Fucoidan Prevents RANKL-Stimulated Osteoclastogenesis and LPS-Induced Inflammatory Bone Loss via Regulation of Akt/GSK3β/PTEN/NFATc1 Signaling Pathway and Calcineurin Activity
Abstract
1. Introduction
2. Results
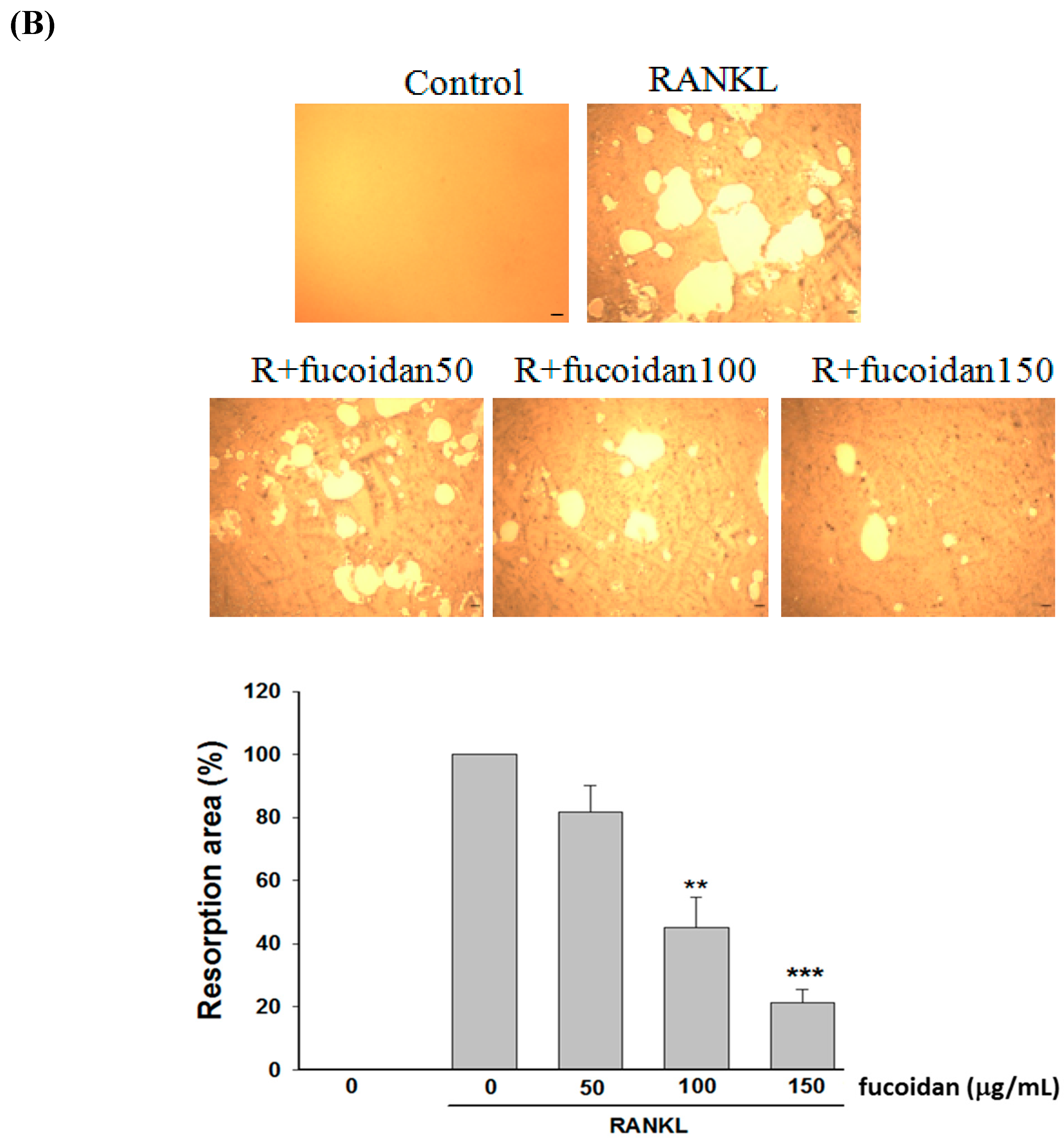
2.1. Fucoidan Inhibited RANKL-Induced Osteoclast Differentiation and Bone Resorbing Activity
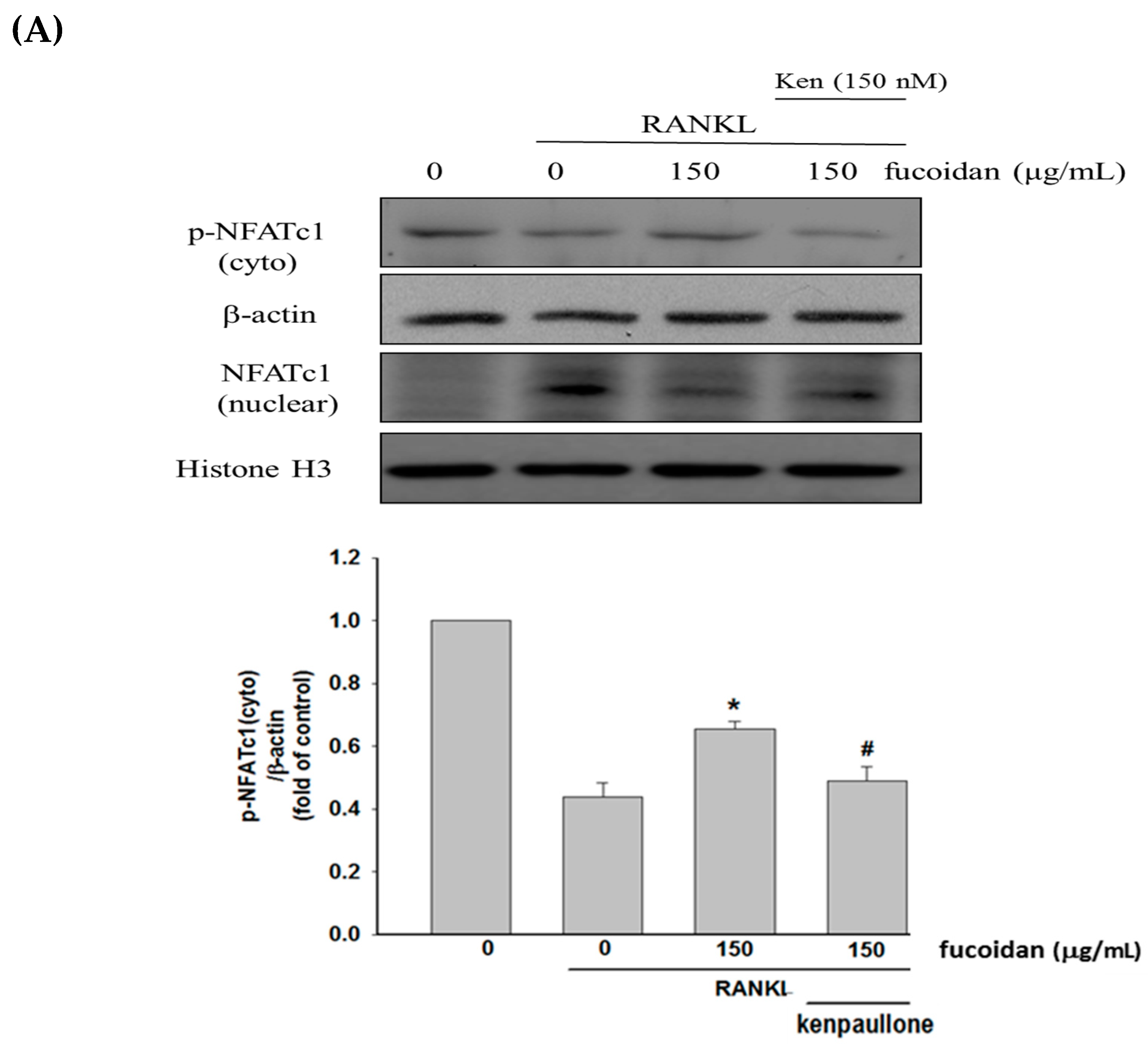
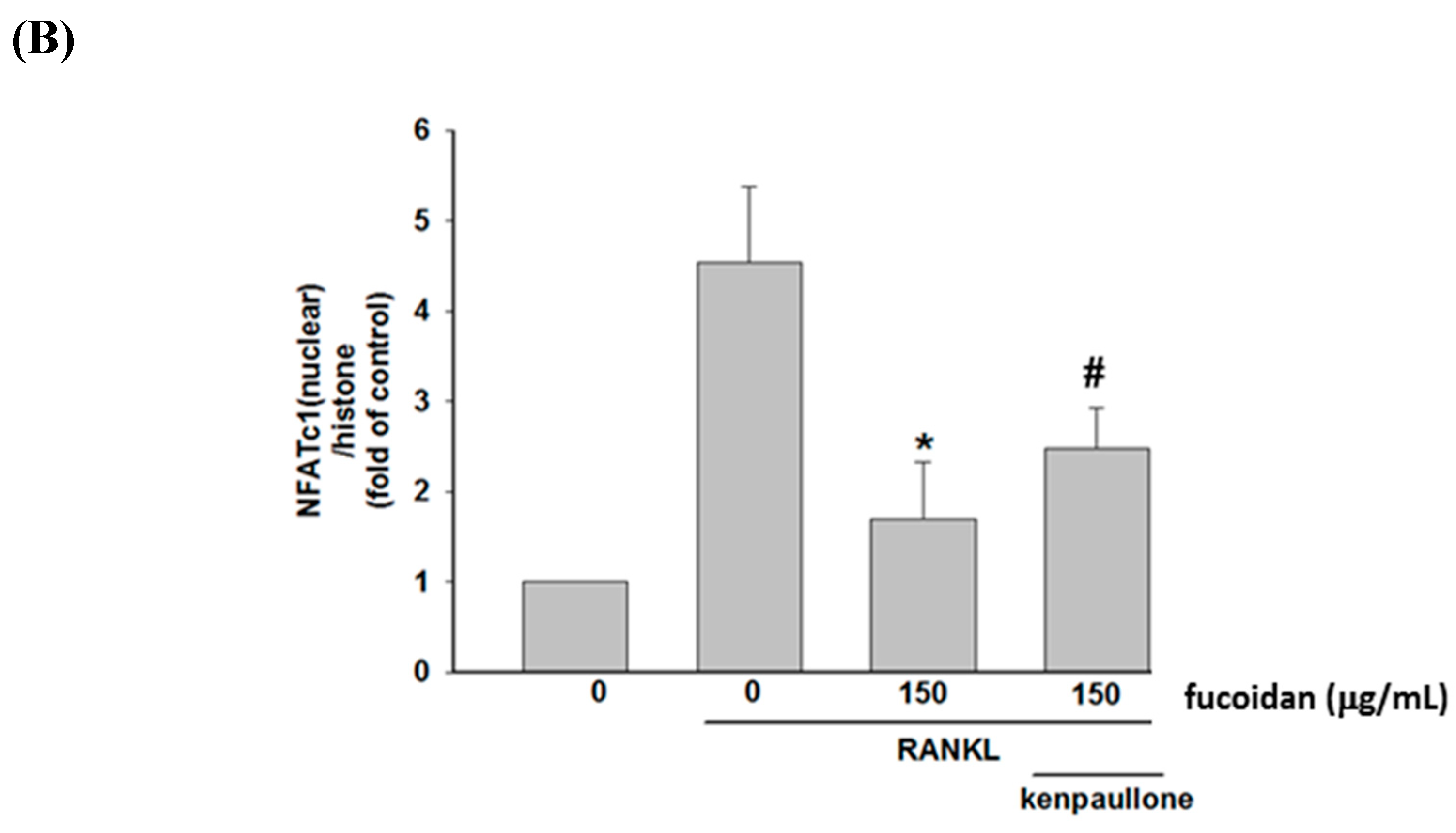
2.2. Fucoidan Inhibited RANKL-Activated NFATc1 Nucleotranslocation
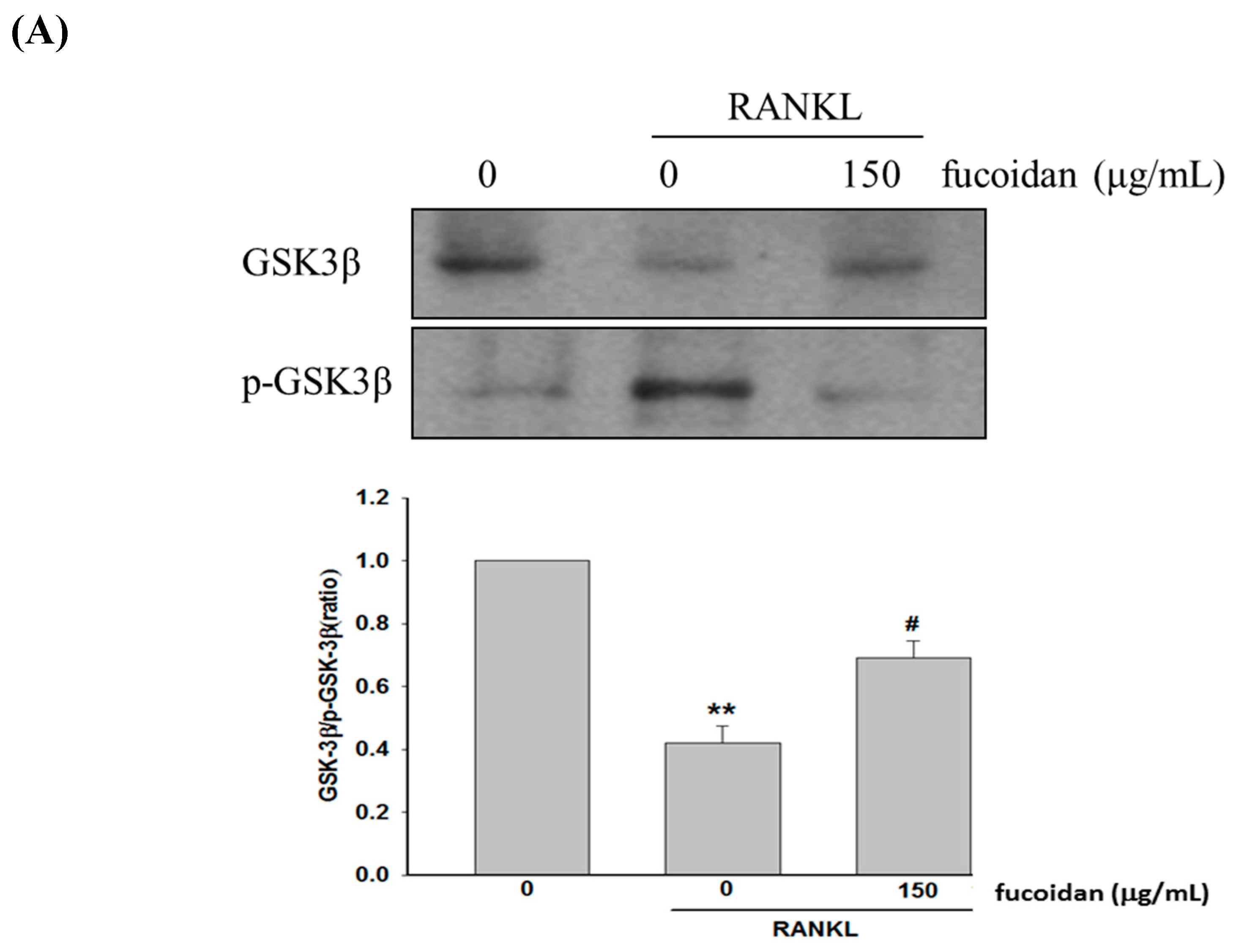
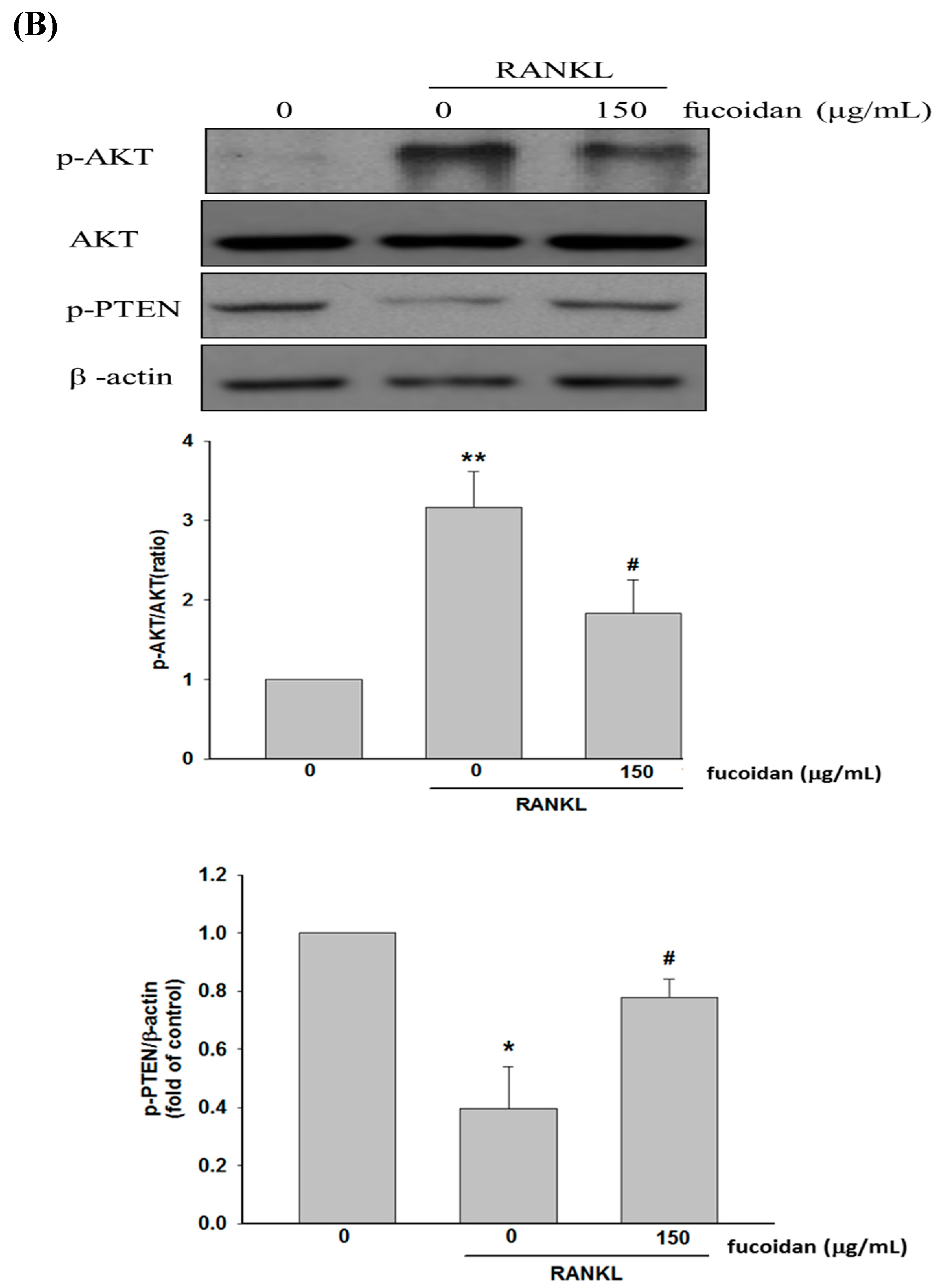
2.3. Fucoidan Regulated the Akt/GSK3β/PTEN Cascade
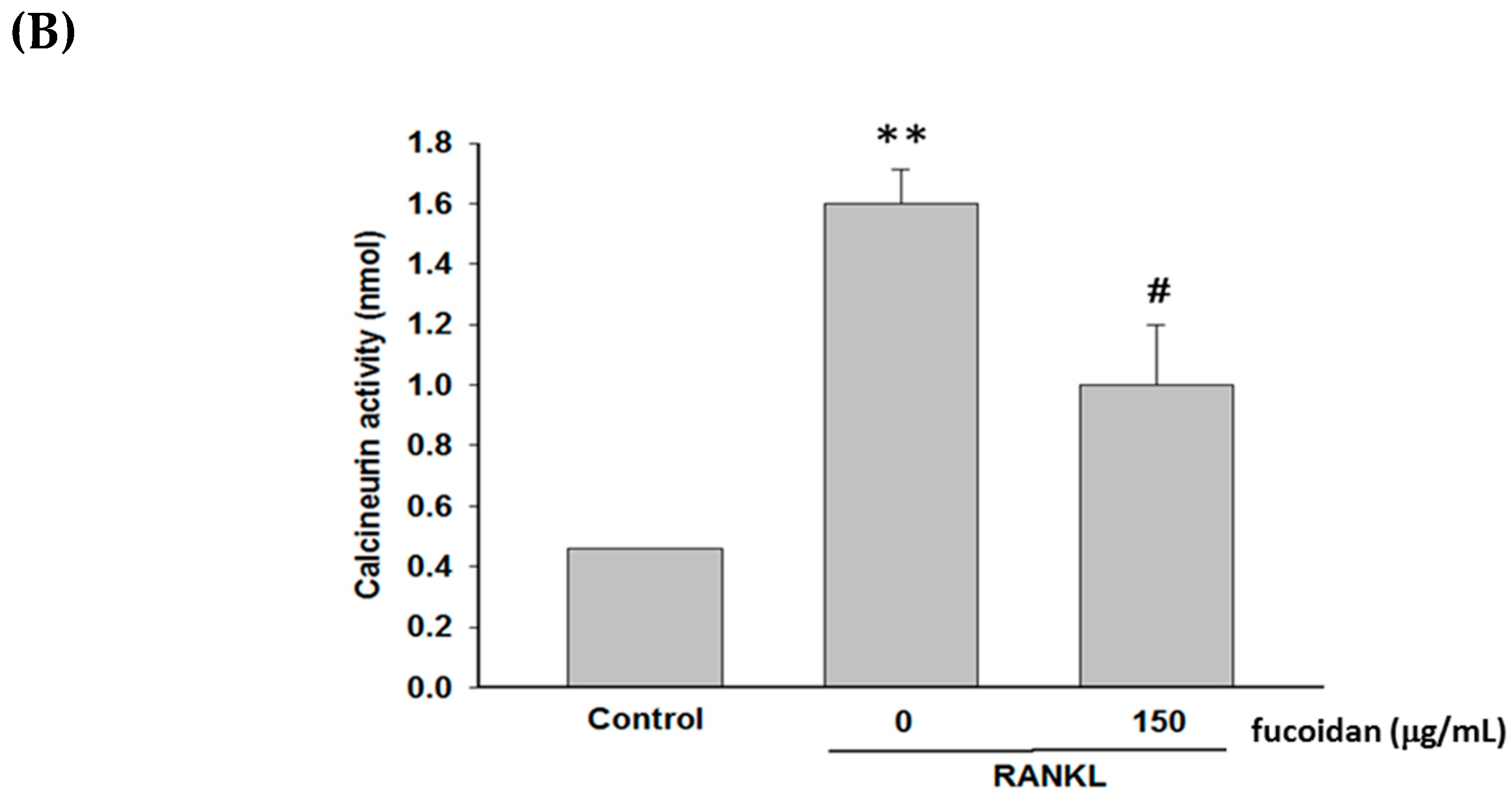
2.4. Fucoidan Inhibited RANKL-Evoked [Ca2+]i and Calcineurin Activity
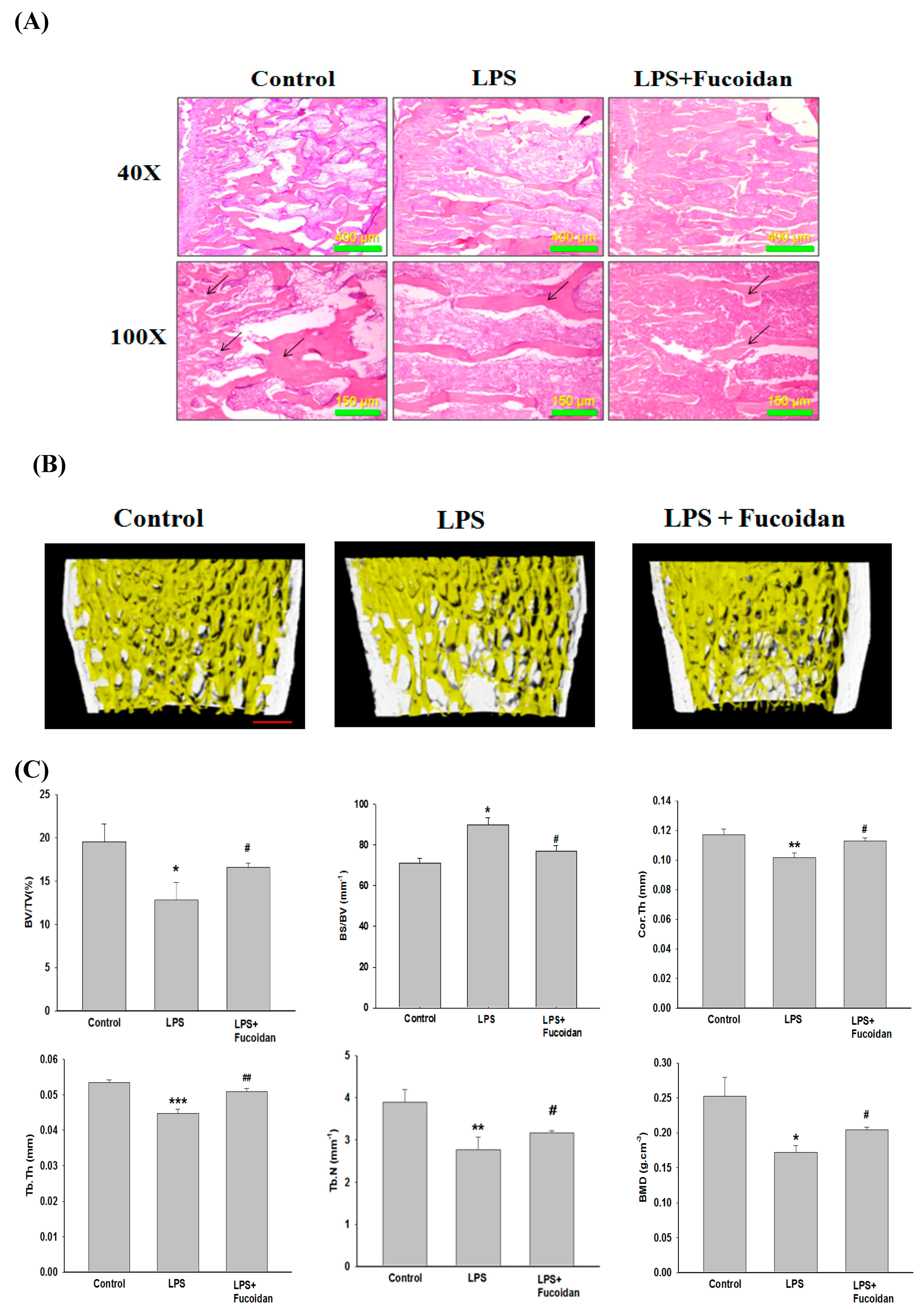
2.5. Fucoidan Prevented Lipopolysaccharide (LPS)-Induced Bone Loss in Mice
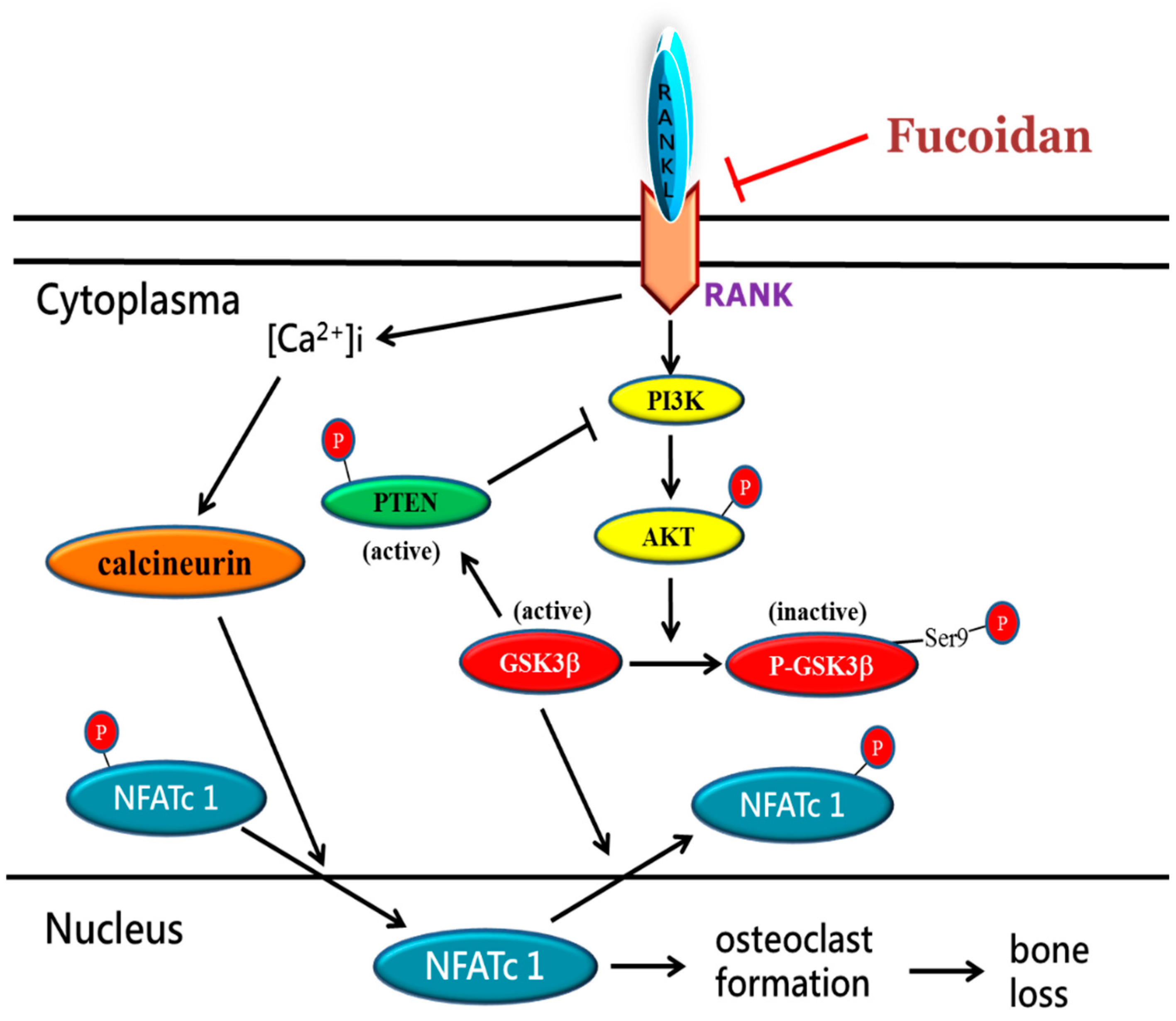
3. Discussion
4. Materials and Methods
4.1. Reagents and Fucoidan Preparation
4.2. Cell Culture and Cell Viability Assay
4.3. TRAP Staining and Activity
4.4. Pit Formation Assay
4.5. Western Blotting
4.6. Analysis of Intracellular Free Ca2+ Oscillation
4.7. Calcineurin Activity Assay
4.8. LPS-Induced Bone Erosion in Mouse Femurs
4.9. Bone Morphometric Analyses
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Ueki, Y.; Sulyanto, R.; Park, A.; Sigrist, K.S.; Sharma, S.M.; Ostrowski, M.C.; Olsen, B.R.; Glimcher, L.H. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Investig. 2008, 118, 3775–3789. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, G.R. Generic signals and specific outcomes: Signaling through Ca2+, calcineurin, and NF-AT. Cell 1999, 96, 611–614. [Google Scholar] [CrossRef]
- Hirotani, H.; Tuohy, N.A.; Woo, J.T.; Stern, P.H.; Clipstone, N.A. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Beals, C.R.; Sheridan, C.M.; Turck, C.W.; Gardner, P.; Crabtree, G.R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 1997, 275, 1930–1934. [Google Scholar] [CrossRef]
- Jang, H.D.; Shin, J.H.; Park, D.R.; Hong, J.H.; Yoon, K.; Ko, R.; Ko, C.Y.; Kim, H.S.; Jeong, D.; Kim, N.; et al. Inactivation of glycogen synthase kinase-3beta is required for osteoclast differentiation. J. Biol. Chem. 2011, 286, 39043–39050. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.B.; Kim, J.H.; Kim, K.; Youn, B.U.; Ko, A.; Lee, S.Y.; Kim, N. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J. Immunol. 2012, 188, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.D.; Noh, J.Y.; Shin, J.H.; Lin, J.J.; Lee, S.Y. PTEN regulation by the Akt/GSK-3β axis during RANKL signaling. Bone 2013, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Pharmacotherapy of osteoporosis in postmenopausal women: Focus on safety. Expert Opin. Drug Saf. 2002, 1, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Kim, Y.W.; Baek, S.H.; Lee, S.H.; Kim, T.H.; Kim, S.Y. Fucoidan, a sulfated polysaccharide, inhibits osteoclast differentiation and function by modulating RANKL signaling. Int. J. Mol. Sci. 2014, 15, 18840–18855. [Google Scholar] [CrossRef]
- Kubota, T.; Hoshino, M.; Aoki, K.; Ohya, K.; Komano, Y.; Nanki, T.; Miyasaka, N.; Umezawa, K. NF-kappaB inhibitor dehydroxymethylepoxyquinomicin suppresses osteoclastogenesis and expression of NFATc1 in mouse arthritis without affecting expression of RANKL, osteoprotegerin or macrophage colony-stimulating factor. Arthritis Res. Ther. 2007, 9, R97. [Google Scholar] [CrossRef]
- Purdue, P.E.; Koulouvaris, P.; Potter, H.G.; Nestor, B.J.; Sculco, T.P. The cellular and molecular biology of periprosthetic osteolysis. Clin. Orthop. Relat. Res. 2007, 454, 251–261. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Abu-Amer, Y.; Nelson, C.A.; Fremont, D.H.; Ross, F.P.; Teitelbaum, S.L. Tumour necrosis factor superfamily cytokines and the pathogenesis of inflammatory osteolysis. Ann. Rheum. Dis. 2002, 61 (Suppl. 2), ii82–ii83. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Murphy, C.; Kirstein, B.; Fox, S.W.; Chambers, T.J. TNF alpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 2002, 143, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Cho, M.L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006, 54, 2152–2163. [Google Scholar] [CrossRef]
- Suda, K.; Woo, J.T.; Takami, M.; Sexton, P.M.; Nagai, K. Lipopolysaccharide supports survival and fusion of preosteoclasts independent of TNF-alpha, IL-1, and RANKL. J. Cell. Physiol. 2002, 190, 101–108. [Google Scholar] [CrossRef]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef]
- Hou, G.Q.; Guo, C.; Song, G.H.; Fang, N.; Fan, W.J.; Chen, X.D.; Yuan, L.; Wang, Z.Q. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX-2 expression in RAW264.7 cells. Int. J. Mol. Med. 2013, 32, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Hung, Y.L.; Phan, N.N.; Hieu, B.T.N.; Chang, P.M.; Li, K.L. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of low molecular weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of Lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Zhai, Z.J.; Li, H.W.; Liu, G.W.; Qu, X.H.; Tian, B.; Yan, W.; Lin, Z.; Tang, T.T.; Qin, A.; Dai, K.R. Andrographolide suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Br. J. Pharmacol. 2014, 171, 663–675. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-H.; Hsia, Y.-J.; Shih, K.-C.; Chou, T.-C. Fucoidan Prevents RANKL-Stimulated Osteoclastogenesis and LPS-Induced Inflammatory Bone Loss via Regulation of Akt/GSK3β/PTEN/NFATc1 Signaling Pathway and Calcineurin Activity. Mar. Drugs 2019, 17, 345. https://doi.org/10.3390/md17060345
Lu S-H, Hsia Y-J, Shih K-C, Chou T-C. Fucoidan Prevents RANKL-Stimulated Osteoclastogenesis and LPS-Induced Inflammatory Bone Loss via Regulation of Akt/GSK3β/PTEN/NFATc1 Signaling Pathway and Calcineurin Activity. Marine Drugs. 2019; 17(6):345. https://doi.org/10.3390/md17060345
Chicago/Turabian StyleLu, Sheng-Hua, Yi-Jan Hsia, Kuang-Chung Shih, and Tz-Chong Chou. 2019. "Fucoidan Prevents RANKL-Stimulated Osteoclastogenesis and LPS-Induced Inflammatory Bone Loss via Regulation of Akt/GSK3β/PTEN/NFATc1 Signaling Pathway and Calcineurin Activity" Marine Drugs 17, no. 6: 345. https://doi.org/10.3390/md17060345
APA StyleLu, S.-H., Hsia, Y.-J., Shih, K.-C., & Chou, T.-C. (2019). Fucoidan Prevents RANKL-Stimulated Osteoclastogenesis and LPS-Induced Inflammatory Bone Loss via Regulation of Akt/GSK3β/PTEN/NFATc1 Signaling Pathway and Calcineurin Activity. Marine Drugs, 17(6), 345. https://doi.org/10.3390/md17060345



