Geoditin A Induces Oxidative Stress and Apoptosis on Human Colon HT29 Cells
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
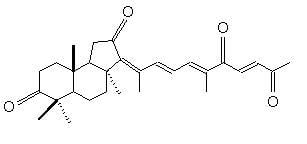
3.1. Test compounds
3.2. Cell cultures
3.3. Cell proliferation assay
3.4. Fluorescence staining for morphological observation
3.5. Flow cytometric cell cycle analysis
3.6. Immunoblotting analysis
3.7. Internalization of transferrin by Geoditin A-treated HT29 cells
Acknowledgements
References and Notes
- Liu, WK; Ho, JC; Che, CT. Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia HL60 cells. Cancer Lett 2005, 230, 102–110. [Google Scholar]
- Sung, JJ; Lau, JY; Goh, KL; Leung, WK. Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005, 6, 871–876. [Google Scholar]
- Hong Kong Cancer Registry. Hospital Authority. 2006. http://www.cancer-fund.org/en/bowel-cancer.html/ (accessed November 2009).
- Kinghorn, AD; Chin, YW; Swanson, SM. Discovery of natural product anticancer agents from biodiverse organisms. Curr Opin Drug Discov Devel 2009, 12, 189–196. [Google Scholar]
- Freudenthal, HD. Fusetani, N, Ed.; Transactions of the Drugs from the Sea Symposium, University of Rhode Island, Kingston, RI, USA. 1967. In Drugs from the Sea; Marine Technology Society: Washington, DC, USA, 1968. [Google Scholar]
- Bhakuni, DS; Rawat, DS. Bioactive Marine Natural Products; Springer: New York, NY, USA, 2005; p. 279. [Google Scholar]
- Report to the Nation Improving Public Health Through Human Drugs, Center for Drug Evaluation and Research, US Department of Health and Human Services, Food and Drug Administration: USA, 2004; p. 23.
- Singh, R; Sharma, M; Joshi, P; Rawat, DS. Clinical status of anti-cancer agents derived from marine sources. Anticancer Agents Med Chem 2008, 8, 603–617. [Google Scholar]
- Tasdemir, D; Mangalindan, GC; Concepción, GP; Verbitski, SM; Rabindran, S; Miranda, M; Greenstein, M; Hooper, JN; Harper, MK; Ireland, CM. Bioactive isomalabaricane triterpenes from the marine sponge Rhabdastrella globostellata. J Nat Prod 2002, 65, 210–214. [Google Scholar]
- Daniels, TR; Delgado, T; Rodriguez, JA; Helguera, G; Penichet, ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol 2006, 121, 144–158. [Google Scholar]
- Sun, X; Ge, R; Cai, Z; Sun, H; He, QY. Iron depletion decreases proliferation and induces apoptosis in a human colonic adenocarcinoma cell line, Caco2. J Inorg Biochem 2009, 103, 1074–1081. [Google Scholar]
- Liu, BPL; Che, Chun-Tao; Liu, WK. Tangutorine induces p21 expression and abnormal mitosis in human colon cancer HT-29 cells. Biochem Pharmacol 2005, 70, 287–299. [Google Scholar]
- McCabe, T; Clardy, J; Minale, L; Pizza, C; Zollo, F; Riccio, R. A triterpenoid pigment with the isomalabaricane skeleton from the marine sponge. Tetrahedron Lett 1982, 23, 3307–3310. [Google Scholar]
- Zhang, WH; Che, CT. Isomalabaricane-type nortriterpenoids and other constituents of the marine sponge Geodia japonica. J Nat Prod 2001, 64, 1489–1492. [Google Scholar]
- Clement, JA; Li, M; Hecht, SM; Kingston, DG. Bioactive isomalabaricane triterpenoids from Rhabdastrella globostellata that stabilize the binding of DNA polymerase beta to DNA. J Nat Prod 2006, 69, 373–376. [Google Scholar]
- Lin, HW; Wang, ZL; Wu, JH; Shi, N; Zhang, HJ; Chen, WS; Morris-Natschke, SL; Lin, AS. Stellettins L and M, cytotoxic isomalabaricane-type triterpenes, and sterols from the marine sponge Stelletta tenuis. J Nat Prod 2007, 70, 1114–1117. [Google Scholar]
- Meragelman, KM; McKee, TC; Boyd, MR. New cytotoxic isomalabaricane triterpenes from the sponge Jaspis Species. J Nat Prod 2001, 64, 389–392. [Google Scholar]
- Sakagami, H; Kawase, M; Wakabayashi, H; Kurihara, T. Factors that affect the type of cell death induced by chemicals. Autophagy 2007, 3, 493–495. [Google Scholar]
- Mukherjee, S; Shields, D. Nuclear Import Is Required for the Pro-apoptotic Function of the Golgi Protein p115. J Biol Chem 2009, 284, 1709–1717. [Google Scholar]
- Mukherjee, S; Chiu, R; Leung, SM; Shields, D. Fragmentation of the Golgi apparatus: An early apoptotic event independent of the cytoskeleton. Traffic 2007, 8, 369–378. [Google Scholar]
- Müller, CI; Miller, CW; Kawabata, H; McKenna, RJ, Jr; Marchevsky, AM; Koeffler, HP. Do cancer cells selectively mutate HFE to increase their intracellular iron? Oncol Rep 2005, 14, 299–303. [Google Scholar]
- Ortiz-Sánchez, E; Daniels, TR; Helguera, G; Martinez-Maza, O; Bonavida, B; Penichet, ML. Enhanced cytotoxicity of an anti-transferrin receptor IgG3-avidin fusion protein in combination with gambogic acid against human malignant hematopoietic cells: functional relevance of iron, the receptor, and reactive oxygen species. Leukemia 2009, 23, 59–70. [Google Scholar]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat Res 2003, 531, 81–92. [Google Scholar]
- Duarte, TL; Jones, GD. Vitamin C modulation of H2O2-induced damage and iron homeostasis in human cells. Free Radic Biol Med 2007, 43, 1165–1175. [Google Scholar]
- Buss, JL; Neuzil, J; Ponka, P. Oxidative stress mediates toxicity of pyridoxal isonicotinoyl hydrazone analogs. Arch Biochem Biophys 2004, 421, 1–9. [Google Scholar]








© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
W. K. Cheung, F.; Li, C.; Che, C.-T.; P. L. Liu, B.; Wang, L.; Liu, W.-K. Geoditin A Induces Oxidative Stress and Apoptosis on Human Colon HT29 Cells. Mar. Drugs 2010, 8, 80-90. https://doi.org/10.3390/md8010080
W. K. Cheung F, Li C, Che C-T, P. L. Liu B, Wang L, Liu W-K. Geoditin A Induces Oxidative Stress and Apoptosis on Human Colon HT29 Cells. Marine Drugs. 2010; 8(1):80-90. https://doi.org/10.3390/md8010080
Chicago/Turabian StyleW. K. Cheung, Florence, Chunman Li, Chun-Tao Che, Bonnie P. L. Liu, Lijun Wang, and Wing-Keung Liu. 2010. "Geoditin A Induces Oxidative Stress and Apoptosis on Human Colon HT29 Cells" Marine Drugs 8, no. 1: 80-90. https://doi.org/10.3390/md8010080
APA StyleW. K. Cheung, F., Li, C., Che, C.-T., P. L. Liu, B., Wang, L., & Liu, W.-K. (2010). Geoditin A Induces Oxidative Stress and Apoptosis on Human Colon HT29 Cells. Marine Drugs, 8(1), 80-90. https://doi.org/10.3390/md8010080