Abstract
Five new sesterterpenes, 14,15-dehydro-6-epi-ophiobolin K (1), 14,15-dehydro- ophiobolin K (2), 14,15-dehydro-6-epi-ophiobolin G (3), 14,15-dehydro-ophiobolin G (4) and 14,15-dehydro-(Z)-14-ophiobolin G (5), together with four known ophiobolins (6–9) were isolated from the marine fungus Aspergillus flocculosus derived from the seaweed Padina sp. collected in Vietnam. The five new ophiobolins were first isolated as ophiobolin derivatives consisting of a fully unsaturated side chain. Their structures were elucidated via spectroscopic methods including 1D, 2D NMR and HR-ESIMS. The absolute configurations were determined by the comparison of chemical shifts and optical rotation values with those of known ophiobolins. All compounds (1–9) were then evaluated for their cytotoxicity against six cancer cell lines, HCT-15, NUGC-3, NCI-H23, ACHN, PC-3 and MDA-MB-231. All the compounds showed potent cytotoxicity with GI50 values ranging from 0.14 to 2.01 μM.
1. Introduction
The marine environment is an enormous reservoir of novel sources of biologically active metabolites, many of which display unique structural skeletons that can be used as lead structures for the development of new drugs [,]. To adapt and live in an environment that is significantly different from terrestrial organisms, marine organisms frequently produce structurally unique chemical compounds [,]. Specifically, secondary metabolites from marine microorganisms are recognized as a novel chemical source for drug discovery and development. Among marine-derived microbes, marine fungi produce a wide range of promising biologically active compounds []. Numerous novel compounds from marine fungi have displayed a wide range of bioactivities such as antiviral, antibacterial, anticancer, antiplasmodial and anti-inflammatory [,,]. In the marine context, Aspergillus and Penicillium represent the best studied fungal genera as depicted in marine contexts [,]. The genus Aspergillus is known as a major contributor of pharmacologically bioactive compounds, including anticancer asperazine, antibacterial varixanthone and antifungal amphotericin B [,].
Ophiobolins are a group of sesterpenoids with an unusual tricyclic 5-8-5 ring system. They show a broad range of inhibitory activities against nematodes, fungi, bacteria and cytotoxic activity against cancer cells [,]. They are produced by the fungal genus Bipolaris, Aspergillus, Sarocladium and Drechslera []. The first ophiobolin, ophiobolin A, was isolated from Biolaris spp. and displays inhibitory activity against calmodulin-activated cyclic nucleotide phosphodiesterase []. These findings made the compound a useful calmodulin probe for research purposes and implied an application in anti–cancer therapy []. Interestingly, more than half of the 49 ophiobolins identified between 1999 and 2016 exhibit cytotoxic activities against human cancer cell lines []. Although their biological properties have been well exploited in recent years, their structure-activity relationship remains unestablished []. Consequently, this study focused on the discovery of bioactive natural products from marine fungi. During our ongoing investigation for new bioactive compounds from marine microorganisms, the fungal 168ST-16.1 strain was isolated from the seaweed Padina sp. collected at Da Nang, Vietnam, and, based on its 28S rRNA gene sequence, it was identified as Aspergillus flocculosus. Subsequent chemical investigations on an EtOAc extract of the fungal culture broth using reversed-phase HPLC led to the isolation of the five new ophiobolins, named, 14,15-dehydro-6-epi-ophiobolins K and G (1 and 3), 14,15-dehydro-ophiobolins K and G (2 and 4) and 14,15-dehydro-(Z)-14-ophiobolin G (5), together with four known ophiobolins, 6-epi-ophiobolins C and N (6 and 8) and ophiobolins C and N (7 and 9) [,,] (Figure 1). Herein, details of the structure elucidation and biological activity of these compounds are described.
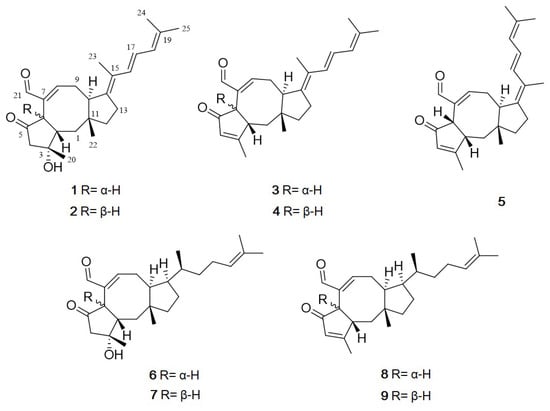
Figure 1.
Structures of 1–9 isolated from Aspergillus flocculosus.
2. Results and Discussion
Compound 1 was obtained as an amorphous powder. The molecular formula of 1 was determined to be C25H34O3 based on HRESIMS. The 1H NMR spectroscopic data of 1 displayed resonances for an aldehyde proton (δH 9.23), four olefinic protons (δH 6.84, 6.42, 6.38 and 5.93), five methylene protons (δH 3.15, 2.47, δH 2.94, 2.22, δH 2.59, 2.25, δH 1.84, 1.63 and δH 1.63, 1.50), three methine protons (δH 3.33, 3.19 and 2.19) and five methyl groups (δH 1.83, 1.81, 1.79, 1.47 and 0.94) (Table 1). The combination of 13C NMR and HSQC spectra revealed the presence of 25 carbon resonances, including one ketone (δC 216.6), one aldehyde carbon (δC 194.0), four olefinic carbons, five methylene, three methine, five methyl and six quaternary carbons (δC 146.8, 142.1, 134.9, 124.9, 76.6 and 43.6) (Table 2). Spin systems and their partial structures were confirmed and assembled by combined analysis of COSY and HMBC correlations (Figure 2). Three spin systems, H2-1/H-2/H-6, H-8/H2-9/H-10 and H2-12/H2-13, and HMBC correlations from H3-22 (δH 0.94) to C-1 (δC 41.4), C-10 (δC 47.8), C-11 (δC 43.6) and C-12 (δC 44.5), and from H-21 (δH 9.23) to C-6 (δC 48.8), C-7 (δC 142.1) and C-8 (δC 158.4) confirmed the presence of an eight-membered ring system with an aldehyde group. The five-membered ring with a ketone was also determined by the HMBC correlations from H3-20 (δH 1.47) to C-2 (δC 49.6), C-3 (δC 76.6) and C-4 (δC 55.0) and from H-6 (δH 3.33) to C-4 (δC 55.0), C-5 (δC 216.6), C-7 (δC 142.1) and C-21 (δC 194.0). Additionally, the HMBC correlations from H2-13 (δH 2.25, 2.59) to C-10 (δC 47.8), C-14 (δC 146.8) and C-15 (δC 124.9) suggested that one additional five-membered ring was connected to the eight-membered ring, which generated a 5-8-5 tricyclic carbon skeleton. The partial structure was closely related to ophiobolin analogs and the 1H and 13C NMR spectra of 1 resembled those of 6-epi-ophiobolin C (6) except for the presence of two olefinic protons (δH 6.38 and 6.42) and two sp2 quaternary carbons (δC 146.8 and 124.9). Finally, the COSY correlation of H-16/H-17/H-18 and the HMBC correlations from H3-24 (δH 1.81) and H3-25 (δH 1.79) to C-18 (δC 125.9) and C-19 (δC 134.9) and from H3-23 (δH 1.83) to C-14 (δC 146.8), C-15 (δC 124.9) and C-16 (δC 130.6) defined a conjugated side chain connected to C-14 of the tricyclic ring. The planar structure of 1 was elucidated to possess a fully unsaturated side chain. To the best of our knowledge, 1 is the first ophiobolin with three double bonds at the side chain and is named 14,15-dehydro-6-epi- ophiobolin K.

Table 1.
1H and 13C NMR data for 1, 2 and 3 at 600 MHz (δ in ppm, J in Hz).

Table 2.
13C NMR data for 1–5 at 150 MHz (δ in ppm).
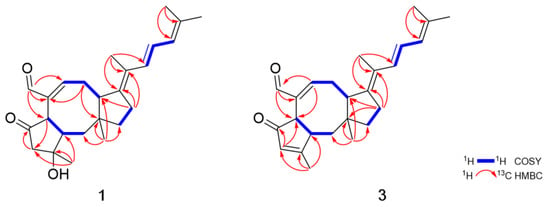
Figure 2.
Key COSY and HMBC correlations of 1 and 3.
The stereochemistry of 1 was determined by the analysis of proton-proton coupling constants and NOESY data. The strong NOESY correlations of H-6/H-10 and H-2/H3-20/H3-22 suggested that H-6 and H-10 were on the same face, and H-2, H3-20 and H3-22 were on opposite faces. Based on a comprehensive literature review, ophiobolin analogs have A/B-cis or A/B-trans isomers at C-2 and C-6 []. It has been reported that H-2 of the 6-epimer having H-6α is shielded by ca. 0.2-0.3 ppm in comparison with the A/B-cis isomer having H-6β []. On the basis of this analysis, the H-2 protons of 1 and 2 were observed at δH 2.19 and δH 2.57, respectively, indicating that 1 has an A/B-trans ring structure. The lack of a NOESY correlation of H-2/H-6 and a comparison of the 1H NMR data of 1 with those of 6-epi-ophiobolin C (6) also supported the fact that the A/B ring junction is trans in 1 (Figure 3). The relative configuration of the side chain was confirmed by comprehensive NOESY and 1H NMR analyses. The NOESY correlations of H-9α/H3-23 and H-13β/H-16 indicated the relative configuration of Δ14,15 was E conformation (Figure 3a). The geometry of the Δ16,17 was confirmed as E by the large coupling constants of H-16 (d, J = 15.3 Hz) and H-17 (dd, J = 15.3, 9.7 Hz) and NOESY correlations of H-13β/H-16, H-17/H3-23 and H-16/H-18. Moreover, a combination of literature review and comparison of the NMR spectral data and spectral properties of 1 with those of 6, suggested that 14, 15-dehydro-6-epi-ophiobolin K (1) has the same absolute configuration of a 5-8-5 core structure in 6-epi-ophiobolin K [,,].
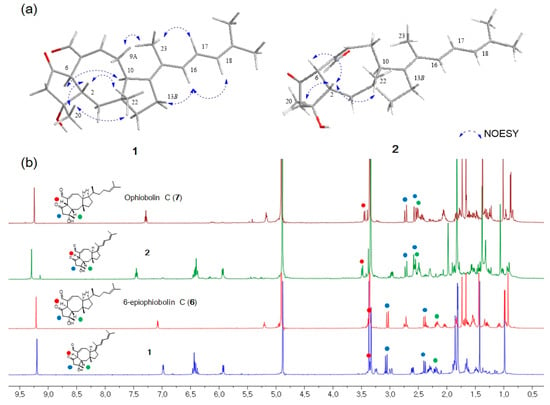
Figure 3.
(a) Key NOESY correlations of 1 and 2. (b) Comparison of chemical shifts of H2-4 and H-6 in 1 (H-6α), 2 (H-6β), 6 (H-6α) and 7 (H-6β).
Compound 2 had the same molecular formula C25H32O3 as 1. Its 1H and 13C NMR data were similar to those of 1, differing only by slightly shifted proton and carbon signals. It has been reported that H-2 of the 6-epi isomer having H-6α (A/B-trans) is upfield-shifted in comparison with the A/B-cis ophiobolin [] (Figure 3b). The H-2 proton (δH 2.57) of 2 is downfield-shifted than that (δH 2.19) of 1, indicating that 2 has an A/B-cis ring structure. This study also revealed that the chemical shifts of the geminal proton H2-4 are closer to each other when the A/B ring junction is cis than when it is trans (Figure 3b). The key NOE correlations of H-2/H-6, H-2/H3-20 and H-2/H3-22 suggested that 2 has an A/B-cis ring structure and is a stereoisomer of 1 (Figure 3a). Based on these results, the structure of 2 was determined and named 14,15-dehydro-ophiobolin K.
Compound 3 was obtained as an amorphous powder with the molecular formula of C25H32O2 based on HRESIMS. The molecular formula of 3 has one less CH2 and one less oxygen compared to that of 1. The 1H and 13C NMR data of 3 were quite similar to those of 1, displaying one additional singlet olefin signal (δH 6.01) and a sp2 quaternary carbon at C-3 (δC 179.9), while lacking a methylene and sp3 quaternary carbon signal. The HMBC correlations from H3-20 (δH 2.12) to C-2 (δC 49.5), C-3 (δC 179.9) and C-4 (δC 129.4) revealed that a double bond existed between C-3 and C-4 by the dehydroxylation of the tertiary alcohol at C-3 in 1 (Figure 2). NOESY correlations from H-6/H-10 and H-2/H3-20/H3-22, the lack of NOESY correlation of H-2/H-6 and, comparison of the NMR spectral data and spectral properties of 3 with those of 6-epi-ophiobolin N (8), suggested that 3 has the same ring system as the A/B-trans ophiobolin [,] (Figure 4). On the basis of detailed data analysis, the structure of 3 was elucidated and named 14,15-dehydro-6-epi-ophiobolin G.
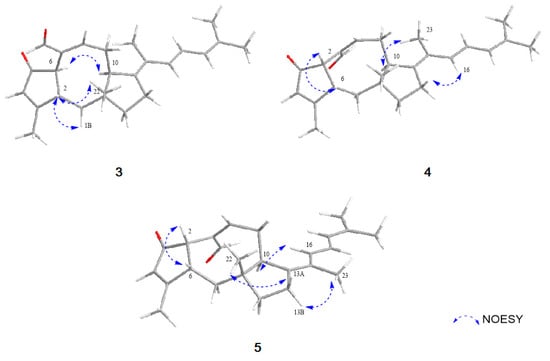
Figure 4.
Key NOESY correlations of 3–5.
Compound 4 was isolated as an amorphous powder with the molecular formula of C25H32O2 as determined by HRESIMS. Its 1H NMR data were similar to those of 3, differing only by slightly shifted signals. In contrast to the data for 3, the NOESY correlation of H-2/H-6 indicated that 4 has an A/B-cis ring structure (Figure 4). Thus, compound 4 was identified as a stereoisomer of 3 and named 14,15-dehydro-ophiobolin G.
Compound 5 was isolated as an amorphous powder with the same molecular formula C25H32O2 as compound 4, as determined by HRESIMS. The 1H NMR data of 5 and 4 were nearly identical except for the H-16 proton which was slightly downfield-shifted than that of 4. By comprehensive analysis of its 1D and 2D NMR data, the planar structure of 5 was elucidated to be the same as that of 4, differing only in the orientation of H3-23. The NOESY correlations of H-16/H-10 and H-23/H2-13 suggested the relative configuration of Δ14,15 in 5 was Z conformation, which is different from that of compound 4 (Figure 4). Therefore, the structure of 5 was elucidated to be as shown in Figure 1, and named 14, 15-dehydro-(Z)-14-ophiobolin G.
The structures of the four known compounds were determined as 6-epi-ophiobolin C (6), ophiobolin C (7), 6-epi-ophiobolin N (8) and ophiobolin N (9) by comparing of their 1H, 13C NMR and MS data with those reported in literature (Supplementary Materials).
The cytotoxicity of all the isolated compounds (1–9) against cancer cell lines, such as HCT-15, NUGC-3, NCI-H23, ACHN, PC-3 and MDA-MB-231, was investigated using the sulforhodamine B (SRB) assay, with adriamycin as a positive control. The results showed that all compounds were strongly active against 6 cancer cell lines with GI50 values in the range of 0.14 to 2.01 μM (Table 3). Compound 1 displayed the strongest cytotoxicity against the HCT-15, NUGC-3 and MDA-MB-231 cell lines with GI50 values of 0.21, 0.19 and 0.14 μM, respectively. Based on the cytotoxicity results, the analogs with one double bond (6–9) in the side chain seemed to be slightly more active than those with three double bonds (1–5). 5 was least active against all cell lines, even with GI50 values ranging from 1.53 to2.01 μM, indicating that the geometry of C-14/C-15 might appear to have a slight influence on their activities. In addition, results for all the strongly active compounds indicated that the stereochemistry of C-6 and the hydroxyl group at C-3 might not noticeably affect the cytotoxicity.

Table 3.
Growth Inhibition (GI50, μM) Values of 1–9 against Human Tumor Cell Lines.
3. Materials and Methods
3.1. General Experimental Procedures
The 1D (1H and 13C) and 2D (COSY, HSQC, HMBC, and NOESY) NMR spectra were obtained on a Bruker 600 MHz spectrometer. Specific optical rotations were obtained on a Rudolph Research Analytical (Autopol III) polarimeter. UV-visible spectra were acquired on a Shimadzu UV-1650PC spectrophotometer in 1 mm quartz cells. IR spectra were recorded on a JASCO FT/IR-4100 spectrophotometer. High-resolution ESIMS were recorded on a hybrid ion-trap time-of-flight mass spectrometer (Shimadzu LC/MS-IT-TOF). HPLC was performed using a PrimeLine Binary pump with RI-101(Shodex). RP-HPLC was performed using a semi-prep ODS column (YMC-Triart C18, 250 × 10 mm i.d, 5 µm) and an analytical ODS column (YMC-Triart C18, 250 × 4.6 mm i.d, 5 µm).
3.2. Fungal Material and Fermentation
The fungus 168ST-16.1 was isolated from the algae Padina sp., collected at a depth of 10 m in Son Tra peninsular, Da Nang, Vietnam (16°09′97.8″ N, 108°29′96.1″ E), in August 2016. The fungal strain was identified as Aspergillus flocculosus (GenBank accession number MG920345) by DNA amplification and ITS region sequencing and named Aspergillus flocculosus 168ST-16.1.
The isolated fungi were cultured on rice media at 28 °C for three weeks in 100 Erlenmeyer flasks (500 mL), each containing rice (20.0 g), yeast extract (20.0 mg), KH2PO4 (10 mg), and natural sea water (40 mL).
3.3. Isolation of Compounds 1–9
The whole fermentation media were extracted with EtOAc and evaporated in vacuo to give the crude extract (22 g), which was fractionated by flash column chromatography on ODS using a gradient of MeOH/ H2O (1:4, 2:3, 3:2, 4:1 and 100% MeOH, each fraction 300× 3). The second fraction eluted with 100% MeOH was separated into ten subfractions (Fr. A-J) by column chromatography on ODS eluting with a step gradient of MeCN/H2O (70:30 to 100:0, v/v). Fr. E (200 mg) was further purified by an analytical reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 4.6 mm i.d, 5 µm, flow rate 2.5 mL/ min, isocratic elution with 55% MeCN in H2O, RI detector) to yield 1 (7.5 mg, tR = 18 min) and 2 (1.5 mg, tR = 21 min). Compounds 3 (2.2 mg, tR = 30 min), 4 (3.1 mg, tR = 33 min), 5 (1.2 mg, tR = 36 min), 6 (3.5 mg, tR = 50 min) and 7 (3.2 mg, tR = 53 min) were isolated from Fr. F (210 mg) by a semi-preparative reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 10 mm i.d, 5 µm, flow rate 6.0 mL/ min, isocratic elution with 60% MeCN in H2O, RI detector). Fr G (136 mg) was subjected to a semi-preparative reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 10 mm i.d, 5 µm, flow rate 5.5 mL/ min, isocratic elution with 65% MeCN in H2O, RI detector) to obtain 8 (3.6 mg, tR = 40 min) and 9 (2.9 mg, tR = 43 min).
14,15-dehydro-6-epi-ophiobolin K (1): amorphous powder; +74.0(c 1.0, MeOH); IR νmax 3442, 2931, 2852, 1736, 1683, 1640, 1454, 1379 cm−1; UV(MeOH) λmax (log ε) 286 (3.62), 236 (3.04) nm; HRESIMS m/z 405.2405 [M + Na]+ (calcd for 405.2406, C25H34O3Na); 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) see Table 1.
14,15-dehydro-ophiobolin K (2): amorphous powder; +94.0(c 1.0, MeOH); IR νmax 3451, 2967, 2897, 1734, 1688, 1448, 1377, 1233 cm−1; UV(MeOH) λmax (log ε) 289 (3.58), 238 (3.36) nm; HRESIMS m/z 405.2404 [M + Na]+ (calcd for 405.2406, C25H34O3Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) see Table 1.
14,15-dehydro-6-epi-ophiobolin G (3): amorphous powder; +87.0(c 1.0, MeOH); IR νmax 2922, 2858, 1683, 1625, 1455, 1377 cm−1; UV(MeOH) λmax (log ε) 286 (3.53), 227 (3.24) nm; HRESIMS m/z 387.2301 [M + Na]+ (calcd for 387.2300, C25H32O2Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) see Table 1.
14,15-dehydro-ophiobolin G (4): amorphous powder; +85.0(c 1.0, MeOH); IR νmax 2925, 2858, 1689, 1636, 1441, 1377 cm−1; UV(MeOH) λmax (log ε) 291 (3.59), 231 (3.37) nm; HRESIMS m/z 387.2299 [M + Na]+ (calcd for 387.2300, C25H32O2Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) see Table 1.
14,15-dehydro-(Z)-14-ophiobolin G (5): amorphous powder; +132.0(c 1.0, MeOH); IR νmax 2922, 2855, 1692, 1632, 1437, 1377 cm−1; UV(MeOH) λmax (log ε) 289 (3.61), 231 (3.54) nm; HRESIMS m/z 387.2299 [M + Na]+ (calcd for 387.2300, C25H32O2Na); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) see Table 1.
3.4. Cytotoxicity Test by SRB Assay
The human cancer cell lines, HCT-15 (colon), NUGC-3 (stomach), NCI-H23 (lung), ACHN (renal), PC-3 (prostate) and MDA-MB-231 (breast), were purchased from American Type Culture Collection (Manassas, VA, USA). They were then cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Cell cultures were maintained at 37 °C under a humidified atmosphere of 5% CO2. The growth inhibition assay against human cancer cell lines was performed in accordance with the sulforhodamine B (SRB) assay []. In brief, 8,000 cells/well were seeded onto a 96-well plate. On the following, the cells were treated with compounds 1–9, vehicle control (0.1% DMSO) and positive control (adriamycin). After incubation for 48 h, the cultures were fixed with 50% trichloroactetic acid (50 μg/mL) and stained with 0.4% sulforhodamine B in 1% acetic acid. Unbound dye was removed by washing with 1% acetic acid, and protein-bound dye was extracted with 10 mM Tris base (pH 10.5) for optical density determination. Absorbance at 540 nm was determined using a VersaMax microplate reader from Molecular Devices (LLC, Sunnyvale, CA, USA). GI50 values were calculated using GraphPad Prism 4.0 software from GraphPad Software, Inc. (San Diego, CA, USA).
4. Conclusions
Chemical investigation of the marine-derived fungus Aspergillus flocculosus 168ST-16.1 led to the isolation and identification of five new (1–5) and four known (6–9) ophiobolin derivatives. The five new ophiobolins possessed a fully unsaturated side chain, and 5 had a Z-conformation at C-14/C-15. To the best of our knowledge, the new compounds 1–5 are the first ophiobolins with three double bonds at the side chain. All compounds (1–9) exhibited potent growth inhibitory activities against the HCT-15, NUGC-3, NCI-H23, ACHN, PC-3 and MDA-MB-231 cancer cell lines. The cytotoxicities of the new ophiobolins 4 and 5 were slightly weaker or similar to those of the known compounds (6–9). These results suggest that dehydration at C-14 and C-15 might not significantly affect the cytotoxicity against cancer cell lines. This study is the first report to describe the effect of the side chain of ophiobolins by evaluating the anticancer activity of five new and four known compounds together.
Supplementary Materials
The followings are available online at https://www.mdpi.com/1660-3397/17/6/346/s1, Figures S1–S35: HRESI-MS data, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY and experimental spectra of 1–5, Figures S36–S43: LRMS data, 1H NMR, 13C NMR and experimental spectra of 6–9.
Author Contributions
H.J.S. was the principal investigator, who proposed ideas for the present work, managed and supervised the whole research work, prepared and corrected the manuscript, and contributed to the structure elucidation of the new and known compounds. B.-K.C. achieved all experiments for compounds 1–9, including fermentation, isolation, and structure elucidation, and prepared the manuscript. P.T.H.T., H.-S.L., B.-W.C., N.T.D.N. and T.T.T.V. contributed to analyzing data. J.S.K. evaluated the cytotoxicity of 1–9.
Funding
This research was supported in part by the Korea Institute of Ocean Science and Technology (Grant PE99752 to H.J.S.).
Acknowledgments
The authors express gratitude to Young Hye Kim, Korea Basic Science Institute, Ochang, Korea, for providing mass data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Putz, A.; Ortlepp, S.; Kjer, J.; Bayer, M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010, 9, 475–489. [Google Scholar] [CrossRef]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Qadir, M.I.; Janbaz, K.H.; Ali, M. Novel drugs from marine microorganisms. Crit. Rev. Microbiol. 2011, 37, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug. Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs. 2016, 14, 19. [Google Scholar] [CrossRef]
- Nicoletti, R.; Vinale, F. Bioactive Compounds from Marine-Derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs. 2018, 16, 408. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Borah, N.; Yellusani, K.R.; Gade, S.; Reddy, P.; Rajamanikyam, M.; Vempati, L.N.S.; Gubbala, S.P.; Chopra, P.; Upadhyayula, S.M.; et al. Aspergillus Secondary Metabolite Database, a resource to understand the Secondary metabolome of Aspergillus genus. Sci. Rep. 2017, 7, 7325. [Google Scholar] [CrossRef] [PubMed]
- Trianto, A.; Widyaningsih, S.; Radjasa, O.K.; Pribadi, R. Symbiotic Fungus of Marine Sponge Axinella sp. Producing Antibacterial Agent. Environ. Earth. Sci. 2017, 55, 012005. [Google Scholar] [CrossRef]
- Wei, H.; Itoh, T.; Kinoshita, M.; Nakai, Y.; Kurotaki, M.; Kobayashi, M. Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron 2004, 60, 6015–6019. [Google Scholar] [CrossRef]
- Au, T.K.; Chick, W.S.; Leung, P.C. The biology of ophiobolins. Life Sci. 2000, 67, 733–742. [Google Scholar] [CrossRef]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.L.; William, A.T.; Wang, J.H.; Carl, L.T. Role of Calmodulin Inhibition in the Mode of Action of Ophiobolin A. Plant Physiol. 1985, 77, 303–308. [Google Scholar]
- Chai, H.; Yin, R.; Liu, Y.; Meng, H.; Zhou, X.; Zhou, G.; Bi, X.; Yang, X.; Zhu, T.; Zhu, W.; et al. Sesterterpene ophiobolin biosynthesis involving multiple gene clusters in Aspergillus ustus. Sci. Rep. 2016, 6, 27181. [Google Scholar] [CrossRef]
- Tian, W.; Deng, Z.; Hong, K. The Biological Activities of Sesterterpenoid-Type Ophiobolins. Mar. Drugs. 2017, 15, 7. [Google Scholar]
- Tsipouras, A.; Adefarati, A.A.; Tkacz, J.S.; Frazier, E.G.; Rohrer, S.P.; Birzin, E.; Rosegay, A.; Zink, D.L.; Goetz, M.A.; Singh, S.B.; et al. Ophiobolin M and analogues, noncompetitive inhibitors of ivermectin binding with nematocidal activity. Bioorg. Med. Chem. 1996, 4, 531–536. [Google Scholar] [CrossRef]
- Bladt, T.T.; Dürr, C.; Knudsen, P.B.; Kildgaard, S.; Frisvad, J.C.; Gotfredsen, C.H.; Seiffert, M.; Larsen, T.O. Bio-Activity and Dereplication-Based Discovery of Ophiobolins and Other Fungal Secondary Metabolites Targeting Leukemia Cells. Molecules 2013, 18, 14629–14650. [Google Scholar] [CrossRef]
- Nozoe, S.; Hirai, K.; Tsuda, K. The structure of zizannin-A and -B, C25-terpenoids isolated from helminthosporiumzizaniae. Tetrahedron Lett. 1966, 7, 2211–2216. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer. Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).