Abstract
Cystoseira barbata is an edible brown seaweed, traditionally used in the Black Sea area as functional food. Both alginate and brown seaweed biomass are well known for their potential use as adsorbents for heavy metals. Alginate was extracted from C. barbata recovered from the Romanian coast on the Black Sea with a yield of 19 ± 1.5% (w/w). The structural data for the polysaccharide was obtained by HPSEC-MALS, 1H-NMR. The M/G ratio was determined to be 0.64 with a molecular weight of 126.6 kDa with an intrinsic viscosity of 406.2 mL/g. Alginate beads were used and their adsorption capacity with respect to Pb2+ and Cu2+ ions was determined. The adsorption kinetics of C. barbata dry biomass was evaluated and it was shown to have an adsorption capacity of 279.2 ± 7.5 mg/g with respect to Pb2+, and 69.3 ± 2 with respect to Cu2+. Alginate in the form of beads adsorbs a maximum of 454 ± 4.7 mg/g of Pb2+ ions and 107.3 ± 1.7 mg/g of Cu2+ ions.
1. Introduction
Edible seaweeds have significant potential as functional food, especially as they are active against various human non-communicable diseases such as cardiovascular diseases, cancers, type 2 diabetes/metabolic syndrome, auto-immune diseases, due to their high level of bioactive components—polysaccharides, peptides, polyunsaturated fatty acids, polyphenols, vitamins and minerals [1,2]. One of the main active components of edible brown seaweed is alginate, a polysaccharide composed of two different uronic acids, mannuronic and guluronic [3]. Due to the fact that alginates are not digested by human enzymes, they act as prebiotics, supporting the production of short-chain fatty acids, and as potential immunomodulators [4].
Traditionally, alginates have been used as tablet excipients and for the treatment of stomach ulcers, gastric reflux and heartburn [5]. Alginates’ absorptive, swelling and haemostatic features are involved in their mode of action against such health conditions. These features also substantiate the use of alginate in wound treatment [6]. Their absorptive and swelling features have also been linked to many other health-related effects. For example, binding of glucose and α-amylase inhibition, which reduce post-prandial glucose levels [7,8]. Alginates (calcium) have the ability to absorb bile acids and lipids and therefore to lower cholesterol [9] and lipid levels [10].
The adsorption of heavy metal ions (biosorption) from the intestinal system is related to organism detoxification. Treatment with calcium alginate in a dose of 500 mg/kg removes the lead accumulated in rats after intoxication with lead acetate [11]. Simultaneous intoxication of lead acetate and treatment with calcium alginate significantly reduced lead accumulation in rat organs [12]. Administration of alginate in combination with modified citrus pectin reduces the total body burden of heavy metals [13].
Heavy metals are highly toxic to the environment and to humans. The recommended values for human consumption are in the very low ppm ranges: Pb, 0.01 ppm; Cu, 2 ppm; Hg, 0.001 ppm; As, 0.01 ppm; Ni, 0.02 ppm; etc. [14]. High levels of heavy metals have been reported in sources of drinking water [15], which have been contaminated from a variety of sources such as pipe corrosion [16,17] or industrial activities [18,19,20]. Other activities that have been associated with high contamination risks are petrochemistry [21] and electronics waste disposal (E-waste) [22]. In many cases, the recovery of heavy metal contaminants using adsorbents [15,23] is mainly due to their reduced costs as compared to other methods such as membrane filtration, chemical precipitation, ion exchange and others [15,24,25,26].
Biosorption is the process by which some type of biological material is used as an adsorbent to bind certain compounds [27]. It is regarded as a promising alternative to classical methods due to its cost-effectiveness and environment-friendly nature [21]. The biosorbents that are currently under development are mostly trying to take advantage of the adsorption properties of natural biomasses [28], or of composite materials based on natural sources [21,29]. Apart from bacteria [30,31,32] and fungi [21,33,34], agricultural [35] and algae-based adsorbents [28,36] have also been used. Among macroalgae, brown seaweeds are of particular interest because they contain alginate, which has a chemical affinity for divalent metals [37,38]. In fact, alginate chains form a tridimensional matrix in the presence of divalent metals by ionic crosslinking following the egg-box model [39]. Ca2+ ions are used for this purpose. However, divalent metals of higher atomic mass replace the calcium ions as the active sites in the alginate hydrogel have increased affinity for larger divalent metal ions [40]. For decades, alginate has been extracted from brown seaweed with a good yield at an industrial level to be used as a thickening and gelling agent in the food and cosmetics industry [41,42]. The main species that are currently exploited industrially are Macrocystis pyrifera, Laminaria hyperborea, Laminaria digitata and Asphyllum nodosum [43]. However, alternative uses are proposed for alginate in the biomedical field as a drug delivery system, for wound healing and tissue engineering [6], or as a biosorbent material. In fact, both brown seaweed biomass and alginate, in the form of gel beads have been extensively studied as biosorbents [28,37,44,45,46,47]. Recently, composite materials based on alginate have been derived with improved properties. For instance, including magnetite has enhanced the recovery of biosorbents. In this case, adsorption of Cd2+ ions was improved by also including activated carbon in the alginate matrix leading to an improved material in terms of adsorption capacity and costs [48]. Another approach is to obtain a compressive alginate sponge where the contaminant is recovered after adsorption simply by compressing the biosorbent before reuse. The efficiency of this system was proven in the case of treating methylene blue contaminated wastewaters [49]. The crosslinked alginate matrix has also been used as a coating for composite materials that are to be used as controlled drug release systems [50].
Cystoseira is a polyphyletic genus of brown seaweed, included in the Sargassaceae family, which is found extensively on the coasts of the Mediterranean Sea and the eastern Atlantic Ocean and also in the Black Sea [51,52] Most studies of Cystoseira sp. have shown a large variety of secondary metabolites with biological activity including phlorotannins, terpenoids and carbohydrates [53]. Cystoseira barbata recovered from the coasts of Tunisia has been proven to contain compounds that show biological activity, including laminarin, which has antioxidant, antibacterial and wound healing properties [54]; fucoxanthin, used as a color enhancer and oxidative stability enhancer of meat products [55]; and polyphenolic-protein-polysaccharide ternary conjugates, which are used as biopreservatives [56]. In the Black Sea, C. barbata and Cystoseira crinita are the only representatives of Cystoseira sp., although a Cystoseira bosphorica member has sometimes been reported [51,57]. C. barbata is traditionally used in the Black Sea area as functional food [2,58]. Recent studies have been based on ecological interest in the levels of heavy elements in certain parts of the Black Sea coast [59,60,61], as well as the structure of certain metabolites found in C. barbata and C. crinita [62]. The use of Cystoseira spp. biomass as a biosorbent has been shown in one study involving C. amentacea var. stricta (formerly C. stricta) [47]. Dried C. barbata biomass from the Turkish coast of the Black Sea has also been investigated as a biosorbent [46]. To the best of our knowledge, the structure of alginate extracted from C. barbata and its use as a biosorbent in the form of beads to adsorb heavy metals has never been studied.
The aim of this paper is to characterize alginate extracted from C. barbata recovered from the Romanian Black Sea and to prove that this bioactive component presents heavy metal adsorption properties compatible with its use as an adsorbent/biosorbent for heavy metal detoxification. The adsorption properties of the initial seaweed powder were also evaluated.
2. Results and Discussion
2.1. Extraction and Structural Characterization
In recent years, many studies on the characterization of polysaccharides extracted from brown algae, and more specifically on Cystoseira sp. have been conducted and described in the literature [63,64,65]. Nevertheless, alginate extracted from C. barbata recovered from the Romanian Black Sea coast has never been investigated from a structural point of view.
The extraction method (Figure 1) that was employed for the extraction of alginate had a yield of 19 ± 1.5% (w/w) and relative to the algae dry matter estimated at 95 ± 2%. This method is presented in detail in Section 3.2. The final alginate product is obtained as a fine powder (under 0.2 mm), which is referred to as CBA UF.
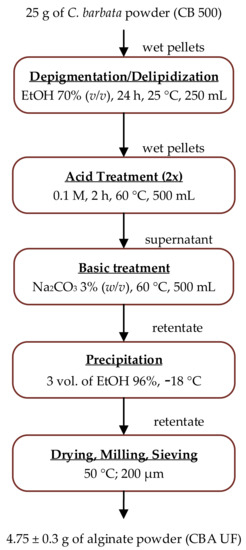
Figure 1.
Processing steps for the extraction of alginate from C. barbata dry seaweed biomass.
2.2. HPSEC-MALS
The average molecular weight in mass (Mw), average molecular weight in number (Mn) and the intrinsic viscosity of alginate (CBA UF) extracted from C. barbata were determined by high performance size-exclusion chromatography equipped with a multi-angle light diffusion detector coupled to a differential refractometer and an in-line viscometer. The recovery rate of the sample was estimated at 90%.
The values of the determined average molecular weights and hydrodynamic radius are reported in Table 1. The Mn and Mw of alginate (CBA UF) were estimated at 85.2 (±2.7%) kDa and 126.6 (±1.0%) kDa, indicating a low polydispersity index (PDI = Mw/Mn =1.49). These results are similar to those for other alginates obtained from brown algae, and especially from Cystoseira sp. such as Cystoseira sedoides [66], Cystoseira compressa [63,66], C. crinita [66]. In fact, our present results for the Mw values were very consistent with earlier investigations on alginates from Fucales algae families, for example, Fucus vesiculosus, A. nodosum, C. compressa or C. sedoides, which have Mw values ranging from around 100 kDa to 200 kDa [66,67]. Nevertheless, compared to other brown algae from Sargassum species, where Mw values ranged from 300 to 1000 kDa, the Mw of alginate fractions (CBA UF) extracted from Romanian C. barbata were lower [67,68].

Table 1.
Characterization of alginate (CBA UF) extracted from C. barbata collected from the Romanian Black Sea.
Compared with other alginates from brown algae, and especially from Cystoseiraceae species, the PDI value of 1.49 is very close to the alginate from Tunisian C. compressa [63]. This PDI value of alginate from Romanian C. barbata confirms a good Mw polysaccharides distribution, and indicates that there is no depolymerization of polysaccharides during the extraction/purification process steps. Finally, the intrinsic viscosity, which signifies the hydrodynamic volume occupied by the macromolecules in a dilute solution, was estimated for CBA UF at 406 mL/g. This value is lower than the alginate [ƞ] values from Sargassum (800–1300 mL/g) brown algae but is close to other intrinsic viscosity values of alginate extracted from Cystoseiraceae species [63,64,68]. Notably, a similar [ƞ] was observed with alginate extracted from Tunisian brown algae C. compressa [63]. As generally described, these observed differences are related to the origin of the algae and the extraction / purification processes used, which affect molecular weight and intrinsic viscosity.
2.3. NMR Analysis
Alginate extracted from C. barbata (CBA UF) was analyzed by 1H NMR. As observed in Figure 2, the 1D 1H-NMR spectrum showed the specific signal characteristics of the sodium alginate fraction, revealing high purity [69,70].
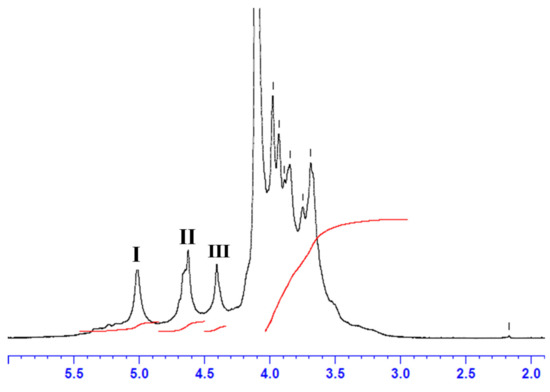
Figure 2.
1H NMR analysis of CBA UF extracted from C. barbata with: signal I = guluronic acid anomeric proton (G-1), signal II = overlap between the mannuronic acid anomeric proton (M-1) and the H-5 of alternating blocks (GM-5), signal III = guluronic acid H-5 position (block GG-5G), G-6 the C-6 from guluronic acid residue and M-6 the C-6 from mannuronic residue.
As well defined in the literature [69,70], 1H-NMR analysis identifies the alginate structure with three signals in the anomeric region: Signal I corresponds to the anomeric proton of the guluronic acid residue (G-1), Signal II corresponds to the overlap between the mannuronic acid anomeric proton (M-1) and the H-5 of alternating blocks (GM-5), and Signal III corresponds to proton H-5 guluronic acid from the GG-5G block (G-5). As a general rule, by using the NMR method we could estimate the proportions of each individual block of guluronic and mannuronic acids (FG and FM), the homogeneous (FGG and FMM) and heterogeneous (FGM and FMG) blocks of alginate [70] extracted from C. barbata (CBA UF) using the areas (A) of signals I, II and III and the Equations (1), (2) and (3):
Regarding the estimation of the block FGM, FMM and M/G molar ratio of alginate extracted from C. barbata (CBA UF), we used the following equations:
Consequently, Equations (4), (5) and (6) allow the complete structural characterization [70] of CBA UF, which is summarized in Table 2.

Table 2.
Structural characterization of alginate (CBA UF) extracted from C. barbata from Romanian Black Sea.
The frequencies of structural blocks shown in Table 2 provide information about the alginate composition in C. barbata. For example, the ratio between mannuronic and guluronic acid gives information about the quality of Ca2+ reticulated gels which, in this case, has a strong and rigid quality [71]. This value is generally higher than those reported for other species from Cystoseira genus such as Sirophysalis trinodis (formerly C. trinodis) (0.59), C. myrica [68], although it is lower in some cases, C. compressa (0.77) [63], C. humilis (1.46) [72]. Compared to other species from Laminaria or Sargassum, this value is higher in some cases (L. hyperborea, 0.41 [70]; Sargassum filipendula, 0.19 [73]), and lower in other cases (S. vulgare, 1.27 [69]; L. digitata, 1.12 [74]).
Structural information can be derived by evaluating the parameter η = 1.13 obtained using Equation (7). The value suggests that alginate extracted from C. barbata is of an alternate block type since it is greater than 1 [75]. Surprisingly, alginates extracted from other species from the Cystoseira genus present η < 1, a feature of alginates that have predominantly homopolymeric blocks [63,68,72].
2.4. Kinetics of Adsorption
Sodium alginate beads were obtained as described in Section 3.5. Photos (at 10× magnification) of the beads (not shown here) were taken and analyzed digitally in order to determine the diameter. Twenty beads were analyzed, and the mean diameter was determined to be 4413 ± 134 µm.
Initially, the kinetics of adsorption was studied by contacting sodium alginate beads and dry seaweed powder (<500 µm) with Cu2+ and Pb2+ solutions at 20 ppm and 74 ppm, respectively (Ci). [14] Four pairs of substrate/heavy metal were obtained. The instantaneous sorption capacity (qt) was determined as defined in Equation (8) where Ct is the concentration of heavy metals at time t. This equation is deduced from mass balance. Sm represents the value of substrate mass divided by the volume of solution that was used.
The kinetic curves for copper and lead adsorption of each of the two substrates are shown in Figure 3 and Figure 4.
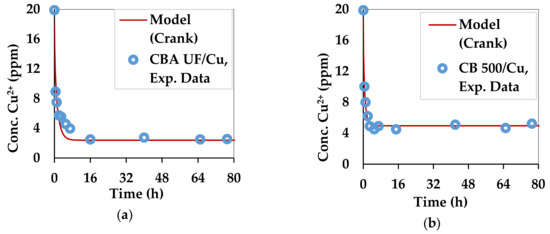
Figure 3.
Kinetics of copper adsorption by the two substrates: (a) C. barbata alginate (CBA UF) and (b) C. barbata powder (CB 500).
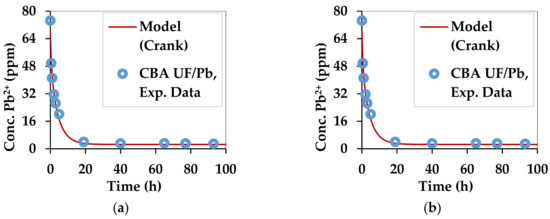
Figure 4.
Kinetics of lead adsorption by the two substrates: (a) C. barbata alginate (CBA UF) and (b) C. barbata powder (CB 500).
The diffusion equation represented by Equation (9) [76] was used to simulate the experimental results of adsorption of Pb2+ and Cu2+ and is based on the diffusion of ions from a solution at a given ion concentration until the solution is solid free or has a constant negligible concentration of ions, which is the case of the present study. α in Equations (9) and (10) represents the ratio between the volume of the liquid and the volume of the solid. In the case of the alginate beads, the volume of solid is represented by the total volume of the beads used. When algal powder is used, the volume of the solute can be approximated by the equivalent volume of water and powder, which is taken up by the powder when swelling. This value is 4.14 times greater than the mass of the used powder. The ratio of a sphere equivalent in volume to the substrate needs to be found for both the alginate and the powder. This term is named a. The effective diffusivity of the solute in the solid is termed Deff (m2/s). qn represents the six non-zero roots of Equation 10. qe represents the equilibrium value of the adsorption while qt is the value of the ion adsorption at time t.
Figure 3 and Figure 4 compare the experimental and theoretical results from using Equation (9) by regression. The parameters that were optimized are qe and Deff. The optimization algorithm, GRG Nonlinear was applied in Excel 2013 by the Solver tool. The Multistart feature was used with a population size of 100, using central derivatives to converge towards the solution. Upper and lower bounds with physical significance were applied for each optimized parameter. The combinations of Deff and qe which best fitted the experimental data for all 4 combinations of substrate and divalent metal ions are summarized in Table 3.

Table 3.
Crank diffusion model for a sphere. Model coefficients for 4 pairs of substrate / heavy metal.
The values of Deff in all cases are similar to the self-diffusivity of Cu2+ and Pb2+ in water: 0.71 × 10−9 m2/s and 0.95 × 10−9 m2/s [77]. This can be explained by the high content of water in the beads and the swollen biomass. Alginate gels are generally nanoporous [6,78], leading to high diffusion rates of small solutes [6]. However, in this case Deff is accelerated by the affinity of divalent metal ions towards the egg-box structure of gels [39] formed by the G fractions in the alginate structure. The Ca2+ ions, which occupy this site initially, are replaced by the Pb2+ and Cu2+ ions, which have a higher affinity [40]. The qe is the maximum adsorption capacity in the given setup, which was not meant to saturate the substrate. The diffusivities obtained for the substrates used in this work are higher than those obtained for materials used in a similar work for Pb2+ and Cu2+ [79].
2.5. Adsorption Isotherms
The experimental values for the maximum sorption capacities deduced from the adsorption isotherms are represented in Figure 5 and Figure 6 and are presented in Table 4. Such levels of Pb2+ concentration are in the range of contaminated mine waters [80,81].
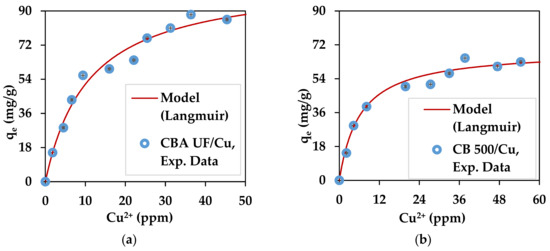
Figure 5.
Copper adsorption isotherms for: (a) C. barbata alginate (CBA UF) and (b) C. barbata powder (CB500).
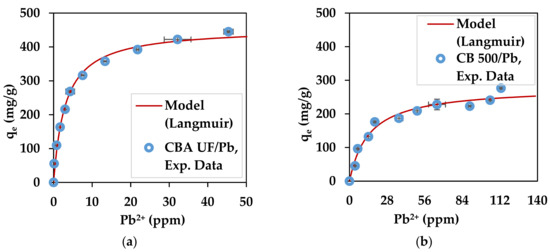
Figure 6.
Lead adsorption isotherms for: (a) C. barbata alginate (CBA) and (b) C. barbata powder (CB500).

Table 4.
Adsorption isotherms - model parameters (Langmuir).
On the same figures, the Langmuir adsorption model is shown for optimized parameters: qmax and KL. This model has been used in many studies that involve the adsorption of pollutants such as heavy metals, dyes and phenol [24,82,83]. This model is represented by Equation (11). Ce represents the concentration of metal ions still in the solution.
The optimized parameters for each substrate/metal pair are shown in Table 4.
In all cases the capacity of the substrates to adsorb the heavy metals used in this work remains high and reliable because the standard deviation is very low. Interestingly, the seaweed powder without any treatment also had excellent capacity for the adsorption of heavy metals. This is an interesting result because it shows that the brown seaweed recovered from the Romanian Black Sea can be used in its native form for heavy metal adsorption. The values obtained for qmax show that the substrates used for both metal ions have good theoretical adsorption capacity according to Langmuir (infinite equilibrium time considered). These values are similar to results obtained in a previous work [79] where for Pb2+, alginate beads were found to have a qmax of 390.3 mg/g, while brown seaweed biomass (L. digitata) was shown to have a qmax value of 264.2 mg/g [79]. For Cu2,+ similar results can be found in the literature with, for example, values of 107.5 mg/g and 74.5 mg/g for alginate and algal biomass (L. digitata), respectively [79]. The algal biomass can also be compared to other agricultural wastes that have been investigated as heavy metal adsorbents, such as treated green coconut (Cocos nucifera) shells, Pb2+: 54.62 mg/g, Cu2+: 41.36 mg/g [84]; apricot stone activated carbon, Pb2+ 22.85 mg/g, Cu2+ 24.21 mg/g [85]; tea waste, Cu2+: 48 mg/g [86]; and rose waste biomass, Pb2+: 151.51 mg/g [87]. As can be observed, the algal powder has a better adsorption capacity than other dried biomasses. The heavy metals adsorption capacity of C. barbata from the Black Sea has been studied before on samples recovered from the Turkish Black Sea Coast [46], where dried C. barbata biomass was found to have a maximum sorption capacity of 253 mg/g for lead, which is only slightly lower than the value obtained in this study. Other seaweed from Cystoseira sp. have also shown to have heavy metal adsorption properties when used directly as dried biomass. Cystoseira amentacea var. stricta (formerly C. stricta) recovered on the Algerian coast proved to adsorb 64.5 mg/g of lead ions after undergoing several chemical treatments [47].
The good performance of alginate from C. barbata can be explained by the low value of the M/G ratio (0.64), which indicates the higher availability of G blocks [40,88]. This could explain the better performance compared to other studies where alginate from L. digitata was used [79]. L. digitata was shown to have an M/G value of 1.12 [74] or 1.63 [44].
The natural capacity of C. barbata to adsorb heavy metals is proven in this study and confirms the high adsorption capacity which was also proven in a previous study [46]. The affinity of brown seaweed for heavy metals can be explained by the presence of alginate, especially the G homogeneous block fractions, which chelate divalent metal ions. This is why alginate extracted from C. barbata has an increased capacity.
The good heavy metal absorption characteristics of the edible brown seaweed C. barbata and of its major bioactive component, alginic acid, offer a possible explanation regarding the traditional use of this seaweed as functional food. Further studies on biological systems are needed.
Beside its use as nutraceutical/functional food, the biomass of C. barbata and/or of its bioactive component, alginic acid, could be used for water treatment to recover heavy metals ions. In this case, this seaweed and the alginate extracted from it have the potential to be used to develop systems such as cartridges, which could facilitate their use in processes under continuous conditions [44]. Also, the behavior during sorption/desorption cycles needs to be determined to prove reusability.
3. Materials and Methods
3.1. Raw Material and Chemicals
C. barbata seaweed was recovered from the Romanian seashore of the Black Sea in the city of Mangalia (GPS coordinates at latitude: N 43° 49.2′, longitude: E 28° 35.4′). The biomass was thoroughly washed with tap water to remove sand particles and epiphytes and dried at room temperature for one week in the dark to prevent any possible degradation associated with sunlight. Afterwards, the dry biomass was milled and sieved at 500 µm to obtain the material referred to as CB 500. The powder recovered under the sieve was used for further manipulation. The chemicals used for this work were analytical grade and were purchased from Sigma-Aldrich (Sigma-Aldrich, Saint-Louis, MO, USA).
3.2. Extraction of Alginate
Alginate was extracted by adapting several methods used in the literature [64]. Initially, 25 g of dried seaweed powder underwent a mild depigmentation and defatting in EtOH (70% v/v, 24 h, 250 mL). The solid was removed by vacuum filtration (Whatman filter paper, 25 µm, Whatman, Maidstone, UK) and added to HCl (0.1 M, 2 h, 60 °C, 500 mL). After vacuum filtration, this operation was repeated for the recovered wet pellets. The excess acid was washed away with distilled water before extracting the alginate in a Na2CO3 solution (3% (w/v), 2 h, 60 °C). The sodium alginate extract was left to cool before separating it from the solid waste by centrifugation (15,000 g, 30 min, 4 °C). After neutralization with dilute HCl, the extract was treated by ultrafiltration on a Vivaflow 200 crossflow cassette module (100 kDa, polyethersulfone, Sartorius, Göttingen, Germany) fitted with a peristaltic pump. Following the treatment in diafiltration mode with 5 diavolumes, alginate remained in solution in the retentate while most of contaminants were removed in the permeate. This procedure was followed by a concentration process, which reduced the volume of the retentate 3 times before precipitating the alginate by adding 3 volumes of EtOH (96%, −18 °C). The alginate pellets were dehydrated twice with 20 mL of acetone at −18 °C. The pellets were then dried at 50 °C, milled and sieved (0.2 mm) to obtain a fine powder called CBA UF.
3.3. NMR Analysis
The sample was prepared and analyzed under conditions described in the literature [63]. CBA UF was dissolved in D2O (99.9% D) at a concentration of 50 g/L. After dissolution the sample was freeze-dried resulting in CBA UF alginate with exchangeable protons replaced by deuterium. In total, this operation was done 3 times. Before analysis, the CBA UF lyophilized sample was dissolved again in D2O (99.9% D) at a concentration of 40 g/L. NMR spectra were obtained at 80 °C using a 400 MHz Bruker Avance spectrometer (Bruker, Billerica, MA, USA), equipped with a BBFO probe. A spectral width of 3000 Hz was used for acquiring the data obtained under the following acquisition parameters: acquisition mode = 2 s, pulse 90° = 8 μsec, scans = 64, recovery = 5 s (for a complete return after the 90° pulse).
3.4. HPSEC-MALS
High pressure size exclusion chromatography (HPSEC) was used to determine the number average molar mass, (Mn), the mass average molar mass (Mw), intrinsic viscosity ([η]), hydrodynamic radius (Rh) and gyration radius (Rg) for CBA UF. Three detectors were used in parallel, and were coupled to the chromatograph: multi-angle light scattering (MALS, DAWN-EOS from Wyatt Technology Corp., Santa Barbara, CA, USA) with a Ga-As laser (690 nm) and a K5 cell (50 µL) (HELEOS II Wyatt Technology Corp., USA), a viscosimeter (Viscostar II, Wyatt Technology Corp., USA) and refractive index detector (RID, RID10A Shimadzu, Kyoto, Japan). One OHPAK SB-G, two OHPAK SB 804 and 806 HQ columns were used in series for the HPSEC line (Shodex Showa Denko K.K., Tokyo, Japan). The system was eluted with LiNO3 0.1 M, filtered using a 0.1 µm unit (Millipore, Merck Group, Darmstadt, Germany) and degassed (DGU-20A3 Shimadzu, Kyoto, Japan). The flow rate was set at a value of 0.5 mL/min (LC10Ai Shimadzu, Kyoto, Japan). The sample was diluted to 1 mg/mL in LiNO3 0.1M under stirring for 24h, filtered (0.45 µm, Millipore) before 500 µL was placed onto the analytical line of the instrument with an automatic injector (SIL-20A Shimadzu, Kyoto, Japan).
3.5. Preparation of Alginate Beads
CBA UF was dissolved in MilliQ water to obtain a solution with a concentration of 3% (w/v). The solution was then pumped through a capillary (d = 1 mm) with its other end above a 0.5 M CaCl2 solution under stirring. Alginate beads are formed drop by drop as they contact the calcium solution. They were stored for 12 h at 4 °C in a 0.5 M CaCl2 solution. A digital camera (Euromex CMEX-5000, Arnhem, The Netherlands) was used to take photos of the alginate beads. The diameter of the beads was measured using the ImageFocus software.
3.6. Adsorption Kinetics
The initial conditions for the kinetic experiments were adapted from the literature [79] and are shown in Table 5. The substrate mass represents the quantity of alginate used to produce the corresponding number of beads used or the mass of dried powder added. The initial concentration of heavy metal ions is also given in Table 5 and was obtained from PbCl2 and CuSO4. The corresponding quantity of substrate was added under stirring. The kinetics experiments were run for 100 h in order to achieve equilibrium. The concentration of metal ions was determined using MP-AES (microwave plasma-atomic emission spectrometry, Agilent 4200, Santa Clara, CA, USA).

Table 5.
Initial conditions for adsorption kinetics experiments.
3.7. Adsorption Isotherms
Following the adsorption experiments, it was observed that the equilibrium was well reached after ~65 h. This value is used as the time necessary to achieve equilibrium in the adsorption isotherm experiments. Ten solutions of 50 mL each were obtained for each substrate/heavy metal pair with a concentration of heavy metal ions varying from 22.3 to 223 ppm for Pb2+ and from 8 to 79.6 ppm for Cu2+(Ci). In this case, the mass of alginate contained in the beads with respect to the heavy metal solution (Sm) was fixed at 0.4 g/L of solution. The corresponding mass of substrate was added to the heavy metal solutions. The solutions are kept under agitation using a plate shaker until equilibrium was reached. Afterwards, the final concentration of heavy metals was determined by MP-AES.
Author Contributions
Conceptualization, B.T., C.D., A.-V.U., P.M., and G.D.; Investigation, B.T., F.G., P.M. and F.O.; Methodology, B.T., F.G., A.-V.U. and G.D.; Supervision, C.D., T.D. and G.D.; Writing—original draft, B.T., C.D. and G.D.; Writing—review & editing, B.T., C.D., T.D. and F.O.
Funding
This research was founded by a grant from the Hubert Curien (PHC) Brancusi 2017 Programme, project number 38365QK entitled "On the advanced processing of marine algae from Romanian Black Sea edge" and by the project PN19.23.01.01 “Integrated platform for smart valorization of the biomass” SmartBi, from Nucleu Programme ChemEmergent of NIRDCP-ICECHIM, founded by the Ministry of Research and Innovation, Romania. The APC was funded by the project PFE 31/2018, Enhancing NIRDCP-ICECHIM research & innovation potential within the inter-disciplinary and cross-sectoral field of key enabling technologies - TRANS-CHEM, founded by Ministry of Research and Innovation, Romania.
Acknowledgments
We would like to thank Didier Le Cerf for the HPSEC-MALS analysis (Normandie Univ, UNIROUEN, INSA Rouen, CNRS, PBS, 76000 Rouen, France), Jacques Desbrières (Université de Pau et des Pays de l’Adour (UPPA), IPREM, UMR 5254 CNRS/UPPA, Helioparc Pau Pyrénées, 2 avenue P. Angot, 64053 PAU CEDEX 09, France) for the 1H-NMR and Diana Constantinescu-Aruxandei, for helpful discussion and English editing.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef]
- Pereira, L. Therapeutic and Nutritional Uses of Slgae; CRC Press: Boca Raton, FL, USA, 2018; p. 672. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Okolie, C.L.; Rajendran, S.R.C.K.; Udenigwe, C.C.; Aryee, A.N.A.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Idota, Y.; Kato, T.; Shiragami, K.; Koike, M.; Yokoyama, A.; Takahashi, H.; Yano, K.; Ogihara, T. Mechanism of suppression of blood glucose level by calcium alginate in rats. Biol. Pharm. Bull. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Georg Jensen, M.; Pedersen, C.; Kristensen, M.; Frost, G.; Astrup, A. Review: Efficacy of alginate supplementation in relation to appetite regulation and metabolic risk factors: Evidence from animal and human studies. Obes. Rev. 2013, 14, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, F.; Kato, T.; Idota, Y.; Takahashi, H.; Kakinuma, C.; Yano, K.; Arakawa, H.; Hara, K.; Miyajima, C.; Ogihara, T. Reduction Effect of Calcium Alginate on Blood Triglyceride Levels Causing the Inhibition of Hepatic and Total Body Accumulation of Fat in Rats. Biol. Pharm. Bull. 2019, 42, 365–372. [Google Scholar] [CrossRef]
- Savchenko, O.V.; Sgrebneva, M.N.; Kiselev, V.I.; Khotimchenko, Y.S. Lead removal in rats using calcium alginate. Environ. Sci. Pollut. Res. 2015, 22, 293–304. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Serguschenko, I.; Khotimchenko, Y. Lead absorption and excretion in rats given insoluble salts of pectin and alginate. Int. J. Toxicol. 2006, 25, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, I.; Weil, E.; Wilk, B. Integrative Medicine and the Role of Modified Citrus Pectin/Alginates in Heavy MetIntegrative Medicine and the Role of Modified Citrus Pectin/Alginates in Heavy Metal Chelation and Detoxification—Five Case Reports. Complement. Med. Res. 2007, 14, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Asher, C.J.; Kopittke, R.A.; Menzies, N.W. Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environ. Pollut. 2007, 150, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.A.; Sadiq, M. Metal contamination of drinking water from corrosion of distribution pipes. Environ. Pollut. 1989, 57, 167–178. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Al-Doush, I. Survey of trace elements in household and bottled drinking water samples collected in Riyadh, Saudi Arabia. Sci. Total Environ. 1998, 216, 181–192. [Google Scholar] [CrossRef]
- Simeonov, V.; Stratis, J.A.; Samara, C.; Zachariadis, G.; Voutsa, D.; Anthemidis, A.; Sofoniou, M.; Kouimtzis, T. Assessment of the surface water quality in Northern Greece. Water Res. 2003, 37, 4119–4124. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Islam, S.; Rahman, M.S.; Haque, M.R.; Islam, M.M. Heavy Metals in Water, Sediment and Some Fishes of Buriganga River, Bangladesh. Int. J. Environ. Res. 2010, 4, 321–332. [Google Scholar] [CrossRef]
- Jane Wyatt, C.; Fimbres, C.; Romo, L.; Méndez, R.O.; Grijalva, M. Incidence of Heavy Metal Contamination in Water Supplies in Northern Mexico. Environ. Res. 1998, 76, 114–119. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Wu, Q.; Leung, J.Y.S.; Du, Y.; Kong, D.; Shi, Y.; Wang, Y.; Xiao, T. Trace metals in e-waste lead to serious health risk through consumption of rice growing near an abandoned e-waste recycling site: Comparisons with PBDEs and AHFRs. Environ. Pollut. 2019, 247, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-Z.; Wang, M.-H.; Ho, Y.-S. Mapping of drinking water research: A bibliometric analysis of research output during 1992–2011. Sci. Total Environ. 2013, 443, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Pal, P. Groundwater Arsenic Remediation: Treatment Technology and Scale UP; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Holan, Z.R.; Volesky, B. Biosorption of lead and nickel by biomass of marine algae. Biotechnol. Bioeng. 1994, 43, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Yadanaparthi, S.K.; Graybill, D.; von Wandruszka, R. Adsorbents for the removal of arsenic, cadmium, and lead from contaminated waters. J. Hazard. Mater. 2009, 171, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Puyen, Z.M.; Villagrasa, E.; Maldonado, J.; Diestra, E.; Esteve, I.; Solé, A. Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour. Technol. 2012, 126, 233–237. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, S.; Teng, C.; Song, T.; Dong, L.; Liang, J.; Bai, X.; Xu, X.; Qu, J. Biosorption characteristic of Alcaligenes sp. BAPb.1 for removal of lead(II) from aqueous solution. 3 Biotech. 2017, 7, 123. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhana, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 2003, 154, 233–238. [Google Scholar] [CrossRef]
- Kariuki, Z.; Kiptoo, J.; Onyancha, D. Biosorption studies of lead and copper using rogers mushroom biomass ‘Lepiota hystrix’. S. Afr. J. Chem. Eng. 2017, 23, 62–70. [Google Scholar] [CrossRef]
- Abia, A.A.; Asuquo, E.D. Lead (II) and nickel (II) adsorption kinetics from aqueous metal solutions using chemically modified and unmodified agricultural adsorbents. Afr. J. Biotechnol. 2006, 5, 1475–1482. [Google Scholar]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.J.; Sepehr, S. Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater. 2002, 92, 253–262. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Zhang, P.; Gu, Q.; Gao, C. Absorption of Heavy Metal Ions by Alginate. In Bioact. Seaweeds Food Appl. 2018, 255–268. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Q.; Luo, F.; Chen, J. Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J. Hazard. Mater. 2009, 163, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Smidsrod, O.; Skjakbrk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Haug, A.; Bjerrum, J.; Buchardt, O.; Olsen, G.E.; Pedersen, C.; Toft, J. Affinity of some divalent metals to different types of alginates. Acta Chem. Scand. 1961, 15, 1794–1795. [Google Scholar] [CrossRef]
- Draget, K.I.; Moe, S.T.; Skjak-Braek, G.; Smidsrod, O. Alginates, Food Ppolysaccharides and Their Applications (Second Edition); CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hentati, F.; Ursu, A.V.; Pierre, G.; Delattre, C.; Trică, B.; Abdelkafi, S.; Djelveh, G.; Dobre, T.; Michaud, P. Production, Extraction and Characterization of Alginates from Seaweeds; Université Clermont Auvergne: AUBIÈRE, France, 2018. [Google Scholar]
- Kim, S.-K.; Chojnacka, K. Processes, Products, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Algal Foams Applied in Fixed-Bed Process for Lead(II) Removal Using Recirculation or One-Pass Modes. Mar. Drugs 2017, 15, 315. [Google Scholar] [CrossRef]
- Esteves, A.J.P.; Valdman, E.; Leite, S.G.F. Repeated removal of cadmium and zinc from an industrial effluent by waste biomass Sargassum sp. Biotechnol. Lett. 2000, 22, 499–502. [Google Scholar] [CrossRef]
- Yalcin, S.; Sezer, S.; Apak, R. Characterization and lead(II), cadmium(II), nickel(II) biosorption of dried marine brown macro algae Cystoseira barbata. Environ. Sci. Pollut. Res. Int. 2012, 19, 3118–3125. [Google Scholar] [CrossRef]
- Iddou, A.; Hadj Youcef, M.; Aziz, A.; Ouali, M.S. Biosorptive removal of lead (II) ions from aqueous solutions using Cystoseira stricta biomass: Study of the surface modification effect. J. Saudi Chem. Soc. 2011, 15, 83–88. [Google Scholar] [CrossRef]
- De Castro Alves, L.; Yáñez-Vilar, S.; Piñeiro-Redondo, Y.; Rivas, J. Novel Magnetic Nanostructured Beads for Cadmium(II) Removal. Nanomaterials 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Huang, P.; Li, F.; Wang, X.; Yuan, T.; Sun, R. Compressive Alginate Sponge Derived from Seaweed Biomass Resources for Methylene Blue Removal from Wastewater. Polymers 2019, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/Shell Gel Beads with Embedded Halloysite Nanotubes for Controlled Drug Release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef]
- Marin, O.A.; Timofte, F. Atlasul Macrofitelor de la Litoralul Romanesc; Editura Boldas: Constanta, Romania, 2011. [Google Scholar]
- Algae Base. Available online: http://www.algaebase.org (accessed on 24 May 2019).
- Bruno de Sousa, C.; Gangadhar, K.N.; Macridachis, J.; Pavão, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron Asymmetry 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Ksouda, G.; Benslima, A.; Nasri, R.; Rinaudo, M.; Nasri, M.; Hajji, M. Enhancing colour and oxidative stabilities of reduced-nitrite turkey meat sausages during refrigerated storage using fucoxanthin purified from the Tunisian seaweed Cystoseira barbata. Food Chem. Toxicol. 2017, 107, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Benslima, A.; Barragan-Montero, V.; Hajji, M.; Nasri, M. Polyphenolic-protein-polysaccharide ternary conjugates from Cystoseira barbata Tunisian seaweed as potential biopreservatives: Chemical, antioxidant and antimicrobial properties. Int. J. Biol. Macromol. 2017, 105, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Berov, D.; Ballesteros, E.; Sales, M.; Verlaque, M. Reinstatement of Species Rank for Cystoseira bosphorica Sauvageau (Sargassaceae, Phaeophyceae). BIOONE 2015, 36, 65–80. [Google Scholar]
- Milchakova, N. Marine plants of the Black Sea. An Illustrated Field Guide; DigitPrint: Sevastopol, Russia, 2011. [Google Scholar]
- Nonova, T.; Tosheva, Z. Cesium and strontium in Black Sea macroalgae. J. Environ. Radioact. 2014, 129, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Jordanova, A.; Strezov, A.; Ayranov, M.; Petkov, N.; Stoilova, T. Heavy metal assessment in algae, sediments and water from the bulgarian black sea coast. Water Sci. Technol. 1999, 39, 207–212. [Google Scholar] [CrossRef]
- Strezov, A.; Nonova, T. Influence of macroalgal diversity on accumulation of radionuclides and heavy metals in Bulgarian Black Sea ecosystems. J. Environ. Radioact. 2009, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Milkova, T.; Talev, G.; Christov, R.; Dimitrova-Konaklieva, S.; Popov, S. Sterols and volatiles in Cystoseira barbata and Cystoseira crinita from the black sea. Phytochemistry 1997, 45, 93–95. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Kadri, N.; Barragan-Montero, V.; Laouer, H.; Hajji, M.; Nasri, M. Fucans from a Tunisian brown seaweed Cystoseira barbata: structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 2014, 66, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hadj Ammar, H.; Lajili, S.; Ben Said, R.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2015, 23. [Google Scholar] [CrossRef] [PubMed]
- Fourest, E.; Volesky, B. Alginate Properties and Heavy Metal Biosorption by Marine Algae. Appl. Biochem. Biotechnol. 1997, 67, 215–226. [Google Scholar] [CrossRef]
- Larsen, C.K.; Gåserød, O.; Smidsrød, O. A novel method for measuring hydration and dissolution kinetics of alginate powders. Carbohydr. Polym. 2003, 51, 125–134. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.; Silva Filho, E.A.; Melo, D.F.; Feitosa, J.P.; de Paula, R.C.; Lima, M.G. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Grasdalen, H. High-field, 1H-n.m.r. spectroscopy of alginate: sequential structure and linkage conformations. Carbohydr. Res. 1983, 118, 255–260. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Zrid, R.; Bentiss, F.; Ali, R.A.B.; Belattmania, Z.; Zarrouk, A.; Elatouani, S.; Eddaoui, A.; Reani, A.; Sabour, B. Potential uses of the brown seaweed Cystoseira humilis biomass: 1-Sodium alginate yield, FT-IR, H NMR and rheological analyses. J. Mater. Environ. Sci. 2016, 7, 613–620. [Google Scholar]
- Davis, T.A.; Llanes, F.; Volesky, B.; Mucci, A. Metal Selectivity of Sargassum spp. and Their Alginates in Relation to Their α-l-Guluronic Acid Content and Conformation. Environ. Sci. Technol. 2003, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Dahmane, E.m.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Rheological characterisation of polysaccharides extracted from brown seaweeds. J. Sci. Food Agric. 2007, 87, 1630–1638. [Google Scholar] [CrossRef]
- Crank, J. Diffusion in a sphere. In The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975; pp. 89–103. [Google Scholar]
- Marcus, Y. Ion Properties; CRC Press: Boca Raton, FL, USA, 1997; Volume 1. [Google Scholar]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and Algal-Based Beads for the Sorption of Metal Cations: Cu(II) and Pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Peña, R.C.; Cornejo, L.; Bertotti, M.; Brett, C.M.A. Electrochemical determination of Cd(ii) and Pb(ii) in mining effluents using a bismuth-coated carbon fiber microelectrode. Anal. Methods 2018, 10, 3624–3630. [Google Scholar] [CrossRef]
- Briso, A.; Quintana, G.; Ide, V.; Basualto, C.; Molina, L.; Montes, G.; Valenzuela, F. Integrated use of magnetic nanostructured calcium silicate hydrate and magnetic manganese dioxide adsorbents for remediation of an acidic mine water. J. Water Process Eng. 2018, 25, 247–257. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.; Karim, W.A. Langmuir model application on solid–liquid adsorption using agricultural wastes: environmental application review. J. Pur. Util. React. Environs. 2012, 1, 170–199. [Google Scholar]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Sousa, F.W.; Oliveira, A.G.; Ribeiro, J.P.; Rosa, M.F.; Keukeleire, D.; Nascimento, R.F. Green coconut shells applied as adsorbent for removal of toxic metal ions using fixed-bed column technology. J. Environ. Manage. 2010, 91, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Javed, M.A.; Bhatti, H.N.; Hanif, M.A.; Nadeem, R. Kinetic and Equilibrium Modeling of Pb(II) and Co(II) Sorption onto Rose Waste Biomass. Sep. Sci. Technol. 2007, 42, 3641–3656. [Google Scholar] [CrossRef]
- Persin, Z.; Stana-Kleinschek, K.; Foster, T.J.; van Dam, J.E.G.; Boeriu, C.G.; Navard, P. Challenges and opportunities in polysaccharides research and technology: The EPNOE views for the next decade in the areas of materials, food and health care. Carbohydr. Polym. 2011, 84, 22–32. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).