Abstract
Microbes such as the White Spot Syndrome Virus account for severe losses in the shrimp farming industry globally. This review examines the literature on the mangrove plants of Asia and the Pacific with antibacterial, antifungal, or antiviral activities. All of the available data published on this subject were collected from Google Scholar, PubMed, Science Direct, Web of Science, ChemSpider, PubChem, and a library search from 1968 to 2022. Out of about 286 plant species, 119 exhibited antimicrobial effects, and a total of 114 antimicrobial natural products have been identified including 12 with MIC values below 1 µg/mL. Most of these plants are medicinal. The mangrove plants of Asia and the Pacific yield secondary metabolites with the potential to mitigate infectious diseases in shrimp aquaculture.
Keywords:
mangrove plants; shrimp farming; natural products; antibacterial; antifungal; antiviral; Asia; Pacific 1. Introduction
The global shrimp and prawn aquaculture industry is regularly threatened by outbreaks of microbial infections [1] that require antibiotics, antifungals, and antiviral agents participating in the selection of multidrug-resistant strains of microbes, pausing the grim scenario of the emergence of a “superbug” that could wipe out the global supply of penaeids [2]. In this context, there is an urgent necessity to search for antimicrobial agents with original chemical frameworks, and such molecules could come from the flora of Asia and the Pacific, which is the oldest, largest, and richest on Earth, especially seashores, tidal rivers, and mangrove plants.
Mangroves are ecosystems of the tropical and subtropical seashores, estuaries, and tidal rivers characterized by a halophytic flora of mainly trees and shrubs divided into true mangrove or mangrove-associated species. True mangrove species are restricted to mangroves whereas mangrove-associated species are found along the seashores, and even inland. There are estimates of about 54 true mangrove plant species and 60 mangrove-associated species globally, which are home to shrimps, prawns, crabs, and fish [3]. Most mangrove species grow in Asia and the Pacific [4]. Examples of true mangrove plant species are Excoecaria agallocha L. (land zone), Bruguiera gymnorhiza (L.) Savigny, Rhizophora stylosa Griff. (intermediate zone), Avicennia alba Bl, and Aegiceras corniculatum (L.) Blanco (fringing zone) [5]. Even though most of the global fish catches are directly or indirectly dependent on mangroves, these are on their way to extinction due to logging, agriculture, aquaculture, and urbanization, with an estimate of about 2–8% loss of surface per year [6]. Shrimps, prawns, and fish farming are the greatest threat to mangroves with, for example, approximately half of the 279,000 ha of mangroves in the Philippines lost from 1951 to 1988 [7]. Another aggravating factor is global warming, and consequently, a rise in sea levels that interfere with the growth of true mangrove plants.
Most plants in mangroves are Angiosperms organized phylogenetically into 11 major taxa or clades organized in three groups: (i) Basal Angiosperms: Protomagnoliids, Magnoliids, Monocots, Eudicots; (ii) Core Angiosperms: Core Eudicots, Rosids, Fabids, Malvids; and (iii) Upper Angiosperms: Asterids, Lamiids, and Campanulids. Within each clade, plants yield specific secondary metabolites to control and even communicate with phytopathogenic bacteria and fungi. Plants are challenged by phytopathogenic bacteria, fungi, and viruses and produce a vast array of antimicrobial secondary metabolites [8]. These antimicrobial principles fall into two main categories: phytoanticipins and phytoalexins. Phytoanticipins are antimicrobials present in plant tissues before pathogen challenges or inactive immediate precursors of phytoalexins [8].
Phytoanticipins and phytoalexins are mainly either phenolics, terpenes, or alkaloids with various levels of solubility in water and are extractable with water, polar organic (methanol, ethanol), mid-polar solvents (chloroform, dichloromethane, ethyl acetate), and non-polar solvents (hexane, petroleum ether) [9]. The measurement of the antibacterial and antifungal strength of extracts and secondary metabolites in vitro is quantitatively based on the minimum inhibiting concentration (MIC) and several thresholds of activity have been proposed [10]. Qualitatively, antibacterial and antifungal strength are appreciated by halos developed around a paper disc or an agar well expressed in the inhibition zone diameter (IZD) [10].
Colette et al. (2022) noted that the presence of Atriplex jubata S. Moore evoked some levels of remediation in the shrimp farms of New Caledonia [11] and this review aims to attempt to answer the following points: What is the current knowledge on the distribution of antibacterial, antifungal, and antiviral principles from the mangrove plants of Asia and the Pacific? What are the strongest antimicrobial principles isolated thus far from these plants? What is the spectrum of activity of the antimicrobial principles? What are the medicinal values of these plants? What is the potential usefulness of these plants as remediation of shrimp farming? We hypothesize that a shrimp or prawn farming system preserving healthy mangroves could be a mean to solve the increasing problem of infection.
3. Antimicrobial Extracts and Compounds from Mangrove and Mangrove-Associated Plants with the Potential to Be Used for Shrimp Farming
According to Kuete (2010), crude extracts with MIC values less than 100 µg/mL are antimicrobial [10]. Here, we define a very strongly active extract with a MIC value below 10 µg/mL. An isolated compound is defined as very strongly active for a MIC value below or equal to 1 µg/mL (as well as less than 1 µg/thin layer chromatography), strongly antibacterial (or antifungal) for a MIC value above 1 µg/mL and equal to or below 50 µg/mL, moderately antibacterial (or antifungal) for a MIC from 50 and below 100 µg/mL, weakly antibacterial (or antifungal) for a MIC from 100 and below 500 µg/mL, very weakly antibacterial (or antifungal) for a MIC ranging from 500 to below 2500 µg/mL, and inactive for a MIC value above 2500 µg/mL.
For antiviral principles, we suggest that a compound with an IC50 value below or equal to 1 µg/mL is very strongly active, for an IC50 value above 1 and equal to or below 20 µg/mL strongly antiviral, for an IC50 above 20 and below or equal to 100 µg/mL moderately antiviral, for an IC50 above 100 and below or equal to 500 µg/mL weakly antiviral, for an IC50 ranging from above 500 to below or equal to 2500 µg/mL very weakly antiviral, and inactive with an IC50 value above 2500 µg/mL.
Using these criteria, the strongest antimicrobial extracts from mangrove and mangrove-associated plants that could be of value for shrimp farming are from C. inophyllum (S. aureus, T. rubrum) [63], T. catappa (E. faecalis) [129]., C. manghas (E. coli, P. aeruginosa, VSV) [17,220], and C. odollam (HSV) [223].
The strongest antimicrobial principles identified from the mangrove and mangrove-associated plants that could be of value for shrimp farming are as follows (Figure 1):
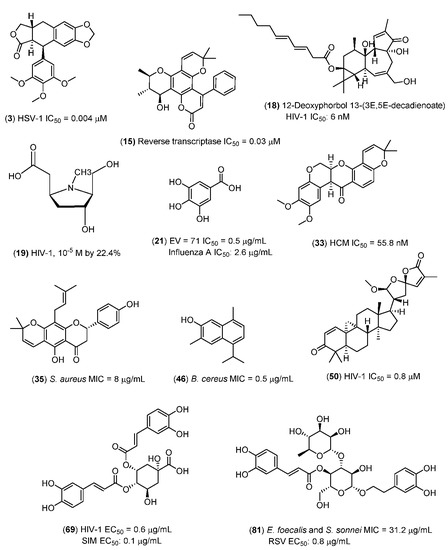
Figure 1.
Natural products from mangrove plants with very strong antimicrobial activities.
(i) Antibacterial: Lupinifolin (35) (Gram-positive and Gram-negative) [116]; 7-hydroxycadalene (46) [152].
(ii) Antifungal: Lupinifolin (35) (Yeasts) [116].
(iii) Antiviral: Naringenin (9) [50], verbascoside (81) [248], inophyllum B (15) [61], 12-deoxyphorbol 13-(3E,5E-decadienoate) (18) [69], 5β-carboxymethyl-3α-hydroxy-2β-hydroxymethyl-1- methylpyrrolidine (19) [70], deguelin (33) [117], deoxypodophyllotoxin (3) [25,116] (9R,10R, 23R)-21,23:23,27-diepoxycycloarta-1,24-diene-3,27-dione (49) [156], gallic acid (21) [83], and 4,5-di-O-caffeoylquinic acid (69).
(iv) We note that most of these principles are hydrophilic or amphiphilic (Figure 1).
4. Spectrum of Activity of Antimicrobial Extracts and Principles from Mangrove and Mangrove-Associated Plants
The following observations can be made:
- (i)
- No reports on the only lycopod associated with mangrove are available.
- (ii)
- Of the 26 ferns, nine had antibacterial effects and six are antifungal, and no antiviral activities were reported. The only antimicrobial principle from ferns thus far is the strong antibacterial (Gram-positive) stenopalustroside A [13] (Table 1).
- (iii)
- The cycad associated with mangroves has antibacterial effects.
- (iv)
- No reports on the only pine tree associated with mangrove are available.
- (v)
- Of the 51 monocots, 11 displayed antimicrobial effects, of which eight had antibacterial activity, six with antiviral activity, and none reported with antiviral properties. Active principles isolated were phenolics such as the flavanol naringenin (9) in the Pandanaceae, antibacterial, antifungal, and antiviral orchidaceous phenanthrenes as well as the flavones and antifungal hydroxycinnamic acid of Poaceae (Table 2)
- (vi)
- Of the 207 dicots, 92 had antimicrobial effects including 78 antibacterial, 39 antifungal, and 25 antiviral effects. A total of about 80 antimicrobial principles were isolated (Table S2).
- (vii)
- Aqueous and organic polar extracts of plants from the mangrove presented activity against Gram-positive and Gram-negative bacteria, filamentous fungi and yeasts, enveloped and non-enveloped viruses, DNA, and RNA viruses (Table S2).
- (viii)
- The extract of P. pinnata [262] and gallic acid (21) abounds notably in the true mangrove trees Rhizophora apiculata Bl. and Aegiceras corniculatum (L.) Blanco is a protected shrimp against WSSV [84] as well as an aqueous extract of the true mangrove tree C. tagal (Perr.) C.B. Rob. [263]
5. Medicinal Use of Mangrove and Mangrove-Associated Plants
One could suggest the use of medicinal plants as a more sustainable alternative to chemotherapy in paenid aquaculture. Therefore, the possible beneficial effect of mangrove and mangrove-associated plants for the sanitation of shrimp farms is reinforced by the observation that 85 plants were used for the treatment of infectious diseases including mainly diarrhea, dysentery, and wounds [264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294] (Table S1). The pharmacological effect of these plants involves active principles that are potentially able to act on paenids, which could be examined further.
6. Mangrove and Mangrove-Associate Plants as Remediation of Shrimp Farming?
Shrimp and prawn farms are regularly affected by (+)-RNA viruses such as the Taura syndrome virus, Yellow head virus, and Gill-associated virus as well as DNA viruses (WSSV, Monodon Baculovirus) and Gram-negative bacteria such as Hepatobacterium penaei and Vibrio spp. [295]. Synthetic drugs are being used in an attempt to evade economic losses but threaten the environment and contribute to the selection of multidrug-resistant pathogenic microorganisms. Being able to produce antimicrobial principles (some of them water soluble like ellagic acid), mangrove and mangrove-associated plants could be used as a source of natural agents and/or afford ecological systems to combat the infections with shrimps and prawns. Polar organic and aqueous extracts of most mangrove and mangrove-associated plants exhibit broad-spectrum antibacterial, antifungal, or antiviral properties in vitro, suggesting that antimicrobial secondary metabolites from plants and plant litter in the sea and brackish waters could afford some control against the overgrowth of pathogenic microbes. Of note, P. pinnata ethanol extract of leaves given to Penaeus monodon as part of feed at the dose of 300 μg/g of body weight/day evoked some levels of protection against WSSV [262]. Gallic acid (21), which abounds notably in the true mangrove trees R. apiculata and A. corniculatum is strongly antiviral and protected shrimps against WSSV [84]. Gallic acid (21) may, at least in part, account for the fact the aqueous extract of the true mangrove tree C. tagal given at the dose of 10% of the body weight, twice a day, protected shrimps against WSSV [263]. Furthermore, gallic acid (21) decreases microbial proliferation in mangrove soil [296] as well as the growth of microalgae [297], which contribute to a decreased production in shrimp aquaculture [298], at least in part, to the alteration in the shrimp’s immune system [299]. The control of pathogenic bacteria may have some beneficial effects for the symbiotic bacteria of shrimp against pathogenic microorganisms [300]. Furthermore, phenolic acids from mangrove and mangrove-associated plants could, by chelation, protect shrimps against toxic metals including cadmium [301,302]. Therefore, it is possible to extend the protective effect of mangrove and mangrove-associated plants to fisheries and crab farming, the latter being affected by Vibrio species [165]. Another interesting feature of mangrove plants is that they are a host for microorganisms for Actinomyces producing antibacterial principles [303].
7. Conclusions
Plants from the Mangroves of Asia and the Pacific produce a vast array of antimicrobial secondary metabolites that could be further examined for their possible development into medications to control microbial outbreaks in aquaculture. In parallel, growing plant mangroves in aquacultures and promoting mangrove-associated aquaculture could be beneficial. The rise in the global population and the imperative to supply shrimps, prawns, crabs, and fish globally requires the preservation of mangroves.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20100643/s1, Figure S1: Natural products from the plants of the mangroves, tidal rivers, and the seashores of Asia and the Pacific; Table S1: Plants from the mangroves, tidal rivers, and the seashores of Asia and the Pacific, Table S2: Antimicrobial activity of extracts and isolates from the Dicotyledons from the mangroves, tidal rivers, and the seashores of Asia and the Pacific.
Author Contributions
Conceptualization, C.W., M.S. (Mazdida Sulaiman), and V.N.; Methodology, C.W.; Validation, M.R. (Mohammed Rahmatullah), M.R. (Mogana Rajagopal), A.K.P. and V.N.; Formal analysis, M.S. (Mazdida Sulaiman) and M.S. (Monica Suleiman); Investigation, J.S.S.S. and N.A.R.; Resources, C.W.; Writing—original draft preparation, C.W. and M.Sulaiman; Writing—review and editing, V.N., M.R. (Mohammed Rahmatullah), A.K.P., M.R. (Mogana Rajagopal), M.R. (Mohammed Rahmatullah), N.A.R., J.S.S.S., C.W., Z.A.Z. and M.S. (Monica Suleiman); Visualization, M.S. (Mazdida Sulaiman) and C.W.; Supervision, C.W.; Project administration, C.W.; Funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lightner, D.V.; Redman, R.M.; Pantoja, C.R.; Tang, K.F.J.; Noble, B.L.; Schofield, P.; Mohney, L.L.; Nunan, L.M.; Navarro, S.A. Historic emergence, impact and current status of shrimp pathogens in the Americas. J. Invertebr. Pathol. 2012, 110, 174–183. [Google Scholar] [CrossRef]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquacult. 2020, 12, 966–986. [Google Scholar] [CrossRef]
- Giesen, W.; Wulfraat, S.; Zieren, M.; Scholten, L. Mangrove Guidebook for Southeast Asia; FAO: Bangkok, Thailand; Wetlands International: Wageningen, The Netherlands, 2007. [Google Scholar]
- Ricklefs, R.E.; Latham, R.E. Global patterns of diversity in mangrove floras. In Species Diversity in Ecological Communities: Historical and Geographical Perspectives; University of Chicago Press: Chicago, IL, USA, 1993; pp. 215–229. [Google Scholar]
- Stewart, M.; Fairfull, S. Mangroves. In Industries; NDOP, Ed.; NSW Government: Parramatta, Sydney, 2008. [Google Scholar]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Rafael, A.; Calumpong, H.P. Fungal infections of mangroves in natural forests and reforestation sites from Philippines. Aquacul Aquar. Conserv. Legisl. 2019, 12, 2062–2074. [Google Scholar]
- Tiku, A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Colette, M.; Guentas, L.; Gunkel-Grillon, P.; Callac, N.; Della Patrona, L. Is halophyte species growing in the vicinity of the shrimp ponds a promising agri-aquaculture system for shrimp ponds remediation in New Caledonia? Marine Pollution Bull. 2022, 177, 13563. [Google Scholar] [CrossRef]
- Zuraini, Z.; Sasidharan, S.; Kaur, S.R.; Nithiyayini, M. Antimicrobial and antifungal activities of local edible fern Stenochlaena palustris (Burm. F.) Bedd. Pharmacol.Online 2010, 1, 233–237. [Google Scholar]
- Liu, H.; Orjala, J.; Sticher, O.; Rali, T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J. Nat. Prod. 1999, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Padhy, R.; Dash, S.K. Antibacterial evaluation of methanolic Rhizome extract from an in vivo and in vitro grown Pteridophyte, Drynaria quercifolia (Linn.) J. Smith. Asian J. Pharm. Clin. Res. 2015, 8, 130–138. [Google Scholar]
- Amoroso, V.B.; Antesa, D.A.; Buenavista, D.P.; Coritico, F.P. Antimicrobial, antipyretic, and anti-inflammatory activities of selected Philippine medicinal pteridophytes. Asian J. Biodivers. 2014, 5, 1. [Google Scholar] [CrossRef][Green Version]
- Somchit, M.N.; Hassan, H.; Zuraini, A.; Chong, L.C.; Mohamed, Z.; Zakaria, Z.A. In vitro anti-fungal and anti-bacterial activity of Drymoglossum piloselloides L. Presl. against several fungi responsible for athletes foot and common pathogenic bacteria. Afr. J. Microbiol. Res. 2011, 5, 3537–3541. [Google Scholar]
- Frankova, A.; Vistejnova, L.; Merinas-Amo, T.; Leheckova, Z.; Doskocil, I.; Soon, J.W.; Kudera, T.; Laupua, F.; Alonso-Moraga, A.; Kokoska, L. In vitro antibacterial activity of extracts from Samoan medicinal plants and their effect on proliferation and migration of human fibroblasts. J. Ethnopharmacol. 2021, 264, 113220. [Google Scholar] [CrossRef]
- Desnilasari, D.; Iwansyah, A.C.; Fauziah, R. From local wisdom: Preliminary antibacterial activity of “Tanduk rusa” Fern (Platycerium coronarium). In Proceedings of the 1st International Conference on Appropriate Technology Development 2015, Bandung, Indonesia, 5–7 October 2015. [Google Scholar]
- Thomas, T. In vitro evaluation of antibacterial activity of Acrostichum aureum Linn. Ind. J. Nat. Prod. 2012, 3, 135–138. [Google Scholar]
- Shamsuddin, A.A.; Najiah, M.; Suvik, A.; Azariyah, M.N.; Kamaruzzaman, B.Y.; Effendy, A.W.; John, B.A. Antibacterial properties of selected mangrove plants against Vibrio species and its cytotoxicity against Artemia salina. World Appl. Sci. J. 2013, 25, 333–340. [Google Scholar]
- Saad, S.; Taher, M.; Susanti, D.; Qaralleh, H.; Awang, A.F.I.B. In vitro antimicrobial activity of mangrove plant Sonneratia alba. Asian Pacific J. Trop. Biomed. 2012, 2, 427–429. [Google Scholar] [CrossRef]
- Khan, A.V.; Ahmed, Q.U.; Khan, A.A.; Shukla, I. Antibacterial activity of Cycas rumphii Miq. leaves extracts against some tropical human pathogenic bacteria. Res. J. Microbiol. 2011, 6, 761. [Google Scholar] [CrossRef]
- Amin, E. Phytochemical content and biological activity of the genus Cycas, Family Cycadaceae: A review. Pharm. Sci. Asia 2021, 4, 300–319. [Google Scholar]
- Puvanendran, S.; Wickramasinghe, A.; Karunaratne, D.N.; Carr, G.; Wijesundara, D.S.A.; Andersen, R.; Karunaratne, V. Antioxidant constituents from Xylopia championii. Pharm. Biol. 2008, 46, 352–355. [Google Scholar] [CrossRef][Green Version]
- Sudo, K.; Konno, K.; Shigeta, S.; Yokota, T. Inhibitory effects of podophyllotoxin derivatives on herpes simplex virus replication. Antiv. Chem. Chemothery 1998, 9, 263–267. [Google Scholar] [CrossRef]
- Padmaja, V.; Thankamany, V.; Hara, N.; Fujimoto, Y.; Hisham, A. Biological activities of Annona glabra. J. Ethnopharmacol. 1995, 48, 21–24. [Google Scholar] [CrossRef]
- Uddin, S.J.; Rouf, R.; Shilpi, J.A.; Alamgir, M.; Nahar, L.; Sarker, S.D. Screening of some Bangladeshi medicinal plants for in vitro antibacterial activity. Orient. Pharm. Exp. Med. 2008, 8, 316–321. [Google Scholar] [CrossRef]
- Goshwami, D.; Rahman, M.M.; Muhit, M.A.; Islam, M.S.; Anasri, M. Antioxidant property, cytotoxicity and antimicrobial activity of Lasia spinosa leaves. Nepal J. Sci. Technol. 2012, 13, 215–218. [Google Scholar] [CrossRef]
- Mondal, B.; Hore, M.; Baishakhi, F.S.; Ramproshad, S.; Sadhu, S.K. Study of antioxidant, antidiabetic and antibacterial activities of mangrove plant Phoenix paludosa. Asian J. Med. Health Res. 2018, 3, 12. [Google Scholar]
- Paul, S.R.; Sayeed, M.; Hakim, M. Antibacterial and cytotoxic activity of the bark of Phoenix paludosa in different solvents. Jordan J. Biol. Sci. 2017, 10, 213–217. [Google Scholar]
- Essien, E.E.; Antia, B.S.; Etuk, E.I. Phytoconstituents, antioxidant and antimicrobial activities of Livistona chinensis (Jacquin), Saribus rotundifolius (Lam.) Blume and Areca catechu Linnaeus Nuts. Pharm. Biosci. J. 2017, 10, 59–67. [Google Scholar] [CrossRef]
- Khan, W.U.; Khan, R.A.; Ahmed, M.; Khan, L.U.; Khan, M.W. Pharmacological evaluation of methanolic extract of Cyperus scariosus. Bangladesh J. Pharmacol. 2016, 11, 353–358. [Google Scholar] [CrossRef]
- Zhan, G.; Pan, L.Q.; Mao, S.B.; Zhang, W.; Wei, Y.Y.; Tu, K. Study on antibacterial properties and major bioactive constituents of Chinese water chestnut (Eleocharis dulcis) peels extracts/fractions. Eur. Food Res. Technol. 2014, 238, 789–796. [Google Scholar] [CrossRef]
- Ramadhani, D.A.; Novita, H.; Rajamuddin, M.A.L.; Elya, B. Chemical compounds screening of leaves extract from Eleocharis dulcis (Burm. f.) trin. ex hensch and in vitro antibacterial pathogenic test for fish. AACL Bioflux. 2020, 13, 2770–2778. [Google Scholar]
- Ebana, R.U.B.; Etok, C.A.; Edet, U.O. Phytochemical screening and antimicrobial activity of Nypa fruticans harvested from Oporo River in the Niger Delta Region of Nigeria. Int. J. Innov. Appl. Stud. 2015, 10, 1120. [Google Scholar]
- Joshi, B.; Panda, S.K.; Jouneghani, R.S.; Liu, M.; Parajuli, N.; Leyssen, P.; Neyts, J.; Luyten, W. Antibacterial, antifungal, antiviral, and anthelmintic activities of medicinal plants of Nepal selected based on ethnobotanical evidence. Evidence-Based Compl. Alt. Med. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bushmann, P.J.; Ailstock, M.S. Antibacterial compounds in estuarine submersed aquatic plants. J. Exp. Marine Biol. Ecol. 2006, 331, 41–50. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Permatasari, A.E.N.; Kadarwenny, C.P.; Pratoko, D.K.; Triatmoko, B.; Rosyidi, V.A.; Norcahyanti, I.; Dewi, I.P.; Dianasari, D.; Sary, I.P.; et al. Phytochemical screening and the antimicrobial and antioxidant activities of medicinal plants of Meru Betiri National park–Indonesia. J. Herbs Spices Med. Plants 2020, 26, 303–314. [Google Scholar] [CrossRef]
- Klawikkan, N.; Nukoolkarn, V.; Jirakanjanakit, N.; Yoksan, S.; Wiwat, C. Effect of Thai medicinal plant extracts against Dengue virus in vitro. Mahidol Univ. J. Pharm. Sci. 2011, 38, 13–18. [Google Scholar]
- Kaushik, S.; Kaushik, S.; Sharma, V.; Yadav, J. Antiviral and therapeutic uses of medicinal plants and their derivatives against Dengue viruses. Pharm. Rev. 2018, 12, 13–18. [Google Scholar]
- Paul, P.; Chowdhury, A.; Nath, D.; Bhattacharjee, M.K. Antimicrobial efficacy of orchid extracts as potential inhibitors of antibiotic resistant strains of E. coli. Asian J. Pharm. Clin. Res. 2013, 6, 108–111. [Google Scholar]
- Juneja, R.K.; Sharma, S.C.; Tandon, J.S. Two substituted bibenzyls and a dihydrophenanthrene from Cymbidium aloifolium. Phytochemistry 1987, 26, 1123–1125. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Jiang, L.; Xie, Q.; Yuan, H.; Yang, Y.; Zafar, S.; Liu, Y.; Jian, Y.; Li, B.; et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 280, 114263. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zheng, C.J.; Gan, L.S.; Chen, G.Y.; Zhang, X.P.; Song, X.P.; Li, G.N.; Sun, C.G. Bioactive phenanthrene and bibenzyl derivatives from the stems of Dendrobium nobile. J. Nat. Prod. 2016, 79, 1791–1797. [Google Scholar] [CrossRef]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat. Prod. Commun. 2014, 9, 1934578X1400900625. [Google Scholar] [CrossRef]
- Ren, J.; Qian, X.P.; Guo, Y.G.; Li, T.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. Two new phenanthrene glycosides from Liparis regnieri Finet and their antibacterial activities. Phytochem. Lett. 2016, 18, 64–67. [Google Scholar] [CrossRef]
- Hasan, C.M.; Alam, F.; Haque, M.; Sohrab, M.H.; Monsur, M.A.; Ahmed, N. Antimicrobial and cytotoxic activity from Lasia spinosa and isolated lignan. Lat. Am. J. Pharm. 2011, 30, 550–553. [Google Scholar]
- Pagning, A.L.N.; Lateef, M.; Tapondjou, L.A.; Kuiate, J.R.; Ngnokam, D.; Ali, M.S. New triterpene and new flavone glucoside from Rhynchospora corymbosa (Cyperaceae) with their antimicrobial, tyrosinase and butyrylcholinesterase inhibitory activities. Phytochem. Lett. 2016, 2016, 121–128. [Google Scholar] [CrossRef]
- Yazawa, K.; Kurokawa, M.; Obuchi, M.; Li, Y.; Yamada, R.; Sadanari, H.; Matsubara, K.; Watanabe, K.; Koketsu, M.; Tuchida, Y.; et al. Anti-influenza virus activity of tricin, 4′, 5, 7-trihydroxy-3′, 5′-dimethoxyflavone. Antiv. Chem. Chemother. 2011, 22, 1–11. [Google Scholar] [CrossRef]
- Castrillo, M.; Córdova, T.; Cabrera, G.; Rodríguez-Ortega, M. Effect of naringenin, hesperetin and their glycosides forms on the replication of the 17D strain of yellow fever virus. Av. Biomed. 2015, 4, 69–78. [Google Scholar]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 2021, 163, 105255. [Google Scholar] [CrossRef]
- Sari, R.S.E.; Soegianto, L.; Liliek, S. Uji aktivitas antimikroba ekstrak etanol daun Cayratia trifolia terhadap S. aureus dan Candida albicans. J. Farm. Sains Dan Terap. 2018, 5, 23–29. [Google Scholar]
- Nguyen, T.N.A.; Dao, T.T.; Tung, B.T.; Choi, H.; Kim, E.; Park, J.; Lim, S.I.; Oh, W.K. Influenza A (H1N1) neuraminidase inhibitors from Vitis amurensis. Food Chem. 2011, 124, 437–443. [Google Scholar] [CrossRef]
- Lee, S.; Mailar, K.; Kim, M.I.; Park, M.; Kim, J.; Min, D.H.; Heo, T.H.; Bae, S.K.; Choi, W.; Lee, C. Plant-derived purification, chemical synthesis, and in vitro/in vivo evaluation of a resveratrol dimer, viniferin, as an HCV replication inhibitor. Viruses 2019, 11, 890. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, B.; Navarro, V.; Leon-Rivera, I.; Rios, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef]
- Sahidin, I.; Wahyuni, W.; Malaka, M.H.; Imran, I. Antibacterial and cytotoxic potencies of stilbene oligomers from stem barks of baoti (Dryobalanops lanceolata) growing in Kendari, Indonesia. Asian J. Pharm. Clin. Res. 2017, 10, 139–143. [Google Scholar]
- Basri, D.F.; Xian, L.W.; Abdul Shukor, N.I.; Latip, J. Bacteriostatic antimicrobial combination: Antagonistic interaction between epsilon-viniferin and vancomycin against methicillin-resistant Staphylococcus aureus. BioMed Res. Int. 2014, 2014, 461756. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.; Trung, T.N.; Kim, J.P.; Lee, S.; Na, M.; Jung, H.; Kim, H.S.; Kim, Y.H.; Bae, K. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 1165–1168. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef]
- Yimdjo, M.C.; Azebaze, A.G.; Nkengfack, A.E.; Meyer, A.M.; Bodo, B.; Fomum, Z.T. Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry 2004, 65, 2789–2795. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef]
- Hamdillah, A.; Isnansetyo, A.; Istiqomah, I.; Puspita, I.D.; Handayani, D.P.; Kaneko, T. Antibacterial activity of coastal plants and marine sponges from Kei Island Indonesia against bacterial fish pathogens. Pharmacog. J. 2019, 11, 812–817. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Oubada, A.; Bello, A.; Maes, L.; Cos, P.; Monzote, L. Antimicrobial assessment of resins from Calophyllum antillanum and Calophyllum inophyllum. Phytother. Res. 2015, 29, 1991–1994. [Google Scholar] [CrossRef] [PubMed]
- Spino, C.; Dodier, M.; Sotheeswaran, S. Anti-HIV coumarins from Calophyllum seed oil. Bioorg. Med. Chem. Lett. 1998, 8, 475–3478. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Ravikumar, S.; Gnanadesigan, M.; Vijayakumar, V. In vitro antibacterial activity of diterpene and benzoxazole derivatives from Excoecaria agallocha L. Int. J. Biol. Chem. Sci. 2010, 4, 692–701. [Google Scholar]
- Sabu, K.R.; Sugathan, S.; Idhayadhulla, A.; Woldemariam, M.; Aklilu, A.; Biresaw, G.; Tsegaye, B.; Manilal, A. Antibacterial, antifungal, and cytotoxic activity of Excoecaria agallocha leaf extract. J. Exp. Pharmacol. 2022, 14, 692–707. [Google Scholar]
- Premanathan, M.; Rajendran, S.; Ramanathan, T.; Kathiresan, K. A survey of some Indian medicinal plants for anti-human immunodeficiency virus (HIV) activity. Indian. J. Med. Res. 2000, 112, 73. [Google Scholar] [PubMed]
- Erickson, K.L.; Beutler, J.A.; Cardellina, J.H.; McMahon, J.B.; Newman, D.J.; Boyd, M.R. A novel phorbol ester from Excoecaria agallocha. J. Nat. Prod. 1995, 58, 769–772. [Google Scholar] [CrossRef]
- Yan, R.Y.; Wang, H.Q.; Liu, C.; Chen, R.Y.; Yu, D.Q. Three new water-soluble alkaloids from the leaves of Suregada glomerulata (Blume) Baill. Fitoter 2011, 82, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Millat, M.S.; Islam, S.; Hussain, M.S.; Moghal, M.M.R.; Islam, T. Antibacterial profiling of Launaea sarmentosa (Willd.) and Bruguiera cylindrica (L.): Two distinct ethno medicinal plants of Bangladesh. Eur. Exp. Biol. 2017, 7, 1–5. [Google Scholar]
- Bibi Sadeer, N.; Haddad, J.G.; Oday Ezzat, M.; Desprès, P.; Abdallah, H.H.; Zengin, G.; Uysal, A.; El Kalamouni, C.; Gallo, M.; Montesano, D.; et al. Bruguiera gymnorhiza (L.) Lam. at the forefront of pharma to confront Zika virus and microbial infections—An in vitro and in silico perspective. Molecules 2021, 26, 5768. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Gnanadesigan, M.; Suganthi, P.; Ramalakshmi, A. Antibacterial potential of chosen mangrove plants against isolated urinary tract infectious bacterial pathogens. Int. J. Med. Sci. 2010, 2, 94–99. [Google Scholar]
- Jasna, T.K.; Khaleel, K.M.; Rajina, M. In vitro antibacterial activity of mangrove plant Kandelia candel (L.) druce (Rhizophoraceae). World J. Pharma Res. 2017, 6, 470–477. [Google Scholar]
- Mahalakshmi, G.; Vengadeshkumar, L.; Rajamohan, K.; Sanjaygandhi, S.; Sharmila, A.M. Leaf extract of Rhizophora apiculata as a potential bio-inducer of early blight disease resistance in tomato plant. Nov. Res. Microbiol. J. 2020, 4, 714. [Google Scholar]
- Burhanuddin, B.; Saru, A.; Rantetondok, A.; Zainuddin, E.N. Antibacterial activity Rhizophora stylosa and Avicennia marina of mangrove fruit extraction on vibriosis of mangrove crab larvae (Scylla serrata Forsskal). Int. J. Environ. Agric. Biotechnol. 2019, 4, 1242–1248. [Google Scholar] [CrossRef]
- Lim, S.H.; Darah, I.; Jain, K. Antimicrobial activities of tannins extracted from Rhizophora apiculata barks. J. Trop. Forest Sci. 2006, 18, 59–65. [Google Scholar]
- Kokpol, U.; Chavasiri, W.; Chittawong, V.; Bruce, M.; Cunningham, G.N.; Miles, D.H. Long chain aliphatic alcohols and saturated carboxylic acids from heartwood of Rhizophora apiculata. Phytochemistry 1993, 33, 1129–1131. [Google Scholar] [CrossRef]
- Thiem, B.; Goślińska, O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoter 2004, 75, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Chacha, M.; Mapitse, R.; Afolayan, A.J.; Majinda, R.R. Antibacterial diterpenes from the roots of Ceriops tagal. Nat. Prod. Commun. 2008, 3, 17–20. [Google Scholar] [CrossRef]
- Fogliani, B.; Raharivelomanana, P.; Bianchini, J.P.; Bouraı, S.; Hnawia, E. Bioactive ellagitannins from Cunonia macrophylla, an endemic Cunoniaceae from New Caledonia. Phytochem. 2005, 66, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.K.; Manoharan, M.S.; Illanchezian, S. Antibacterial, antifungal and tumor cell suppression potential of Morinda citrifolia fruit extracts. Int. J. Integr. Biol. 2008, 3, 44–49. [Google Scholar]
- Choi, H.J.; Song, J.H.; Bhatt, L.R.; Baek, S.H. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother. Res. 2010, 24, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.P.; Zhang, X.; Hu, Y.; Liu, L.; Chen, J. Antiviral activity of esculin against white spot syndrome virus: A new starting point for prevention and control of white spot disease outbreaks in shrimp seedling culture. J. Fish Dis. 2022, 45, 59–68. [Google Scholar] [CrossRef]
- Hatano, T.; Kusuda, M.; Hori, M.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Theasinensin A, a tea polyphenol formed from Epigallocatechin gallate, suppresses antibiotic resistance of methicillin-resistant S. aureus. Planta Med. 2003, 69, 984–989. [Google Scholar] [PubMed]
- Xu, T.; Wang, Z.; Lei, T.; Lv, C.; Wang, J.; Lu, J. New flavonoid glycosides from Sedum aizoon L. Fitoter 2015, 101, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hafid, A.F.; Wahyuni, T.S.; Tumewu, L.; Apryani, E.V.H.Y.; Permanasari, A.A.; Adianti, M.; Kawahara, N. Antihepatitis C virus activity of Indonesian Mahogany (Toona Sureni). Asian J. Pharm. Clin. Res. 2018, 11, 87–90. [Google Scholar] [CrossRef][Green Version]
- Hsu, W.C.; Chang, S.P.; Lin, L.C.; Li, C.L.; Richardson, C.D.; Lin, C.C.; Lin, L.T. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antiv. Res. 2015, 118, 139–147. [Google Scholar] [CrossRef]
- You, H.L.; Huang, C.C.; Chen, C.J.; Chang, C.C.; Liao, P.L.; Huang, S.T. Anti-pandemic influenza A (H1N1) virus potential of catechin and gallic acid. J. Chin. Med. Assoc. 2018, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Lin, C.S.; Lai, H.C.; Lin, Y.P.; Wang, C.Y.; Tsai, Y.C.; Wu, K.C.; Huang, S.H.; Lin, C.W. Antiviral activity of Sambucus ormosana Nakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019, 273, 197767. [Google Scholar] [CrossRef] [PubMed]
- Afifi, F.Ü.; Al-Khalil, S.; Abdul-Haq, B.K.; Mahasneh, A.; Al-Eisawi, D.M.; Sharaf, M.; Wong, L.K.; Schiff, P.L., Jr. Antifungal flavonoids from Varthemia iphionoides. Phytother. Res. 1991, 5, 173–175. [Google Scholar] [CrossRef]
- Wang, J.; Qin, X.; Chen, Z.; Ju, Z.; He, W.; Tan, Y.; Zhou, X.; Tu, Z.; Lu, F.; Liu, Y. Two new anthraquinones with antiviral activities from the barks of Morinda citrifolia (Noni). Phytochem. Lett. 2016, 15, 13–15. [Google Scholar] [CrossRef]
- Saludes, J.P.; Garson, M.J.; Franzblau, S.G.; Aguinaldo, A.M. Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn. (Rubiaceae). Phytother. Res. 2002, 16, 683–685. [Google Scholar] [CrossRef]
- Simlai, A.; Mukherjee, K.; Mandal, A.; Bhattacharya, K.; Samanta, A.; Roy, A. Partial purification and characterization of an antimicrobial activity from the wood extract of mangrove plant Ceriops decandra. EXCLI J. 2016, 15, 103–112. [Google Scholar]
- Saeed, M.A.; Sabir, A.W. Antibacterial activity of Caesalpinia bonducella seeds. Fitoter 2001, 72, 807–809. [Google Scholar] [CrossRef]
- Simin, K.; Khaliq-uz-Zaman, S.M.; Ahmad, V.U. Antimicrobial activity of seed extracts and bondenolide from Caesalpinia bonduc (L.) Roxb. Phytother. Res. 2001, 15, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Mandal, T.K.; Kumar, N.; Bhosale, J.D.; Hole, A.; Sharma, G.L.; Padhi, M.M.; Lavekar, G.S.; Dabur, R. In vitro and in vivo antimicrobial activities of seeds of Caesalpinia bonduc (Lin.) Roxb. J. Ethnopharmacol. 2009, 123, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.; Udenigwe, C.C.; Gale, E.M.; Samarasekera, R. Minor chemical constituents of Caesalpinia bonduc. Nat. Prod. Commun. 2009, 4, 311–314. [Google Scholar] [CrossRef]
- Idrus, I.; Kurniawan, F.; Mustapa, F.; Wibowo, D. Concentration Effect of Leaf Extract from Kekara Laut (Canavalia Maritima Thou.) in Inhibiting of Staphylococcus Epidermidis Bacteria With a Statistical Science Approach. Indo. J. Chem. Res. 2021, 8, 180–185. [Google Scholar] [CrossRef]
- Powar, P. Antifungal activity of Mangrove bark. Int. J. Pharm. Bio. Sci. 2011, 4, 484–488. [Google Scholar]
- Suhendi, A.; Indrayudha, P.; Azizah, T. Antibacterial activity of ethanol extract of steam bark of Cynometra ramiflora Linn against various bacterial. Open Conf. Proc. J. 2013, 4, 1. [Google Scholar] [CrossRef]
- Kumar, V.A.; Ammani, K.; Siddhardha, B. In vitro antimicrobial activity of leaf extracts of certain mangrove plants collected from Godavari estuarine of Konaseema delta, India. Int. J. Med. Arom. Plants. 2011, 1, 132–136. [Google Scholar]
- Xin, L.Y.; Min, T.H.; Zin, P.N.L.M.; Pulingam, T.; Appaturi, J.N.; Parumasivam, T. Antibacterial potential of Malaysian ethnomedicinal plants against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA). Saudi J. Biol.Sci. 2021, 28, 5884–5889. [Google Scholar] [CrossRef]
- Gunasekara, T.D.C.P.; Radhika, N.D.M.; Ragunathan, K.K.; Gunathilaka, D.P.P.; Weerasekera, M.M.; Hewageegana, H.G.S.P.; Arawwawala, L.A.D.M.; Fernando, S.S.N. Determination of antimicrobial potential of five herbs used in ayurveda practices against Candida albicans, Candida parapsilosis and methicillin resistant S. aureus. Anc. Sci. Life. 2017, 36, 187. [Google Scholar] [CrossRef]
- Rattanasuk, S.; Boongapim, R.; Phiwthong, T. Antibacterial activity of Cathormion umbellatum. Bangladesh J. Pharmacol. 2021, 16, 91–95. [Google Scholar] [CrossRef]
- Hamburger, M.O.; Cordell, G.A.; Tantivatana, P.; Ruangrungsi, N. Traditional medicinal plants of Thailand, VIII. Isoflavonoids of Dalbergia candenatensis. J. Nat. Prod. 1987, 50, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Inagaki, M.; Ikegami, F.; Fujii, Y.; Ruangrungsi, N. Six diprenylisoflavones, derrisisoflavones A–F, from Derris scandens. Phytochemistry 1999, 52, 87–94. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Krohn, K.; Kouam, S.F.; Abbas, G.; Shah, A.; Raees, M.A.; Ullah, R.; Aziz, S.; Schulz, B. Phytochemical investigation and antimicrobial activity of Derris scandens. J. King Saud Univ.-Sci. 2015, 27, 375–378. [Google Scholar] [CrossRef]
- Mohotti, S.; Rajendran, S.; Muhammad, T.; Strömstedt, A.A.; Adhikari, A.; Burman, R.; De Silva, E.D.; Göransson, U.; Hettiarachchi, C.M.; Gunasekera, S. Screening for bioactive secondary metabolites in Sri Lankan medicinal plants by microfractionation and targeted isolation of antimicrobial flavonoids from Derris scandens. J. Ethnopharmacol. 2020, 246, 112158. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Deachathai, S.; Phongpaichit, S.; Jansakul, C.; Taylor, W.C. A benzil and isoflavone derivatives from Derris scandens Benth. Phytochemistry 2004, 65, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Mathaiyan, M.; Suresh, A.; Balamurugan, R. Binding property of HIV p24 and reverse transcriptase by chalcones from Pongamia pinnata seeds. Bioinformation 2018, 14, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Nukui, M.; O’Connor, C.M.; Murphy, E.A. The natural flavonoid compound deguelin inhibits HCMV lytic replication within fibroblasts. Viruses 2018, 10, 614. [Google Scholar] [CrossRef]
- Yusuf, A.I.; Dewi, B.E.; Sjatha, F. Antiviral activity of Cynometra ramiflora Linn leaves extract against replication of Dengue virus serotype 2 on Huh 7.5 cell in vitro. In Proceedings of the BROMO Conference (BROMO 2018)—Symposium on Natural Product and Biodiversity, Surabaya, Indonesia, 11–12 July 2018; pp. 1–4. [Google Scholar]
- Takatsuki, A.; Nakatani, N.; Morimoto, M.; Tamura, G.; Matsui, M.; Arima, K.; Yamaguchi, I.; Misato, T. Antiviral and antitumor antibiotics. XX. Effects of rotenone, deguelin, and related compounds on animal and plant viruses. Appl. Microbiol. 1969, 18, 660–667. [Google Scholar] [CrossRef]
- Yusook, K.; Weeranantanapan, O.; Hua, Y.; Kumkrai, P.; Chudapongse, N. Lupinifolin from Derris reticulata possesses bactericidal activity on S. aureus by disrupting bacterial cell membrane. J. Nat. Med. 2017, 71, 357–366. [Google Scholar] [CrossRef]
- Mazimba, O.; Masesane, I.B.; Majinda, R.R. A flavanone and antimicrobial activities of the constituents of extracts from Mundulea sericea. Nat. Prod. Res. 2012, 26, 1817–1823. [Google Scholar] [CrossRef]
- Phrutivorapongkul, A.; Lipipun, V.; Ruangrungsi, N.; Watanabe, T.; Ishikawa, T. Studies on the constituents of seeds of Pachyrrhizus erosus and their anti herpes simplex virus (HSV) activities. Chem. Pharm. Bull. 2002, 50, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Nehad, M.G.; Abdulrahaman, S.H. Antimicrobial efficacy of Casuarina equisetifolia extracts against some pathogenic microorganisms. J. Med. Plants Res. 2012, 6, 5819–5825. [Google Scholar]
- Lagnika, L.; Amoussa, A.M.O.; Oketokoun, S.A.; Adjovi, Y.; Sanni, A. In vitro antifungal and antioxidant activities of two Benin medicinal plants. J. Med. Plants Res. 2014, 8, 513–519. [Google Scholar]
- Kumar, U.M.N.; Panneerselvam, T. Efficacy of aqueous and ethanol extracts Casuarina equisetifolia for potential antimicrobial activity. World J. Pharm. Pharm. Sci. 2015, 4, 1877–1882. [Google Scholar]
- Ao, C.; Li, A.; Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 2008, 19, 940–948. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.; Liu, N.; Zhang, F.; Lu, Y.; Zhang, Y.; Fu, L. Flavans with anti-HSV activity from the leaves of Ficus microcarpa L. J. Trop. Subtrop. Bot. 2010, 18, 559–563. [Google Scholar]
- Nantachit, K.; Sirilun, S.; Nobsathian, S.; Rungjang, S. Three new polycyclic containing sulfur compounds from the seeds of Combretum quadrangulare kurz (Combretaceae), antifungal and anti-mycobacterium activities. Chiang Mai J. Sci. 2017, 44, 157–167. [Google Scholar]
- Pawar, S.P.; Pal, S.C. Antimicrobial activity of extracts of Terminalia catappa root. Indian J Med. Sci. 2002, 56, 276–278. [Google Scholar]
- Terças, A.G.; Monteiro, A.D.S.; Moffa, E.B.; Dos Santos, J.R.; Sousa, E.M.D.; Pinto, A.R.; Costa, P.C.; Borges, A.C.; Torres, L.; Barros Filho, A.K.; et al. Phytochemical characterization of Terminalia catappa linn. extracts and their antifungal activities against candida spp. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Adesina, S.K.; Idowu, O.; Ogundaini, A.O.; Oladimeji, H.; Olugbade, T.A.; Onawunmi, G.O.; Pais, M. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Acalypha hispida. Phytother. Res. 2000, 14, 371–374. [Google Scholar] [CrossRef]
- Yeo, S.G.; Song, J.H.; Hong, E.H.; Lee, B.R.; Kwon, Y.S.; Chang, S.Y.; Kim, S.H.; Won Lee, S.; Park, J.H.; Ko, H.J. Antiviral effects of Phyllanthus urinaria containing corilagin against human enterovirus 71 and Coxsackievirus A16 in vitro. Arch. Pharm. Res. 2015, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Dao, N.T.; Jang, Y.; Kim, M.; Nguyen, H.H.; Pham, D.Q.; Le Dang, Q.; Van Nguyen, M.; Yun, B.S.; Pham, Q.M.; Kim, J.C.; et al. Chemical constituents and anti-influenza viral activity of the leaves of Vietnamese plant Elaeocarpus tonkinensis. Rec. Nat. Prod. 2019, 13, 71–80. [Google Scholar] [CrossRef]
- Aman, S.; Naim, A.; Siddiqi, R.; Naz, S. Antimicrobial polyphenols from small tropical fruits, tea and spice oilseeds. Food Sci. Technol. Int. 2014, 20, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Premnathan, M.; Chandra, K.; Bajpai, S.K.; Kathiresan, K. A survey of some Indian marine plants for antiviral activity. Bot. Mar. 1992, 35, 321–324. [Google Scholar] [CrossRef]
- Arunkumar, J.; Rajarajan, S.A. Study on the in vitro Cytotoxicity and Anti-HSV-2 Activity of lyophilized extracts of Terminalia catappa Lin., Mangifera indica Lin. and phytochemical compound mangiferin. Int. J. Med. Pharm. Virol. 2015, 2, 22–26. [Google Scholar]
- Tewtrakul, S.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T.; Yoshinaga, T.; Fujiwara, T.; Supavita, T.; Yuenyongsawad, S.; et al. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother. Res. 2003, 17, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hardjito, L. Antibacterial, antioxidant and topoisomerase-I inhibitor activities of the coastal ethnomedicinal plant Pemphis acidula. BIOTROPIA-Southeast Asian J. Trop. Biol. 2007, 14, 43–51. [Google Scholar]
- Samidurai, K.; Saravanakumar, A. Antibacterial activity of Pemphis acidula Forst. Glob. J. Pharmacol. 2009, 3, 113–115. [Google Scholar]
- Abd Wahab, N.Z.; Ja’afar, N.S.A.; Ismail, S.B. Evaluation of antibacterial activity of essential oils of Melaleuca cajuputi Powell. J. Pure Appl. Microbiol. 2022, 16, 549–557. [Google Scholar] [CrossRef]
- Bua, A.; Molicotti, P.; Donadu, M.G.; Usai, D.; Le, L.S.; Tran, T.T.T.; Ngo, V.Q.T.; Marchetti, M.; Usai, M.; Cappuccinelli, P.; et al. “In vitro” activity of Melaleuca cajuputi against mycobacterial species. Nat. Prod. Res. 2020, 34, 1494–1497. [Google Scholar] [CrossRef]
- Yala, J.F.; Mabika, R.M.; Camara, B.; Tuo, S.; Souza, A.; Lepengue, A.N.; Koné, D.; M’batchi, B. Assessment of the antibacterial activity of four essential oils and the biobactericide Neco. Int. J. Phytomed 2017, 9, 443–450. [Google Scholar] [CrossRef]
- Keereedach, P.; Hrimpeng, K.; Boonbumrung, K. Antifungal activity of Thai cajuput oil and its effect on efflux-pump gene expression in fluconazole-resistant Candida albicans clinical isolates. Int. J. Microbiol. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.; Shin, S.C.; Lee, S.G.; Park, I.K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flav. Frag. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Pino, O.; Sánchez, Y.; Rojas, M.M.; Rodríguez, H.; Abreu, Y.; Duarte, Y.; Martínez, B.; Peteira, B.; Correa, T.M.; Martínez, D. Composición química y actividad plaguicida del aceite esencial de Melaleuca quinquenervia (Cav) ST Blake. Rev. Protección Veg. 2011, 26, 177–186. [Google Scholar]
- Hossain, S.J.; Basar, M.H.; Rokeya, B.; Arif, K.M.T.; Sultana, M.S.; Rahman, M.H. Evaluation of antioxidant, antidiabetic and antibacterial activities of the fruit of Sonneratia apetala (Buch.-Ham.). Oriental Pharm. Exp. Med. 2013, 13, 95–102. [Google Scholar] [CrossRef]
- Afzali, S.F.; Wong, W.L. In vitro screening of Sonneratia alba extract against the oomycete fish pathogen, Aphanomyces invadans. Iran. J. Fish. Sci. 2017, 16, 1333–1340. [Google Scholar]
- Bobbarala, V.; Vadlapudi, V.; Naidu, K.C. Mangrove plant Sonneratia apetala antimicrobial activity on selected pathogenic microorganisms. Orient. J. Chem. 2009, 25, 445–447. [Google Scholar]
- Khumaidah, L.; Purnomo, A.S.; Fatmawati, S. Antimicrobial activity of Sonneratia ovata backer. Hayati J. Biosci. 2019, 26, 152. [Google Scholar] [CrossRef]
- Limbago, J.S.; Sosas, J.; Gente, A.A.; Maderse, P.; Rocamora, M.M.; Gomez, D.K. Antibacterial effects of mangrove ethanolic leaf extract against zoonotic fish pathogen Salmonella arizonae. J. Fish. 2021, 9, 92205. [Google Scholar] [CrossRef]
- Harizon Pujiastuti, B.; Kurnia, D.; Sumiarsa, D.; Shiono, Y.; Supratman, U. Antibacterial triterpenoids from the bark of Sonneratia alba (Lythraceae). Nat. Prod. Comm. 2015, 10, 277–280. [Google Scholar]
- Gong, K.K.; Li, P.L.; Qiao, D.; Zhang, X.W.; Chu, M.J.; Qin, G.F.; Tang, X.L.; Li, G.Q. Cytotoxic and antiviral triterpenoids from the mangrove plant Sonneratia paracaseolaris. Molecules 2017, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Moe, T.S.; Win, H.H.; Hlaing, T.T.; Lwin, W.W.; Htet, Z.M.; Mya, K.M. Evaluation of in vitro antioxidant, antiglycation and antimicrobial potential of indigenous Myanmar medicinal plants. J. Integr. Med. 2018, 16, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Gnanasekar, M.; Ignacimuthu, S. Antifungal activity of triterpenoid isolated from Azima tetracantha leaves. Folia Histochem. Cytobiol 2010, 48, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Sumardi, S.; Basyuni, M.; Wati, R. Antimicrobial activity of polyisoprenoids of sixteen mangrove species from North Sumatra, Indonesia. Biodiversitas J. Biol. Divers 2018, 19, 1243–1248. [Google Scholar] [CrossRef]
- Abdul-Awal, S.M.; Nazmir, S.; Nasrin, S.; Nurunnabi, T.R.; Uddin, S.J. Evaluation of pharmacological activity of Hibiscus tiliaceus. Springerplus 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Boonsri, S.; Karalai, C.; Ponglimanont, C.; Chantrapromma, S.; Kanjana-Opas, A. Cytotoxic and antibacterial sesquiterpenes from Thespesia populnea. J. Nat. Prod. 2008, 71, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Arthanari, S.K.; Vanitha, J.; Ganesh, M.; Venkateshwaran, K.; Clercq, D. Evaluation of antiviral and cytotoxic activities of methanolic extract of S. grandiflora (Fabaceae) flowers. Asian Pac. J. Trop. Biomed. 2012, 2, S855–S858. [Google Scholar] [CrossRef]
- Chungsamarnyart, N.; Sirinarumitr, T.; Chumsing, W.; Wajjawalku, W. In vitro study of antiviral activity of plant crude-extracts against the foot and mouth disease virus. Agric. Nat. Res. 2007, 41, 97–103. [Google Scholar]
- Dey, M.C.; Roy, R.N.; Sinhababu, A. Fatty acid composition and antibacterial activity of the leaf oil of Kleinhovia hospita Linn. J. Nat. Prod. 2017, 10, 378–384. [Google Scholar]
- Rahim, A.; Saito, Y.; Miyake, K.; Goto, M.; Chen, C.H.; Alam, G.; Morris-Natschke, S.; Lee, K.H.; Nakagawa-Goto, K. Kleinhospitine E and cycloartane triterpenoids from Kleinhovia hospita. J. Nat. Prod. 2018, 81, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Al Muqarrabun, L.M.R. and Ahmat, N.Medicinal uses, phytochemistry and pharmacology of family Sterculiaceae: A review. Eur. J. Med. Chem. 2015, 92, 514–530. [Google Scholar] [CrossRef]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; De Koning, C.B. A new cinnamoylglycoflavonoid, antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; de Dieu Tamokou, J.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.P.; Miranda, M.M.F.S.; Simoni, I.C.; Wigg, M.D.; Lagrota, M.H.C.; Costa, S.S. Flavonol monoglycosides isolated from the antiviral fractions of Persea americana (Lauraceae) leaf infusion. Phytother. Res. 1998, 12, 562–567. [Google Scholar] [CrossRef]
- Kuspradini, H.; Mitsunaga, T.; Ohashi, H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. J. Wood Sci. 2009, 55, 308–313. [Google Scholar] [CrossRef]
- Jiang, R.W.; Ma, S.C.; He, Z.D.; Huang, X.S.; But, P.P.H.; Wang, H.; Chan, S.P.; Ooi, V.E.C.; Xu, H.X.; Mak, T.C. Molecular structurs and antiviral activities of naturally occurring and modified cassane furanoditerpenoids and friedelane triterpenoids from Caesalpinia minax. Bioorg. Med. Chem. 2002, 10, 2161–2170. [Google Scholar] [CrossRef]
- Chang, F.R.; Yen, C.T.; Ei-Shazly, M.; Lin, W.H.; Yen, M.H.; Lin, K.H.; Wu, Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012, 7, 1415–1417. [Google Scholar] [CrossRef]
- Wangensteen, H.; Dang, H.C.T.; Uddin, S.J.; Alamgir, M.; Malterud, K.E. Antioxidant and antimicrobial effects of the mangrove tree Heritiera fomes. Nat. Prod. Comm. 2009, 4, 371–376. [Google Scholar] [CrossRef]
- Choudhury, S.; Sree, A.; Mukherjee, S.C.; Pattnaik, P.; Bapuji, M. In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fish. Sci. 2005, 18, 285. [Google Scholar] [CrossRef]
- Veni, P.S.; Sunita, S.; Srinivasulu, A. Antibacterial and phytochemical screening of Xylocarpus moluccensis leaf and stem on selected drug resistant and sensitive bacteria. Int. J. Microbiol. Res. IJMR 2014, 5, 30–34. [Google Scholar]
- Dai, Y.G.; Wu, J.; Padmakumar, K.P.; Shen, L. Sundarbanxylogranins A–E, five new limonoids from the Sundarban Mangrove, Xylocarpus granatum. Fitoter 2017, 122, 85–89. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.; Shen, L.; Pedpradab, P.; Bruhn, T.; Wu, J.; Bringmann, G. Antiviral limonoids including khayanolides from the Trang mangrove plant Xylocarpus moluccensis. J. Nat. Prod. 2015, 78, 1570–1578. [Google Scholar] [CrossRef]
- Zhang, Q.; Satyanandamurty, T.; Shen, L.; Wu, J. Krishnolides A–D: New 2-ketokhayanolides from the Krishna mangrove, Xylocarpus moluccensis. Mar. Drugs 2017, 15, 333. [Google Scholar] [CrossRef] [PubMed]
- Pudhom, K.; Sommit, D.; Nuclear, P.; Ngamrojanavanich, N.; Petsom, A. Moluccensins H− J, 30-Ketophragmalin Limonoids from Xylocarpus moluccensis. J. Nat. Prod. 2010, 73, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wang, M.; Zhu, W.; Qin, Z. A new fungicidal lactone from Xylocarpus granatum (Meliaceae). Nat. Prod. Res. 2009, 23, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, D.; De Rocca Serra, D.; Bighelli, A.; Minh Hoi, T.; Huy Thai, T.; Casanova, J. Composition and antimicrobial activity of the essential oil of Acronychia pedunculata (L.) Miq. from Vietnam. Nat. Prod. Res. 2008, 22, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasampandan, R.; Gunasekar, R.; Gogulramnath, M. Chemical composition analysis, antioxidant and antibacterial activity evaluation of essential oil of Atalantia monophylla Correa. Pharmacog. Res. 2015, 7, S52. [Google Scholar] [CrossRef] [PubMed]
- Trong Le, N.; Viet Ho, D.; Quoc Doan, T.; Tuan Le, A.; Raal, A.; Usai, D.; Sanna, G.; Carta, A.; Rappelli, P.; Diaz, N.; et al. Biological activities of essential oils from leaves of Paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (Miq.) Swingle. Antibiotics 2020, 9, 207. [Google Scholar] [CrossRef]
- Reddy, K.H.; Sharma, P.V.G.K.; Reddy, O.V.S. A comparative in vitro study on antifungal and antioxidant activities of Nervilia aragoana and Atlantia monophylla. Pharm. Biol. 2010, 48, 595–602. [Google Scholar] [CrossRef]
- Wainwright, M. Acridine—a neglected antibacterial chromophore. J. Antimicrob. Chemother. 2001, 47, 1–13. [Google Scholar] [CrossRef]
- Thimmaiah, K.; Ugarkar, A.G.; Martis, E.F.; Shaikh, M.S.; Coutinho, E.C.; Yergeri, M.C. Drug–DNA interaction studies of acridone-based derivatives. Nucleosides Nucleotides Nucleic Acids 2015, 34, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, M.; Rajkumar, T.; Vimal, S.; Taju, G.; Majeed, S.A.; Hameed, A.S.; Kannabiran, K. Antiviral activity of 9 (10H)-Acridanone extracted from marine Streptomyces fradiae strain VITMK2 in Litopenaeus vannamei infected with white spot syndrome virus. Aquaculture 2018, 488, 66–73. [Google Scholar] [CrossRef]
- Sripisut, T.; Deachathai, S.; Chang, L.C.; Laphookhieo, S. Antibacterial compounds from Atalantia monophylla roots and stems. Planta Med. 2013, 79, PN11. [Google Scholar] [CrossRef]
- Wisetsai, A.; Lekphrom, R.; Suebrasri, T.; Schevenels, F.T. Acroflavone A, a new prenylated flavone from the fruit of Acronychia pedunculata (L.) Miq. Nat. Prod. Res. 2021, 36, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Noor, M.A.; Karon, B.; de Freitas, R.M. In vitro antimicrobial and brine shrimp lethality of Allophylus cobbe L. Ayu 2012, 33, 299. [Google Scholar] [CrossRef]
- Raghavendra, H.L.; Prashith kekuda T., R.; Karthik K., N.; Ankith G., N. Antiradical, antibacterial, and antifungal activity of Harpullia arborea (Blanco) Radlk. (Sapindaceae). Int. J. Curr. Pharm. Res. 2017, 9, 32–36. [Google Scholar]
- Tumewu, L.; Apryiani, E.; Santi, M.R. Anti Hepatitis C virus activity screening on Harpullia arborea extracts and isolated compound. In Proceeding the 1st International Conference on Pharmaceutics and Pharmaceutical Sciences; Fakultas Farmasi Universitas Airlangga: Surabaya, Indonesia, 2014; pp. 165–167. [Google Scholar]
- Abdelkader, M.S.A.; Rateb, M.E.; Mohamed, G.A.; Jaspars, M. Harpulliasides A and B: Two new benzeneacetic acid derivatives from Harpullia pendula. Phytochem. Lett. 2016, 15, 131–135. [Google Scholar] [CrossRef]
- Anusha, P.; Sudha Bai, R. Phytochemical profile and antimicrobial potential of methanolic extracts of bark and leaf of Quassia indica (Gaertn.) Nooteb. J. Phytopharmacol. 2017, 6, 269–276. [Google Scholar] [CrossRef]
- Hardiyanti, R.; Marpaung, L.; Adnyana, I.K.; Simanjuntak, P. Isolation of quercitrin from Dendrophthoe pentandra (L.) Miq leaves and it’s antioxidant and antibacterial activities. Rasayan J. Chem. 2019, 12, 1822–1827. [Google Scholar] [CrossRef]
- Tripathi, S.; Ray, S.; Das, P.K.; Mondal, A.K.; Verma, N.K. Antimicrobial activities of some rare aerial hemi parasitic taxa of south West Bengal, India. Int. J. Phytopharm. 2013, 4, 106–112. [Google Scholar]
- Satish, S.; Raveesha, K.A.; Janardhana, G.R. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett. Appl. Microbiol. 1999, 28, 145–147. [Google Scholar] [CrossRef]
- Omer, M.E.F.A.; Elnima, E.I. Antimicrobial activity of Ximenia americana. Fitoter 2003, 74, 122–126. [Google Scholar] [CrossRef]
- Owk, A.K.; Lagudu, M.N. Evaluation of antimicrobial activity and phytochemicals in Olax scandens Roxb. roots. Pharma Sci. Monit. 2016, 7, 232–239. [Google Scholar]
- Kone, W.M.; Atindehou, K.K.; Terreaux, C.; Hostettmann, K.; Traore, D.; Dosso, M. Traditional medicine in North Côte-d’Ivoire: Screening of 50 medicinal plants for antibacterial activity. J. Ethnopharmacol. 2004, 93, 43–49. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Compl. Altern. Med. 2006, 6, 1–7. [Google Scholar] [CrossRef]
- Asres, K.; Bucar, F.; Kartnig, T.; Witvrouw, M.; Pannecouque, C.; De Clercq, E. Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother. Res. 2001, 15, 62–69. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar]
- Magwa, M.L.; Gundidza, M.; Gweru, N.; Humphrey, G. Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef]
- Bhosale, S.H.; Jagtap, T.G.; Naik, C.G. Antifungal activity of some marine organisms from India, against food spoilage Aspergillus strains. Mycopathologia 1999, 147, 133–138. [Google Scholar] [CrossRef]
- Banerjee, M.B.; Ravikumar, S.; Gnanadesigan, M.; Rajakumar, B.; Anand, M. Antiviral, antioxidant and toxicological evaluation of mangrove associate from South East coast of India. Asian Pac. J. Trop. Biomed. 2012, 2, S1775–S1779. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Nat. C 2008, 63, 331–336. [Google Scholar] [CrossRef]
- Ghosh, D.; Mondal, S.; Ramakrishna, K. Spectroscopic characterization of phytoconstituents isolated from a rare mangrove Aegialitis rotundifolia Roxb., leaves and evaluation of antimicrobial activity of the crude extract. Asian J. Pharm. Clin. Res. 2019, 12, 220–224. [Google Scholar] [CrossRef]
- Sett, S.; Hazra, J.; Datta, S.; Mitra, A.; Mitra, A.K. Screening the Indian Sundarban mangrove for antimicrobial activity. Int. J. Sci. Innov. Disc. 2014, 4, 17–25. [Google Scholar]
- Min, B.S.; Kim, Y.H.; Tomiyama, M.; Nakamura, N.; Miyashiro, H.; Otake, T.; Hattori, M. Inhibitory effects of Korean plants on HIV-1 activities. Phytother. Res. 2001, 15, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Panda, P.K.; Mishra, S.R.; Parida, R.K.; Ellaiah, P.; Dash, S.K. Antibacterial activity of Barringtonia acutangula against selected urinary tract pathogens. Indian J. Pharma Sci. 2008, 70, 677. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sahoo, S.; Mishra, S.K.; Rout, K.K.; Nayak, S.K.; Panda, P.K.; Dhal, N.K. Antimicrobial investigation of leaves of Barringtonia acutangula Linn. Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 55–58. [Google Scholar]
- Khan, M.R.; Omoloso, A.D. Antibacterial, antifungal activities of Barringtonia asiatica. Fitoter 2002, 73, 255–260. [Google Scholar] [CrossRef]
- Hussin, N.M.; Muse, R.; Ahmad, S.; Ramli, J.; Mahmood, M.; Sulaiman, M.R.; Shukor, M.Y.A.; Rahman, M.F.A.; Aziz, K.N.K. Antifungal activity of extracts and phenolic compounds from Barringtonia racemosa L.(Lecythidaceae). African J. Biotechnol. 2009, 8, 2835–2842. [Google Scholar]
- Saha, S.; Sarkar, K.K.; Hossain, M.L.; Hossin, A.; Barman, A.K.; Ahmed, M.I.; Sadhu, S.K. Bioactivity studies on Barringtonia racemosa (Lam.) bark. Pharmacologyonline 2013, 1, 93–100. [Google Scholar]
- Ragasa, C.Y.; Espineli, D.L.; Shen, C.C. New triterpenes from Barringtonia asiatica. Chem. Pharm. Bull. 2011, 59, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Antognoni, F.; Mandrone, M.; Protti, M.; Mercolini, L.; Lianza, M.; Gentilomi, G.A.; Poli, F. Phytochemical analysis and antibacterial activity towards methicillin-resistant Staphylococcus aureus of leaf extracts from Argania spinosa (L.) Skeels. Plant Biosystem. 2017, 151, 649–656. [Google Scholar] [CrossRef]
- Rahman, M.M.; Polfreman, D.; MacGeachan, J.; Gray, A.I. Antimicrobial activities of Barringtonia acutangula. Phytother. Res. 2005, 19, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Pefile, S.C. A Study of the Antiherpes Simplex Virus Type 1 Properties of Barringtonia racemosa. Ph.D. Thesis, University of Cape, Cape Town, Africa, 2001. [Google Scholar]
- Janmanchi, H.; Raju, A.; Degani, M.S.; Ray, M.K.; Rajan, M.G.R. Antituberculosis, antibacterial and antioxidant activities of Aegiceras corniculatum, a mangrove plant and effect of various extraction processes on its phytoconstituents and bioactivity. S. Afr. J. Bot. 2017, 113, 421–427. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mukherjee, K.; Roy, A. Two novel antifungals, acornine 1 and acornine 2, from the bark of mangrove plant Aegiceras corniculatum (Linn.) Blanco from Sundarban Estuary. Pharmacog. Mag. 2014, 10, S342. [Google Scholar]
- Nariya, P.B.; Bhalodia, N.R.; Shukla, V.J.; Acharya, R.N. Antimicrobial and antifungal activities of Cordia dichotoma (Forster F.) bark extracts. Ayu 2011, 32, 585. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J. Agric. Food Chem. 2005, 53, 32–37. [Google Scholar] [CrossRef]
- Horwitz, S.B.; Chang, C.K.; Grollman, A.P. Antiviral action of camptothecin. Antimicrob. Agents Chemother. 1972, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.X.; Chu, J.J.H. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antiv. Res. 2017, 143, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Avery, R.J.; Dimmock, N.J. Camptothecin: An inhibitor of influenza virus replication. J. Gen. Virol. 1974, 25, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, L.; Rein, A. Effect of camptothecin on Simian virus 40 DNA. Nature 1974, 248, 226–228. [Google Scholar] [CrossRef]
- Pantazis, P. Camptothecin: A promising antiretroviral drug. J. Biomed. Sci. 1996, 3, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Mackeen, M.M.; El-Sharkawy, S.H.; Hamid, J.A.; Ismail, N.H.; Ahmad, F.; Lajis, N.H. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J. Trop. Agric. Sci. 1996, 19, 129–136. [Google Scholar]
- Ahmed, F.; Amin, R.; Shahid, I.Z.; Sobhani, M.M.E. Antibacterial, cytotoxic and neuropharmacological activities of Cerbera odollam seeds. Adv. Trad. Med. 2008, 8, 323–328. [Google Scholar] [CrossRef]
- Chu, S.Y.; Singh, H.; Ahmad, M.S.; Mamat, A.S. Phytochemical screening of antifungal biocompounds from fruits and leaves extract of Cerbera odollam Gaertn. Malays. Appl. Biol. 2015, 44, 75–79. [Google Scholar]
- Pájaro-González, Y.; Cabrera-Barraza, J.; Martelo-Ramírez, G.; Oliveros-Díaz, A.F.; Urrego-Álvarez, J.; Quiñones-Fletcher, W.; Díaz-Castillo, F. In Vitro and In Silico Antistaphylococcal Activity of Indole Alkaloids Isolated from Tabernaemontana cymosa Jacq (Apocynaceae). Sci. Pharm. 2022, 90, 38. [Google Scholar] [CrossRef]
- Ahmed, F.; Reza, M.S.H.; Shahid, I.Z.; Khatun, A.; Islam, K.K.; Ali, M.R. Antibacterial and antinociceptive activity of Hoya parasitica. Hamdard Med. 2008, 51, 22–26. [Google Scholar]
- Muangrom, W.; Vajrodaya, S. Comparative phytochemistry and antibacterial properties of Guettarda speciosa L. In Proceedings of the 56th Kasetsart University Annual Conference, Bangkok, Thailand, 30 January–2 February 2018. [Google Scholar]
- Prachayasittikul, S.; Buraparuangsang, P.; Worachartcheewan, A.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar] [CrossRef] [PubMed]
- Bachala, T. Antibacterial and Antifungal activities of various extracts of Guettarda speciosa L. Int. J. Phytopharmacol. 2010, 1, 20–22. [Google Scholar]
- Zhang, H.; Rothwangl, K.; Mesecar, A.D.; Sabahi, A.; Rong, L.; Fong, H.H. Lamiridosins, hepatitis C virus entry inhibitors from Lamium album. J. Nat. Prod. 2009, 72, 2158–2162. [Google Scholar] [CrossRef]
- Ge, L.; Wan, H.; Tang, S.; Chen, H.; Li, J.; Zhang, K.; Zhou, B.; Fei, J.; Wu, S.; Zeng, X. Novel caffeoylquinic acid derivatives from Lonicera japonica Thunb. flower buds exert pronounced anti-HBV activities. RSC Adv. 2018, 8, 35374–35385. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Junior, A.R.; Oliveira Ferreira, R.; de Souza Passos, M.; da Silva Boeno, S.I.; Glória das Virgens, L.D.L.; Ventura, T.L.B.; Calixto, S.D.; Lassounskaia, E.; de Carvalho, M.G.; Braz-Filho, R.; et al. Antimycobacterial and nitric oxide production inhibitory activities of triterpenes and alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra. Molecules 2019, 24, 1026. [Google Scholar]
- Hanh, N.P.; Phan, N.H.T.; Thuan, N.T.D.; Hanh, T.T.H.; Vien, L.T.; Thao, N.P.; Thanh, N.V.; Cuong, N.X.; Binh, N.Q.; Nam, N.H.; et al. Two new simple iridoids from the ant-plant Myrmecodia tuberosa and their antimicrobial effects. Nat. Prod. Res. 2016, 30, 2071–2076. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Sharma, S.C.; Tokuda, H.; Nishino, H.; Ueda, S. Inhibitory effect of iridoids on Epstein-Barr virus activation by a short-term in vitro assay for anti-tumor promoters. Cancer Lett. 1996, 102, 223–226. [Google Scholar] [CrossRef]
- Pollo, L.A.; Martin, E.F.; Machado, V.R.; Cantillon, D.; Wildner, L.M.; Bazzo, M.L.; Waddell, S.J.; Biavatti, M.W.; Sandjo, L.P. Search for antimicrobial activity among fifty-two natural and synthetic compounds identifies anthraquinone and polyacetylene classes that inhibit Mycobacterium tuberculosis. Front. Microbiol. 2021, 11, 622629. [Google Scholar] [CrossRef] [PubMed]
- Manzione, M.G.; Martorell, M.; Sharopov, F.; Bhat, N.G.; Kumar, N.V.A.; Fokou, P.V.T.; Pezzani, R. Phytochemical and pharmacological properties of asperuloside, a systematic review. Eur. J. Pharmacol. 2020, 883, 173344. [Google Scholar] [CrossRef]
- Mahmood, N.; Moore, P.S.; De Tommasi, N.; De Simone, F.; Colman, S.; Hay, A.J.; Pizza, C. Inhibition of HIV infection by caffeoylquinic acid derivatives. Antiv. Chem. Chemother. 1993, 4, 235–240. [Google Scholar] [CrossRef]
- Ticona, L.A.; Bermejo, P.; Guerra, J.A.; Abad, M.J.; Beltran, M.; Lázaro, R.M.; Alcamí, J.; Bedoya, L.M. Ethanolic extract of Artemisia campestris subsp. glutinosa (Besser) Batt. inhibits HIV–1 replication in vitro through the activity of terpenes and flavonoids on viral entry and NF–κB pathway. J. Ethnopharmacol. 2020, 263, 113163. [Google Scholar] [CrossRef]
- Chokchaisiri, R.; Suaisom, C.; Sriphota, S.; Chindaduang, A.; Chuprajob, T.; Suksamrarn, A. Bioactive flavonoids of the flowers of Butea monosperma. Chem. Pharm. Bull. 2009, 57, 428–432. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chen, C.C.; Tsai, W.J.; Ho, Y.H. Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens: Relationship to gene expression, DNA replication, and protein synthesis. Antiv. Res. 2001, 51, 95–109. [Google Scholar] [CrossRef]
- Yu, S.; Liu, B.C.; Lai, P.X. Chemical composition, antibacterial and antioxidant activities of the essential oil of Psychotria serpens. Chem. Nat. Comp. 2020, 56, 748–750. [Google Scholar] [CrossRef]
- Bose, S.; Bose, A. Antimicrobial activity of Acanthus ilicifolius (L.). Indian J. Pharm. Sci. 2008, 70, 821. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Raja, M.; Gnanadesigan, M. Antibacterial potential of benzoate and phenylethanoid derivatives isolated from Acanthus ilicifolius L. leaf extracts. Nat. Prod. Res. 2012, 26, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.H.; Wu, S.Z.; Mu, X.M.; Xu, B.; Su, Q.J.; Wei, J.L.; Yang, Y.; Qin, B.; Xie, Z.C. Effect of alcohol extract of Acanthus ilicifolius L. on anti-duck hepatitis B virus and protection of liver. J. Ethnopharmacol. 2015, 160, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Illian, D.N.; Basyuni, M.; Wati, R.; Hasibuan, P.A.Z. Polyisoprenoids from Avicennia marina and Avicennia lanata inhibit WiDr cells proliferation. Pharmacog. Mag. 2018, 14, 513. [Google Scholar]
- Lalitha, P.; Parthiban, A.; Sachithanandam, V.; Purvaja, R.; Ramesh, R. Antibacterial and antioxidant potential of GC-MS analysis of crude ethyl acetate extract from the tropical mangrove plant Avicennia officinalis L. S. Afr. J. Bot. 2021, 142, 149–155. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Naidu, K.C. Bioactivity of marine mangrove plant Avicennia alba on selected plant and oral pathogens. Int. J. ChemTech Res. 2009, 1, 1213–1216. [Google Scholar]
- Subrahmanyam, C.; Kumar, S.R.; Reddy, G.D. Bioactive diterpenes from the mangrove Avicennia officinalis Linn. Indian J. Chem. 2006, 45, 2556–2557. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Xia, N.H.; Van Chu, T.; Van Sam, H. Ethnobotanical study on medicinal plants in traditional markets of Son La province, Vietnam. For. Soc. 2019, 3, 171–192. [Google Scholar] [CrossRef]
- Chen, J.L.; Blanc, P.; Stoddart, C.A.; Bogan, M.; Rozhon, E.J.; Parkinson, N.; Ye, Z.; Cooper, R.; Balick, M.; Nanakorn, W.; et al. New iridoids from the medicinal plant Barleria prionitis with potent activity against respiratory syncytial virus. J. Nat. Prod. 1998, 61, 1295–1297. [Google Scholar] [CrossRef]
- Yecheng, D.; Zhen, Y.; Yanzhen, Y.; Xiulian, B. Inhibitory activity against plant pathogenic fungi of extracts from Myoporum bontioides A. Gray and indentification of active ingredients. Pest Manag. Sci. 2008, 64, 203–207. [Google Scholar] [CrossRef]
- Dong, L.M.; Huang, L.L.; Dai, H.; Xu, Q.L.; Ouyang, J.K.; Jia, X.C.; Gu, W.X.; Tan, J.W. Anti-MRSA sesquiterpenes from the semi-mangrove plant Myoporum bontioides A. Gray. Mar. Drugs 2018, 16, 438. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.T.; Toan, H.K.; Quang, L.D.; Hoang, V.D. Myobontioids AD and antifungal metabolites from the leaves of Myoporum bontioides A. Gray. Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lirio, S.B.; Macabeo, A.P.G.; Paragas, E.M.; Knorn, M.; Kohls, P.; Franzblau, S.G.; Wang, Y.; Aguinaldo, M.A.M. Antitubercular constituents from Premna odorata Blanco. J. Ethnopharmacol. 2014, 154, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Tangarife-Castaño, V.; Roa-Linares, V.; Betancur-Galvis, L.A.; Durán García, D.C.; Stashenko, E.; Mesa-Arango, A.C. Antifungal activity of Verbenaceae and Labiatae families essential oils. Pharmacologyonline 2012, 1, 133–145. [Google Scholar]
- Rahman, A.; Shanta, Z.S.; Rashid, M.A.; Parvin, T.; Afrin, S.; Khatun, M.K.; Sattar, M.A. In vitro antibacterial properties of essential oil and organic extracts of Premna integrifolia Linn. Arabian J. Chem. 2016, 9, S475–S479. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Eun, J.B. Antimicrobial activity of some Vietnamese medicinal plants extracts. J. Med. Plants Res. 2013, 4, 2597–2605. [Google Scholar]
- Sheeba, S.N.; Ariharan, V.N.; Mary, J.V.J.; Bai, S.M.M.; Paul, J.V. Phytochemical and biological screening of organic Solvent extracts of Ipomoea pes-caprae flower. Ann. Rom. Soc. Cell Biol. 2021, 25, 7800–7821. [Google Scholar]
- Ryan, D.H.; Katherine, H.H.; Taylor, J.W.; Gajendra, S. Traditional Tongan treatments for infections: Bioassays and ethnobotanical leads for activity. J. Med. Plants Res. 2014, 8, 1215–1222. [Google Scholar]
- Srimoon, R.; Ngiewthaisong, S. Antioxidant and antibacterial activities of Indian marsh fleabane (Pluchea indica (L.) Less). Asia-Pac. J. Sci. Technol. 2015, 20, 144–154. [Google Scholar]
- Locher, C.P.; Witvrouw, M.; De Béthune, M.P.; Burch, M.T.; Mower, H.F.; Davis, H.; Lasure, A.; Pauwels, R.; De Clercq, E.; Vlietinck, A.J. Antiviral activity of Hawaiian medicinal plants against human immunodeficiency virus type-1 (HIV-1). Phytomed 1996, 2, 259–264. [Google Scholar] [CrossRef]
- Manimegalai, B.; Inbathamizh, L.; Ponnu, T.M. In vitro studies on antimicrobial activity and phytochemical screening of leaf extracts of Scaevola taccada. Int. J. Pharm. Pharm. Sci. 2012, 4, 367–370. [Google Scholar]
- Suthiwong, J.; Thongsri, Y.; Yenjai, C. A new furanocoumarin from the fruits of Scaevola taccada and antifungal activity against Pythium insidiosum. Nat. Prod. Res. 2017, 31, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rameshthangam, P.A.; Ramasamy, P. Antiviral activity of bis (2-methylheptyl) phthalate isolated from Pongamia pinnata leaves against White Spot Syndrome Virus of Penaeus monodon Fabricius. Virus Res. 2007, 126, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, N.S.; Philip, R.; Singh, I.B. In vivo screening of mangrove plants for anti WSSV activity in Penaeus monodon, and evaluation of Ceriops tagal as a potential source of antiviral molecules. Aquacult 2011, 311, 36–41. [Google Scholar] [CrossRef]
- Rahmawati, N.; Mustofa, F.I.; Haryanti, S. Diversity of medicinal plants utilized by To Manui ethnic of Central Sulawesi, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 375–392. [Google Scholar] [CrossRef]
- Chusri, S.; Sinvaraphan, N.; Chaipak, P.; Luxsananuwong, A.; Voravuthikunchai, S.P. Evaluation of antibacterial activity, phytochemical constituents, and cytotoxicity effects of Thai household ancient remedies. J. Altern. Compl. Med. 2014, 20, 909–918. [Google Scholar] [CrossRef]
- Swamy, V.; Ninge, K.; Sudhakar, R. Antimicrobial activity of Casuarina equisetifolia. Int. J. Innov. Pharm. Dev. 2013, 1, 49–57. [Google Scholar]
- Buenz, E.J. Hepatocytes detoxify Atuna racemosa extract. Exp. Biol. Med. 2006, 231, 1739–1743. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants in the Asia Pacific for Zoonotic Pandemics; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Brijesh, S.; Daswani, P.G.; Tetali, P.; Rojatkar, S.R.; Antia, N.H.; Birdi, T.J. Studies on Pongamia pinnata (L.) Pierre leaves: Understanding the mechanism(s) of action in infectious diarrhea. J. Zhejiang Univ. Sci. B. 2006, 7, 665–674. [Google Scholar] [CrossRef]
- Thaman, R.R.; Thomson, L.A.; DeMeo, R.; Areki, F.; Elevitch, C.R. Intsia bijuga (vesi). In Species Profiles for Pacific Island Agroforestry; Academic Publishing: Cambridge, MA, USA, 2006. [Google Scholar]
- Islam, A.R.; Hasan, M.M.; Islam, M.T.; Tanaka, N. Ethnobotanical study of plants used by the Munda ethnic group living around the Sundarbans, the world’s largest mangrove forest in southwestern Bangladesh. J. Ethnopharmacol. 2022, 285, 114853. [Google Scholar] [CrossRef]
- Walker, T. An Examination of Medicinal Ethnobotany and Biomedicine Use in Two Villages on the Phnom Kulen Plateau; Hollins University: Roanoke, VA, USA, 2017. [Google Scholar]
- Li, D.L.; Xing, F.W. Ethnobotanical study on medicinal plants used by local Hoklos people on Hainan Island, China. J. Ethnopharmacol. 2016, 194, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Ignacimuthu, S. Antifungal activity of traditional medicinal plants from Tamil Nadu, India. Asian Pac. J. Trop. Biomed 2011, 1, S204–S215. [Google Scholar] [CrossRef]
- Ju, S.K.; Semotiuk, A.J.; Krishna, V. Indigenous knowledge on medicinal plants used by ethnic communities of South India. Ethnobot. Res. Appl. 2019, 18, 1–112. [Google Scholar]
- Janni, K. Plants in Samoan Culture. The Ethnobotany of Samoa. Econ. Bot. 2002, 56, 100. [Google Scholar] [CrossRef]
- Herlina, R.; Rahayuningsih, M.; Iswari, R.S. Species richness of medicinal plants in the Dieng Plateau. J. Innov. Sci. Educ. 2019, 8, 116–122. [Google Scholar]
- Uy, M.M.; Garcia, K.I. Evaluation of the antioxidant properties of the leaf extracts of Philippine medicinal plants Casuarina equisetifolia Linn, Cyperus brevifolius (Rottb) Hassk, Drymoglossum piloselloides Linn, Ixora chinensis Lam, and Piper abbreviatum Opiz. Adv. Agric. Bot. 2015, 7, 71–79. [Google Scholar]
- Tomar, S.; Jawanjal, P. Overview of Pentatropis capensis (Asclepiadaceae)—An extra pharmacopoeial plant. J. Ayu Herb. Med. 2019, 5, 66–69. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants of Asia and the Pacific; Drugs for the Future? World Scientific Publishing Co. Inc.: Singapore, 2006. [Google Scholar]
- Thomson, L.A.; Englberger, L.; Guarino, L.; Thaman, R.R.; Elevitch, C.R. Pandanus tectorius (pandanus). In Species Profiles for Pacific Island Agroforestry; ResearchGate GmbH: Berlin, Germany, 2006; p. 28. [Google Scholar]
- Medhi, R.P.; Chakrabarti, S. Traditional knowledge of NE people on conservation of wild orchids. Indian J. Tradit. Knowl. 2009, 8, 11–16. [Google Scholar]
- Akhter, M.; Hoque, M.M.; Rahman, M.; Huda, M.K. Ethnobotanical investigation of some orchids used by five communities of Cox’s Bazar and Chittagong hill tracts districts of Bangladesh. J. Med. Plants Stud. 2017, 5, 265–268. [Google Scholar]
- Rahmatullah, M.; Mollik, M.A.H.; Islam, M.K.; Islam, M.R.; Jahan, F.I.; Khatun, Z.; Seraj, S.; Chowdhury, M.H.; Islam, F.; Miajee, Z.U.M.; et al. A survey of medicinal and functional food plants used by the folk medicinal practitioners of three villages in Sreepur Upazilla, Magura district, Bangladesh. Am. Eurasian J. Sustain. Agric. 2010, 4, 363–373. [Google Scholar]
- Primavera, J.; Sadaba, R.; Lebata, M.; Hazel, J.; Altamirano, J. Handbook of Mangroves in the Philippines-Panay; Aquaculture Department, Southeast Asian Fisheries Development Center: Tigbauan, Philippines, 2004. [Google Scholar]
- Latayada, F.S.; Uy, M.M. Screening of the antioxidant properties of the leaf extracts of Philippine medicinal plants Ficus nota (Blanco) Merr., Metroxylon sagu Rottb., Mussaenda philippica A. Rich., Inocarpus fagifer, and Cinnamomum mercadoi Vidal. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 18–24. [Google Scholar]
- Wiart, C. Ethnopharmacology of Medicinal Plants: Asia and the Pacific; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Motley, T.J. The ethnobotany of Fagraea Thunb. (Gentianaceae): The timber of malesia and the scent of polynesia. Econ. Bot. 2004, 58, 396–409. [Google Scholar] [CrossRef]
- Paramita, S. Tahongai (Kleinhovia hospita L.): Review sebuah tumbuhan obat dari Kalimantan Timur. Indones. J. Plant Med. 2016, 9, 29–36. [Google Scholar] [CrossRef][Green Version]
- Duke, N.C. Australia’s Mangroves: The Authoritative Guide to Australia’s Mangrove Plants; MER: Brisbane, Australia, 2006. [Google Scholar]
- Liebezeit, G.; Rau, M.T. New Guinean mangroves—Traditional usage and chemistry of natural products. Senckenberg. Marit. 2006, 36, 1–10. [Google Scholar] [CrossRef]
- Cambie, R.C.; Ash, J. Fijian Medicinal Plants; CSIRO Publishing: Clayton, Australia, 1994. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Reverter, M.; Bontemps, N.N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano, R.; del Rio-Rodríguez, R.l.; Diéguez, A.L.; Romalde, J.L. Virulence of Vibrio harveyi responsible for the “Bright-red” Syndrome in the Pacific white shrimp Litopenaeus vannamei. J. Invertebr. Pathol. 2012, 109, 307–317. [Google Scholar] [CrossRef]
- Davies, T.K.; Lovelock, C.E.; Pettit, N.E.; Grierson, P.F. Short-term microbial respiration in an arid zone mangrove soil is limited by availability of gallic acid, phosphorus and ammonium. Soil Biol. Biochem. 2017, 115, 73–81. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Huang, Q. Allelopathic effects of gallic acid from Aegiceras corniculatum on Cyclotella caspia. J. Env. Sci. 2013, 25, 776–784. [Google Scholar] [CrossRef]
- Alonso-Rodrıguez, R.; Páez-Osuna, F. Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: A review with special reference to the situation in the Gulf of California. Aquaculture 2003, 219, 317–336. [Google Scholar] [CrossRef]
- Gao, J.; Zuo, H.; Yang, L.; He, J.H.; Niu, S.; Weng, S.; He, J.; Xu, X. Long-term influence of cyanobacterial bloom on the immune system of Litopenaeus vannamei. Fish Shellfish. Immunol. 2017, 61, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gil-Turnes, M.S.; Hay, M.E.; Fenical, W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 1989, 246, 116–118. [Google Scholar] [CrossRef]
- Haoliang, L.; Chongling, Y.; Jingchun, L. Low-molecular-weight organic acids exuded by Mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environ. Exp. Bot. 2007, 61, 159–166. [Google Scholar] [CrossRef]
- White, S.L.; Rainbow, P.S. Regulation and accumulation of copper, zinc and cadmium by the shrimp Palaemon elegans. Mar. Ecol. Prog. Ser. 1982, 8, 95–101. [Google Scholar] [CrossRef]
- Rozirwan, R.; Muda, H.I.; Ulqodry, T.Z. Antibacterial potential of Actinomycetes isolated from mangrove sediment in Tanjung Api-Api, South Sumatra, Indonesia. Biodiversitas J. Biol. Divers 2020, 21, 5723–5728. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
