Abstract
The demand for sustainable and environmentally friendly food sources and food ingredients is increasing, and microalgae are promoted as a sustainable source of essential and bioactive lipids, with high levels of omega-3 fatty acids (ω-3 FA), comparable to those of fish. However, most FA screening studies on algae are scattered or use different methodologies, preventing a true comparison of its content between microalgae. In this work, we used gas-chromatography mass-spectrometry (GC-MS) to characterize the FA profile of seven different commercial microalgae with biotechnological applications (Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica). Screening for antioxidant activity was also performed to understand the relationship between FA profile and bioactivity. Microalgae exhibited specific FA profiles with a different composition, namely in the ω-3 FA profile, but with species of the same phylum showing similar tendencies. The different lipid extracts showed similar antioxidant activities, but with a low activity of the extracts of Nannochloropsis oceanica. Overall, this study provides a direct comparison of FA profiles between microalgae species, supporting the role of these species as alternative, sustainable, and healthy sources of essential lipids.
1. Introduction
Microalgae are considered a sustainable source of essential and bioactive lipids [1]. Moreover, they are quite diverse in their fatty acid (FA) profile and abundances, which shows exploitable potential [2]. In fact, different saturated FA (SFA), monounsaturated FA (MUFA), and polyunsaturated FA (PUFA) were described in microalgae. However, there are some common FAs observed among different microalgae, for example, hexadecanoic acid (C16:0) and oleic acid (C18:1). Some microalgae are rich in omega-3 (ω-3) FA), and are often considered as the main producers of these healthy lipids, representing an alternative source to fish [3]. Depending on the microalgae, they can provide essential FAs, such as alpha-linolenic acid (C18:3 ω-3; ALA), which can be metabolized in mammals to eicosapentaenoic acid (C20:5 ω-3; EPA) and docosahexaenoic acid (C22:6 ω-3; DHA) [4]. Some microalgae also provide EPA directly and are therefore a direct source of this healthy and bioactive FA [5]. This is of the utmost importance as EPA is not found at high levels in terrestrial plants but is found in some seaweeds, such as Palmaria palmata [6], but in lower amounts compared to microalgae [5].
Omega-3 FAs are important nutrients for the central nervous system in humans and other mammals [7,8]. They are also important antioxidant compounds and are recognized precursors of anti-inflammatory eicosanoids [9], thus playing a key role in the prevention of neurodegenerative diseases and memory loss [10,11] and in the regulation of inflammation [9]. Their ability to participate in the modulation of inflammation is quite important in the prevention of non-communicable diseases (NCDs) [12], such as cardiovascular disease, diabetes, and obesity. These NCDs are among the main causes of morbidity and mortality in the world, being associated with chronic low-grade inflammation characterized by continuously elevated levels of circulating pro-inflammatory cytokines, chemokines and acute inflammatory phase proteins [13], and continuous oxidative stress [14], leading to impaired bodily function. In this context, the use of ω-3 FA to prevent and mitigate chronic inflammation offers an opportunity to reduce the prevalence of NCDs.
Modern diets often have an imbalance in the intake of these essential and healthy FA [15]. Microalgae are considered important new foods for the prevention of the global burden of 21st century chronic diseases, such as NCDs [16,17], and therefore should be included in healthy and sustainable diets [18,19]. The recommendation for microalgae intake is also supported by their sustainable production in aquaculture [20], as it does not compete with water or land and, consequently, does not compete with other terrestrial plants, reducing ocean pollution and overexploitation of marine resources [21]. The disadvantages of consuming fish oil, for example, the significant abundance of heavy metals or antibiotics in fish oils, also bolster microalgae as a promising source of healthy lipids [22]. Microalgae have the advantage of being single-cell factories, producing sustainable and safe biomass and ingredients with reproducible nutritional and health value for food, ingredients or supplements, feed or pharmaceuticals, and cosmetics [3].
Nowadays, most of the microalgae used in the food industry are Chlorella vulgaris, Odontella aurita, and Spirulina, typically as food supplements, and Tetraselmis chui, as a flavoring agent for seafood [23]. Other microalgae are used to obtain extracts for a wide range of uses (such as supplementation with essential nutrients, pharmaceutical and cosmetic applications, production of biodiesel, among others), thus they have been studied for many years [24,25,26]. The presence of high abundances of ω-3 FA in microalgae is essential to valorize them as food products and food ingredients [27]. Omega-3 FA are sensitive to oxidation and could, therefore, work as food preservatives to prevent spoilage [28]. Moreover, microalgae are recognized as a source of antioxidant compounds, namely, polyphenols [29]. However, despite the antioxidant potential of lipids in microalgae, they remain scarcely recognized, and most studies explore the use of aqueous extracts of microalgae, rich in phenolic compounds [29,30]. Nevertheless, individual studies on microalgae have highlighted and recognized the antioxidant potential of their healthy lipids [31,32,33], for example, FA from different microalgae from different phyla.
Over the years, numerous studies have reported that microalgae have different FA profiles, some of them considered important for algal exploitation and nutritional valorization as well as for taxonomic classifications [34]. Indeed, it has already been reported that the level of production of ω-3 FA and FA species differs between microalgae. For example, the taxa of Diatoms and Eustigmatophytes, such as Phaeodcatylum tricornutum and Nannochloropsis sp., respectively, are important synthesizers of EPA while microalgae belonging to the taxa of Dinophytes, Haptophytes, and Thraustochytrids are considered to be producers of DHA- [35,36,37]. The production of long-chain ω-3 FA (EPA and DHA) in Cyanobacteria (e.g., Spirulina platensis) and Chlorophytes (e.g., Chlorella vulgaris) is considered negligible and, in the case of C. vulgaris, only interesting amounts of ALA or stearidonic acid (C18:4 ω-3) are found [38]. Although some microalgae produce lower levels of ω-3 FA, these amounts can be increased by manipulating growing conditions [39], since the production of ω-3 FA is also dependent on the surrounding environment [40]. Therefore, the characterization of the FA profile from microalgae is of the utmost importance.
Nevertheless, published work focused on the study of microalgae FA is scattered because different authors have used different extraction methods, FA derivatization techniques, and data acquisition [31,32,41,42,43]. For example, published work has often analyzed crude extracts, obtained using different mixtures of solvents with different extraction capacities, or different extraction methodologies. In fact, the vast majority of studies have used chloroform:methanol extractions and differences seem to stem from other ancillary procedures of mechanical cell disruption (e.g., ultrasound-assisted extraction, supercritical CO2 extraction) that are necessary with some species of hard cell walls and to enhance the extraction of lower yield solvents (e.g., ethanol) [44]. Additionally, most studies used a gas-chromatography flame ionization detector (GC-FID); however, GC-mass spectrometry (MS) is considered a more powerful and accurate methodology currently available to detect FA (e.g., gas-chromatography mass spectrometry (GC-MS)) [45].
Thus, in this work, an overview of the microalgae most commonly used as biomass or ingredients will be given, using an efficient lipid extraction method, aimed at clarifying the FA profiles according to the species of microalgae, grown under saline (Nannochloropsis oceanica, Phaeodactylum tricornutum, Tetraselmis chui) or fresh water media (Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Spirulina sp.), but also looking for putative correlation between the fatty acid profile and antioxidant activity of the extracts. The selected microalgae are among the most cultivated species in Europe [46]. The present work will thus promote the valorization of microalgae as food and feed ingredients with added value or products of pharmacological interest.
2. Results
2.1. Lipid Content in Different Microalgae
The lipid content of the microalgae Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica were determined by gravimetry and are described in Figure 1 and Table S1. N. oceanica was the microalgae with the highest lipid content (20.9 ± 3.4%) followed by C. amblystomatis, P. tricornutum, S. obliquus, and Spirulina sp., respectively. The microalgae C. vulgaris and T. chui had the lowest lipid contents (8.8 ± 0.7% and 6.5 ± 0.4%, respectively). The lipid content of N. oceanica was significantly different from that of C. vulgaris, T. chui, and Spirulina sp. (q < 0.05). In addition, the lipid content of C. vulgaris was significantly lower than that of C. amblystomatis, and the latter was significantly higher than that of T. chui. The microalgae T. chui had a significantly lower lipid content than that of P. tricornutum.
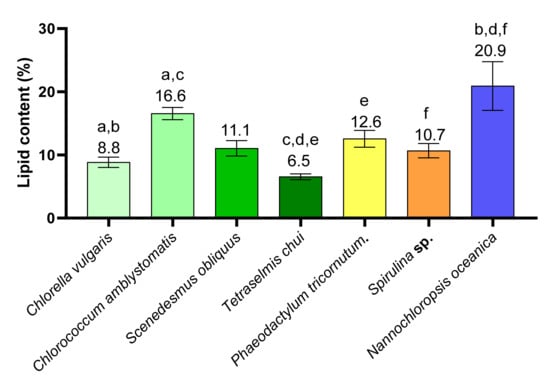
Figure 1.
Lipid content of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica (expressed in percentage % of biomass). Matching letters (a–f) indicate statistically significant differences between microalgae species, i.e., the same letter represents significant differences (q < 0.05, Kruskal–Wallis test followed by Dunn’s post-hoc comparisons).
2.2. Fatty Acid Profile of Different Microalgae and Nutritional Indices
The FA profiles identified in the total lipid extracts of C. vulgaris, C amblystomatis, S. obliquus, T. chui, P. tricornutum, Spirulina sp., and N. oceanica are summarized in Table 1. The FAs were identified after transesterification reactions and analyzed as fatty acid methyl esters (FAMEs) using gas-chromatography mass spectrometry (GC-MS). One of the most abundant FAs identified in all microalgae was palmitic acid (C16:0), with a higher abundance in Spirulina sp. extracts (38.6 ± 0.4%). Other FAs were common among the selected microalgae, such as C14:0, C16:1 ∆9 (ω-7), C17:0, C18:0, C18:1 ∆11 (ω-7), C18:1 ∆9 (ω-9), and C18:2 ∆9,12 (ω-6). Some of the common FAs displayed a higher relative abundance in different species, namely C16:1 ∆9 (ω-7) in P. tricornutum (16.4 ± 0.3%) and N. oceanica (21.6 ± 1.3%), and both C18:2 ∆9,12 (ω-6) and C18:3 ∆6,9,12 (ω-6) in Spirulina sp. (21.4 ± 0.8% and 23.3 ± 0.8%, respectively). In addition to these FAs, C16:4 ∆4,7,10,13 (ω-3) was identified with a high abundance in C. amblystomatis, S. obliquus, and T. chui (12.7 ± 0.6%, 15.5 ± 1.4%, and 14.5 ± 1.8%).

Table 1.
Fatty acid (FA) profile of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica by GC-MS. FAs are expressed in relative abundance (%) and the values are the means of five analytical samples (n = 5) ± standard deviation (SD).
With the exception of Spirulina sp., all microalgae had different but interesting amounts of distinct ω-3 FA, namely C18:4 ∆6,9,12,15 (ω-3), α-linolenic acid (C18:3 ∆9,12,15 (ω-3), ALA) and eicosapentaenoic acid (C20:5 ∆5,8,11,14,17 (ω-3), EPA). The lipid extract of S. obliquus had the highest relative abundance of ALA (35.0 ± 2.7%) followed by C. vulgaris (26.3 ± 1.4%), C. amblystomatis (21.9 ± 0.9%), T.chui (18.3 ± 2.4%), and P. tricornutum (1.8 ± 0.1%), which had the lowest abundance. EPA was identified in four microalgae, with N. oceanica having the highest relative abundance (30.8 ± 2.4%) followed by P. tricornutum (27.3 ± 1.5%), C. amblystomatis (10.7 ± 0.6%), and T.chui (4.2 ± 0.6%). Among the selected microalgae, only P. tricornutum had docosahexaenoic acid (C22:6 ∆4,7,10,13,16,19 (ω-3), DHA), with a relative abundance of 0.6 ± 0.1%.
The relative abundance of all FAs detected in the seven microalgae was used for multivariate statistical analysis. The hierarchical clustering and principal component analysis (PCA) plots are shown in Figure 2 and Figure S1, respectively. Hierarchical clustering showed that the microalgae of the Chlorophytes phylum clustered together at the first level of the tree and the other phylum (Ochophytes, Bacillariophytes, and Cyanobaceria) were merged. At the second level of the tree, T. chui and S. obliquus, and C. amblystomatis clustered together and C. vulgaris formed its own meta-class. With levels three and four, the other microalgae of the Chlorophytes formed their own meta-class. The other phyla were clustered into different branches, with Cyanobaceria (Spirulina sp.) forming its own meta-class at level two and Ochophytes (N. oceanica) and Bacillariophytes (P. tricornutum) forming their own meta-class at level three of the tree.
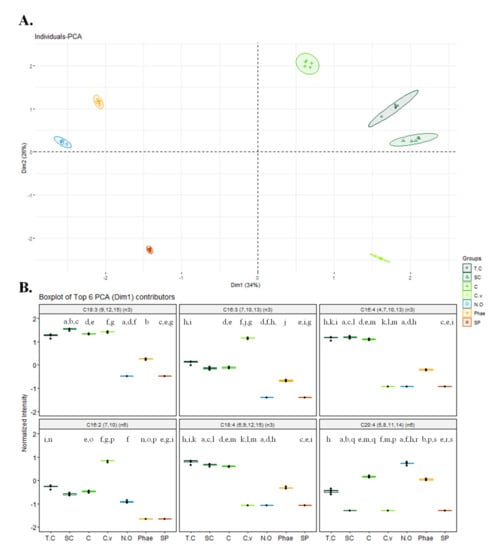
Figure 2.
(A) Principal component analysis (PCA) scores plot and (B) Boxplots of the 6 major contributors for PC1 using the relative abundance after log normalization of all fatty acids identified in Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica. Abbreviations: C, Chlorococcum amblystomatis; C.v, Chlorella vulgaris; SC, Scenedesmus obliquus; T.C, Tetraselmis chui; Phae, Phaeodactylum tricornutum; SP, Spirulina sp.; and N.O, Nannochloropsis oceanica. Matching letters (a–s) indicate statistically significant differences between microalgae species, i.e., the same letter represents significant differences (q < 0.05, Kruskal–Wallis test followed by Dunn’s post-hoc comparisons).
PCA analysis showed that the first two principal components accounted for 60% of the total variance (PC1 34%; PC2 26%). PCA results also showed an association of four microalgae belonging to the same phylum Chlorophyta (C. vulgaris, C. amblystomatis, S. obliquus, T. chui) along PC1, as shown in Figure 2. Among the six major contributors to discrimination, four were omega-3 PUFA: C18:3 ω-3, C16:3 ω-3, C16:4 ω-3, and C18:4 ω-3. The relative abundance of these FAs was higher in species of green microalgae but specific for each microalga, namely C18:3 ω-3 in C. vulgaris, C. amblystomatis, S. obliquus, and T. chui; C16:3 ω-3 in C. vulgaris; and C16:4 and C18:4 ω-3 in C. amblystomatis, S. obliquus, and T. chui.
The Thrombogenicity Index (TI), Atherogenicity Index (AI), PUFA ω-6/ω-3, and hypocholesterolemic/hypercholesterolemic (h/H) ratios were calculated from FA data obtained to assess the nutritional index and the potential health benefits of these seven microalgae (Table 2). The values of the AI and TI indices varied between 0.2 and 0.7, and 0.1 and 1.6, respectively. S. obliquus (0.2 ± 0.1) and C. vulgaris (0.2 ± 0.0) recorded the lowest AI, with significant differences detected between these values and those of microalgae belonging to different phylum (Bacillariophyta, Cyanobacteria, and Ochrophyta). The lowest TI value also belonged to S. obliquus extracts (0.1 ± 0.0), being significantly different from N. oceanica and Spirulina sp. Regarding the h/H ratio, the highest value was obtained for S. obliquus followed by C. vulgaris and P. tricornutum. There are significant differences between S. obliquus and the other two microalgae, Spirulina sp. and T. chui. The microalgae Spirulina sp. had the lowest h/H value, being significantly different from C. vulgaris, P. tricornutum, and S. obliquus.

Table 2.
Fatty acid (FA) indicators of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica. Values correspond to relative abundances (except for AI, TI, and h/H calculations) and are the means of five analytical samples (n = 5) ± standard deviation (SD). Matching letters (a–l) indicate statistically significant differences between microalga species, i.e., the same letter represents significant differences (q < 0.05, Kruskal–Wallis test followed by Dunn’s post-hoc comparisons).
2.3. In Chemico Evaluation of Antioxidant Activity
The antioxidant activity of the lipid extracts of C. vulgaris, C. amblystomatis, S. obliquus, T. chui, P. tricornutum, Spirulina sp., and N. oceanica was evaluated using the ABTS●+ and DPPH● scavenging assays of the free radicals, and the results are shown in Figure 3 and Figure S2.
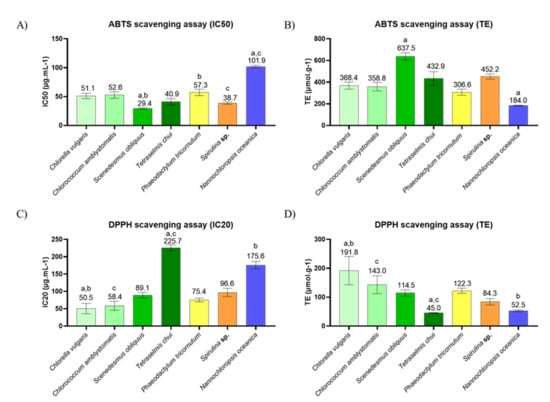
Figure 3.
Evaluation of the antioxidant activity of lipid extracts from different microalgae. Concentration of lipid extract (µg.mL−1) that provided: (A) 50% inhibition of the ABTS●+ radical, (B) 20% inhibition of the DPPH● radical, and (C,D) the Trolox equivalents (TE; µmol.g−1). The values are displayed as the mean (n = 3) ± standard deviation. Matching letters (a–c) represent significant differences between microalgae, i.e., the same letter represents significant differences (q < 0.05, Kruskal–Wallis test followed by Dunn’s post-hoc comparisons).
The lipid extracts of all the microalgae studied had an inhibition of 50% (IC50) in the ABTS●+ assay, at the tested concentrations. The lowest IC50 was obtained with S. obliquus (29.4 ± 1.2 μg/mL) and Trolox equivalents (TE) of 637.5 ± 27.4, followed by Spirulina sp., T. chui, C. vulgaris, C. amblystomatis, and P.tricornutum. N. oceanica extracts showed the highest IC50 (101.9 ± 1.7 µg.mL−1) and the lowest TE values (184.0 ± 3.2 µmol.g−1), compared to other extracts.
In the DPPH● assay, all the microalgae studied were found to inhibit up to 20% (IC20) of the radical. The C. vulgaris extracts were responsible for the lowest IC20 value (50.5 ± 12.3, with a TE of 191.8 ± 40.0). The two extracts of T. chui and N. oceanica showed the highest IC20 (225.7 ± 6.9 µg.mL−1 and 175.6 ± 8.7 µg.mL−1, respectively) and the lowest TE (45.0 ± 1.4 µmol.g−1 and 52.5 ± 2.7 µmol.g−1, respectively) of microalgae extract.
3. Discussion
Microalgae are innovative food products with high nutritional value and are a rich source of essential nutrients, such as proteins, vitamins, minerals, carbohydrates, or lipids, contributing to a healthy and sustainable diet [19]. They are primary producers of ω-3 FA, thus representing a sustainable alternative to fish sources, and have current food applications [3]. Modern diets are known to be rich in saturated lipids and refined sugars but lacking in essential and healthy lipids, such as ω-3 FA [15]. These unbalanced diets cause malnutrition and increase the risk factor for noncommunicable diseases. Significant efforts are being made to encourage consumers to adopt healthier diets and new food products that provide essential, safe, and bioactive nutrients, such as algae.
The seven microalgae selected for this study belong to four different phylum, Chlorophyta (C. vulgaris, C. amblystomatis, S. obliquus, and T.chui), Bacillariophyta (P. tricornutum), Cyanobacteria (Spirulina sp.), and Ochrophyta (N. oceanica). Three of them (C. vulgaris, T.chui, and Spirulina sp.) have food safety approval for human consumption [23]. The microalga with the highest lipid content was N. oceanica (20.9 ± 3.4%), and the one with the lowest amount was T. chui (6.5 ± 0.4%), highlighting the great differences existing among the species. Microalgae lipid content is typically described as within 20–50% of the microalgae dry weight, which was not observed in the present work [47]. Probably, the difference in results could be due to the different growth conditions of the microalgae or due to the use of crude organic extracts, thus other molecules, other than lipids, could have contributed to the weight of the extract [48,49].
The FA described in this work correspond to esterified FAs, which are considered to be more bioaccessible when compared to their free forms [50]. The FAs profiled for these different microalgae were consistent with what has been reported in previous studies for each alga, concerning FA, but with differences in the FA contents [31,51,52,53,54,55,56], namely the present work reports higher amounts of ω-3 FA. These differences may be due to the different growth conditions for the microalgae studied, the use of different extraction methodologies (e.g., using different solvents and mixtures of solvents), different derivatization methodologies, or different platforms for data acquisition (e.g., GC-FID) [57,58]. Despite a few common FA species, each microalga had a unique FA signature (Table 2), with microalgae from the same phylum showing similar profiles and clustering at higher levels, as observed in the principal component analysis (PCA) (Figure 2) and in the hierarchical clustering analysis (Figure S1). Omega-3 FAs have been identified with significantly elevated abundances in all microalgae, except Spirulina sp. The absence of ω-3 FA in Spirulina species has been extensively reported [51,59,60,61,62,63]. However, some studies described the presence of some of these PUFA, although in very low abundances [64,65,66,67,68]. These differences might be dependent on growth conditions, as ω-3 production is tightly dependent on them [3]. The microalga S. obliquus had the highest abundance of ω-3 PUFA (55.9 ± 4.5%), showing the highest abundance of α-linolenic acid (ALA, C18:3 ω-3) (35.0 ± 2.7%). This ω-3 is an essential FA of the greatest importance for humans and mammals, and can be metabolized into another longer ω-3 PUFA, such as eicosapentaenoic acid (EPA, C20:5 ω-3) and docosahexaenoic acid (DHA, C22:6 ω-3) [4]. These results are in line with what was previously reported for S. obliquus; however, some studies have reported high abundances of EPA in this microorganism, which we do not report [69,70,71,72]. Nonetheless, variations in cultivation might justify the absence of EPA, as salinity, nitrogen, and nutrient stress affect the composition and yield of FA [73,74,75,76]. Additionally, the way S. obliquus biomass is processed has been shown to affect the abundance of FA [69]. The high abundance of this essential FA in S. obliquus contributes to the valorization of this microalga as a source of this essential ω-3 FA as a food ingredient. However, the synthesis of EPA and DHA from ALA is limited in mammals, and they must also be absorbed through the diet [5]. These two ω-3 FAs were not found in S. obliquus. Nonetheless, EPA was found in significant high abundances in other microalgae, particularly in N. oceanica, which lacks ALA. In line with our results, previous studies reported a total absence of ALA and high abundances of EPA [77,78,79,80,81,82]. One study reported the presence of negligible amounts of ALA; however, the authors were evaluating the effects of the use of phytohormones and nitrogen depletion on biomass and lipid production, which might justify the presence of negligible amounts of this FA in N. oceanica [83]. Regarding these FAs, an interesting correlation was observed between the abundances of ALA and EPA. Namely, when ALA was the most abundant FA, EPA was either absent (S. obliquus) or in lower amounts (C. amblystomatis and T. chui), suggesting a lower synthesis of EPA from ALA. The reverse was also observed in microalgae with EPA as the most abundant FA having no ALA (N. oceanica) or negligible amounts (P. tricornutum), suggesting increased production of EPA. This relationship between ALA and EPA appears to be unrelated to saline and freshwater growth. P. tricornutum was the only microalga in this study containing DHA, but with negligible amounts (0.6 ± 0.1%). EPA and DHA FA are important for good neurological development, as well as a regulated inflammatory response, as they are precursors of anti-inflammatory eicosanoids [9,10]. Other authors also observed small abundances of DHA in P. tricornutum when compared to ALA and EPA [84,85,86,87,88]. Few studies even reported an absence of DHA in this microalga [89,90,91].
Omega-3 FA are also very important for the prevention of atherosclerosis and cardiovascular disease [92]. The use of cardiovascular disease risk predictors, such as the atherogenic index (AI) and the thrombogenic index (TI), facilitates evaluation of the nutritional quality of lipids since the AI and TI measure the probability of reducing the risk of atherogenic plaques and blood clots, respectively [93]. These indices have already been used to assess these benefits in seaweeds, fish, and even microalgae [67,94,95,96,97]. Among the microalgae studied, S. obliquus showed both the lowest AI (0.2 ± 0.1) and TI (0.1 ± 0.0) values. The index values reported in this study were similar to those reported for fish and other seafood [98,99]. Compared to the indices reported for other microalgae, S. obliquus showed lower AI and TI than Spirulina platensis, Nannochloropsis gaditana, Nannochloropsis oculata, and Porphyridium cruentum (values among 0.49–1.70 and 0.22–3.82, respectively) [67].
Antioxidant compounds are in great demand as food ingredients to prevent damage caused by oxidative stress and, at the same time, the development of various diseases, including NCDs [100]. They are also of interest as natural preservatives to prevent oxidation and spoilage of food. Seaweed extracts have already shown potential to prevent food spoilage [101,102]. Microalgae are natural sources of antioxidant agents because they produce phenolic compounds [103], described as powerful antioxidants [104], and antioxidant lipids [105], in particular PUFA [49], which scavenge oxidative radicals and prevent damage caused by oxidation [106]. Most studies have focused on the antioxidant potential of phenolic compounds from microalgae; however, individual studies have highlighted the antioxidant activity of their lipids [29,30,31,32,33]. The ABTS●+ and DPPH● radical scavenging assays were used to assess this biological potential of lipid extracts from seven different microalgae. For the evaluation of the antioxidant potential, it is useful to use distinct methods as distinct compounds and different mechanisms may underly the antioxidant potential [107,108]. Among the different methods for antioxidant activity screening, the DPPH and ABTS assays are the most used ones due to their methodological simplicity and stability of the radicals. The ABTS assay evaluates the capacity of the extracts to reduce the ABTS●+ radical, which is generated previously through the oxidation of the ABTS, while the DPPH assay evaluates the capacity of the extracts to reduce the DPPH● radical, which is a stabilized radical by itself [108,109]. Both assays were used to evaluate the antioxidant properties of the microalgae lipid extracts, with T. chui showing the highest IC20 in the DPPH assay. The obtained results suggest that each microalga has antioxidant potential but at different levels. S. obliquus showed the highest antioxidant potential of all the microalgae studied, with an IC50 for the ABTS●+ scavenging assay of 29.4 ± 1.2 μg/mL (TE of 637.5 ± 27.4), and with a DPPH● IC20 value of 89.1 ± 6.6 μg/mL−1 (TE of 114.5 ± 8.9 µmol.g−1). A previous study reported the DPPH● radical scavenging activity for methanol extracts of different microalgae, including S. obliquus, which promoted 20% inhibition at around 200 μg/mL−1, showing lower antioxidant power compared to dichloromethane:methanol extracts [49]. Our extracts also showed lower IC20 compared to seven other microalgae used in the same study. Another study using ultrasound-assisted ethanol extracts of S. obliquus reached IC50 of DPPH● radical at 12.75 ± 0.31 µg.mL−1, showing higher antioxidant power compared to the extracts used in our study [110]. This microalga currently has no food safety approval [23]; however, the high content of ω-3 FA, favorable nutritional indicators, and the potent ability to scavenge the ABTS●+ radical indicate a promising food ingredient with health benefits, and a source of antioxidants to prevent spoilage of food. The highest IC50 for the ABTS●+ radical scavenging assay was attributed to extracts of N. oceanica (101.9 ± 1.7 µg.mL−1), with poorer performance than the remaining six microalgae. This could be associated with the lower abundance of PUFA in this microalga, when compared with the others in this study (Table 2), since the antioxidant activity in lipid extracts has been attributed to PUFA [49]. In fact, the microalgae with the best antioxidant activities (Figure 3) were the ones with a higher abundance of PUFA (C. vulgaris, C. amblystomatis, S. obliquus, and P. tricornutum). This was confirmed when calculating the correlation coefficient between PUFA and antioxidant activity (Figure S2), where PUFA were highly correlated with the TE obtained in the DPPH and ABTS assays, but TE was not correlated with the amount of MUFA. This can be justified since PUFAs are more prone to oxidation, as they are the first targets of free radicals [111,112,113]. In addition, despite the nutritional importance of the balance between omega-3 and omega-6 FAs, the relation with antioxidant activity through inhibition of free radicals appears to depend on the amount of PUFA but does not depend on the amount of omega 3 or omega 6 FAs. In the literature, few published works have reported a similar trend in the susceptibility to oxidation of both omega-3 and omega-6 FAs, while others suggested a dissimilar behavior [106,111,112]. In fact, N. oceanica have the high content in EPA (Table 1) but showed the lowest antioxidant capabilities in both ABTS and DPPH assays, when compared to the other microalgae (Figure 3). Nonetheless, N. oceanica might have antioxidant potential using other mechanisms. For instance, it was reported to enhance the expression of the antioxidant enzymes glutathione s-transferase, glutathione peroxidase, and glutathione reductase in Cornish Giant cockerels [113]. On the other hand, the lowest IC20 value for the DPPH● assay was found for C. vulgaris of 50.5 ± 12.3 µg.mL−1 (TE 191.8 ± 40.0 µmol.g−1). Compared to hexane and hexane:chloroform extracts from another study, these extracts did not fare so well [114]. This microalga has current applications in the food industry, being used as a supplement or directly as food. The antioxidant potential described here is beneficial for its valorization as a new natural source of antioxidant agents, namely antioxidant lipids. In our study, the extracts with the highest IC20 were those of T.chui (225.7 ± 6.9 µg.mL−1). Previous studies using methanolic extracts of this microalga had a lower DPPH● IC20 [49,115]. Another study reported 90.7% DPPH inhibition for ethanol extracts but at 10 mg/mL [116]. However, this microalga had the third lowest IC50 for the ABTS●+ radical, nevertheless suggesting that this microalga is able to prevent the oxidation of other radicals.
In summary, this study supports already existing studies in order to better understand the FA composition in microalgae. Different microalgae have different lipid composition, which can be valued and explored for different biotechnology applications and industries. With the exception of Spirulina sp., the microalgae in this study had high amounts of ω-3 FA, with S. obliquus showing the greatest abundance. This was favorable to the nutritional indices calculated for this microalga. In addition, S. obliquus had a better performance in the antioxidant assay, which strengthens the nutritional potential of this sustainable bio-factory for the implementation of food-based ingredients and preservatives. However, despite the extraction approach generating extracts rich in lipids, one cannot exclude the possible contribution of other compounds to the described antioxidant activity. Therefore, further studies are needed to explore and establish the relationship between microalgae lipids and biological activities. Nevertheless, the results described here reinforce the use of microalgae as a source of value-added lipids for the food, feed, and pharmaceutical industries.
4. Materials and Methods
4.1. Reagents
HPLC-grade methanol (MeOH), ethanol absolute, hexane, and dichloromethane (CH2Cl2) were purchased from Fisher Scientific Ltd. (Loughborough, UK). All other reagents were purchased from major commercial sources. Milli-Q water (Synergy, Millipore Corporation, Billerica, MA, USA) was used. Furthermore, 2,2-diphenyl-1-picrylhydrazy radical (DPPH●) was purchased from Aldrich (Milwaukee, WI), 2,20-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS●+) was obtained from Fluka (Buchs, Switzerland), and 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) was purchased from Sigma-Aldrich (St Louis, MO, USA). All the other reagents and chemicals used were of the highest grade of purity commercially available.
4.2. Microalgae Material
The spray-dried biomass of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica was supplied by Allmicroalgae, Natural Products S.A. located in Pataias, Portugal.
The specimens are internally deposited at Allmicroalgae’s culture collection. All species were autotrophically grown in Guillard’s F2 culture medium, the composition of which was adapted to the local water (REF below). Phaeodactylum tricornutum, Tetraselmis chui, and Nannochloropsis oceanica were further supplemented with magnesium mixture (Necton, Olhão, Portugal) and NaCl (Salexpor, Coimbra, Portugal) at 30 g/L salinity. Spirulina sp. was supplemented with 16.8 g/L sodium bicarbonate (Quimitécnica, Barreiro, Portugal). Then, 5-L flask reactors were cultivated from 7 to 15 days, under continuous 700 μmol photons.m2/s light exposition. Five 5-L flask reactors were used to inoculate one 0.1 m3 L outdoor flat panel (FP) reactor, which was later sequentially scaled until 1 m3 FPs was reached. Except in the case of Spirulina sp., which was collected directly from the FPs, four of the later reactors were used as inoculum of a 10 m3 tubular photobioreactor (PBR). The reactor was operated for the time necessary to reach the stationary phase, exposed to the environmental light and temperature conditions. Constant pH was maintained by pulse injections of CO2 and the temperature was kept under the limit by a sprinkler-like irrigation system. Table 3 describes the pH and temperature at which PBRs were operated. After the growing period, the biomass was industrially collected by centrifugation and further spray-drying.

Table 3.
Description of the limiting temperature and pH conditions operated in the tubular photobioreactors for microalgae cultivation.
4.3. Lipid Extraction Procedure
Lipids were extracted from 25 mg of lyophilized biomass of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica, based on Folch extraction [44,117], using dichloromethane instead of chloroform. The phase separation was achieved by centrifugation at 2000 rpm for 10 min, and the organic phase was collected. The aqueous phase was reextracted with 2 mL of dichloromethane, two more times. The combined organic phases were dried under a stream of nitrogen and weighted.
Each series of extracts was repeated five times and the total lipid content was determined by gravimetry.
4.4. Analysis of Fatty Acids by Gas Chromatography-Mass Spectrometry (GC-MS)
The fatty acid methyl esters (FAMEs) were prepared from total lipid extracts of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp., and Nannochloropsis oceanica by transmethylation reaction using a methanolic solution of potassium hydroxide (2.0 M) according to the methodology previously described [118]. A volume of 2.0 μL of a hexane solution containing FAMEs and 1.0 μg mL−1 of methyl nonadecanoate (Sigma, St. Louis, MO, USA) as internal standard was injected in a chromatography-mass spectrometry (GC–MS) (Agilent Technologies 8860 GC System, Santa Clara, CA, USA) equipped with a DB-FFAP column with the following specifications: 30 m long, 0.32 mm internal diameter, and 0.25 μm film thickness (J&W Scientific, Folsom, CA, USA). The GC equipment was connected to an Agilent 5977B Mass Selective Detector operating with electron impact ionization at 70 eV and a scanning range of m/z 50–550 (1 s cycle in a full scan mode). The following conditions were used: helium as carrier gas (constant flow 1.4 mL min−1), inlet temperature 220 °C, detector temperature 230 °C, and injection volume 2 μL (splitless). The oven temperature was programmed as follows: 58 °C for 2 min, 25 °C min−1 to 160 °C, 2 °C min−1 to 210 °C, and 30 °C min−1 to 225 °C (held for 20 min). The data acquisition software used was GCMS 5977B/Enhanced MassHunter.
4.5. Data Analysis
The acquired data were analyzed using Agilent MassHunter Qualitative Analysis 10.0 software. The identification of FA was carried out by the retention time and comparison of the MS spectrum with the NIST chemical database library and confirmed with the literature reports [119]. Five independent replicates were injected. The atherogenic (AI), thrombogenic (TI) and hypocholesterolemic/hypercholesterolemic indexes (h/H) were calculated using the following formula (Equations (1)–(3)), as proposed by Ulbricht and Southgate [120]:
4.6. Statistical Analysis
Multivariate and univariate analyses were performed using R version 4.0.2 [121] in Rstudio version 1.3.1093 [122]. Data were glog transformed using the Metaboanalyst software [123]. Principal component analysis (PCA) was conducted for exploratory data analysis, with the R built-in function. PCA and ellipses (level of 0.95) were created using R libraries FactoMineR [124] and factoextra [125]. Hierarchical clustering was generated by the Metaboanalyst [126]. The Kruskal–Wallis test followed by Dunn’s post-hoc comparisons were performed with the R built-in function. P-values were corrected for multiple testing using the BH Benjamini, Hochberg, and Yekutieli method (q values) [127]. A q-value < 0.05 was considered an indicator of statistical significance. All graphics and boxplots were created using the R package ggplot2 [128]. Other R packages used for data management and graphics included plyr [129], dplyr [130], and tidyr [131].
4.7. DPPH Radical Scavenging Assay
The antioxidant scavenging activity against the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH●) was evaluated as described in previous studies [107,132], with some modifications. Firstly, 150 µL of an ethanolic dilution of the extracts (25, 125, 250, 500 µg mL−1) or 150 µL of the Trolox standard solution (5, 12.5, 25, 37.5 µmol L−1 in ethanol) were mixed in triplicate with 150 µL of a DPPH● working solution in ethanol (absorbance ~0.9, 517 nm). The mixture was incubated for 120 min and the absorbance was measured at 517 nm every 5 min (Multiskan GO 1.00.38, Thermo Scientific, Hudson, NH, USA). A control was prepared by replacing the DPPH● solution with ethanol. The antioxidant activity, expressed as a percentage of inhibition of the DPPH radical, was calculated using the following equation (Equation (4)):
The concentration of sample able to scavenge 20% of DPPH radical (IC20) after 120 min of reaction was calculated by linear regression plotting the concentration of lipid extract versus the percentage of the inhibition curve. The activity is expressed in Trolox Equivalents, which were calculated using Equation (5), where IC20 values are the concentration of sample or of Trolox that induces the reduction of the DPPH• radical to 20%:
4.8. ABTS Cation Radical Scavenging Assay
The antioxidant scavenging activity against the 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid radical cation (ABTS●+) was evaluated using a method previously described [132,133] with some modifications. Firstly, 150 µL of an ethanolic dilution of the extracts (25, 125, 250, 500 µg mL−1) or 150 µL of the Trolox standard solution (5, 12.5, 25, 37.5 µmol L−1 in ethanol) were mixed in triplicate with 150 µL of an ABTS●+ working solution in ethanol (absorbance ≈0.9, 734 nm). The mixture was incubated for 120 min and the absorbance was measured at 734 nm every 5 min (Multiskan GO 1.00.38, Thermo Scientific, Hudson, NH, USA). A control was prepared by replacing the ABTS●+ solution with ethanol. The antioxidant activity, expressed as a percentage of inhibition of the ABTS radical, was calculated using Equations (1) and (2) (Abs_DPPH● substituted by Abs_ABTS●+) and expressed in IC50 and Trolox Equivalents (Equation (2)).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md19070357/s1 Table S1. Lipid content of Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum, Spirulina sp. and Nannochloropsis oceanica. Kruskal–Wallis test followed by Dunn’s post-hoc comparisons). Table S2. Evaluation of the antioxidant activity for the extracts of different microalgae. Concentration of lipid extract (µg.mL−1) providing 50% inhibition of the ABTS●+ radical and 20% inhibition of the DPPH● radical, and their respective Trolox equivalents (TE; µmol.g−1). The values are displayed as the mean (n = 3) ± standard deviation. Matching letters (a–f) indicate statistically significant differences between microalgae species, i.e., the same letter repre-sents significant differences (q < 0.05, Kruskal–Wallis test followed by Dunn’s post-hoc comparisons). Figure S1. (A) Hierarchical cluster analysis using relative abundance after Glog normalization of all fat-ty acids identified in Chlorella vulgaris, Chlorococcum amblystomatis, Scenedesmus obliquus, Tetraselmis chui, Phaeodactylum tricornutum. Spirulina sp. and Nannochloropsis oceanica. The green, blue, yellow, and orange boxes show microalgae from the phylum Chlorophyta, Ochrophyta, Bacillariophyta and Cyanobacteria, respectively. Abbreviations: C, Chlorococcum amblystomatis; C.v, Chlorella vulgaris; SC, Scenedesmus obliquus; T.C, Tetraselmis chui; Phae, Phaeodactylum tricornutum; SP, Spirulina sp.; and N.O, Nannochloropsis oceanica., Figure S2. Correlation coefficients analysis using the sum of relative abundances from saturated fatty acids (SFA), monounsaturated FA (MUFA), polyunsaturated FA (PUFA), omega-3 PUFA and omega-6 PUFA, and antioxidant activity. (A) Correlation coefficients with ABTS assay. (B) Correlation coefficients with DPPH assay.
Author Contributions
Conceptualization and design: T.A.C., B.F.N., D.C. and M.R.D.; methodology and formal analysis: T.A.C., B.F.N., D.C., P.D., M.R.D. and T.M.; validation, T.A.C., B.F.N., D.C., P.D., M.R.D. and T.M.; collection and assembly of data: T.A.C., B.F.N., D.C., J.S., M.C., P.D., M.R.D. and T.M.; writing—original draft preparation: T.A.C., B.F.N., D.C., M.C., J.S., B.N., P.D., M.R.D. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received funding under the project AlgaValor, from the Portugal 2020 program (grant agreement nº POCI-01-0247-FEDER-035234; LISBOA-01-0247-FEDER-035234; ALG-01-0247-FEDER-035234).
Data Availability Statement
Raw datasets generated during this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors would like to acknowledge all staff members of Allmicroalgae Natural Products, S.A. for the kind support in microalgae growth. Thanks are due to the University of Aveiro and FCT/MCT for the financial support to CESAM (UIDB/50017/2020 + UIDP/50017/2020), LAQV/REQUIMTE (UIDB/50006/2020), iBIMED (UIDB/04501/2020) and to RNEM, Portuguese Mass Spectrometry Network, (LISBOA-01-0145-FEDER-402-022125) through national funds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement. Tiago Alexandre Conde (2020.05678.BD) is grateful to FCT for his grant. Bruna Neves is grateful for her research fellowship under the project AlgaValor (POCI-01-0247-FEDER-035234). Daniela Couto (SFRH/BD/138992/2018) is grateful to FCT for her grant. Tânia Melo thanks the research contract under the project Omics 4 Algae: Lipidomic tools for chemical phenotyping, traceability and valorisation of seaweeds from aquaculture as a sustainable source of high added-value compounds (POCI-01-0145-FEDER-030962), funded by Centro2020, through FEDER and PT2020. This is a contribution of Marine Lipidomics laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Bio. Technol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Felicitas, V.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Innis, S.M. Omega-3 Fatty Acid Biochemistry: Perspectives from Human Nutrition. Mil. Med. 2014, 179, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Krupanidhi, S.; Sanjeevi, C.B. Omega-3 Fatty Acids for Nutrition and Medicine: Considering Microalgae Oil as a Vegetarian Source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, M.R.; Lillebø, A.I.; Calado, R. A New Look for the Red Macroalga Palmaria palmata: A Seafood with Polar Lipids Rich in EPA and with Antioxidant Properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 Fatty Acids and their Role in Central Nervous System—A Review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef]
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 39–103. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Eckert, G.P.; Lipka, U.; Muller, W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 105–114. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, D. Omega-3 polyunsaturated fatty acids and non-communicable diseases: Meta-analysis based systematic review. Asia Pac. J. Clin. Nutr. 2015, 24, 10–15. [Google Scholar] [PubMed]
- Phillips, C.M.; Chen, L.-W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Melo, H.M.; Santos, L.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef] [Green Version]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar]
- FAO; WHO. Sustainable and Healhy Diets: Guiding Principles; FAO: Rome, Italy, 2019. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Sustainability Analysis of Microalgae Production Systems: A Review on Resource with Unexploited High-Value Reserves. Environ. Sci. Technol. 2018, 52, 14031–14049. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Charles, C.N.; Msagati, T.; Swai, H.; Chacha, M. Microalgae: An alternative natural source of bioavailable omega-3 DHA for promotion of mental health in East Africa. Sci. Afr. 2019, 6, e00187. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Pina-Pérez, M.; Brück, W.; Brück, T.; Beyrer, M. Microalgae as healthy ingredients for functional foods. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–137. [Google Scholar]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; Mackay, D. Oxidation in EPA- and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Pinchetti, J.L.G. Phenolic Profile and Antioxidant Activity of Crude Extracts from Microalgae and Cyanobacteria Strains. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar lipidomic profile shows Chlorococcum amblystomatis as a promising source of value-added lipids. Sci. Rep. 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the polar lipid profile of the microalgae Chlorella vulgaris grown under heterotrophic and autotrophic conditions. Algal Res. 2020, 53, 102128. [Google Scholar] [CrossRef]
- da Costa, E.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for polar lipids, antioxidant, and anti-inflammatory activities of Gloeothece sp. lipid extracts pursuing new phytochemicals from cyanobacteria. J. Appl. Phycol. 2020, 32, 3015–3030. [Google Scholar] [CrossRef]
- Jeong, E.-Y.; Seo, P.J.; Woo, J.C.; Park, C.-M. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 1–16. [Google Scholar]
- Peltomaa, E.; Johnson, M.D.; Taipale, S.J. Marine Cryptophytes Are Great Sources of EPA and DHA. Mar. Drugs 2017, 16, 3. [Google Scholar] [CrossRef] [Green Version]
- Peltomaa, E.; Hällfors, H.; Taipale, S.J. Comparison of Diatoms and Dinoflagellates from Different Habitats as Sources of PUFAs. Mar. Drugs 2019, 17, 233. [Google Scholar] [CrossRef] [Green Version]
- Jónasdóttir, S.H. Fatty Acid Profiles and Production in Marine Phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Taipale, S.; Peltomaa, E.; Salmi, P. Variation in ω-3 and ω-6 Polyunsaturated Fatty Acids Produced by Different Phytoplankton Taxa at Early and Late Growth Phase. Biomolecules 2020, 10, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladyshev, M.I.; Sushchik, N.N. Long-chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges. Biomolecules 2019, 9, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, M.L.; Warwick, J.; Terry, A.; Allen, M.J.; Napier, J.A.; Sayanova, O. Towards the Industrial Production of Omega-3 Long Chain Polyunsaturated Fatty Acids from a Genetically Modified Diatom Phaeodactylum tricornutum. PLoS ONE 2015, 10, e0144054. [Google Scholar] [CrossRef] [PubMed]
- El Baky, H.H.A.; El-Baroty, G.S.; Bouaid, A.; Martinez, M.; Aracil, J. Enhancement of lipid accumulation in Scenedesmus obliquus by Optimizing CO2 and Fe3+ levels for biodiesel production. Bioresour. Technol. 2012, 119, 429–432. [Google Scholar] [CrossRef] [PubMed]
- El Baky, H.H.A.; El Baroty, G.S.; Mostafa, E.M. Optimization Growth of Spirulina (Arthrospira) Platensis in Photobioreactor under Varied Nitrogen Concentration for Maximized Biomass, Carotenoids and Lipid Contents. Recent Pat. Food Nutr. Agric. 2018, 11, 40–48. [Google Scholar] [CrossRef]
- Li, Y.; Naghdi, F.G.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A comparative study: The impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Factories 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.; da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Q.; Li, N.; Pu, Y.; Wang, B.; Zhang, T. Comparison of critical methods developed for fatty acid analysis: A review. J. Sep. Sci. 2016, 40, 288–298. [Google Scholar] [CrossRef]
- Araújo, R.; Calderón, F.V.; López, J.S.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1–24. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Biotechnology for Biofuels Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. Environ. Boil. Fishes 2018, 31, 309–318. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Tudor, C.; Gherasim, E.C.; Dulf, F.V.; Pintea, A. In vitro bioaccessibility of macular xanthophylls from commercial microalgal powders of Arthrospira platensis and Chlorella pyrenoidosa. Food Sci. Nutr. 2021, 9, 1896–1906. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cui, J.; Feng, Y.; Cui, Q. Metabolic profiles of Nannochloropsis oceanica IMET1 under nitrogen-deficiency stress. Bioresour. Technol. 2013, 130, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, B.; She, X.; Zhao, F.; Cao, Y.; Ren, D.; Lu, J. Lipid production and composition of fatty acids in Chlorella vulgaris cultured using different methods: Photoautotrophic, heterotrophic, and pure and mixed conditions. Ann. Microbiol. 2013, 64, 1239–1246. [Google Scholar] [CrossRef]
- de Oliveira, C.Y.B.; Viegas, T.L.; Lopes, R.G.; Cella, H.; Menezes, R.S.; Soares, A.T.; Filho, N.R.A.; Derner, R. A comparison of harvesting and drying methodologies on fatty acids composition of the green microalga Scenedesmus obliquus. Biomass Bioenergy 2020, 132, 105437. [Google Scholar] [CrossRef]
- Matsui, H.; Sasaki, T.; Kobari, T.; Waqalevu, V.; Kikuchi, K.; Ishikawa, M.; Kotani, T. DHA Accumulation in the Polar Lipids of the Euryhaline Copepod Pseudodiaptomus inopinus and Its Transfer to Red Sea Bream Pagrus major Larvae. Front. Mar. Sci. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazeroni, N.; Baboli, M.J. Fatty acid composition of the marine micro alga Tetraselmis chuii Butcher in response to culture conditions. J. Algal. Biomass Utln. 2015, 6, 49–55. [Google Scholar]
- Olaizola, M.; Grewe, C. Commercial Microalgal Cultivation Systems. In Grand Challenges in Algae Biotechnology; Hallmann, A., Rampelotto, P.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–34. [Google Scholar] [CrossRef]
- Nguyen, H.T.D.; Ramli, A.; Kee, L.M. A Review on Methods Used in Analysis of Microalgae Lipid Composition. J. Jpn. Inst. Energy 2017, 96, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Mar. Drugs 2019, 17, 203. [Google Scholar] [CrossRef] [Green Version]
- de Morais, E.G.; Nunes, I.L.; Druzian, J.I.; de Morais, M.G.; da Rosa, A.P.C.; Costa, J.A.V. Increase in biomass productivity and protein content of Spirulina sp. LEB 18 (Arthrospira) cultivated with crude glycerol. Biomass Convers. Biorefinery 2020, 18, 1–9. [Google Scholar] [CrossRef]
- de Morais, E.G.; Druzian, J.I.; Nunes, I.L.; de Morais, M.G.; Costa, J.A.V. Glycerol increases growth, protein production and alters the fatty acids profile of Spirulina (Arthrospira) sp LEB 18. Process. Biochem. 2019, 76, 40–45. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, J.; Nie, C.; Li, Y.; Jenkins, J.; Pei, H. Filamentous cyanobacteria triples oil production in seawater-based medium supplemented with industrial waste: Monosodium glutamate residue. Biotechnol. Biofuels 2019, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Andrade, B.B.; Cardoso, L.G.; Assis, D.D.J.; Costa, J.A.V.; Druzian, J.I.; Lima, S.T.D.C. Production and characterization of Spirulina sp. LEB 18 cultured in reused Zarrouk’s medium in a raceway-type bioreactor. Bioresour. Technol. 2019, 284, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Roohani, A.M.; Abedian Kenari, A.; Fallahi Kapoorchali, M.; Borani, M.S.; Zoriezahra, S.J.; Smiley, A.H.; Esmaeili, M.; Rombenso, A.N. Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juvenil. Aquac. Nutr. 2019, 25, 633–645. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Sireesha, E.; Chauhan, V.S.; Sarada, R. Evaluation of microalgae as vegetarian source of dietary polyunsaturated fatty acids under autotrophic growth conditions. J. Food Sci. Technol. 2015, 52, 7070–7080. [Google Scholar] [CrossRef]
- Perez-Velazquez, M.; Gatlin, D.M.; González-Félix, M.L.; García-Ortega, A.; de Cruz, C.R.; Juárez-Gómez, M.L.; Chen, K. Effect of fishmeal and fish oil replacement by algal meals on biological performance and fatty acid profile of hybrid striped bass (Morone crhysops ♀ × M. saxatilis ♂). Aquaculture 2019, 507, 83–90. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; De Oliveira, J.V.; Junior, A.F.; Derner, R.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Darwish, R.; Gedi, M.; Eakpetch, P.; Assaye, H.; Zaky, A.; Gray, D. Chlamydomonas reinhardtii Is a Potential Food Supplement with the Capacity to Outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Hassan, M.E.; Mohafrash, S.M.M.; Fallatah, S.A.; El-Sayed, A.E.K.B.; Mossa, A.T.H. Eco-friendly larvicide of Amphora coffeaeformis and Scenedesmus obliquus microalgae extracts against Culex pipiens. J. Appl. Phycol. 2021, 1–11. [Google Scholar]
- Darki, B.Z.; Seyfabadi, J.; Fayazi, S. Effect of nutrients on total lipid content and fatty acids profile of Scenedesmus obliquus. Braz. Arch. Biol. Technol. 2017, 60, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Salama, E.-S.; Kim, H.-C.; Abou-Shanab, R.; Ji, M.-K.; Oh, Y.-K.; Kim, S.-H.; Jeon, B.-H. Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst. Eng. 2013, 36, 827–833. [Google Scholar] [CrossRef]
- Chu, F.-F.; Chu, P.-N.; Shen, X.-F.; Lam, P.K.; Zeng, R.J. Effect of phosphorus on biodiesel production from Scenedesmus obliquus under nitrogen-deficiency stress. Bioresour. Technol. 2014, 152, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-F.; Hu, H.; Ma, L.-L.; Lam, P.K.S.; Yan, S.-K.; Zhou, S.-B.; Zeng, R.J. FAMEs production from Scenedesmus obliquus in autotrophic, heterotrophic and mixotrophic cultures under different nitrogen conditions. Environ. Sci. Water Res. Technol. 2018, 4, 461–468. [Google Scholar] [CrossRef]
- An, M.; Gao, L.; Zhao, W.; Chen, W.; Li, M. Effects of Nitrogen Forms and Supply Mode on Lipid Production of Microalga Scenedesmus obliquus. Energies 2020, 13, 697. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Wang, L.; Zhang, Y.; Zhang, J.; Zhang, X.; Ye, N. The effects of elevated CO2 concentrations on changes in fatty acids and amino acids of three species of microalgae. Phycologia 2020, 59, 208–217. [Google Scholar] [CrossRef]
- Guerra, I.; Pereira, H.; Costa, M.; Silva, J.; Santos, T.; Varela, J.; Mateus, M.; Silva, J. Operation Regimes: A Comparison Based on Nannochloropsis oceanica Biomass and Lipid Productivity. Energies 2021, 14, 1542. [Google Scholar] [CrossRef]
- Osorio, H.; Jara, C.; Fuenzalida, K.; Rey-Jurado, E.; Vásquez, M. High-efficiency nuclear transformation of the microalgae Nannochloropsis oceanica using Tn5 Transposome for the generation of altered lipid accumulation phenotypes. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-J. Omega-3 fatty acid obtained from Nannochloropsis oceanica cultures grown under low urea protect against Abeta-induced neural damage. J. Food Sci. Technol. 2015, 52, 2982–2989. [Google Scholar] [CrossRef] [Green Version]
- Bongiovani, N.; Popovich, C.A.; Martínez, A.M.; Constenla, D.; Leonardi, P.I. Biorefinery Approach from Nannochloropsis oceanica CCALA 978: Neutral Lipid and Carotenoid Co-Production Under Nitrate or Phosphate Deprivation. Bioenergy Res. 2019, 13, 518–529. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol. Biofuels 2018, 11, 174. [Google Scholar] [CrossRef]
- Touliabah, H.; Almutairi, A. Effect of Phytohormones Supplementation under Nitrogen Depletion on Biomass and Lipid Production of Nannochloropsis oceanica for Integrated Application in Nutrition and Biodiesel. Sustainability 2021, 13, 592. [Google Scholar] [CrossRef]
- Pudney, A.; Gandini, C.; Economou, C.K.; Smith, R.; Goddard, P.; Napier, J.A.; Spicer, A.; Sayanova, O. Multifunctionalizing the marine diatom Phaeodactylum tricornutum for sustainable co-production of omega-3 long chain polyunsaturated fatty acids and recombinant phytase. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.L.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab. Eng. 2014, 22, 3–9. [Google Scholar] [CrossRef]
- Shen, P.-L.; Wang, H.-T.; Pan, Y.-F.; Meng, Y.-Y.; Wu, P.-C.; Xue, S. Identification of Characteristic Fatty Acids to Quantify Triacylglycerols in Microalgae. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.; Du, L.; Hosokawa, M.; Miyashita, K.; Kokubun, Y.; Arai, H.; Taroda, H. Fatty Acid and Lipid Class Composition of the Microalga Phaeodactylum tricornutum. J. Oleo Sci. 2017, 66, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Siron, R.; Giusti, G.; Berland, B. Changes in the fatty acid composition of Phaeodactylum tricornutum and Dunaliella tertiolecta during growth and under phosphorus deficiency. Mar. Ecol. Prog. Ser. 1989, 55, 95–100. [Google Scholar] [CrossRef]
- Steinrücken, P.; Prestegard, S.K.; de Vree, J.H.; Storesund, J.E.; Pree, B.; Mjøs, S.A.; Erga, S.R. Comparing EPA production and fatty acid profiles of three Phaeodactylum tricornutum strains under western Norwegian climate conditions. Algal Res. 2018, 30, 11–22. [Google Scholar] [CrossRef]
- Domergue, F.; Spiekermann, P.; Lerchl, J.; Beckmann, C.; Kilian, O.; Kroth, P.G.; Boland, W.; Zähringer, U.; Heinz, E. New Insight into Phaeodactylum tricornutum Fatty Acid Metabolism. Cloning and Functional Characterization of Plastidial and Microsomal Δ12-Fatty Acid Desaturases. Plant Physiol. 2003, 131, 1648–1660. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Thomas-Hall, S.R.; Chua, E.T.; Schenk, P.M. Development of High-Level Omega-3 Eicosapentaenoic Acid (EPA) Production from Phaeodactylum tricornutum. J. Phycol. 2021, 57, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Šimat, V.; Bogdanović, T.; Poljak, V.; Petričević, S. Changes in fatty acid composition, atherogenic and thrombogenic health lipid indices and lipid stability of bogue (Boops boops Linnaeus, 1758) during storage on ice: Effect of fish farming activities. J. Food Compos. Anal. 2015, 40, 120–125. [Google Scholar] [CrossRef]
- Marques, B.; Lillebø, A.I.; Domingues, M.D.R.M.; Saraiva, J.A.; Calado, R. Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System. Molecules 2019, 24, 4503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M.J. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An Analysis of the Nutritional and Health Values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two Chlorophyta Collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef] [PubMed]
- Rueda, F.M.; Hernández, M.D.; Egea, M.A.; Aguado, F.; García, B.; Martínez, F.J. Differences in tissue fatty acid composition between reared and wild sharpsnout sea bream, Diplodus puntazzo (Cetti, 1777). Br. J. Nutr. 2001, 86, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valfré, F.; Caprino, F.; Turchini, G. The Health Benefit of Seafood. Vet. Res. Commun. 2003, 27, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Puchau, B.; Ochoa, M.C.; Zulet, M. Ángeles; Marti, A.; Martínez, J.A.; Members, G. Dietary total antioxidant capacity and obesity in children and adolescents. Int. J. Food Sci. Nutr. 2010, 61, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Athukorala, Y.; Lee, K.-W.; Park, E.-J.; Heo, M.-S.; Yeo, I.-K.; Lee, Y.-D.; Jeon, Y.-J. Reduction of lipid peroxidation and H2O2-mediated DNA damage by a red alga (Grateloupia filicina) methanolic extract. J. Sci. Food Agric. 2005, 85, 2341–2348. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Incorporation of himanthalia elongata seaweed to enhance the phytochemical content of breadsticks using response surface methodology (RSM). Int. Food Res. J. 2013, 20, 1537–1545. [Google Scholar]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R.R.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R.R. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [Green Version]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.; Marques, S.; Ferreira, I.; Cruz, M.T.; Domingues, P.; Segundo, M.; Domingues, M.R.M. New Insights into the Anti-Inflammatory and Antioxidant Properties of Nitrated Phospholipids. Lipids 2018, 53, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Gouda, M.; Chen, K.; Li, X.; Liu, Y.; He, Y. Detection of microalgae single-cell antioxidant and electrochemical potentials by gold microelectrode and Raman micro-spectroscopy combined with chemometrics. Sens. Actuators B Chem. 2021, 329, 129229. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Sun, T.; Magnuson, A.D.; Qamar, T.R.; Lei, X.G. Defatted Microalgae-Mediated Enrichment of n–3 Polyunsaturated Fatty Acids in Chicken Muscle Is Not Affected by Dietary Selenium, Vitamin E, or Corn Oil. J. Nutr. 2018, 148, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- El-Fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Albericio, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Gangadhar, K.N.; Pereira, H.; Rodrigues, M.J.; Custódio, L.; Barreira, L.; Malcata, F.X.; Varela, J. Microalgae-based unsaponifiable matter as source of natural antioxidants and metal chelators to enhance the value of wet Tetraselmis chuii biomass. Open Chem. 2016, 14, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef] [PubMed]
- LIPID MAPS® Lipidomics Gateway [Internet]. Available online: https://lipidmaps.org/resources/lipidweb/index.php?page=ms/methesters/me-arch/index.htm (accessed on 4 June 2021).
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2016. [Google Scholar]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [Green Version]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 36249. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. CRAN R-Project, Package Version 1.0.7; R Packag Version. 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 14 May 2021).
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S.; Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2–Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps. CRAN R-Project, R Package Version 1.0.12. 2019. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 14 May 2021).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R package version; Media; GitHub, Inc.: San Francisco, CA, USA, 2019. [Google Scholar]
- Wickham, H.; Henry, L. Tidyr: Easily Tidy Data with “Spread()” and “Gather()” Functions. R Packag Version 080. 2018. Available online: https//CRANR-project.org/package=tidyr (accessed on 22 April 2021).
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Automatic method for determination of total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Anal. Chim. Acta 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Maia, M.; Reis, S.; Segundo, M. Rapid assessment of endpoint antioxidant capacity of red wines through microchemical methods using a kinetic matching approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).