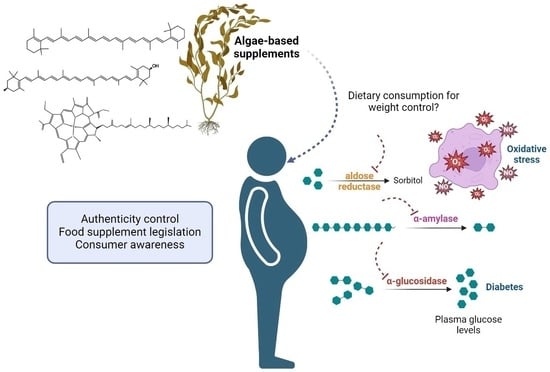
Algae-Based Supplements Claiming Weight Loss Properties: Authenticity Control and Scientific-Based Evidence on Their Effectiveness
Abstract
1. Introduction
2. Results and Discussion
2.1. Pigment Fingerprint
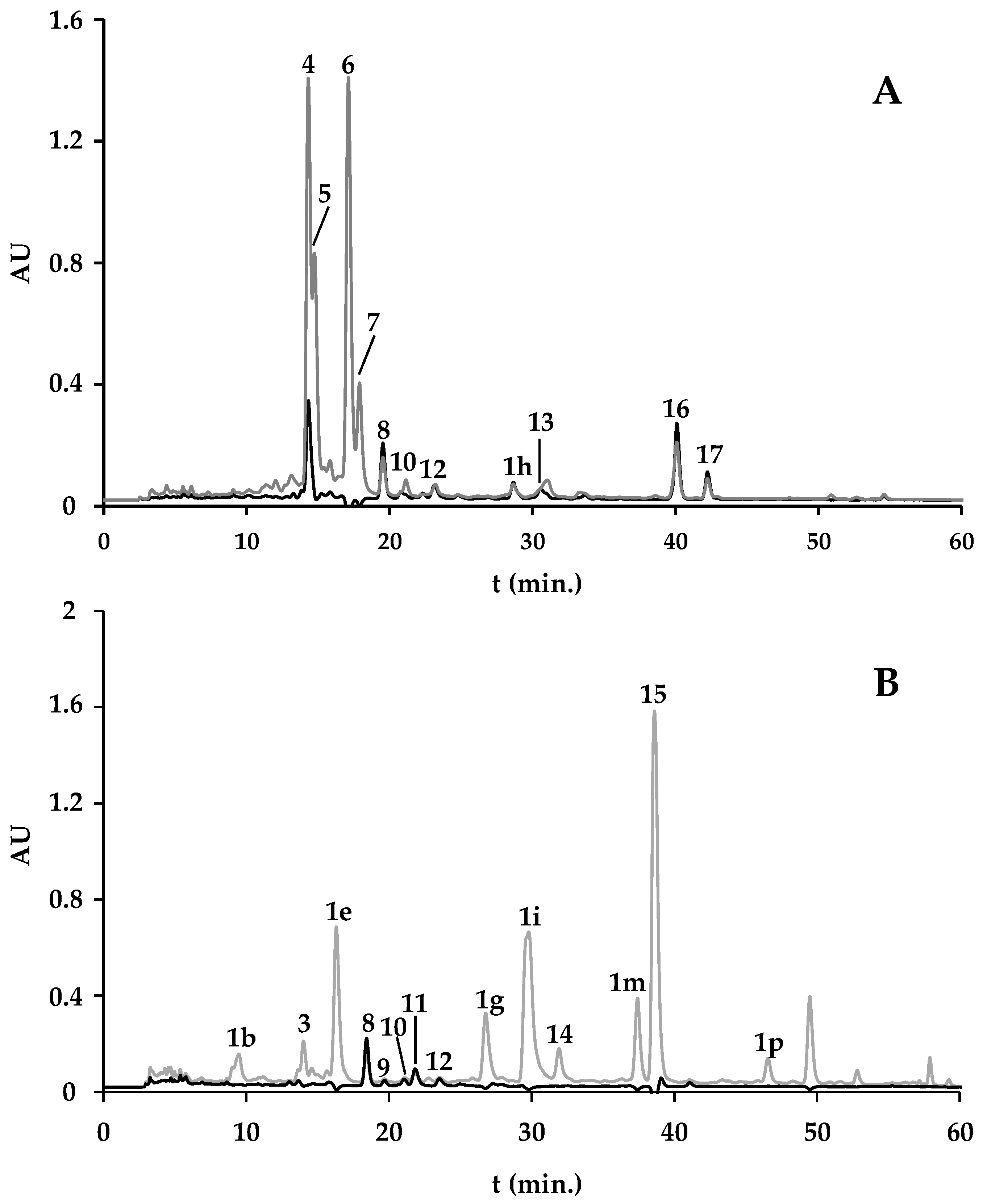
2.1.1. Spirulina and Spirulina-Based Supplements
2.1.2. F. vesiculosus and F. vesiculosus-Based Supplements
| Algae Material/Supplement | Pheoph. a der. (1a–1v) | Chlor. a (4) | Unkn. Chlor. (2, 3, 5, 6, 7, 14) | Zeax. (8) | Unkn. Xant. (9–12) | β-Carot. (16) | Unkn. Carot. (13, 17) | Pheoph. A (15) | Total | Extraction Yield 2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A. platensis from A | 3.04 (0.14) | 39.13 (0.94) | 92.86 (1.77) | 1.42 (0.09) | nq | 2.08 (0.35) | 1.37 (0.24) | − | 139.91 (0.14) | 4.28 (0.48) |
| Spirulina-based supplement from B | 9.99 (1.71) | − | 94.87 (1.14) | 0.82 (0.02) | nq | 0.62 (0.12) | 0.18 (0.03) | − | 106.48 (2.99) | 2.31 (0.12) |
| Spirulina-based supplement from C | 48.95 (4.56) | − | 5.95 (0.93) | 0.95 (0.03) | 1.11 (0.05) | − | − | 30.48 (0.48) | 87.45 (6.05) | 5.79 (0.39) |
| F. vesiculosus wild | 3.77 (0.08) | − | 0.24 (0.01) | − | − | − | − | 1.59 (0.08) | 5.82 (0.30) | 4.20 (0.13) |
| F. vesiculosus from aquaculture | 22.10 (1.46) | − | − | − | − | − | − | − | 22.10 (1.46) | 3.76 (0.14) |
| F. vesiculosus from D | 9.90 (0.28) | − | − | − | − | − | − | − | 9.90 (0.28) | 2.78 (0.04) |
| F. vesiculosus-based supplement from B | 4.34 (0.66) | − | − | − | − | − | − | − | 4.34 (0.66) | 3.28 (0.04) |
| F. vesiculosus-based supplement from E | nq | nq | nq | − | − | − | − | − | nq | * |
| F. vesiculosus & Spirulina-based supplement from F | 21.30 (2.87) | − | 86.21 (3.25) | 0.97 (0.15) | nq | 0.19 (0.00) | 3.52 (1.79) | − | 108.75 (4.94) | 4.48 (0.28) |
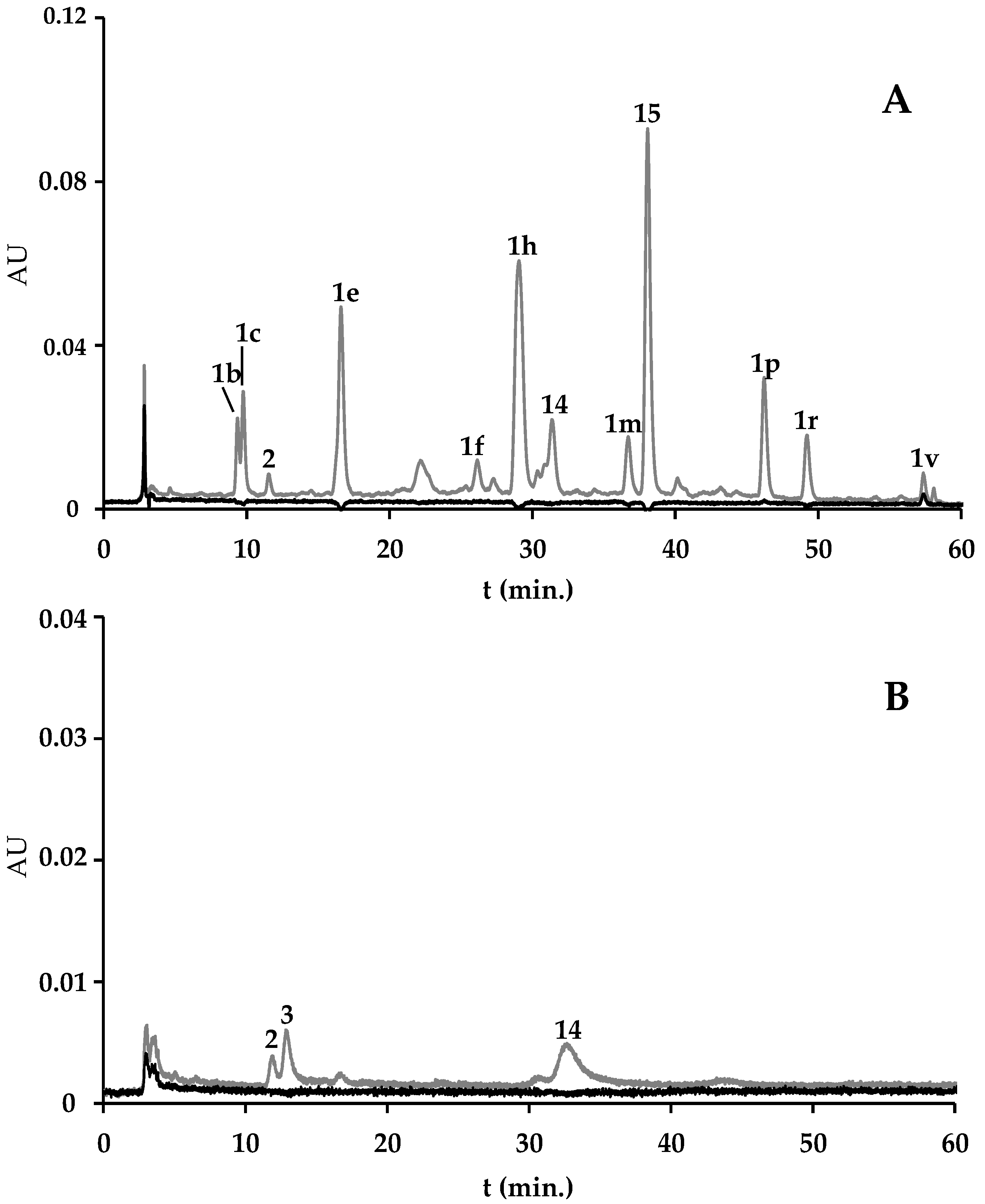
2.1.3. F. vesiculosus and Spirulina-Based Supplement
2.1.4. Labeling Analysis
| Algae Material/Supplement | Pharmaceutical Formulation | Instructions for Consumption/ Recommended Daily Dose | Daily Content (mg) of the Compounds Ingested Following the Supplier’s Recommendations | |||||
|---|---|---|---|---|---|---|---|---|
| Chlor. a | Zeax. | β-Carot. | Pheoph. a | Pheoph. a der. | Unkn. Chlor. | |||
| A. platensis from A | Powder | 1 teaspoon/day | 2.24 (0.05) | 0.08 (0.01) | 0.12 (0.02) | − | 0.17 (0.01) | 5.32 (0.10) |
| Spirulina-based supplement from B | Capsules | 2 capsules, 3 times/day | − | 0.07 (0.00) | 0.05 (0.01) | − | 0.75 (0.12) | 7.95 (0.93) |
| Spirulina-based supplement from C | Pills | 4 pills/day | − | 0.23 (0.01) | − | 7.37 (0.12) | 11.84 (1.10) | 1.44 (0.23) |
| F. vesiculosus-based supplement from B | Pills | 2 pills, 3 times/day | − | − | − | − | 0.51 (0.09) | − |
| F. vesiculosus-based supplement from E | Drops | 30 drops, 3 times/day | nq | − | − | − | − | nq |
| F. vesiculosus & Spirulina-based supplement from F | Capsules | 2 capsules, 3 times/day | − | 0.13 (0.02) | 0.03 (0.00) | − | 2.77 (0.37) | 11.22 (0.42) |
2.2. Total Phenolic Content (TPC)
| Sample | TPC (µg GAE/mg Dry Extract) |
|---|---|
| A. platensis from A | 6.50 ± 0.45 |
| Spirulina-based supplement from B | 6.85 ± 0.41 |
| Spirulina-based supplement from C | 7.80 ± 0.78 |
| F. vesiculosus wild | 8.96 ± 0.92 |
| F. vesiculosus aquaculture | 11.34 ± 0.02 |
| F. vesiculosus from D | 7.0 ± 0.39 |
| F. vesiculosus-based supplement from B | 6.56 ± 0.43 |
| F. vesiculosus-based supplement from E | 12.33 ± 0.19 |
| F. vesiculosus & spirulina-based supplement from F | 7.68 ± 0.29 |
2.3. Biological Assays
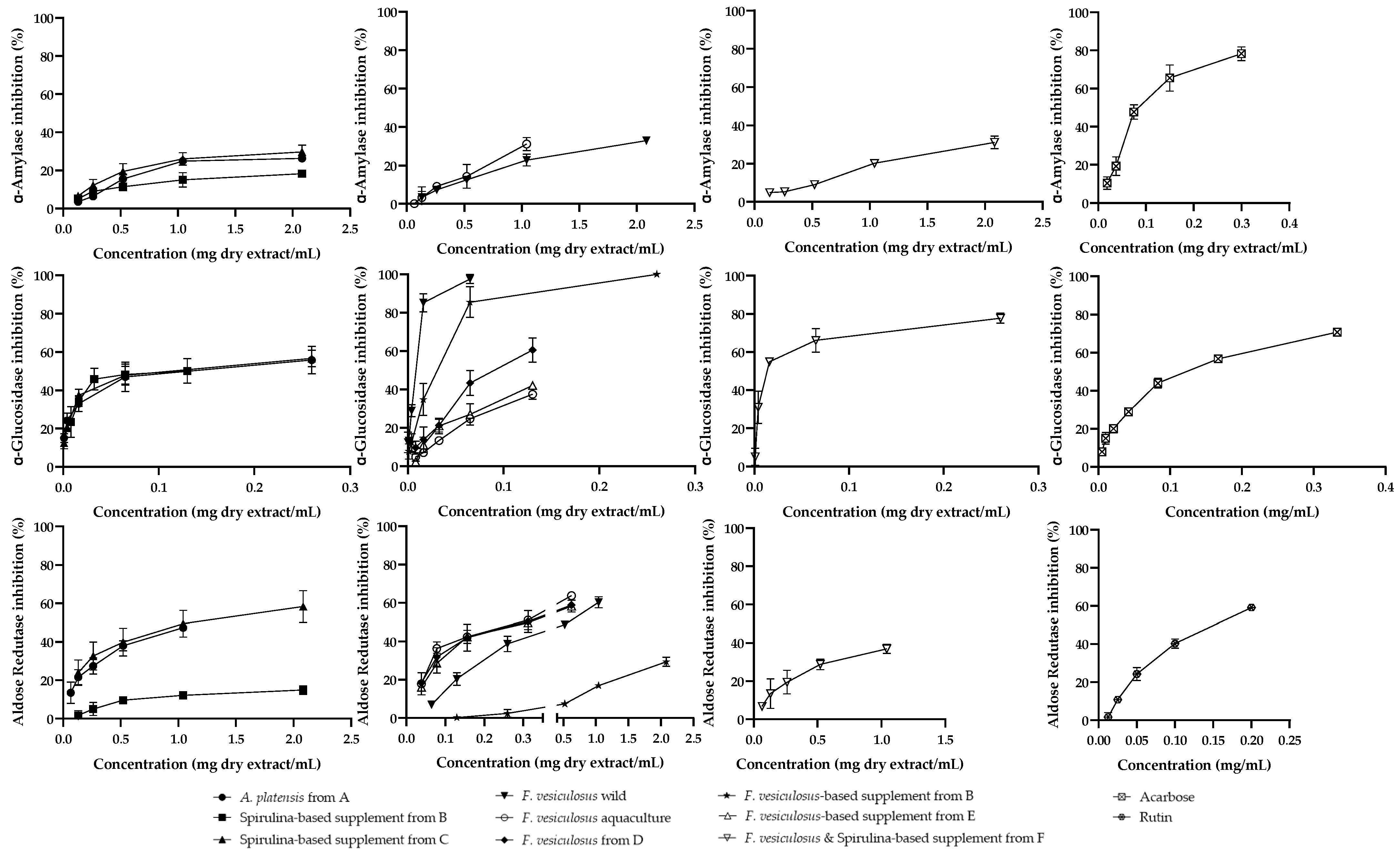
2.3.1. Metabolism of Carbohydrates
2.3.2. Aldose Reductase
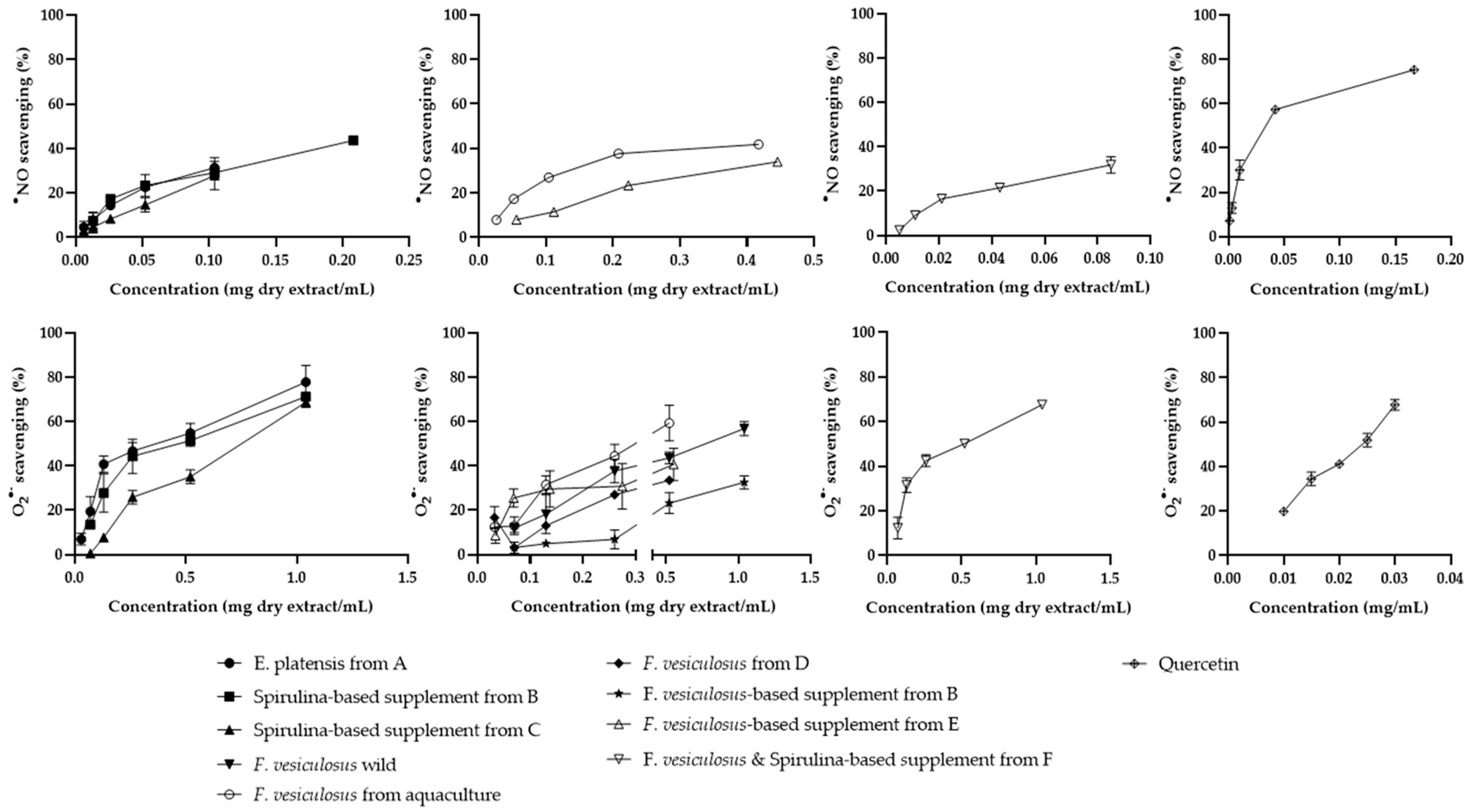
2.3.3. Antiradical Activity
3. Materials and Methods
3.1. Standards and Reagents
3.2. Sampling
3.3. Extraction
3.4. Chemical Analysis
3.4.1. HPLC-DAD Analysis
3.4.2. Linearity
3.4.3. Total Phenolic Content
3.5. Biological Assays
3.5.1. α-Glucosidase Inhibition
3.5.2. α-Amylase Inhibition
3.5.3. Aldose Reductase Inhibition
3.5.4. Superoxide Anion Radical Scavenging
3.5.5. Nitric Oxide Radical Scavenging
3.5.6. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Van Guilder, G.P.; Hoetzer, G.L.; Greiner, J.J.; Stauffer, B.L.; Desouza, C.A. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity 2006, 14, 2127–2131. [Google Scholar] [CrossRef]
- Gletsu-Miller, N.; Hansen, J.M.; Jones, D.P.; Go, Y.M.; Torres, W.E.; Ziegler, T.R.; Lin, E. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity 2009, 17, 439–446. [Google Scholar] [CrossRef]
- Tolochko, P.; Vadrot, A.B.M. Selective world-building: Collaboration and regional specificities in the marine biodiversity field. Environ. Sci. Policy 2021, 126, 79–89. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Bailey, R.L. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit. Ver. Food Sci. Nutr. 2020, 60, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Barbosa, M.; Oliveira, A.P.; Azevedo, I.C.; Sousa-Pinto, I.; Valentão, P.; Andrade, P.B. The pigments of kelps (Ochrophyta) as part of the flexible response to highly variable marine environments. J. Appl. Phycol. 2016, 28, 3689–3696. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Abd El Baky, H.; El-Baz, F.; El Baroty, G. Spirulina Species as a Source of Carotenoids and α-Tocopherol and its anticarcinoma factors. Biotechnology 2003, 2, 222–240. [Google Scholar] [CrossRef]
- Davani, L.; Terenzi, C.; Tumiatti, V.; De Simone, A.; Andrisano, V.; Montanari, S. Integrated analytical approaches for the characterization of Spirulina and Chlorella microalgae. J. Pharm. Biomed. Anal. 2022, 219, 114943. [Google Scholar] [CrossRef]
- Anniva, C.; Grigoriadou, D.; Psomiadou, E.; Tsimidou, M. Pheophytin α degradation products as useful indices in the quality control of virgin olive oil. J. Am. Oil Chem. Soc. 2006, 83, 371–375. [Google Scholar] [CrossRef]
- Colla, L.M.; Bertol, C.D.; Ferreira, D.J.; Bavaresco, J.; Costa, J.A.V.; Bertolin, T.E. Thermal and photo-stability of the antioxidant potential of Spirulina platensis powder. Braz. J. Biol. 2017, 77, 332–339. [Google Scholar] [CrossRef]
- Hurd, C.; Harrison, P.J.; Bischof, K.; Lobban, C. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Andersen, G.S.; Pedersen, M.F.; Nielsen, S.L. Temperature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). J. Phycol. 2013, 49, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Fernandes, F.; Pereira, D.M.; Azevedo, I.C.; Sousa-Pinto, I.; Andrade, P.B.; Valentão, P. Fatty acid patterns of the kelps Saccharina latissima, Saccorhiza polyschides and Laminaria ochroleuca: Influence of changing environmental conditions. Arab. J. Chem. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Boderskov, T.; Schmedes, P.; Bruhn, A.; Rasmussen, M.; Nielsen, M.; Pedersen, M. The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. J. Appl. Phycol. 2015, 28, 1153–1165. [Google Scholar] [CrossRef]
- Martins, C.D.L.; Lhullier, C.; Ramlov, F.; Simonassi, J.C.; Gouvea, L.P.; Noernberg, M.; Maraschin, M.; Colepicolo, P.; Hall-Spencer, J.M.; Horta, P.A. Seaweed chemical diversity: An additional and efficient tool for coastal evaluation. J. Appl. Phycol. 2014, 26, 2037–2045. [Google Scholar] [CrossRef]
- Olischläger, M.; Iñiguez, C.; Gordillo, F.J.; Wiencke, C. Biochemical composition of temperate and Arctic populations of Saccharina latissima after exposure to increased pCO2 and temperature reveals ecotypic variation. Planta 2014, 240, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, T.; Kautsky, L.; Argyrou, M. Dominant chlorophylls and carotenoids in macroalgae of the Baltic Sea (Baltic proper): Their use as potential biomarkers. Sarsia 1997, 82, 55–62. [Google Scholar] [CrossRef]
- Heavisides, E.; Rouger, C.; Reichel, A.F.; Ulrich, C.; Wenzel-Storjohann, A.; Sebens, S.; Tasdemir, D. Seasonal Variations in the Metabolome and Bioactivity Profile of Fucus vesiculosus Extracted by an Optimised, Pressurised Liquid Extraction Protocol. Mar. Drugs 2018, 16, 503. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Agustini, T.; Rosyadi, S.; Wardani, A. Effects of Different Heat Processing on Fucoxanthin, Antioxidant Activity and Colour of Indonesian Brown Seaweeds. IOP Conf. Ser. Environ. Earth Sci. 2017, 55, 012063. [Google Scholar] [CrossRef]
- Ulbricht, C.; Bramwell, R.; Catapang, M.; Giese, N.; Isaac, R.; Le, T.D.; Montalbano, J.; Tanguay-Colucci, S.; Trelour, N.J.; Weissner, W.; et al. An evidence-based systematic review of chlorophyll by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014, 11, 198–239. [Google Scholar] [CrossRef]
- Egner, P.A.; Muñoz, A.; Kensler, T.W. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat. Res. 2003, 523–524, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Wang, J.B.; Zhu, Y.R.; Zhang, B.C.; Wu, Y.; Zhang, Q.N.; Qian, G.-S.; Kuang, S.-Y.; Gange, S.J.; Jacobson, L.P.; et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef]
- Yoshida, A.; Yokono, O.; Oda, T. Therapeutic effect of chlorophyll-a in the treatment of patients with chronic pancreatitis. Gastroenterol. Jpn. 1980, 15, 49–61. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.; Valentão, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N. A review of α-amylase inhibitors on weight loss and glycemic control in pathological state such as obesity and diabetes. Comp. Clin. Path. 2016, 25, 1253–1264. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhat, A.G.; OKeefe, J. Effects of spirulina on weight loss and blood lipids: A review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef]
- Gabbia, D.; Saponaro, M.; Sarcognato, S.; Guido, M.; Ferri, N.; Carrara, M.; De Martin, S. Fucus vesiculosus and Ascophyllum nodosum Ameliorate Liver Function by Reducing Diet-Induced Steatosis in Rats. Mar. Drugs 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae-a Mini Review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef]
- Thiagarajan, D.; Quadri, N.; Jawahar, S.; Zirpoli, H.; Del Pozo, C.H.; López-Díez, R.; Hasan, S.N.; Yepuri, G.; Gugger, P.F.; Finlin, B.S.; et al. Aldose reductase promotes diet-induced obesity via induction of senescence in subcutaneous adipose tissue. Obesity 2022, 30, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 2), 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Duraisamy, R.; Verma, D.; Kumar, A.; Kumar, N.; Kanak, K.R.; Marwein, B.M.; Mohan, K. Antioxidant and phytonutrient activities of Spirulina platensis. Energy Nexus 2022, 6, 100070. [Google Scholar] [CrossRef]
- Les, F.; Valero, M.S.; Moliner, C.; Weinkove, D.; López, V.; Gómez-Rincón, C. Jasonia glutinosa (L.) DC., a Traditional Herbal Tea, Exerts Antioxidant and Neuroprotective Properties in Different In Vitro and In Vivo Systems. Biology 2021, 10, 443. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar. Drugs 2020, 18, 486. [Google Scholar] [CrossRef]
- Barbosa, M.; Fernandes, F.; Carlos, M.J.; Valentão, P.; Andrade, P.B. Adding value to marine invaders by exploring the potential of Sargassum muticum (Yendo) Fensholt phlorotannin extract on targets underlying metabolic changes in diabetes. Algal Res. 2021, 59, 102455. [Google Scholar] [CrossRef]
| Sample | Origin | Data of Collection */Purchase | Voucher Label |
|---|---|---|---|
| A. platensis powdered | Supplier A | November 2021 | Artplat_A_Nov21 |
| Spirulina-based supplement | Supplier B | Spir-Supplem_B_Nov21 | |
| Spirulina-based supplement | Supplier C | Spir-Supplem_C_Nov21 | |
| Wild F. vesiculosus | Praia Norte | December 2018 | Fves_Wild_Dec21 |
| F. vesiculosus from aquaculture | AlgaPlus | December 2021 | Fves_Aquac_Dec21 |
| Commercial F. vesiculosus | Supplier D | November 2021 | Fves_D_Nov21 |
| F. vesiculosus-based supplement | Supplier B | Fvesic-Supplem_B_Nov21 | |
| F. vesiculosus-based supplement | Supplier E | Fves-Supplem_E_Nov21 | |
| F. vesiculosus & Spirulina-based supplement | Supplier F | Fves&Spir-Supplem_F_Nov21 |
| Compound | Regression Equation (mg/mL) | r2 | Linearity (mg/mL) |
|---|---|---|---|
| Chlorophyll a | y = 9.72 × 108x + 1149069.1 | 0.995 | 0.00078–0.100 |
| Chlorophyll b | y = 8.94 × 108x + 2137228.1 | 0.997 | 0.00078–0.100 |
| Zeaxanthin | y = 3.91 × 109x + 1172649.0 | 0.999 | 0.00041–0.106 |
| β-Carotene | y = 4.33 × 109x + 2869689.8 | 0.999 | 0.0001–0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.; Martins, R.; Barbosa, M.; Valentão, P. Algae-Based Supplements Claiming Weight Loss Properties: Authenticity Control and Scientific-Based Evidence on Their Effectiveness. Mar. Drugs 2024, 22, 123. https://doi.org/10.3390/md22030123
Fernandes F, Martins R, Barbosa M, Valentão P. Algae-Based Supplements Claiming Weight Loss Properties: Authenticity Control and Scientific-Based Evidence on Their Effectiveness. Marine Drugs. 2024; 22(3):123. https://doi.org/10.3390/md22030123
Chicago/Turabian StyleFernandes, Fátima, Raquel Martins, Mariana Barbosa, and Patrícia Valentão. 2024. "Algae-Based Supplements Claiming Weight Loss Properties: Authenticity Control and Scientific-Based Evidence on Their Effectiveness" Marine Drugs 22, no. 3: 123. https://doi.org/10.3390/md22030123
APA StyleFernandes, F., Martins, R., Barbosa, M., & Valentão, P. (2024). Algae-Based Supplements Claiming Weight Loss Properties: Authenticity Control and Scientific-Based Evidence on Their Effectiveness. Marine Drugs, 22(3), 123. https://doi.org/10.3390/md22030123