Identifying Potential Antioxidant Properties from the Viscera of Sea Snails (Turbo cornutus)
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition of T. cornutus
2.2. Amino Acid Composition of T. cornutus
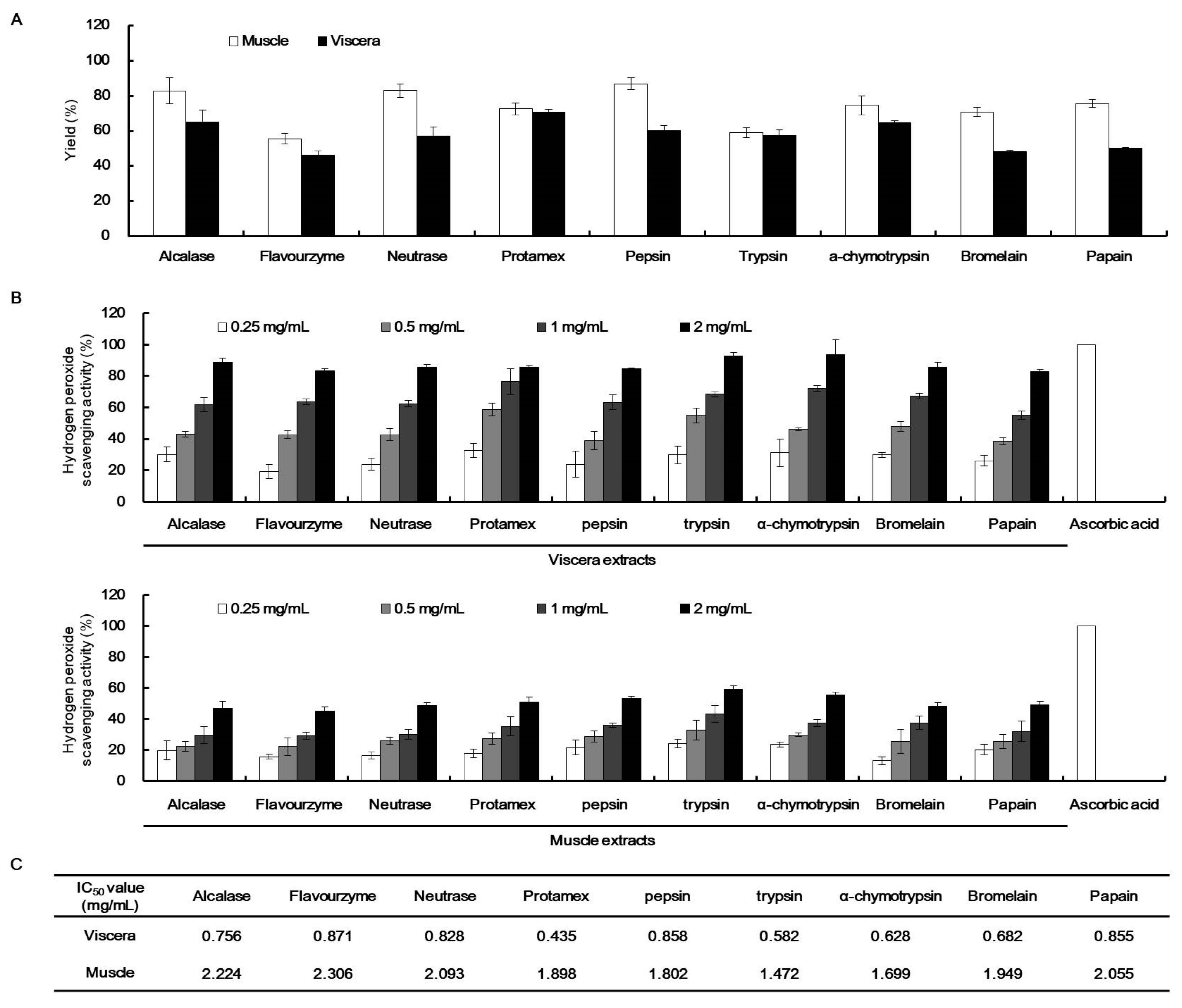
2.3. H2O2 Scavenging Activity of the Enzymatic Extracts of T. cornutus
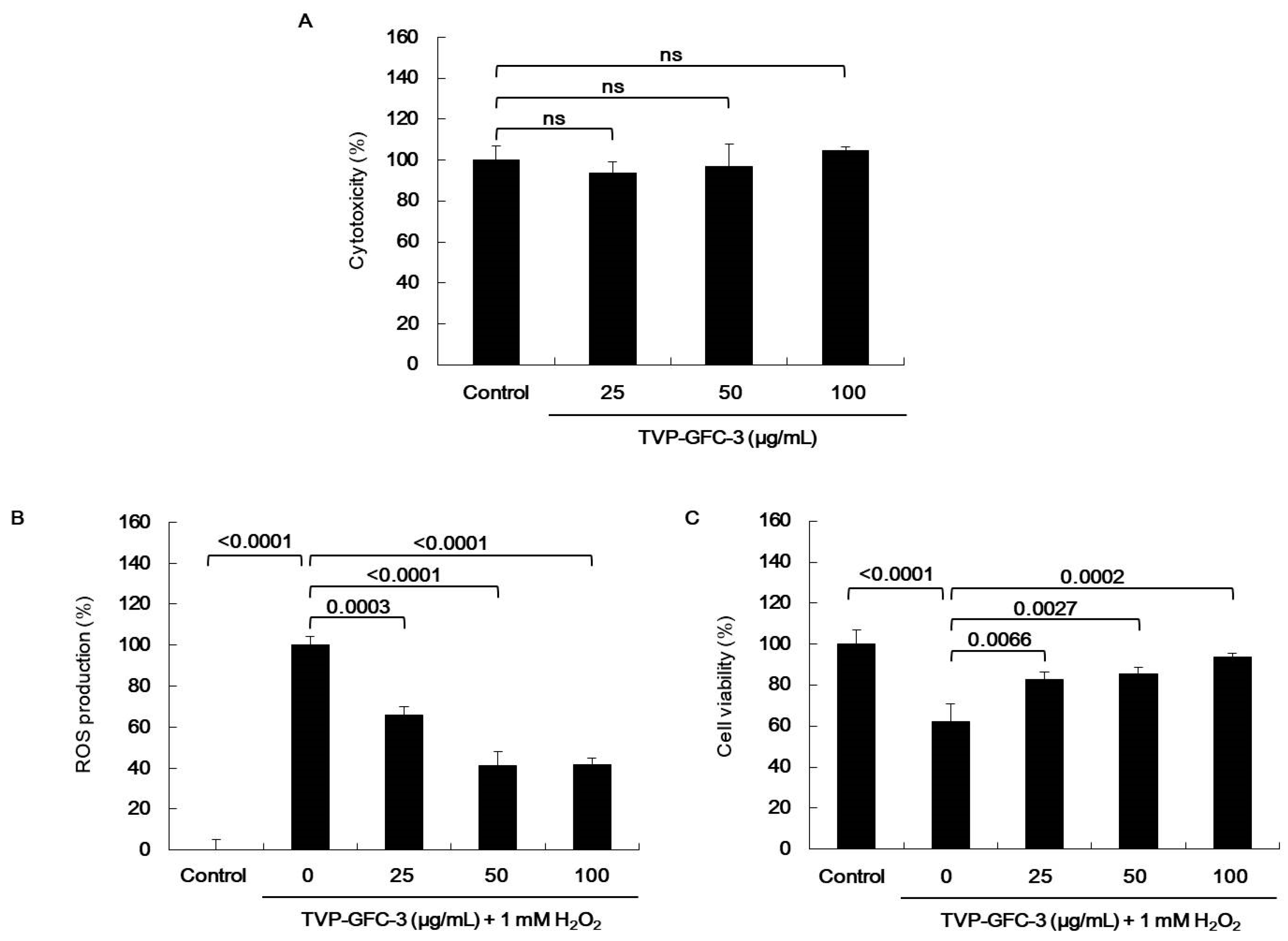
2.4. Effect of Viscera and Muscle Extracts on H2O2-Induced Oxidative Stress in HepG2 Cells
 ), superoxide anion (O2−), singlet oxygen (O2), and H2O2 (H2O2) [5]. ROS react with molecules by reversible oxidative modifications and factors in cellular signaling pathways, such as metabolism, growth, differentiation, and death signaling [5]. However, ROS overproduction without control can result in oxidative damage to cell structures, including lipids and membranes, proteins, and DNA [5,26]. Therefore, the MTT assay was performed in H2O2-exposed HepG2 cells to evaluate the potential antioxidant effect of viscera and muscle extract s. As shown in Figure 2, significant cell death was observed in the H2O2-treated cells. However, TVP and the muscle protamex extract markedly increased cell viability. Especially, TVP showed a higher protective effect than did muscle protamex-assisted extracts against H2O2 in HepG2 cells. In addition, TVP inhibited intracellular ROS production, and aspartate aminotransferase (AST) levels increased by treating H2O2 in HepG2 cells. These results indicated that T. cornutus viscera tissues possess a high value than did T. cornutus muscle tissues by protamex-assisted hydrolysis processing.
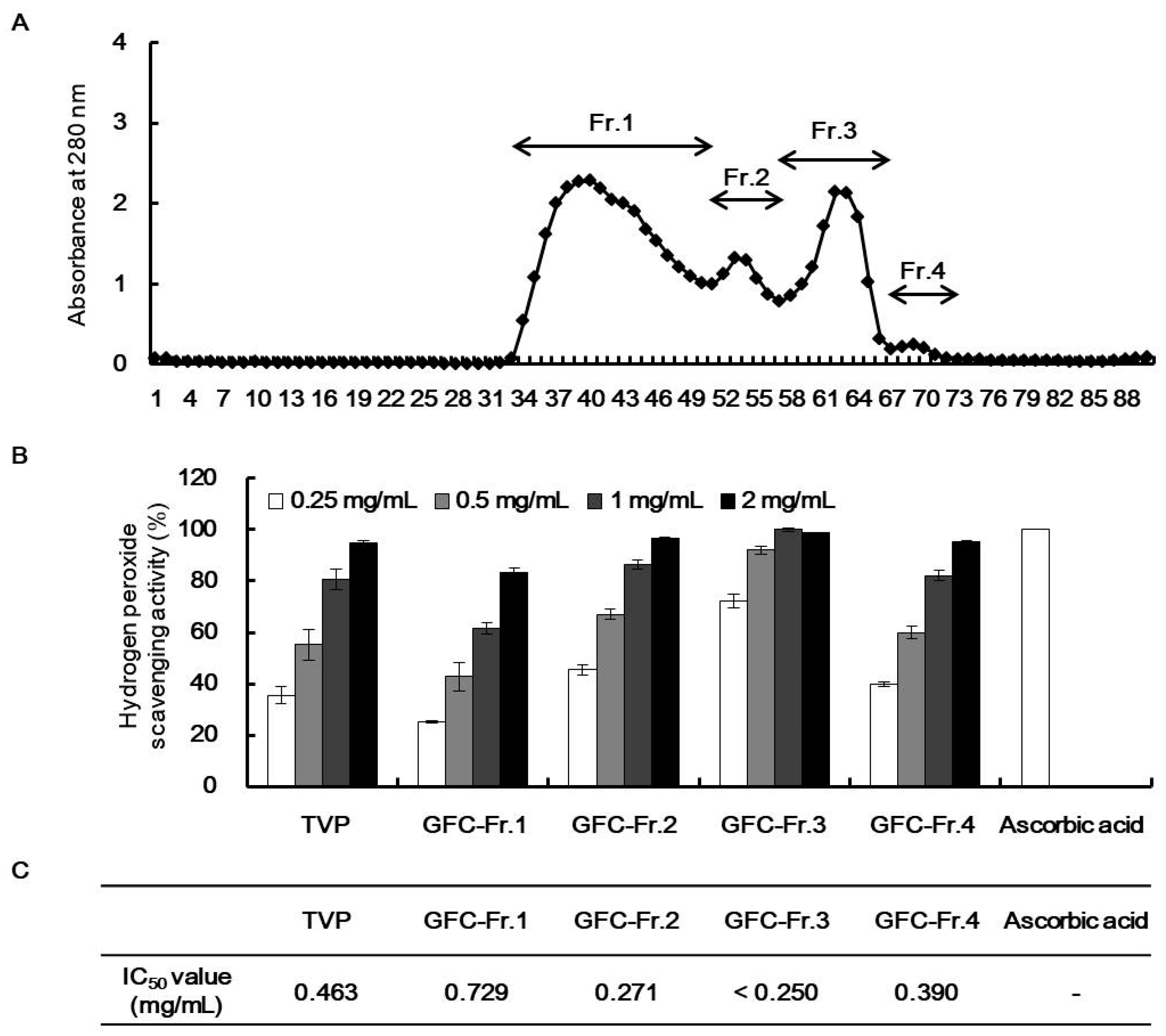
), superoxide anion (O2−), singlet oxygen (O2), and H2O2 (H2O2) [5]. ROS react with molecules by reversible oxidative modifications and factors in cellular signaling pathways, such as metabolism, growth, differentiation, and death signaling [5]. However, ROS overproduction without control can result in oxidative damage to cell structures, including lipids and membranes, proteins, and DNA [5,26]. Therefore, the MTT assay was performed in H2O2-exposed HepG2 cells to evaluate the potential antioxidant effect of viscera and muscle extract s. As shown in Figure 2, significant cell death was observed in the H2O2-treated cells. However, TVP and the muscle protamex extract markedly increased cell viability. Especially, TVP showed a higher protective effect than did muscle protamex-assisted extracts against H2O2 in HepG2 cells. In addition, TVP inhibited intracellular ROS production, and aspartate aminotransferase (AST) levels increased by treating H2O2 in HepG2 cells. These results indicated that T. cornutus viscera tissues possess a high value than did T. cornutus muscle tissues by protamex-assisted hydrolysis processing.2.5. Characterization of Antioxidant Peptides from TVP
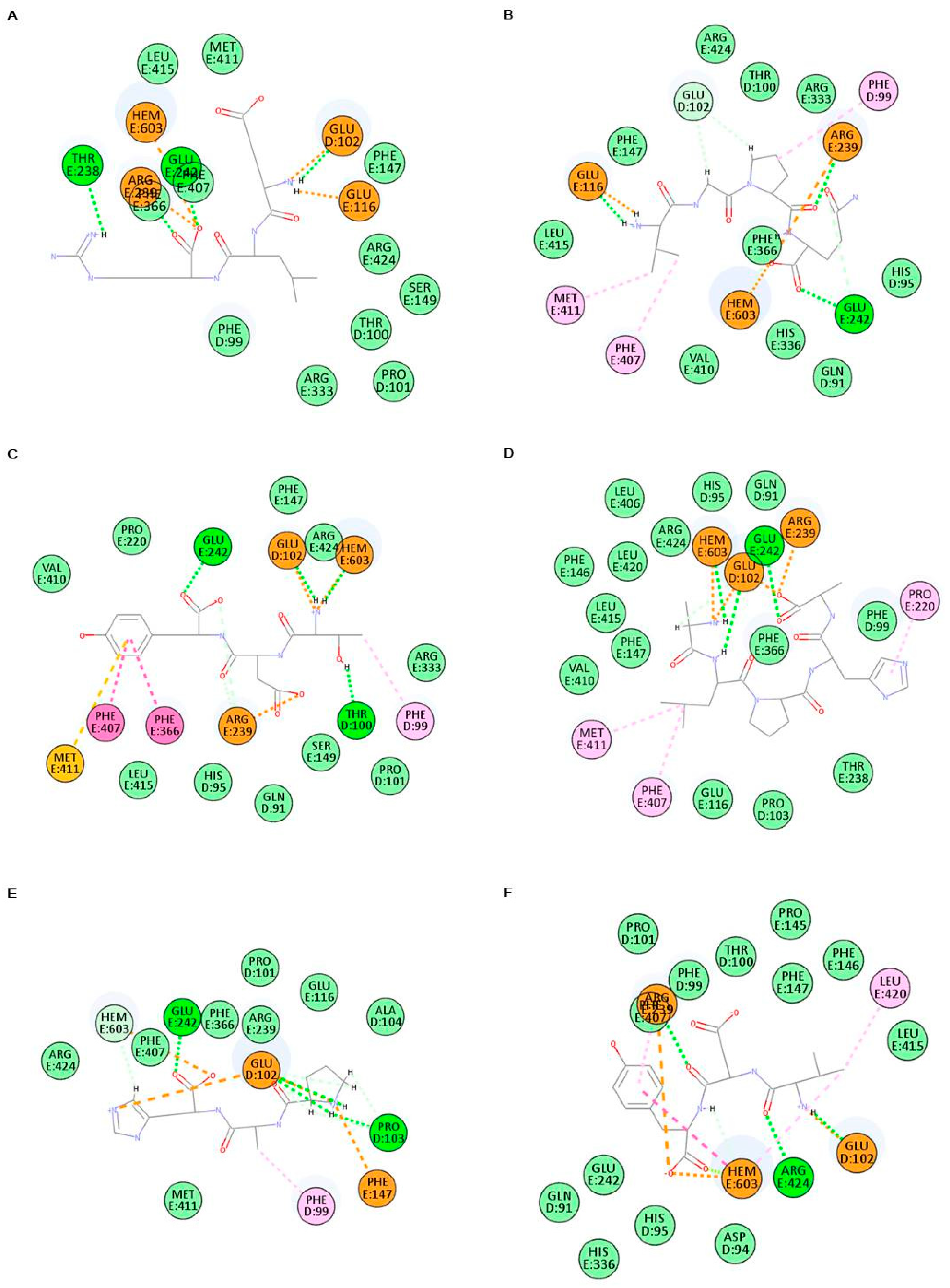
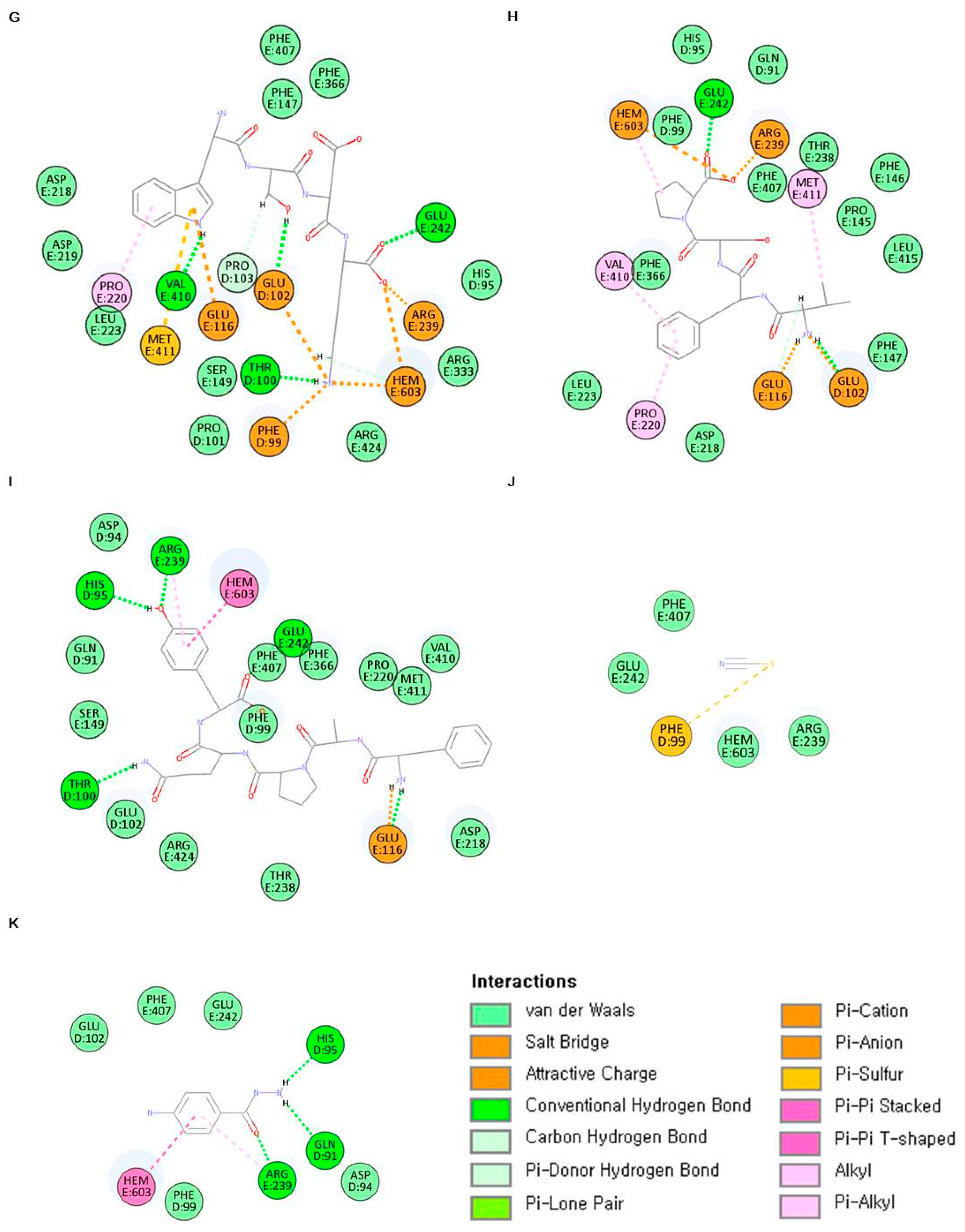
2.6. In Silico Analysis of Antioxidant Peptides on MPO Inhibition
2.7. In Vitro Analysis of Antioxidant Peptides on Myeloperoxidase (MPO) Inhibition
3. Materials and Methods
3.1. Materials
3.2. Proximate Composition of T. cornutus
3.3. Amino Acid Profile
3.4. Preparation of T. cornutus Enzyme-Assisted Extracts
3.5. Separation of Fractions and Radical Scavenging Properties
3.6. Characterization of the Separated Antioxidant Properties
3.7. H2O2 Scavenging Activity
3.8. Cell Line and Cell Culture
3.9. Determination of Cell Viability and ROS Generation in H2O2-Treated HepG2 Cells
3.10. In Silico Analysis of MPO Inhibition
3.11. MPO Inhibition Effect
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chung, S.C.; Lee, J.J.; Lee, C.K. A study on the growth of Jeju Island’s Turban Shell, Turbo cornutus. Bull. Mar. Resour. Res. Inst. 1983, 7, 71–75. [Google Scholar]
- Chang, D.S. Studies on the stock assessment and management of the Turban Shell, Batillus cornutus in Jeju Coastal water, Korea. Ph.D. Thesis, Cheju National University, Cheju, Korea, 2002. [Google Scholar]
- Chung, S.C. Studies on the biometry of the Turbo cornutus solander in the Cheju coastal waters. Bull. Mar. Biol. Stn. 1976, 1, 3–9. [Google Scholar]
- Yoo, J.T.; Oh, B.S.; Chang, D.S. Preference of adult top shell (Batillus cornutus) on specific marine algae in the coastal waters of Jeju Island. Korean J. Malacol. 2011, 27, 299–306. [Google Scholar] [CrossRef][Green Version]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Ko, S.C.; Samarakoon, K.; Kim, E.A.; Kang, M.C.; Lee, S.C.; Kim, J.; Kim, Y.T.; Kim, J.S.; Kim, H. Purification of antioxidative peptide from peptic hydrolysates of Mideodeok (Styela clava) flesh tissue. Food Sci. Biotechnol. 2013, 22, 541–547. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G. Flavonoid composition, cellular antioxidant activity and (myelo) peroxidase inhibition of a Bryonia alba L.(Cucurbitaceae) leaves extract. J. Pharm. Pharmacol. 2019, 71, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, Ý.; Elias, R.; Gepdiremen, A.; Boyer, L.; Köksal, E. A comparative study on the antioxidant activity of fringe tree (Chionanthus virginicus L.) extracts. Afr. J. Biotechnol. 2007, 6, 410–418. [Google Scholar]
- Rees, M.D.; Bottle, S.E.; Fairfull-Smith, K.E.; Malle, E.; Whitelock, J.M.; Davies, M.J. Inhibition of myeloperoxidase-mediated hypochlorous acid production by nitroxides. Biochem. J. 2009, 421, 79–86. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein exerts antioxidant and anti-Inflammatory effects and influences iron utilization of BV-2 microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Kim, S.K.; Venkatesan, J. Introduction to Seafood Processing by-Products. Seafood Processing by-Products; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–9. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S. Utilization of tuna processing byproducts: Protein hydrolysate from skipjack tuna (Katsuwonus pelamis) viscera. J. Food Process. Preserv. 2017, 41, e12970. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, E.A.; Lee, H.; Kim, H.S.; Lee, J.S.; Jeon, Y.J. Antihypertensive effect of surimi prepared from olive flounder (Paralichthys olivaceus) by angiotensin-I converting enzyme (ACE) inhibitory activity and characterization of ACE inhibitory peptides. Process. Biochem. 2019, 80, 164–170. [Google Scholar] [CrossRef]
- Regenstein, J.; Zhou, P. Collagen and gelatin from marine by-products. In Maximising the Value of Marine by-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 279–303. [Google Scholar]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Jang, M.S.; Jang, J.R.; Park, H.Y.; Yoon, H.D. Overall composition, and levels of fatty acids, amino acids, and nucleotide-type compounds in wild abalone Haliotis gigantea and cultured abalone Haliotis discus hannai. Korean J. Food Preserv. 2010, 17, 533–540. [Google Scholar]
- Xia, T.; Yao, J.; Zhang, J.; Zheng, Y.; Song, J.; Wang, M. Protective effects of Shanxi aged vinegar against hydrogen peroxide-induced oxidative damage in LO2 cells through Nrf2-mediated antioxidant responses. RSC Adv. 2017, 7, 17377–17386. [Google Scholar] [CrossRef]
- Kang, N.; Lee, J.H.; Lee, W.; Ko, J.Y.; Kim, E.A.; Kim, J.S.; Heu, M.S.; Kim, G.H.; Jeon, Y.J. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Ko, S.C.; Kim, H.S.; Yang, H.W.; Ahn, G.; Lee, S.C.; Lee, T.G.; Lee, J.S.; Jeon, Y.J. Structural evidence for antihypertensive effect of an antioxidant peptide purified from the edible marine animal Styela clava. J. Med. Food 2020, 23, 132–138. [Google Scholar] [CrossRef]
- Wu, G.; Robertson, D.H.; Brooks, C.L., III; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER—A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Li, W.; Francis, G.A.; Goldstein, J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J. Clin. Investig. 1993, 91, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Baali, N.; Mezrag, A.; Bouheroum, M.; Benayache, F.; Benayache, S.; Souad, A. Anti-inflammatory and Antioxidant Effects of Lotus corniculatus on Paracetamol-induced Hepatitis in Rats. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Herbert, P.; Santos, L.; Alves, A. Simultaneous quantification of primary, secondary amino acids, and biogenic amines in musts and wines using OPA/3-MPA/FMOC-CI fluorescent derivatives. J. Food Sci. 2001, 66, 1319–1325. [Google Scholar] [CrossRef]
- Ko, S.C.; Lee, J.K.; Byun, H.G.; Lee, S.C.; Jeon, Y.J. Purification and characterization of angiotensin I-converting enzyme inhibitory peptide from enzymatic hydrolysates of Styela clava flesh tissue. Process Biochem. 2012, 47, 34–40. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.E. Detection of hydrogen peroxide produced by microorganisms on an ABTS peroxidase medium. Zent. Bakteriol. 1985, 259, 151–154. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, E.A.; Kim, J.; Jung, K.; Kim, S.Y.; Cha, S.H.; Kim, K.N.; Heo, S.J.; Ahn, G. 5-Bromo-3, 4-dihydroxybenzaldehyde from Polysiphonia morrowii attenuate IgE/BSA-stimulated mast cell activation and passive cutaneous anaphylaxis in mice. Biochem. Pharmacol. 2020, 178, 114087–114096. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiratchayamaethasakul, C.; Kim, E.A.; Kim, J.; Heo, S.J.; Lee, S.H. Hyaluronidase inhibitory and antioxidant activities of enzymatic hydrolysate from Jeju Island red sea cucumber (Stichopus japonicus) for novel anti-aging cosmeceuticals. J. Mar. Biosci. Biotechnol. 2018, 10, 62–72. [Google Scholar]


| Viscera | Muscle | |
|---|---|---|
| Proteins | 52.68 ± 0.28 | 78.28 ± 2.23 |
| Lipids | 28.40 ± 1.20 | 10.90 ± 0.81 |
| Moisture | 1.03 ± 0.35 | 4.25 ± 3.66 |
| Ash | 14.79 ± 0.80 | 4.86 ± 0.40 |
| Carbohydrates | 3.12 ± 1.93 | 1.73 ± 1.85 |
| Total | 100 | 100 |
| Viscera | Muscle | |
|---|---|---|
| Aspartic acid | 10.3 ± 0.0 | 9.5 ± 0.2 |
| Glutamic acid | 13.1 ± 0.2 | 16.4 ± 0.1 |
| Serine | 4.9 ± 0.1 | 4.7 ± 0.1 |
| Histidine | 1.8 ± 0.0 | 1.1 ± 0.1 |
| Glycine | 5.5 ± 0.4 | 8.8 ± 0.2 |
| Threonine | 5.2 ± 0.1 | 4.4 ± 0.0 |
| Arginine | 7.1 ± 0.5 | 9.6 ± 0.0 |
| Alanine | 5.0 ± 0.1 | 5.9 ± 0.0 |
| Taurine | 11.3 ± 0.1 | 8.1 ± 0.0 |
| Tyrosine | 3.5 ± 0.0 | 2.6 ± 0.0 |
| Valine | 4.4 ± 0.3 | 3.3 ± 0.2 |
| Methionine | 2.3 ± 0.1 | 2.2 ± 0.2 |
| Phenylalanine | 4.4 ± 0.1 | 2.8 ± 0.1 |
| Isoleucine | 3.8 ± 0.3 | 3.1 ± 0.2 |
| Leucine | 6.5 ± 0.1 | 6.3 ± 0.1 |
| Lysine | 4.8 ± 0.1 | 4.5 ± 0.9 |
| Proline | 6.2 ± 0.3 | 6.6 ± 1.9 |
| Total | 100 | 100 |
| Sample | Charge | m/z | Sequencing |
|---|---|---|---|
| TVP-GFC-3 | 1 | 417.25 | ELR |
| 1 | 400.22 | VGPQ | |
| 1 | 398.16 | TDY | |
| 1 | 508.29 | ALPAH | |
| 1 | 324.17 | PAH | |
| 1 | 396.18 | VDY | |
| 1 | 535.25 | WSDK | |
| 1 | 449.24 | VFSP | |
| 1 | 625.30 | FAPQY |
| Sample | Characteristic of Peptide-MPO Complexes | |
|---|---|---|
| Binding Energy (kcal/mol) | Main Bonding | |
| ELR | −426.358 | HEM603, GLU102, GLU116, THR238, ARG239, GLU242, PHE366, PHE407 |
| VGPQ | −509.950 | HEM603, PHE99, GLU102, GLU116, ARG239, GLU242, PHE366, PHE407, MET411 |
| TDY | −368.111 | HEM603, PHE99, THR100, GLU102, ARG239, GLU242, PHE366, PHE407, MET411 |
| ALPAH | −360.686 | HEM603, GLU102, PRO220, ARG239, GLU242, PHE407, MET411 |
| PAH | −430.944 | HEM603, PHE99, GLU102, PRO103, PHE147, GLU242 |
| VDY | −398.554 | HEM603, GLU102, ARG239, PHE407, LEU420, ARG424 |
| WSDK | −340.875 | HEM603, PHE99, THR100, GLU102, PRO103, GLU116, PRO220, ARG239, GLU242, VAL410, MET411 |
| VFSP | −442.737 | HEM603, GLU102, GLU116, PRO220, ARG239, GLU242, VAL410, MET411 |
| FAPQY | −387.049 | HEM603, HIS95, THR100, GLU116, ARG239, GLU242 |
| Thiocyanate ion | −33.0451 | PHE99 |
| 4-aminobenzoic acid hydrazide (4-ABH) | −74.8248 | HEM603, GLN91, HIS95, ARG239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.; Kim, E.-A.; Kim, J.; Lee, S.-H.; Heo, S.-J. Identifying Potential Antioxidant Properties from the Viscera of Sea Snails (Turbo cornutus). Mar. Drugs 2021, 19, 567. https://doi.org/10.3390/md19100567
Kang N, Kim E-A, Kim J, Lee S-H, Heo S-J. Identifying Potential Antioxidant Properties from the Viscera of Sea Snails (Turbo cornutus). Marine Drugs. 2021; 19(10):567. https://doi.org/10.3390/md19100567
Chicago/Turabian StyleKang, Nalae, Eun-A Kim, Junseong Kim, Seung-Hong Lee, and Soo-Jin Heo. 2021. "Identifying Potential Antioxidant Properties from the Viscera of Sea Snails (Turbo cornutus)" Marine Drugs 19, no. 10: 567. https://doi.org/10.3390/md19100567
APA StyleKang, N., Kim, E.-A., Kim, J., Lee, S.-H., & Heo, S.-J. (2021). Identifying Potential Antioxidant Properties from the Viscera of Sea Snails (Turbo cornutus). Marine Drugs, 19(10), 567. https://doi.org/10.3390/md19100567







