Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment
Abstract
1. Introduction
2. Results and Discussion
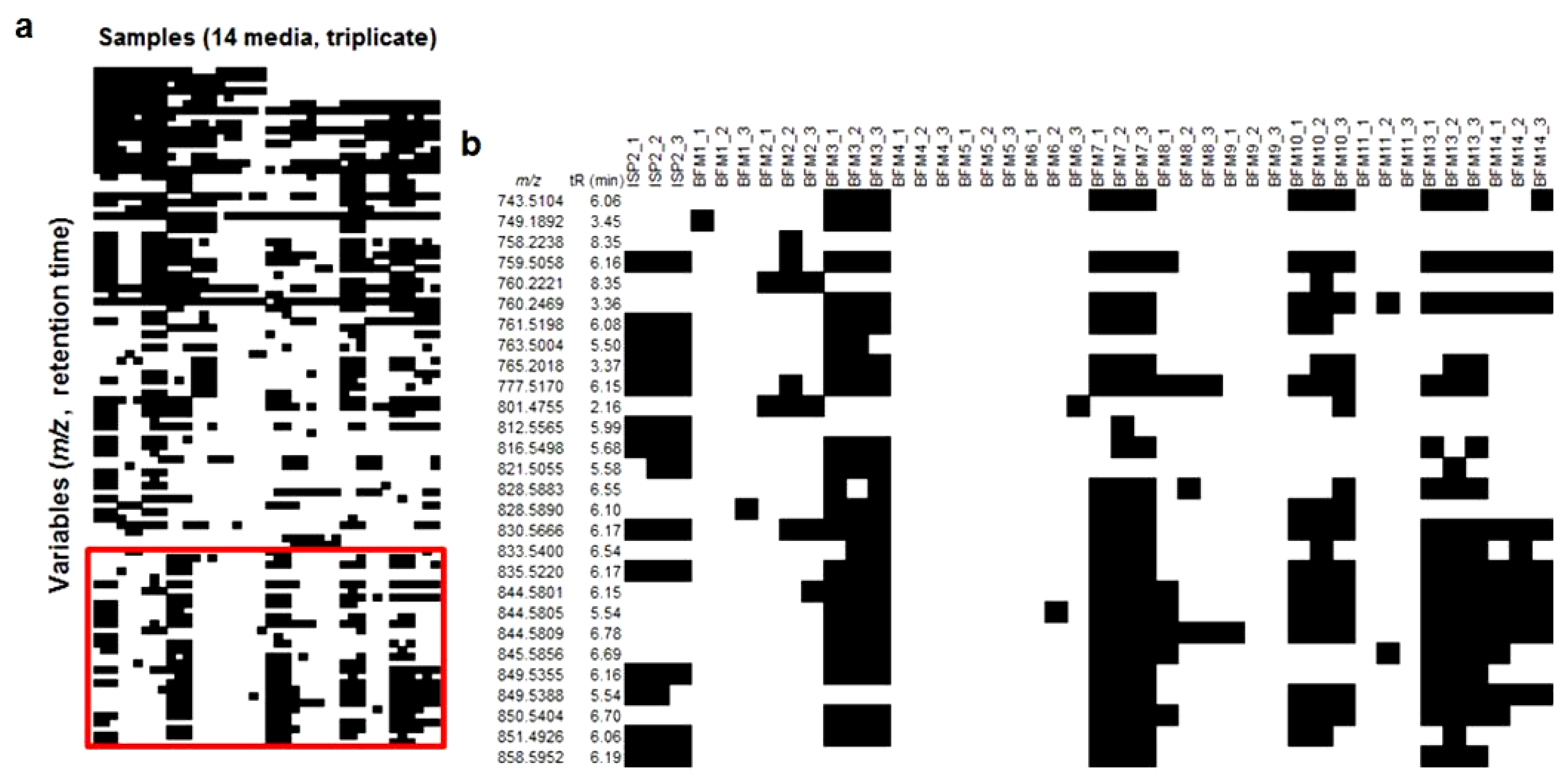
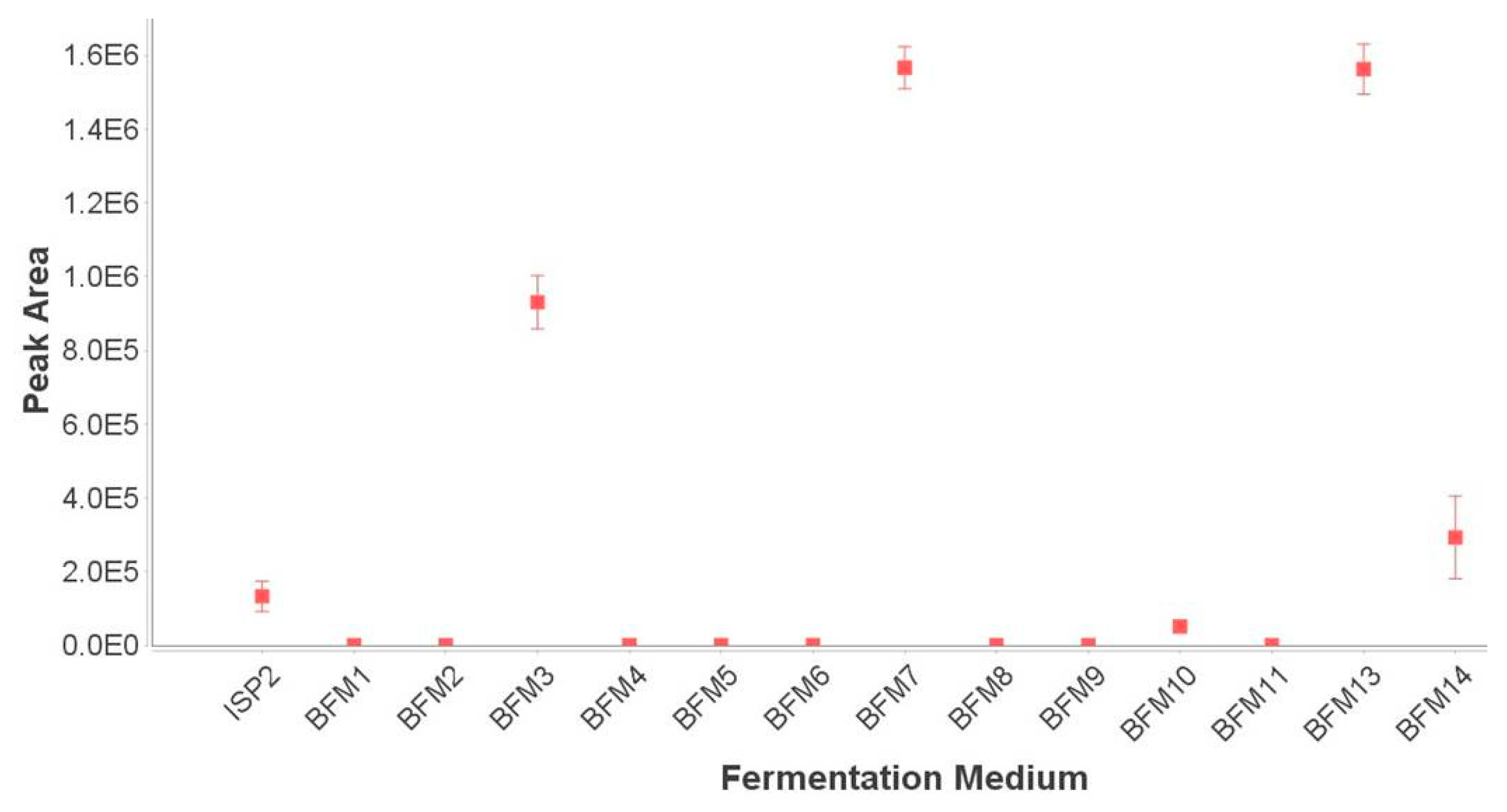
2.1. Fermentations and Chemical Screening
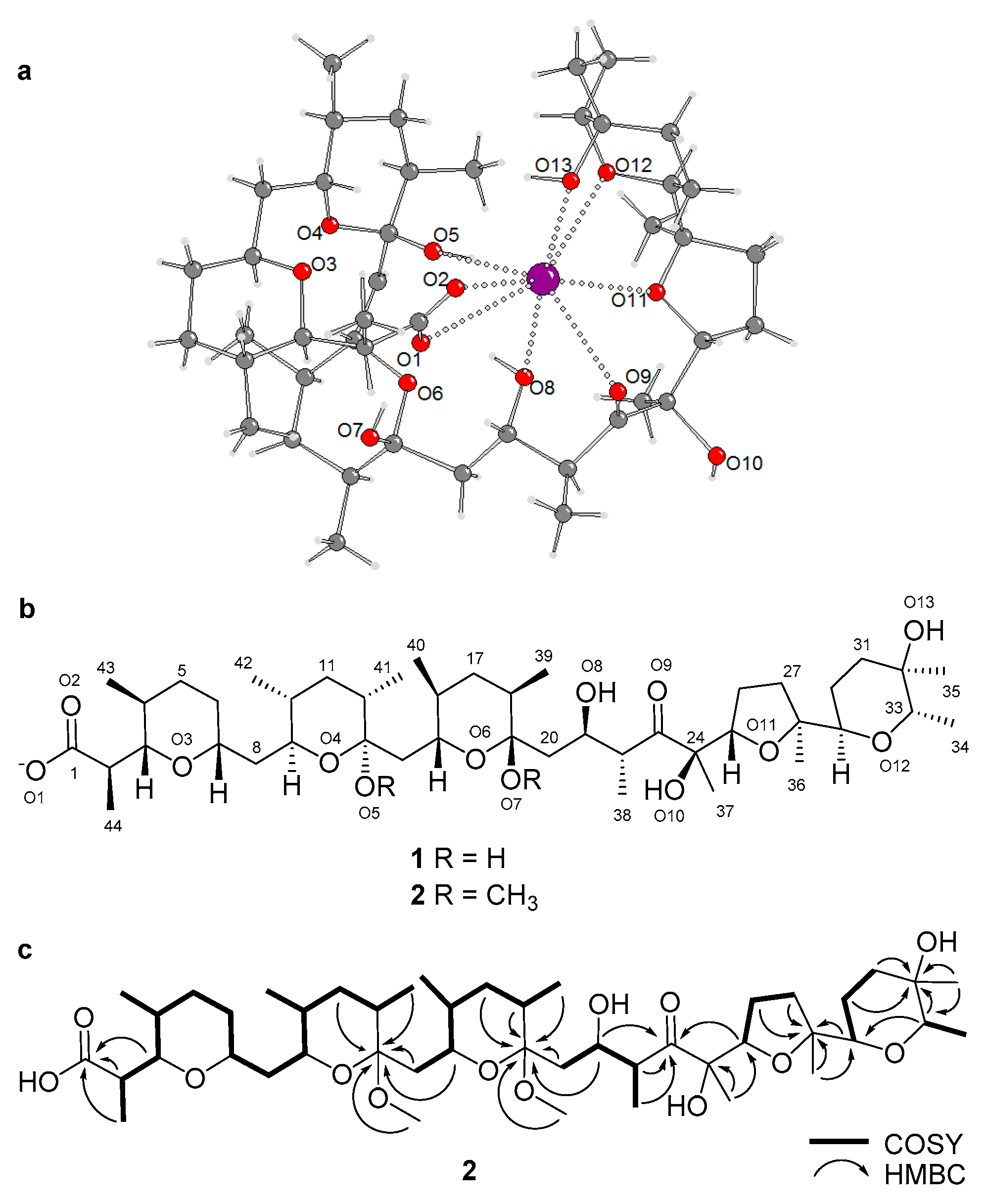
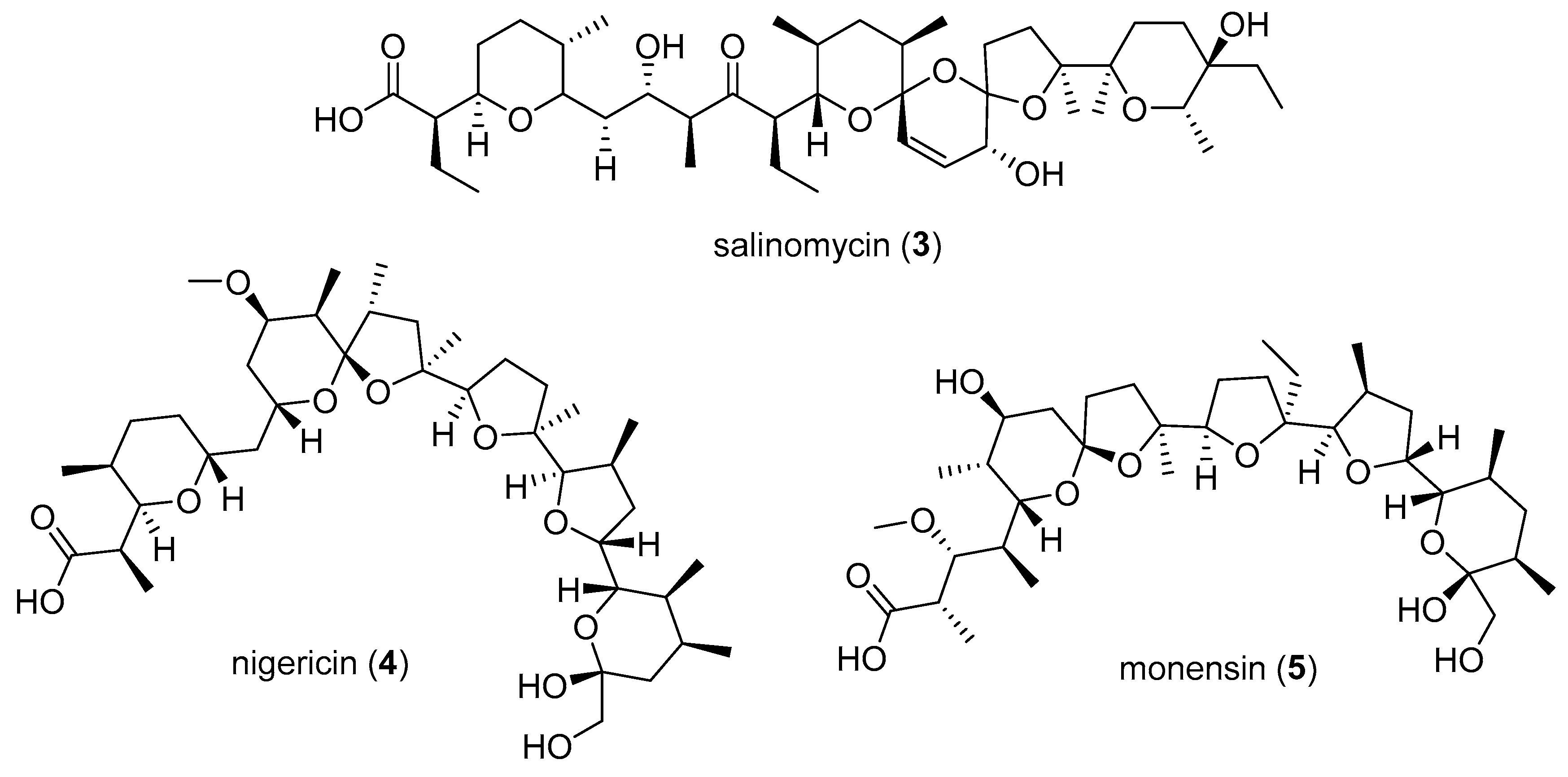
2.2. Purification and Structure Elucidation
2.3. Bioactivity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Microbial Material
3.3. Small-Scale Fermentations and Chemical Screening
3.4. Large-Scale Fermentations of Streptomyces sp. RKND004, Extraction and Isolation
3.5. X-ray Crystallography
3.6. Antimicrobial Assays
3.7. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Thoughts and facts about antibiotics. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Zarins-Tutt, J.S.; Barberi, T.T.; Gao, H.; Mearns-Spragg, A.; Zhang, L.; Newman, D.J.; Goss, R.J.M. Prospecting for new bacterial metabolites: A glossary of approaches for inducing, activating and upregulating the biosynthesis of bacterial cryptic or silent natural products. Nat. Prod. Rep. 2016, 33, 54–72. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Harrison, W.T.; Deng, H.; Okoro, C.K.; Asenjo, J.A.; Andrews, B.A.; Bull, A.T.; Goodfellow, M.; Ebel, R.; et al. Diverse metabolic profiles of a Streptomyces strain isolated from a hyper-arid environment. J. Nat. Prod. 2011, 74, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395–406. [Google Scholar] [CrossRef]
- Dutton, C.J.; Banks, B.J.; Cooper, C.B. Polyether ionophores. Nat. Prod. Rep. 1995, 12, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.; Brzezinski, B. Structures and properties of naturally occurring polyether antibiotics. BioMed. Res. Int. 2013, 2013, 1–31. [Google Scholar] [CrossRef]
- Huczyński, A. Polyether ionophores-promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-F.; Li, C.-X.; Liu, Z.-Q.; Ma, S.; Chen, H.-B. Cancer stem cell targets—A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2045–2051. [Google Scholar] [PubMed]
- Cullen, W.P.; Oscarson, J.R.; Tone, J.; Maeda, H.U.S. Inophore Antibacterial Agent from Streptomyces. U.S. Patent 4751317A, 14 June 1988. [Google Scholar]
- Cullen, W.P.; Oscarson, J.R.; Tone, J.; Maeda, H.U.S. Streptomyces sp. N664-30 Which Produces an Ionophore Antibacterial Agent. U.S. Patent 4908316A, 13 March 1990. [Google Scholar]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Liu, W.; Shen, B. Genes for production of the enediyne antitumor antibiotic C-1027 in Streptomyces globisporus are clustered with the cagA gene that encodes the C-1027 apoprotein. Antimicrob. Agents Chemother. 2000, 44, 382–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagaoka, K.; Matsumoto, M.; Oono, J.; Yokoi, K.; Ishizeki, S.; Nakashima, T. Azinomycins A and B, new antitumor antibiotics. I. Producing organism, fermentation, isolation and characterization. J. Antibiot. 1986, 39, 1527–1532. [Google Scholar] [CrossRef]
- Graziani, E.I.; Ritacco, F.V.; Bernan, V.S.; Telliez, J.-B. Phaeochromycins A–E, anti-inflammatory polyketides isolated from the soil actinomycete Streptomyces phaeochromogenes LL-P018. J. Nat. Prod. 2005, 68, 1262–1265. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Media for Isolation-Cultivation-Maintenance of Medical baCteria; Williams and Wilkins Co.: Baltimore, MD, USA, 1985; Volume 1. [Google Scholar]
- Bushnell, L.D.; Haas, H.F. The utilization of certain hydrocarbons by microorganisms. J. Bacteriol. 1941, 41, 653–673. [Google Scholar]
- ZoBell, C.E. Studies on marine bacteria. I. The cultural requirements of heterotrophic aerobes. J. Mar. Res. 1941, 4, 42–75. [Google Scholar]
- Fernández, E.; Weissbach, U.; Sànchez Reillo, C.; Braña, A.F.; Méndez, C.; Rohr, J.; Salas, J.A. Identification of two genes from Streptomces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J. Bacteriol. 1998, 180, 4929–4937. [Google Scholar]
- Maranesi, G.L.; Baptista-Neto, A.; Hokka, C.O.; Badino, A. Utilization of vegetable oil in the production of clavulanic acid by Streptomyces clavuligerus ATCC 27064. World J. Microbiol. Biotechnol. 2005, 21, 509–514. [Google Scholar] [CrossRef]
- Jensen, P.R.; Williams, P.G.; Oh, D.-C.; Zeigler, L.; Fenical, W. Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl. Environ. Microbiol. 2007, 73, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Moita, C.; Feio, S.S.; Nunes, L.; Curto, M.J.M.; Roseiro, J.C. Optimisation of physical factors on the production of active metabolites by Bacillus subtilis 355 against wood surface contaminant fungi. Int. Biodeterior. Biodegrad. 2005, 55, 261–269. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Yang, S.-Z.; Mu, B.-Z. Production and characterization of a C15-surfactin-O-methyl ester by a lipopeptide producing strain Bacillus subtilis HSO121. Process. Biochem. 2009, 44, 1144–1151. [Google Scholar] [CrossRef]
- Forner, D.; Berrué, F.; Correa, H.; Duncan, K.; Kerr, R.G. Chemical dereplication of marine actinomycetes by liquid chromatography-high resolution mass spectrometry profiling and statistical analysis. Anal. Chim. Acta 2013, 805, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Laatsch, H. AntiBase 2014: The Natural Compound Identifier; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Liu, C.; Hermann, T.E. Characterization of ionomycin as a calcium ionophore. J. Biol. Chem. 1978, 253, 5892–5894. [Google Scholar]
- Liu, C.M.; Hermann, T.E.; Downey, A.; Prosser, B.L.; Schildknecht, E.; Palleroni, N.J.; Westley, J.W.; Miller, P.A. Novel polyether antibiotics X-14868A, B, C, and D produced by a Nocardia. Discovery, fermentation, biological as well as ionophore properties and taxonomy of the producing culture. J. Antibiot. 1983, 36, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, G.A.; Canfield, N.; Leese, R.A.; Morton, G.O.; James, J.C.; Siegel, M.M.; McGahren, W.J. Chemistry of maduramicin I. Salt formation and normal ketalization. J. Antibiot. 1986, 39, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T.; King, H.D.; Kuhn, M. Mutalomycin, a new polyether antibiotic taxonomy, fermentation, isolation and characterization. J. Antibiot. 1977, 30, 903–907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cullen, W.P.; Maeda, H.; Ruddock, J.C.; Tone, J.U.S. Polycyclic Ether Antibiotic. U.S. Patent 4746650, 24 May 1988. [Google Scholar]
- Kevin, D.A., II; Meujo, D.A.F.; Hamann, M.T. Polyether ionophores: Broad spectrum and promising biologically active molecules for the control of drug resistant bacteria and parasites. Expert Opin. Drug Discov. 2009, 4, 106–146. [Google Scholar] [CrossRef] [PubMed]
- Guyot, J.; Jeminet, G.; Prudhomme, M.; Sancelme, M.; Meiniel, R. Interaction of the calcium ionophore A.23187 (Calcimycin) with Bacillus cereus and Escherichia coli. Lett. Appl. Microbiol. 1993, 16, 192–195. [Google Scholar] [CrossRef]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Ketola, K.; Vainio, P.; Fey, V.; Kallioniemi, O.; Iljin, K. Monensin is a potent inducer of oxidative stress and inhibitor of androgen signaling leading to apoptosis in prostate cancer cells Mol. Cancer Ther. 2010, 9, 3175–3185. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-C.; Liang, Y.; Wu, M.-S.; Feng, F.-T.; Hu, W.-R.; Chen, L.-Z.; Feng, Q.-S.; Bei, J.-X.; Zeng, Y.-X. Nigericin selectively targets cancer stem cells in nasopharyngeal carcinoma. Int. J. Biochem. Cell Biol. 2013, 45, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-M.; Dong, T.-T.; Wang, L.-L.; Feng, B.; Zhao, H.-C.; Fan, X.-K.; Zheng, M.-H. Suppression of colorectal cancer metastasis by nigericin through inhibition of epithelial-mesenchymal transition. World J. Gastroenterol. 2012, 18, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Liffers, S.T.; Tilkorn, D.J.; Stricker, I.; Junge, C.G.; Al-Benna, S.; Vogt, M.; Verdoodt, B.; Steinau, H.U.; Tannapfel, A.; Tischoff, I.; et al. Salinomycin increases chemosensitivity to the effects of doxorubicin in soft tissue sarcomas. BMC Cancer 2013, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Kim, C.-J.; Chun, J.; Koh, Y.-H.; Lee, S.-H.; Hyun, J.-W.; Cha, C.-Y.; Kook, Y.-H. Phylogenetic analysis of the genera Streptomyces and Kitasatospora based on partial RNA polymerase B-subunit gene (rpoB) sequences. Int. J. Syst. Evol. Microbiol. 2004, 54, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Gontang, E.; Fenical, W.; Jensen, P.R. Phylogenetic diversity of gram-positive bacteria cultured from marine sediments. Appl. Envrion. Microbiol. 2007, 73, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Blast (basic local alignment search tool). J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- SAINT 7.32A.; Bruker AXS Inc.: Madison, WI, USA, 2006.
- SADABS 2008; George Sheldrick, Bruker AXS, Inc.: Madison, WI, USA, 2008.
- Sheldrick, G.M. SHELXTL. Acta. Cryst. 2008, A64, 12–122. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
| No. | Terrosamycin A (1) | Terrosamycin B (2) | ||||
|---|---|---|---|---|---|---|
| δC, Type | δH, (J, Hz) | HMBC | δC, Type | δH, (J, Hz) | HMBC | |
| 1 | 183.6, C | 179.0, C | ||||
| 2 | 44.5, CH | 2.58, dq (4.4, 7.2, 11.0) | 1, 3, 44 | 42.6, CH | 2.64, dq (3.1, 6.8, 9.7) | 1, 3, 44 |
| 3 | 86.2, CH | 3.66, m | 1, 2, 4, 5, 43, 44 | 84.5, CH | 3.45, dd (3.0, 9.8) | 1, 2, 5, 43, 44 |
| 4 | 33.4, CH | 1.51, m | 3, 5, 43, 44 | 33.6, CH | 1.45, m | 3, 43 |
| 5 | 33.5, CH2 | 1.47, m | 3, 4, 44 | 34.2, CH2 | 1.53, m | 4 |
| 1.28, m | 3, 7, 43 | 1.21, m | 3, 4, 7 | |||
| 6 | 35.2, CH2 | 1.86, m | 3, 5, 7 | 34.3, CH2 | 1.80, m | 3, 7 |
| 1.28, m | 7 | 1.27, m | 5 | |||
| 7 | 75.5, CH | 3.67, m | 3, 6, 8, 9 | 75.3, CH | 3.56, appt a (10.0) | 3, 6, 8, 9 |
| 8 | 39.9, CH2 | 1.67, m | 7, 9, 10 | 41.1, CH2 | 1.62, m | 7, 9 |
| 1.15, m | 6, 7, 10 | 1.34, m | 9 | |||
| 9 | 71.5, CH | 3.65, m | 7, 8, 11, 42 | 73.7, CH | 3.32, td (1.7, 3.3) | 7, 8, 11, 42 |
| 10 | 37.7, CH | 1.29, m | 9, 11, 42 | 36.8, CH | 1.28, m | 11 |
| 11 | 38.6, CH2 | 1.37, m | 10, 12, 13, 41 | 38.6, CH2 | 1.42, m | 13, 9 |
| 1.30, m | 9, 10, 12, 13, 41 | 1.30, m | 9, 14, 41 | |||
| 12 | 40.4, CH | 1.49, m | 11, 41 | 37.0, CH | 1.98, m | 11, 41 |
| 13 | 98.8, C | 102.0, C | ||||
| 14 | 44.2, CH2 | 1.82, dd (7.9, 14.8) | 12, 13, 15, 16 | 38.4, CH2 | 1.92, m | 12, 13, 15, 16 |
| 1.66, m | 13, 16 | 1.83, m | 12, 13, 16 | |||
| 15 | 72.9, CH | 4.10, dd (8.2, 10.1) | 13, 14, 17, 40 | 74.2, CH | 3.38, m | 13, 14, 17, 40 |
| 16 | 36.9, CH | 1.40, m | 13, 15, 17, 40 | 37.1, CH | 1.48, m | 40 |
| 17 | 38.0, CH2 | 1.48, m | 15, 16, 18, 19, 39, 40 | 38.2, CH2 | 1.37, m | 18, 39, 40 |
| 1.29, m | 18, 19, 39, 40 | |||||
| 18 | 40.2, CH | 1.72, m | 17, 19, 20, 39 | 39.5, CH | 1.89, m | 17, 39 |
| 19 | 101.3, C | 102.6, C | ||||
| 20 | 43.1, CH2 | 2.08, dd (1.2, 14.1) | 18, 19, 21, 22 | 39.7, CH2 | 2.08, m | 18, 19, 21, 22 |
| 1.57, dd (10.5, 3.2) | 18, 19, 21, 22 | 1.64, m | 18, 19, 21, 22 | |||
| 21 | 74.8, CH | 4.22, appt a (10.0) | 19, 20, 22, 23, 38 | 72.3, CH | 4.03, appt a (8.5) | 18, 19, 20, 22, 23, 38 |
| 22 | 45.6, CH | 3.5, dq (6.7, 9.8, 13.6) | 20, 21, 23, 38 | 47.8, CH | 3.20, dq (5.5, 6.8, 13.8) | 19, 20, 21, 23, 38 |
| 23 | 220.7, C | 218.0, C | ||||
| 24 | 79.9, C | 80.9, C | ||||
| 25 | 84.8, CH | 4.47, dd (5.6, 10.4) | 23, 24, 26, 27, 37 | 84.8, CH | 4.24, dd (6.0, 8.8) | 23, 24, 26, 27, 37 |
| 26 | 25.8, CH2 | 2.05, m | 24, 25, 27, 28 | 26.7, CH2 | 2.01, m | 24 |
| 1.86, m | 24, 27, 28 | 1.93, m | 24, 27 | |||
| 27 | 33.7, CH2 | 2.05, m | 25, 26, 28, 29, 36 | 34.9, CH2 | 2.08, m | 26, 28, 29, 36 |
| 1.65, m | 25, 26, 28, 36 | 1.63, m | 25, 26, 36 | |||
| 28 | 86.6, C | 86.8, C | ||||
| 29 | 74.9, CH | 3.56, dd (2.3, 11.8) | 27, 28, 30, 31, 33, 36 | 75.0, CH | 3.40, m | 27, 28, 30, 33, 36 |
| 30 | 22.0, CH2 | 1.90, m | 28, 29, 31, 32 | 22.9, CH2 | 1.67, m | |
| 1.60, m | 28, 29, 31, 32 | 1.42, m | 32 | |||
| 31 | 31.8, CH2 | 1.76, td (4.5, 13.5) | 29, 30, 32, 33, 35 | 32.5, CH2 | 1.71, m | 30 |
| 1.66, m | 29, 30, 32, 33, 35 | 1.60, m | 32, 33 | |||
| 32 | 70.3, C | 70.6, C | ||||
| 33 | 79.2, CH | 4.08, q (6.5, 13.7) | 29, 31, 32, 34, 35 | 79.0, CH | 3.72, q (6.6, 13.5) | 29, 34, 35 |
| 34 | 16.1, CH3 | 1.23, d (6.9) | 32, 33 | 15.7, CH3 | 1.19, d (7.3) | 32, 33 |
| 35 | 27.2, CH3 | 1.11, s | 31, 32, 33 | 26.6, CH3 | 1.03, s | 31, 32, 33 |
| 36 | 26.3, CH3 | 1.22, s | 27, 28, 29 | 23.4, CH3 | 1.12, s | 27, 28, 29 |
| 37 | 21.1, CH3 | 1.10, s | 23, 24, 25 | 21.6, CH3 | 1.23, s | 23, 24, 25 |
| 38 | 15.0, CH3 | 0.99, d (6.4) | 21, 22, 23 | 15.6, CH3 | 1.12, d (6.7) | 21, 22, 23 |
| 39 | 17.0, CH3 | 0.91, d (6.9) | 17, 18, 19, 20 | 17.0, CH3 | 0.88, d (6.5) | 17, 18, 19, 20 |
| 40 | 18.7, CH3 | 0.84, d (6.4) | 14, 15, 16, 17 | 19.1, CH3 | 0.86, d (6.5) | 14, 15, 16, 17 |
| 41 | 17.2, CH3 | 0.87, d (6.7) | 10, 11, 12, 13, 14 | 17.2, CH3 | 0.87, d (6.2) | 10, 11, 12, 13, 14 |
| 42 | 18.0, CH3 | 0.78, d (6.2) | 9, 10, 11 | 18.4, CH3 | 0.79, d (5.5) | 9, 10, 11 |
| 43 | 17.5, CH3 | 0.87, d (6.7) | 3, 4, 5, 6 | 18.0, CH3 | 0.84, d (6.8) | 3, 4, 5, 6 |
| 44 | 10.7, CH3 | 1.08, d (7.2) | 1, 2, 3 | 9.7, CH3 | 1.08, d (7.6) | 1, 2, 3 |
| 45 | 47.7, CH3 | 3.14, s | 13 | |||
| 46 | 50.0, CH3 | 3.27, s | 19 | |||
| Compound | MRSA | VRE | S. warneri |
|---|---|---|---|
| terrosamycin A (1) | 0.5 ± 0.2 | 0.58 ± 0.04 | 0.9 ± 0.3 |
| terrosamycin B (2) | 0.34 ± 0.04 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| salinomycin (3) | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.2 |
| monensin (4) | 1.5 ± 0.1 | 8 ± 5 | 2.0 ± 0.2 |
| nigericin (5) | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| vancomycin control | 0.8 ± 0.1 | - | 0.53 ± 0.01 |
| rifampicin control | - | 1.1 ± 0.1 | - |
| Compound | HTB-26 | MCF-7 | Vero | BJ |
|---|---|---|---|---|
| terrosamycin A (1) | 10.1 ± 0.6 | 6 ± 1 | 36 ± 6 | 79 ± 5 |
| terrosamycin B (2) | 6 ± 1 | 3.9 ± 0.4 | 93 ± 4 | 267 ± 28 |
| salinomycin (3) | 13 ± 1 | 10 ± 3 | 78 ± 22 | 196 ± 3 |
| monensin (4) | 10 ± 1 | 7 ± 2 | 1E4 ± 1E3 | 245 ± 11 |
| nigericin (5) | 13 ± 2 | 9 ± 3 | 61 ± 8 | 221 ± 10 |
| doxorubicin control | 4 ± 2 | 0.8 ± 0.1 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sproule, A.; Correa, H.; Decken, A.; Haltli, B.; Berrué, F.; Overy, D.P.; Kerr, R.G. Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment. Mar. Drugs 2019, 17, 347. https://doi.org/10.3390/md17060347
Sproule A, Correa H, Decken A, Haltli B, Berrué F, Overy DP, Kerr RG. Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment. Marine Drugs. 2019; 17(6):347. https://doi.org/10.3390/md17060347
Chicago/Turabian StyleSproule, Amanda, Hebelin Correa, Andreas Decken, Bradley Haltli, Fabrice Berrué, David P. Overy, and Russell G. Kerr. 2019. "Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment" Marine Drugs 17, no. 6: 347. https://doi.org/10.3390/md17060347
APA StyleSproule, A., Correa, H., Decken, A., Haltli, B., Berrué, F., Overy, D. P., & Kerr, R. G. (2019). Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment. Marine Drugs, 17(6), 347. https://doi.org/10.3390/md17060347





