Abstract
Acanthamoeba is a ubiquitous genus of amoebae that can trigger a severe and progressive ocular disease known as Acanthamoeba Keratitis (AK). Furthermore, current treatment protocols are based on the combination of different compounds that are not fully effective. Therefore, an urgent need to find new compounds to treat Acanthamoeba infections is clear. In the present study, we evaluated staurosporine as a potential treatment for Acanthamoeba keratitis using mouse cornea as an ex vivo model, and a comparative proteomic analysis was conducted to elucidate a mechanism of action. The obtained results indicate that staurosporine altered the conformation of actin and tubulin in treated trophozoites of A. castellanii. In addition, proteomic analysis of treated trophozoites revealed that this molecule induced overexpression and a downregulation of proteins related to key functions for Acanthamoeba infection pathways. Additionally, the ex vivo assay used validated this model for the study of the pathogenesis and therapies of AK. Finally, staurosporine eliminated the entire amoebic population and prevented the adhesion and infection of amoebae to the epithelium of treated mouse corneas.
1. Introduction
Free-living amoebae are ubiquitous single-celled living organisms isolated from multiple habitats including water and soil. Until present, six different genera have been reported to be pathogenic to humans and animals, which the most common being Acanthamoeba genus. This amoeba could cause a threatening eye infection known as Acanthamoeba keratitis (AK): a rare eye infection commonly associated with contact lens wearers [1,2]. Although Acanthamoeba keratitis constitutes 2% of the corneal infections, its incidence has been consistently increasing [3]. The worldwide annual prevalence of AK stands at a rate of 2.34 cases per million individuals of all reported clinical cases of microbial keratitis, but it can rise to 1 case in 30,000 in contact lens wearers [4,5]. Notably, published studies confirm the increasing trend in rising AK cases in parallel with the number of contact lens wearers. A national prospective survey conducted through the British Ophthalmic Surveillance Unit in the UK reported a significant increase in the incidence of AK in contact lens wearers from 21.14 in 1997/1998 and 17.53 in 1998/1999 to 26.94 in 2015 [6].
Actual treatment protocols are based on the combination of cationic antiseptics such as polyhexamethylene biguanide (0.02%) or chlorhexidine (0.02%) and aromatic diamidines such as propamidine (0.1%) or hexamidine (0.1%) [7,8,9]. Still, these current therapies are not fully effective in eliminating the parasite because of their variable efficacy among different genotypes, the appearance of highly resistant cyst form, or due to their toxicity generated by a prolonged administration. In addition, a delay in correct diagnosis in most clinical cases implies an increased risk of disease progression and the infection of deeper layers of the corneal epithelium. Therefore, early AK detection is critical; however, AK cases are handled in clinics for other causes, such as bacterial or viral infections due to misdiagnosis, resulting in the worsening of AK infections [9,10]. This leads to an urgent need to discover new drugs and/or drug posology.
Compounds with marine origin have proven to be an interesting source for the development of novel antiparasitic therapies. In this sense, indolocarbazole alkaloids have received the attention of researchers due to their natural abundance, wide structural chemical variety, and broad spectrum of biological activities [11]. The staurosporine alkaloid was first identified from the bacteria Streptomyces staurosporeus in 1977 [12]. This molecule and its analogs are known as potent protein kinase C inhibitors. Beside this, these indolocarbazoles displayed notably antitumor, antimicrobial, antifungal, antiviral, and antiparasitic activities [11,13,14,15,16]. In previous studies, we demonstrated that staurosporine inhibits Acanthamoeba growth and induces programmed cell death (PCD) in trophozoites of A. castellanii Neff [17,18].
On the one hand, in vitro assays are essential for drug discovery against the present infection, because they are simple, reproductible and much more economical than the in vivo assay, but still inefficient for predicting the drug’s action inside the host organism. On the other hand, in vivo assays are more clinically relevant, and the drug effect and side effect could be much more accurate, yet in vivo assays are much more expensive and harder to control as they include a multitude of variables. Ex vivo assays are important preclinical tools between in vitro and in vivo assays. In these ex vivo systems, the cytoarchitecture and intracellular connections and metabolic processes could be conserved, mimicking the in vivo environment [19]. Rabbits, hamsters, and mice were used as models for the in vivo assay of Acanthamoeba Keratitis [20,21]. To the best of our knowledge, only hamster, porcine, and mouse corneas have been used as animal ex vivo models for Acanthamoeba keratitis [22,23,24,25,26,27]. The aim of the present study was to evaluate staurosporine as potential treatment for Acanthamoeba keratitis using mouse cornea as an ex vivo model and to investigate its model of action by comparative proteomic analysis.
2. Results
2.1. Staurosporine Induced Structural Damage of the Acanthamoeba Cytoskeleton
In eukaryotic cells, the cytoskeleton is mainly composed of proteins like microfilaments, microtubules, and intermediate fibers. Microfilaments, composed essentially of actin filaments, play a crucial role in cellular motility and cell interaction with the extracellular and intracellular environment [28]. In Acanthamoeba, targeting the actin network could prevent infection by inhibiting adhesion and cyst formation. A staining using the conjugate phalloidin-TRITC revealed the extent of damage induced by staurosporine on the distribution of actin cytoskeleton: treated cells emitted lower fluorescence, and we observed partly degraded and disorganized acanthopodium, reflecting on the lower expression of actin protein (Figure 1A,B).
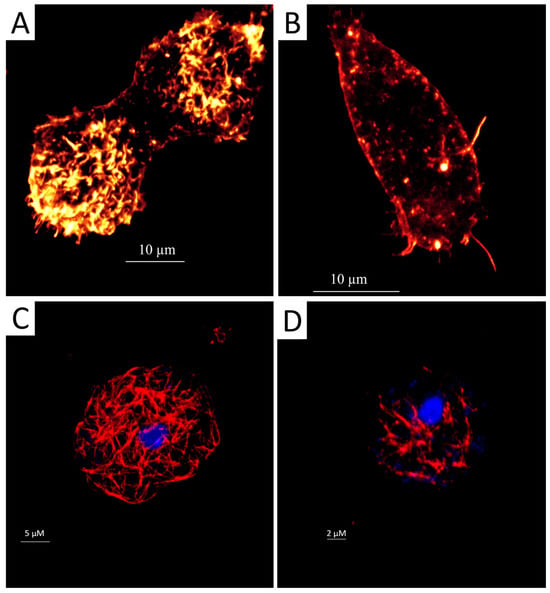
Figure 1.
Evaluation of the effect of IC90 of staurosporine on the actin and tubulin cytoskeleton of Acanthamoeba castellanii trophozoites for 24 h. The phalloidin-TRITC dye stains the polymerised actin cytoskeleton showing the normal organization of the networks with an orange fluorescence in the negative control cells (A); scale bar represents 10 µm. Treated cells emitted a lower orange fluorescence, and trophozoites showed disorganized and degraded acanthopodium (B); scale bar represents 10 µm. Tubulin antibodies bind to microtubules and stain them in control cells showing an intense red fluorescence and demonstrating a normal conformation (C); scale bar represents 5 µm. However, trophozoites incubated with staurosporine show disorganization or destruction of the tubulin microtubules (D); scale bar represents 2 µm. Mounting with DAPI solution for DNA staining shows a blue fluorescence (C,D). All images (63×) were obtained using an inverted confocal light microscope Leica DMI 4000 B (Deerfield, IL, USA).
As for the microtubules that are mainly composed of tubulin, they have been involved in motility, intracellular transport, and cell division [29]. Inhibiting this protein could prevent cell growth and has been suggested as a good target for amoebicidal agents. An indirect immunofluorescence assay for tubulin detection was conducted. After 24 h of incubation with the staurosporine, we observed a decrease in cell shape with a uniformly distributed tubulin network. The affected cells emit lower fluorescence than the untreated cells (Figure 1C,D).
2.2. Proteomic Analysis
To comprehend the effect of staurosporine on Acanthamoeba, a mass spectrometry-based proteomic approach was conducted. Two groups of cells at the trophozoite stage of Acanthamoeba castellanii were prepared: untreated and treated cells with IC50 of the present molecule. After 24 h of treatment, total proteins were extracted. The proteomic profiling of both cultures resulted in the identification of 4566 proteins (Supplementary Materials, Protein analysis). Compared to untreated cells, the proteomic analysis revealed that a total of 812 proteins were differentially expressed at least twofold, in which 97 and 715 proteins were downregulated and upregulated, respectively (Figure 2). The selected affected proteins discussed below are listed in Table 1.
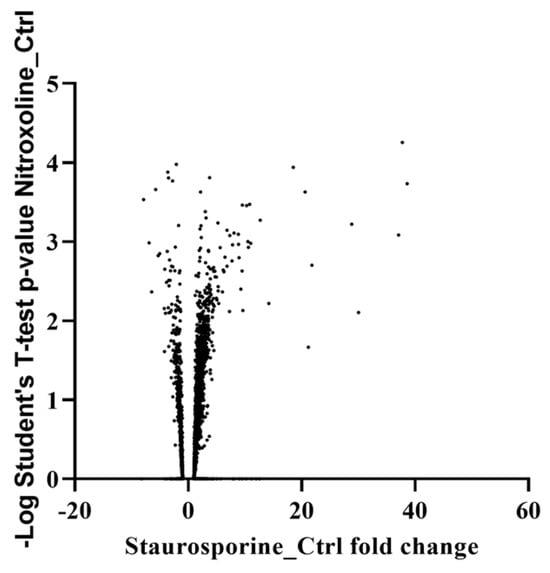
Figure 2.
Volcano graph expressing a logarithmic Student’s t-test p-value as a function of staurosporine protein control fold change.

Table 1.
Changes in the levels of selected proteins upon staurosporine treatment at IC50 for 24 h.
2.3. Ex Vivo Assay in Mouse Corneas Infected with A. castellanii and Treated with Staurosporine
Scanning electron microscopy was used to analyze the effect of staurosporine in the mouse corneas infected with trophozoites of Acanthamoeba castellanii. Mouse corneas incubated alone with staurosporine at IC90 for A. castellanii presented no corneal injury. Furthermore, epithelial cells showed a normal conformation and structure (Figure 3A). In the group of mouse corneas cultured alone with trophozoites, amoebas adhered to the corneal surface and penetrated the junctions of the epithelial cells of the cornea, taking advantage of these junctions to produce infections of the epithelium (Figure 3B). Contact damage was observed without lysis; corneal epithelium showed de-epithelization and destabilization produced by trophozoites; and then, phagocytosis took place.
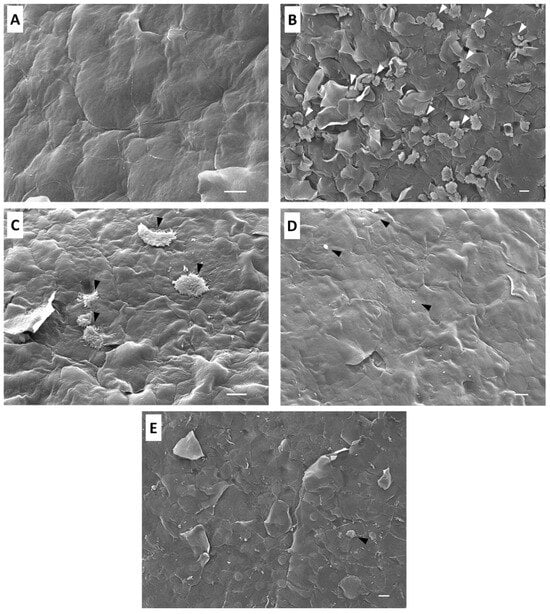
Figure 3.
Scanning electron microscopy analysis of the effect of Acanthamoeba castellanii trophozoites and/or staurosporine on mouse corneas during 3 h. The co-incubation of mouse corneas with staurosporine at IC90 shows no evidence of damage or cell disorganization (A); Group 1, scale bar represents 10 µm. Mouse corneas co-cultured with trophozoites of Acanthamoeba castellanii (B); scale bar represents 10 µm; Group 2 presented de-epithelization of the corneal epithelium by the amoebic infection penetrating between the junctions of the epithelial cells (white arrows). Corneas co-incubated with trophozoites of Acanthamoeba castellanii pretreated with staurosporine 30 min prior (C); scale bar represents 10 µm; Group 3 showed that fewer trophozoites adhered to the corneal epithelium (black arrows), which did not migrate toward the inner layers of the corneal epithelium; therefore, the damage was very limited. In the corneas with simultaneous infection and treatment with staurosporine (D)—Group 4, scale bar represents 10 µm—the cellular debris of Acanthamoeba castellanii trophozoites was observed (black arrows), and the corneal epithelium showed no cell damage or signs of amoebic invasion. Corneas co-cultured with trophozoites and treated with staurosporine 30 min after infection (E); scale bar represents 10 µm; Group 5 showed that no-healthy cells adhered to the corneal surface (black arrow), and the corneal epithelium showed signs of early stages of amoebic infection. Images were obtained using a JEOL-JSM 7100F scanning electron microscope (JEOL Ltd., Tokyo, Japan).
The group co-cultured with trophozoites pretreated with the IC90 of staurosporine before incubation showed that the epithelium cells were not damaged, and scarce amoebas were observed (Figure 3C). The pre-treatment with staurosporine affected the adhesion of the amoebae to the epithelial cells, and consequently, prevented the amoebae from invading the corneal epithelium. Mouse corneas co-incubated simultaneously with trophozoites and staurosporine at IC90 revealed that corneal epithelium was intact without signs of cellular damage. Moreover, no healthy amoebae were observed on the epithelium. The simultaneous treatment with staurosporine eliminated all the amoebas, preventing the amoebic invasion of the corneal epithelium (Figure 3D).
Mouse corneas co-cultured with trophozoites and treated with staurosporine after 30 min of incubation showed no healthy adherent trophozoites on the corneal epithelium. The amoebae presented morphological alterations after staurosporine treatment. The corneal epithelium showed cellular damage caused by the early stages of amoebic, but once staurosporine was administered after 30 min of incubation, it acted directly on the trophozoites, preventing a more severe invasion of the corneal epithelium (Figure 3E).
3. Discussion
Staurosporine was first isolated by Omura, S. et al. in 1977 from a Streptomyces strain [12]. Until present, several authors have confirmed its pharmacological activities like hypotensive, antiprotozoal, anticoagulant, and antifungal properties [11,30]. In a previous work, we proved the amoebicidal activity of staurosporine against various Acanthamoeba strains [18], showing IC50 values of 0.568 ± 0.122 µM and 1.653 ± 0.017 µM in trophozoite and cyst stages of A. castellanii Neff, respectively. The approach was based on molecule isolation and bio-guided fractionation from a Streptomyces sanyensis extract. In addition, we confirmed that the present indolocarbazole could induce program cell death in A. castellanii Neff, evidencing plasma membrane damage, chromatin condensation, the collapse of the ATP level, and mitochondrial membrane potential, as well as increased levels of reactive oxygen species (ROS) [17,18]. In this regard, to confirm the obtained results and to indicate the mechanism of action, we opted to study the effect of the present molecules on the proteomic profile of Acanthamoeba and to confirm its amoebicidal activity using an ex vivo approach.
The main objectives of the present work were to first confirm the amoebicidal effect of staurosporine on this clinical strain of Acanthamoeba castellanii, following protein expression in the early stage of treatment. Second was to establish a new protocol to study the effects of the amoebicidal drug on an amoebic keratitis murine ex vivo model, which turned out to be efficient since data similar to those observed and described in a previous study in the cornea of hamster (Mesocricetus auratus) were obtained.
In our previous work, we highlighted the morphological alterations induced by the staurosporine on Acanthamoeba in the early stage of treatment. To corroborate the effect of the present drug on the cytoskeleton of Acanthamoeba, a specific staining of actin and tubulin was carried out. After 30 min of incubation with the present drug, we observed a dramatic alteration in the cell cytoskeleton; actin staining revealed the formation of long elongation. Various reports confirm the present findings; Hedberg et al. (1990) reported an alteration in the cytoskeleton of several cell lines, including PTK2 epithelial cells, Swiss 3T3 fibroblasts, and human foreskin fibroblasts, by staurosporine [31], while Xie et al. (2017) observed the formation of filaments in the fungal pathogen Candida albicans [32]. In fact, they related the staurosporine-induced filament to a defect in septin ring formation, implicating cell cycle kinases as potential staurosporine targets [32]. As for the microtubules network, we observed that the present drug induces alteration in its rearrangement as a disorganization network with the presence of concentrated points. All those events could be a result of a cell dismantling upon program cell death [33].
The proteomic analysis of cells treated with the staurosporine (IC50) for 24 h revealed that various membrane protein kinases were significantly downregulated, including dual specificity protein kinase shkB and Serine/threonine-protein kinase. Those proteins have been described as regulators of the chemotaxis and phagocytosis processes in Acanthamoeba. Inhibiting these proteins could reduce the pathogenicity and growth of Acanthamoeba. Along with this effect, we noticed the inhibition of cysteine protease implicated in the tissue invasion as well as in the encystation pathway [34].
In treated cells, almost 16% of the total identified proteins were overexpressed. Among those proteins, we observed the upregulation of Profilin. This molecule is implicated in the regulation of actin polymerization and affects the structure of the cytoskeleton. Various reports have confirmed that at lower concentrations, this protein enhances the actin polymerization, while at high concentrations, it would prevent it and trigger the autophagy via the mTOR pathway [35,36]. Among the most upregulated proteins, we found proteins involved in cell survival under oxidative stress.
Although, we have confirmed in vitro the effect of staurosporine on Acanthamoeba, these results are still insufficient to scale up to in vivo. For this reason, one of the main objectives of the present study was to establish an ex vivo model to study the effect of drug therapy on AK infection. Omaña-Molina et al. (2004) established an ex vivo model to study the cytopathic effects of Acanthamoeba castellanii and A. polyphaga on hamster corneas [23]. In this study, the ex vivo assay revealed that in the trophozoites of Acanthamoeba castellanii infected the mouse corneas, showing that the amoebas invaded the corneal epithelium, penetrating the junctions of the epithelial cells without lysis process and only phagocyting the detached cells. This type of invasion suggests that Acanthamoeba infections are contact-dependent. In this sense, Omaña-Molina et al. (2013) demonstrated that the A. castellanii and A. polyphaga invasion and disruption of corneal epithelium in the hamster model is performed by the penetration of the amoebae through cell junctions either by the action of proteases and/or a mechanical effect exerted by trophozoites, suggesting that the contact-dependent activity is an important pathogenic mechanism of these strains of Acanthamoeba [24]. Wang et al. (2021) studied the crucial role of commensals in mitigating A. castellanii pathogenicity [26] using a modified ex vivo mouse model based on the previously reported ex vivo hamster model by Omaña-Molina et al. (2013). This ex vivo mouse study demonstrated that the presence of intact bacteria significantly reduced A. castellanii corneal epithelial cell damage. Moreover, in a previous study, Omaña-Molina et al. (2010) co-cultivated A. castellanii trophozoites with human corneas and reported that the mechanisms of pathogenicity of amoebic infections were very similar to those in the previous study using a hamster model and our current findings obtained using a mouse model, which validates these animal models for the study of the pathogenesis of AK [23,25,37].
Currently, the agents normally recommended to treat AK need to be administered for a prolonged period, which often results in severe ocular surface toxicity [7,38,39,40,41]. In this study, mouse corneas cultured alone with staurosporine at a concentration of 2.70 ± 0.015 µM revelated that this compound causes no adverse effect on the corneal epithelium compared to other drugs, maintaining the mouse corneal tissues intact after 3 h of incubation. In our previous study, the toxicity effect observed when tested in vitro against a macrophage cell line was low (cytotoxicity concentration value of 50, CC50, 8.737 ± 0.718 µM) in comparison to the obtained IC50 and IC90, with values 4-fold higher than the staurosporine IC50 against cysts [18]. Nevertheless, Härtel et al. (2003) reported that staurosporine could induce apoptosis in human corneal epithelial (HCE) cells [42]. HCE cells were incubated in vitro with different concentrations of staurosporine, demonstrating pathological effects after 4 h at 2 µM. In this regard, our ex vivo mouse model has shown that staurosporine with only 30 min pre-treatment affects the adhesion of the amoebae to the corneal epithelium and prevents the trophozoites from invading the corneal tissue. However, a 30 min pre-treatment did not completely eliminate the amoebae population, possibly due to insufficient time for the pre-treatment of the trophozoites. The corneal epithelium was intact in the infected mouse corneas treated simultaneously and 30 min after infection, showing similar results to those of the mouse corneas cultured alone with staurosporine. This compound eliminated the entire amoebic population, preventing the adhesion and infection of amoebae to the epithelium with only 3 h of incubation. Therefore, this study demonstrated that staurosporine could be used to develop a new line of eye drops for the treatment of superficial AK or early stages of amoebic infection.
Nevertheless, our study had limitations. For example, for patients with AK in the chronic stage of the disease, where the infection is located at deeper levels of the corneal epithelium, further studies are needed to determine whether the effect of staurosporine could be observed deeper in the corneal tissue and completely eliminate the amoebic infection, as well as to determine the possibility of toxic effects in longer treatments.
4. Materials and Methods
4.1. Acanthamoeba Strain
Trophozoites of A. castellanii (genotype T4) were isolated from a clinical case of AK at “Asociación para evitar la ceguera en México”, Luis Sánchez Bulnes Hospital, Mexico City. The strain was reported to be invasive in the GAE murine model [43,44,45]. The strain was grown axenically in PYG medium (0.75% (w/v) proteose peptone, 0.75% (w/v) yeast extract, and 1.5% (w/v) glucose) containing 40 μg gentamicin mL−1 (Biochrom AG, Cultek, Granollers, Barcelona, Spain) at 26 °C. After 72 h of incubation (the end of the growth logarithmic phase), the culture was centrifuged (2500 rpm during 5 min), and trophozoites were harvested for the subsequent assays.
4.2. In Vitro Assay
4.2.1. Fluorescent Staining of Actin Distribution
For direct fluorescent staining, trophozoites of A. castellanii were treated first with the IC90 (2.70 ± 0.015 µM) of the staurosporine with a cell concentration of 2.5 × 105 mL−1. After 30 min of incubation, cells were fixed with formaldehyde and deposited on a pre-coated coverslip. Later, cells were treated with Triton (0.1%) for 30 min followed by Phalloidin–tetramethylrhodamine B isothiocyanate (Phalloidin-TRITC; Sigma-Aldrich, Madrid, Spain) for another 30 min at room temperature. Finally, cells were washed with PBS and later examined by Z-stack imaging using an inverted light confocal microscope Leica DMI 4000 B with LAS X software (Version 5.2.2), a 532 nm laser, and a Leica HCX PL Apo 63× Oil Objective (Leica Microsystems, Wetzlar, Germany). Untreated cells were considered the negative control.
4.2.2. Immunofluorescence Staining of the Tubulin of Acanthamoeba Trophozoites
The immunofluorescence staining of tubulin was conducted using the immunofluorescence staining procedure of the manufacturer (Sigma-Aldrich) with slight modifications. Briefly, trophozoites (2.5 × 105 cells mL−1) were first treated with the IC90 of staurosporine for 30 min. In total, 50 µL of the cell suspension was placed on a gelatine precoated coverslip for 30 min. Later, it were fixed with paraformaldehyde (4%). After 15 min, the cells were treated with Triton (0.3%) for 10 min followed by 3 washes with PBS 1×. At this stage, the cells were treated with 5% BSA in PBS 1×/150 mM sucrose for 30 min and washed with glycine 100 mM in PBS 1× for 5 min. Later, the trophozoites were incubated with the first anti-tubulin antibody 1:2000 for 2 h at room temperature (Monoclonal Anti-α-Tubulin antibody produced in mouse, Sigma-Aldrich, Madrid, Spain). After 3 washes with PBS 1×, the cells were incubated with the second antibody labeled with Alexa 594 (1:500) for 1 h at room temperature in darkness (Goat anti-Mouse IgG (H+L) Highly Cross—Adsorbed Secondary Antibody, Alexa Fluor Plus 594; Thermo Fisher Scientific, Rockford, IL, USA). Finally, the cells were washed with PBS 1× and mounted in DAPI (4′,6-Diamidino-2-phenylindole dihydrochloride; Sigma-Aldrich; Madrid, Spain) containing mounting solution. Three-dimensional and maximum projection imaging of the trophozoites were performed by Z-stack imaging using an inverted light confocal microscope Leica DMI 4000 B, LAS X software, a 405 nm laser, a 532 nm laser, and a Leica HCX PL Apo 63× Oil Objective.
4.3. Proteomic Analysis
Comparative label-free proteomic analysis was conducted as described in our recent study [46]. In total, 106 cells of A. castellanii were treated with the IC50 (0.568 ± 0.122 µM) of staurosporine for 24 h and washed 1 time with PBS, and the pellets were subjected to further processing. Untreated cells were prepared as a control group. Both groups were prepared in three biological replicates. Protein identification was made using the data base of https://www.uniprot.org/ (accessed on 1 October 2023).
4.4. Ex Vivo Infection
Co-Incubation and Interaction of A. castellanii Trophozoites with BALB/c Mice Cornea
A total of 12 pathogen-free male BALB/c (Mus musculus) mice were used, with an average age of 21 to 28 days and an average body weight of 35 g. The experiments were based on the protocol previously described for hamster cornea (Mesocricetus auratus) [23,24,25], were approved by the Research Ethics and Animal Welfare Committee of the University of La Laguna, included in the project known as the Evaluation of the in vivo amoebicidal activity of eye drops containing active ingredients administered via the ocular route, with reference number CEIBA2021-3074.
The corneas of each mouse were removed and processed. Then, the corneas were placed in 96-well polystyrene plates and were washed with 1× PBS twice. Five experimental groups were established in the 96-well plates. Group 1 included corneas incubated alone with staurosporine at IC90 of A. castellanii. Corneas in Group 2 were co-cultured alone with 106 Acanthamoeba castellanii trophozoites. Corneas in Group 3 were co-incubated with pretreated A. castellanii with the IC90 of staurosporine for 30 min. Group 4 consisted of corneas co-incubated simultaneously with trophozoites and with IC90 of staurosporine. Corneas of Group 5 were co-cultured alone with A. castellanii trophozoites, and after 30 min of incubation, amoebas were treated with the IC90 of staurosporine. The plates with all groups of mouse corneas were incubated in a humidity chamber at 36 °C during 3 h.
4.5. Scanning Electron Microscopy
After co-incubation, all groups of corneas were fixed at room temperature with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, dehydrated in increasing concentrations of ethanol, and critical-point-dried with liquid CO2 using a Samdri 780 apparatus (Tousimis, Rockville, MD, USA). Then, the corneas were coated with a thin layer (30 nm) of gold in a JEOL-JFC I 100 ion-sputtering device. Finally, all groups of corneas were observed using a JEOL-JSM 7100F scanning electron microscope (JEOL Ltd., Tokyo, Japan) [24].
5. Conclusions
In summary, staurosporine induced structural alterations in the actin and tubulin cytoskeleton in trophozoites of Acanthamoeba castellanii. In addition, the proteomic analysis of treated trophozoites with staurosporine showed that various membrane protein kinases and cysteine proteases were significantly downregulated, mainly involved in the pathogenicity, growth, tissue invasion, and encystation pathway of Acanthamoeba. Furthermore, almost 16% of the total identified proteins were overexpressed. We observed the upregulation of Profilin, which is involved in the regulation of actin polymerization, affecting the structure of the cytoskeleton. On the other hand, the mechanisms of the pathogenicity of amoebic infection reported in previous ex vivo investigations were very similar to our findings using a mouse model, which validate this animal model for the study of the pathogenesis and therapies of AK. Besides this, staurosporine eliminated the entire amoebic population and prevented amoeba adhesion and infection to the epithelium of mouse corneas, demonstrating that staurosporine could be used to develop a new line of eye drops for the treatment of superficial AK or early stages of amoebic infections.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/md22090423/s1, File S1: Experimental conditions for the proteomic analysis of Acanthamoeba trophozoites treated with staurosporine.
Author Contributions
Methodology, R.L.R.-E., I.S., L.S.-V., C.J.B.-E. and R.S.; software, R.L.R.-E., I.S. and R.S.; validation, A.R.D.-M., J.J.F., M.O.-M., R.S., J.E.P. and J.L.-M.; formal analysis, M.O.-M., R.S., J.E.P. and J.L.-M.; investigation, R.L.R.-E., I.S., L.S.-V., C.J.B.-E. and R.S.; resources, J.E.P. and J.L.-M.; data curation, R.L.R.-E. and I.S.; writing—original draft preparation, R.L.R.-E. and I.S.; writing—review and editing, L.S.-V., M.O.-M., R.S., J.E.P. and J.L.-M.; conceptualization, M.O.-M., R.S., J.E.P. and J.L.-M.; visualization, J.E.P. and J.L.-M.; supervision, J.E.P. and J.L.-M.; project administration, J.E.P. and J.L.-M.; funding acquisition, A.R.D.-M., J.J.F., M.O.-M., R.S., J.E.P. and J.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Consorcio Centro de Investigación Biomédica (CIBER) de Enfermedades Infecciosas (CIBERINFEC); Instituto de Salud Carlos III, 28006 Madrid, Spain (CB21/13/00100); and Ministerio de Sanidad, Spain. R.L.R.-E., I.S., and C.J.B.-E. were funded by the Cabildo Insular de Tenerife 2023–2028 PROYECT CC20230222, CABILDO.23. R.S. was supported by CePaViP, provided by ERDF and MEYS CR (CZ.02.1.01/0.0/0.0/16_019/0000759).
Institutional Review Board Statement
This study is approved by the Research Ethics and Animal Welfare Committee of the University of La Laguna, reference number CEIBA2021-3074.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding authors.
Acknowledgments
We acknowledge Karel Harant and Pavel Talacko from the Laboratory of Mass Spectrometry, Biocev, Charles University, Faculty of Science, where proteomic and mass spectrometric analysis were carried out.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction of the information included in the Institutional Review Board Statement. This change does not affect the scientific content of the article.
References
- Henriquez, F.L. Review of “Acanthamoeba: Biology and Pathogenesis” by Naveed Ahmed Khan. Parasit. Vectors 2009, 2, 16. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Martín-Navarro, C.M.; López-Arencibia, A.; Arnalich-Montiel, F.; Piñero, J.E.; Valladares, B. Acanthamoeba Keratitis: An Emerging Disease Gathering Importance Worldwide? Trends Parasitol. 2013, 29, 181–187. [Google Scholar] [CrossRef] [PubMed]
- List, W.; Glatz, W.; Riedl, R.; Mossboeck, G.; Steinwender, G.; Wedrich, A. Evaluation of Acanthamoeba Keratitis Cases in a Tertiary Medical Care Centre over 21 Years. Sci. Rep. 2021, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Afflitto, G.G.; Ceccarelli, F.; Turco, M.V.; Han, Y.; Amescua, G.; Dart, J.K.; Nucci, C. Perspectives on the Incidence of Acanthamoeba Keratitis: A Systematic Review and Meta-Analysis. Ophthalmology 2024. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, F.; Tortori, A.; Vallino, V.; Galdiero, M.; Fea, A.M.; De Sanctis, U.; Reibaldi, M. Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection. Microorganisms 2024, 12, 758. [Google Scholar] [CrossRef]
- Jasim, H.; Grzeda, M.; Foot, B.; Tole, D.; Hoffman, J.J. Incidence of Acanthamoeba Keratitis in the United Kingdom in 2015: A Prospective National Survey. Cornea 2024, 43, 269–276. [Google Scholar] [CrossRef]
- Fanselow, N.; Sirajuddin, N.; Yin, X.-T.; Huang, A.J.W.; Stuart, P.M. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. [Google Scholar] [CrossRef]
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba Keratitis—Clinical Signs, Differential Diagnosis and Treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Posarelli, M.; Passaro, M.L.; Avolio, F.C.; Costagliola, C.; Semeraro, F.; Romano, V. The Incidence of Severe Complications in Acanthamoeba Keratitis: Qualitative and Quantitative Systematic Assessment. Surv. Ophthalmol. 2024, 69, 769–778. [Google Scholar] [CrossRef]
- Nakano, H.; Ōmura, S. Chemical Biology of Natural Indolocarbazole Products: 30 Years since the Discovery of Staurosporine. J. Antibiot. 2009, 62, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchiya, H.; Takahashi, Y.; Asuma, R. A New Alkaloid AM-2282 of Streptomyces Origin Taxonomy, Fermentation, Isolation and Preliminary Characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gani, O.A.B.S.M.; Engh, R.A. Protein Kinase Inhibition of Clinically Important Staurosporine Analogues. Nat. Prod. Rep. 2010, 27, 489. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Zarlenga, D.; Martin, J.C.; Geldhof, P.; Hallsworth-Pepin, K.; Mitreva, M. The Identification of Small Molecule Inhibitors with Anthelmintic Activities That Target Conserved Proteins among Ruminant Gastrointestinal Nematodes. mBio 2024, 15, e00095-24. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.; Sifaoui, I.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Lorenzo-Morales, J.; Piñero, J.E.; Díaz-Marrero, A.R.; Fernández, J.J. Antikinetoplastid Activity of Indolocarbazoles from Streptomyces Sanyensis. Biomolecules 2020, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Elardo, S.M.; Kozytska, S.; Bugni, T.S.; Ireland, C.M.; Moll, H.; Hentschel, U. Anti-Parasitic Compounds from Streptomyces Sp. Strains Isolated from Mediterranean Sponges. Mar. Drugs 2010, 8, 373–380. [Google Scholar] [CrossRef]
- Cartuche, L.; Reyes-Batlle, M.; Sifaoui, I.; Arberas-Jiménez, I.; Piñero, J.E.; Fernández, J.J.; Lorenzo-Morales, J.; Díaz-Marrero, A.R. Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces Sanyensis Cultures. Mar. Drugs 2019, 17, 588. [Google Scholar] [CrossRef]
- Cartuche, L.; Sifaoui, I.; Cruz, D.; Reyes-Batlle, M.; López-Arencibia, A.; Javier Fernández, J.; Díaz-Marrero, A.R.; Piñero, J.E.; Lorenzo-Morales, J. Staurosporine from Streptomyces Sanyensis Activates Programmed Cell Death in Acanthamoeba via the Mitochondrial Pathway and Presents Low in Vitro Cytotoxicity Levels in a Macrophage Cell Line. Sci. Rep. 2019, 9, 11651. [Google Scholar] [CrossRef]
- Dusinska, M.; Rundén-Pran, E.; Schnekenburger, J.; Kanno, J. Toxicity Tests: In Vitro and In Vivo. In Adverse Effects of Engineered Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 51–82. [Google Scholar]
- Ren, M.; Wu, X. Evaluation of Three Different Methods to Establish Animal Models of Acanthamoeba Keratitis. Yonsei Med. J. 2010, 51, 121. [Google Scholar] [CrossRef]
- Dwia Pertiwi, Y.; Chikama, T.; Sueoka, K.; Ko, J.-A.; Kiuchi, Y.; Onodera, M.; Sakaguchi, T. Efficacy of Photodynamic Anti-Microbial Chemotherapy for Acanthamoeba Keratitis In Vivo. Lasers Surg. Med. 2021, 53, 695–702. [Google Scholar] [CrossRef]
- González-Robles, A.; Omaña-Molina, M.; Salazar-Villatoro, L.; Flores-Maldonado, C.; Lorenzo-Morales, J.; Reyes-Batlle, M.; Arnalich-Montiel, F.; Martínez-Palomo, A. Acanthamoeba culbertsoni Isolated from a Clinical Case with Intraocular Dissemination: Structure and In Vitro Analysis of the Interaction with Hamster Cornea and MDCK Epithelial Cell Monolayers. Exp. Parasitol. 2017, 183, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; Navarro-García, F.; González-Robles, A.; Serrano-Luna, J.d.J.; Campos-Rodríguez, R.; Martínez-Palomo, A.; Tsutsumi, V.; Shibayama, M. Induction of Morphological and Electrophysiological Changes in Hamster Cornea after in Vitro Interaction with Trophozoites of Acanthamoeba spp. Infect. Immun. 2004, 72, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; González-Robles, A.; Iliana Salazar-Villatoro, L.; Lorenzo-Morales, J.; Cristóbal-Ramos, A.R.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; Méndez Cruz, A.R.; Martínez-Palomo, A. Reevaluating the Role of Acanthamoeba Proteases in Tissue Invasion: Observation of Cytopathogenic Mechanisms on MDCK Cell Monolayers and Hamster Corneal Cells. Biomed. Res. Int. 2013, 2013, 461329. [Google Scholar] [CrossRef]
- Salazar-Villatoro, L.; Chávez-Munguía, B.; Guevara-Estrada, C.E.; Lagunes-Guillén, A.; Hernández-Martínez, D.; Castelan-Ramírez, I.; Omaña-Molina, M. Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model. Pathogens 2023, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Chen, C.-H.; Chen, J.-W.; Lin, W.-C. Commensals Serve as Natural Barriers to Mammalian Cells during Acanthamoeba castellanii Invasion. Microbiol. Spectr. 2021, 9, e00512-21. [Google Scholar] [CrossRef] [PubMed]
- Teuchner, B.; Wibmer, I.D.; Schaumann, P.; Seifarth, C.; Walochnik, J.; Nagl, M. N-Chlorotaurine Inactivates Acanthamoeba and Candida Albicans in the Porcine Ex Vivo Corneal Infection Model. Cornea 2019, 38, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, S.; Wan, J.; Wang, D.; Pang, X.; Gao, Y.; Ni, N.; Chen, D.; Sun, X. Disturbing Cytoskeleton by Engineered Nanomaterials for Enhanced Cancer Therapeutics. Bioact. Mater. 2023, 29, 50–71. [Google Scholar] [CrossRef]
- Henriquez, F.L.; Ingram, P.R.; Muench, S.P.; Rice, D.W.; Roberts, C.W. Molecular Basis for Resistance of Acanthamoeba Tubulins to All Major Classes of Antitubulin Compounds. Antimicrob. Agents Chemother. 2008, 52, 1133–1135. [Google Scholar] [CrossRef]
- Omura, S.; Sasaki, Y.; Iwai, Y.; Takeshima, H. Staurosporine, a Potentially Important Gift from a Microorganism. J. Antibiot. 1995, 48, 535–548. [Google Scholar] [CrossRef]
- Hedberg, K.K.; Birrell, G.B.; Habliston, D.L.; Griffith, O.H. Staurosporine Induces Dissolution of Microfilament Bundles by a Protein Kinase C-Independent Pathway. Exp. Cell Res. 1990, 188, 199–208. [Google Scholar] [CrossRef]
- Xie, J.L.; O’Meara, T.R.; Polvi, E.J.; Robbins, N.; Cowen, L.E. Staurosporine Induces Filamentation in the Human Fungal Pathogen Candida Albicans via Signaling through Cyr1 and Protein Kinase A. mSphere 2017, 2, e00056-17. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Albuerne, M.; Domínguez, G.; Morán, J. Effect of Staurosporine in the Morphology and Viability of Cerebellar Astrocytes: Role of Reactive Oxygen Species and NADPH Oxidase. Oxid. Med. Cell Longev. 2014, 2014, 678371. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kang, J.-M.; Joo, S.-Y.; Song, S.-M.; Lê, H.G.; Thái, T.L.; Lee, J.; Goo, Y.-K.; Chung, D.-I.; Sohn, W.-M.; et al. Molecular and Biochemical Properties of a Cysteine Protease of Acanthamoeba castellanii. Korean J. Parasitol. 2018, 56, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Saurav, S.; Manna, S.K. Profilin Upregulation Induces Autophagy through Stabilization of AMP-activated Protein Kinase. FEBS Lett. 2022, 596, 1765–1777. [Google Scholar] [CrossRef]
- Pernier, J.; Shekhar, S.; Jegou, A.; Guichard, B.; Carlier, M.-F. Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility. Dev. Cell 2016, 36, 201–214. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Cristóbal-Ramos, A.R.; González-Lázaro, M.; Salinas-Moreno, E.; Méndez-Cruz, R.; Sánchez-Cornejo, M.; De la Torre-González, E.; Martínez-Palomo, A. Acanthamoeba castellanii: Morphological Analysis of the Interaction with Human Cornea. Exp. Parasitol. 2010, 126, 73–78. [Google Scholar] [CrossRef]
- Shing, B.; Balen, M.; McKerrow, J.H.; Debnath, A. Acanthamoeba Keratitis: An Update on Amebicidal and Cysticidal Drug Screening Methodologies and Potential Treatment with Azole Drugs. Expert. Rev. Anti Infect. Ther. 2021, 19, 1427–1441. [Google Scholar] [CrossRef]
- Megha, K.; Sharma, M.; Sharma, C.; Gupta, A.; Sehgal, R.; Khurana, S. Evaluation of in Vitro Activity of Five Antimicrobial Agents on Acanthamoeba Isolates and Their Toxicity on Human Corneal Epithelium. Eye 2022, 36, 1911–1917. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological Characteristics and Pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity Evaluation of Chlorhexidine Gluconate on Human Fibroblasts, Myoblasts, and Osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef]
- Härtel, S.; Zorn-Kruppa, M.; Tykhonova, S.; Alajuuma, P.; Engelke, M.; Diehl, H.A. Staurosporine-induced Apoptosis in Human Cornea Epithelial Cells In Vitro. Cytom. Part A 2003, 55, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jasso, M.; Hernández-Martínez, D.; Avila-Acevedo, J.G.; del Carmen Benítez-Flores, C.; Gallegos-Hernández, I.A.; García-Bores, A.M.; Espinosa-González, A.M.; Villamar-Duque, T.E.; Castelan-Ramírez, I.; del Rosario González-Valle, M.; et al. Morphological Description of the Early Events during the Invasion of Acanthamoeba castellanii Trophozoites in a Murine Model of Skin Irradiated under UV-B Light. Pathogens 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; Hernandez-Martinez, D.; Sanchez-Rocha, R.; Cardenas-Lemus, U.; Salinas-Lara, C.; Mendez-Cruz, A.R.; Colin-Barenque, L.; Aley-Medina, P.; Espinosa-Villanueva, J.; Moreno-Fierros, L.; et al. In Vivo CNS Infection Model of Acanthamoeba Genotype T4: The Early Stages of Infection Lack Presence of Host Inflammatory Response and Are a Slow and Contact-Dependent Process. Parasitol. Res. 2017, 116, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.G.; Smith, J.W.; Cohen, H.K.; Minner, J.R. Experimental Infection of Mice and Monkeys by Acanthamoeba. Am. J. Pathol. 1959, 35, 185–197. [Google Scholar]
- Rodríguez-Expósito, R.L.; Sifaoui, I.; Reyes-Batlle, M.; Fuchs, F.; Scheid, P.L.; Piñero, J.E.; Sutak, R.; Lorenzo-Morales, J. Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline. Antioxidants 2023, 12, 2081. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).