chicago, IL, USA, 2013. [Google Scholar]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Foley, K.T.; Lowman, J.D.; MacLennan, P.A.; Razjouyan, J.; Najafi, B.; Locher, J.; Allman, R.M. Comparison of Posthospitalization Function and Community Mobility in Hospital Mobility Program and Usual Care Patients: A Randomized Clinical Trial. JAMA Int. Med. 2016, 176, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Dall, C.H.; Andersen, H.; Povlsen, T.M.; Henriksen, M. Evaluation of a technology assisted physical activity intervention among hospitalised patients: A randomised study. Eur. J. Intern. Med. 2019, 69, 50–56. [Google Scholar] [CrossRef]
- Killey, B.; Watt, E. The effect of extra walking on the mobility, independence and exercise self-efficacy of elderly hospital in-patients: A pilot study. Contemp. Nurse 2006, 22, 120–133. [Google Scholar] [CrossRef]
- McGowan, T.; Ong, T.; Kumar, A.; Lunt, E.; Sahota, O. The effect of chair-based pedal exercises for older people admitted to an acute hospital compared to standard care: A feasibility study. Age Ageing 2018, 47, 483–486. [Google Scholar] [CrossRef]
- Bogossian, F.; Winters-Chang, P.; Tuckett, A. “The Pure Hard Slog That Nursing Is …”: A Qualitative Analysis of Nursing Work. J. Nurs. Sch. 2014, 46, 377–388. [Google Scholar] [CrossRef]
- Scheerman, K.; Raaijmakers, K.; Otten, R.; Meskers, C.G.; Maier, A.B. Effect of physical interventions on physical performance and physical activity in older patients during hospitalization: A systematic review. BMC Geriatr. 2018, 18, 288. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D. David Oliver: Fighting pyjama paralysis in hospital wards. BMJ 2017, 357, j2096. [Google Scholar] [CrossRef] [PubMed]
- Graf, C. Functional decline in hospitalized older adults. Am. J. Nurs. 2006, 106, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M.; Allore, H.G.; Holford, T.R.; Guo, Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004, 292, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Stocking, J.; Kuhn, B.; Doroy, A.; Blackmon, E.; Young, H.M.; Adams, J.Y. How much do hospitalized adults move? A systematic review and meta-analysis. Appl. Nurs. Res. 2020, 51, 151189. [Google Scholar] [CrossRef] [PubMed]
- Evensen, S.; Sletvold, O.; Lydersen, S.; Taraldsen, K. Physical activity among hospitalized older adults—An observational study. BMC Geriatr. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Fisher, S.R.; Goodwin, J.S.; Protas, E.J.; Kuo, Y.F.; Graham, J.E.; Ottenbacher, K.J.; Ostir, G.V. Ambulatory activity of older adults hospitalized with acute medical illness. J. Am. Geriatr. Soc. 2011, 59, 91–95. [Google Scholar] [CrossRef]
- Buttery, A.K.; Martin, F.C. Knowledge, attitudes and intentions about participation in physical activity of older post-acute hospital inpatients. Physiotherapy 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Tobiano, G.; Bucknall, T.; Marshall, A.; Guinane, J.; Chaboyer, W. Patients’ perceptions of participation in nursing care on medical wards. Scand. J. Caring Sci. 2016, 30, 260–270. [Google Scholar] [CrossRef]
- Seide, S.E.; Röver, C.; Friede, T. Likelihood-based random-effects meta-analysis with few studies: Empirical and simulation studies. BMC Med Res. Methodol. 2019, 19, 16. [Google Scholar] [CrossRef]
- Berney, S.; Haines, K.J.; Skinner, E.H.; Denehy, L. Safety and feasibility of an exercise prescription approach to rehabilitation across the continuum of care for survivors of critical illness. Phys. Ther. 2012, 92, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Koenders, N.; van Oorsouw, R.; Seeger, J.P.; Nijhuis–van der Sanden, M.W.; van de Glind, I.; Hoogeboom, T.J. “I’m not going to walk, just for the sake of walking...”: A qualitative, phenomenological study on physical activity during hospital stay. Disabil. Rehabil. 2020, 42, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Williams, B.R.; Woodby, L.L.; Davis, L.L.; Allman, R.M. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J. Hosp. Med. 2007, 2, 305–313. [Google Scholar] [CrossRef]
- Jonsson, L.R.; Ingelsrud, L.H.; Tengberg, L.T.; Bandholm, T.; Foss, N.B.; Kristensen, M.T. Physical performance following acute high-risk abdominal surgery: A prospective cohort study. Can. J. Surg. 2018, 61, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Dieppe, P.; MacIntyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef]
- Moore, G.F.; Audrey, S.; Barker, M.; Bond, L.; Bonell, C.; Hardeman, W.; Moore, L.; O’Cathain, A.; Tinati, T.; Wight, D.; et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015, 350, h1258. [Google Scholar] [CrossRef]
- Sourdet, S.; Lafont, C.; Rolland, Y.; Nourhashemi, F.; Andrieu, S.; Vellas, B. Preventable Iatrogenic Disability in Elderly Patients during Hospitalization. J. Am. Med Dir. Assoc. 2015, 16, 674–681. [Google Scholar] [CrossRef]
- Baztan, J.J.; Suárez-García, F.M.; López-Arrieta, J.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: Meta-analysis. BMJ 2009, 338, b50. [Google Scholar] [CrossRef]
- Liu, B.; Moore, J.E.; Almaawiy, U.; Chan, W.H.; Khan, S.; Ewusie, J.; Hamid, J.S.; Straus, S.E. MOVE ON Collaboration. Outcomes of Mobilisation of Vulnerable Elders in Ontario (MOVE ON): A multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018, 47, 112–119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).