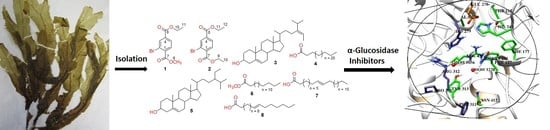
α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii
Abstract
1. Introduction
2. Results and Discussion
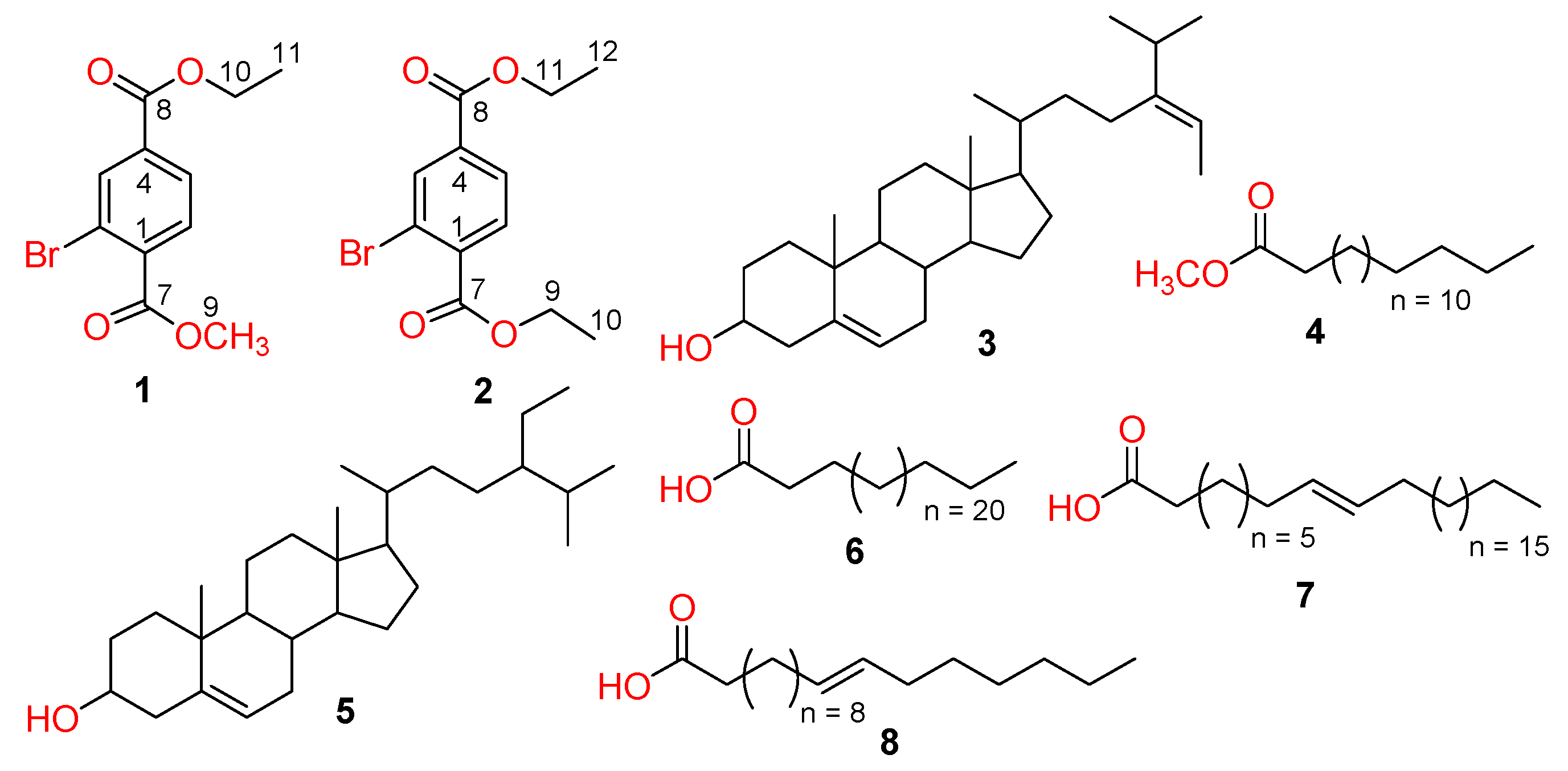
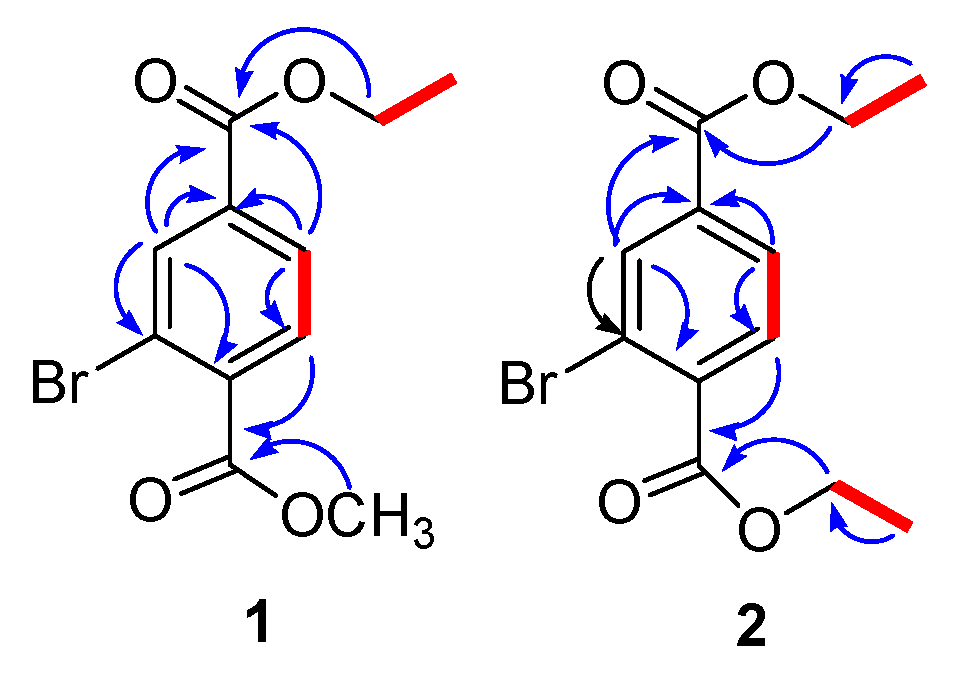
2.1. Structure Elucidation of Compounds 1–8
2.2. α-Glucosidase Inhibition
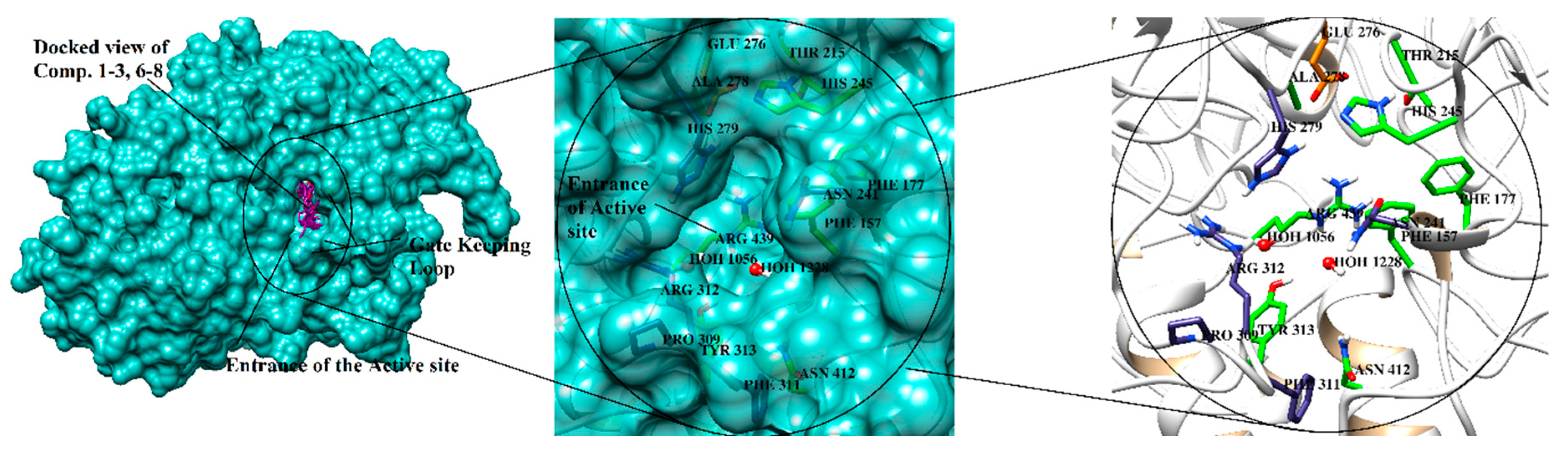
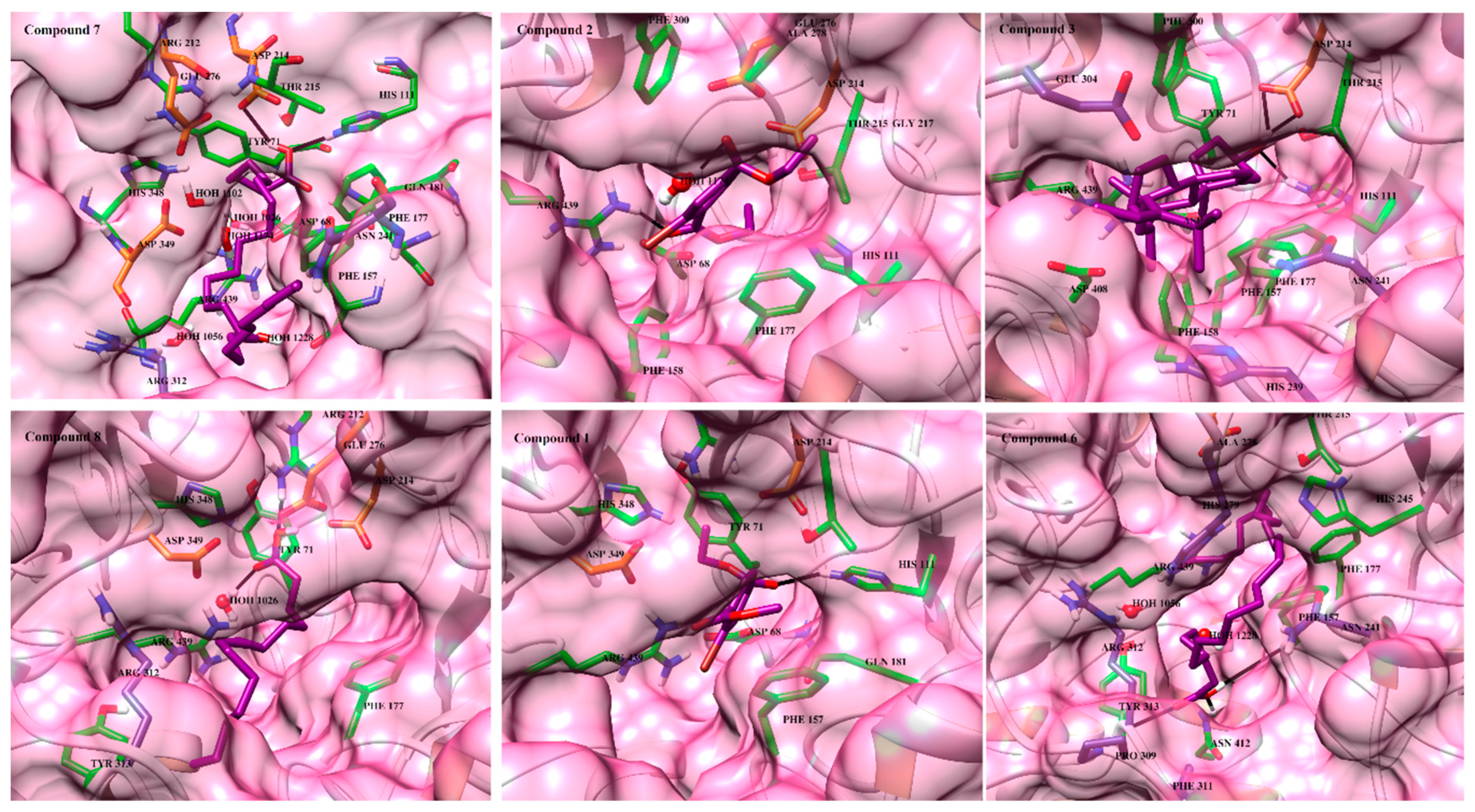
2.3. Molecular Docking of α-Glucosidase Inhibitors
2.4. Pharmakinetic Prediction of α-Glucosidase Inhibitors
3. Material and Methods
3.1. General
3.2. Sample Collection and Identification
3.3. Extraction, Isolation and Purification
3.4. In Vitro α-Glucosidase Inhibition
3.5. Molecular Docking and Pharmacokinetic Prediction
3.6. Pharmacophore Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Global Health Estimates: Deaths by Cause, Age, Sex and Country,2000–2012; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Rehman, N.U.; Khan, A.; Al-Harrasi, A.; Hussain, H.; Wadood, A.; Riaz, M.; Al-Abri, Z. New α-glucosidase inhibitors from the resins of Boswellia species with structure–glucosidase activity and molecular docking studies. Bioorg. Chem. 2018, 79, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Nam, K.A.; Kurihara, H.; Kim, S.M. Potent alpha-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Montgomery, P.A. Acarbose: An α-glucosidase inhibitor. American Journal of Health System Pharmacy. Am. J. Health. Syst. Pharm. 1996, 53, 2277–2290. [Google Scholar] [CrossRef] [PubMed]
- Chougale, A.D.; Ghadyale, V.A.; Panaskar, S.N.; Arvindekar, A.U. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enyzm. Inhib. Med. Chem. 2009, 24, 998–1001. [Google Scholar] [CrossRef]
- Koren, S.; Fantus, I.G. Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 621–640. [Google Scholar] [CrossRef]
- Shi, D.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L. Inhibition of bromophenols against PTP1B and anti-hyperglycemic effect of Rhodomela confervoides extract in diabetic rats. Chin. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef]
- Fisch, K.M.; Bohm, V.; Wright, A.D.; Konig, G.M. Antioxidative meroterpenoids from the brown alga Cystoseira crinita. J. Nat. Prod. 2003, 66, 968–975. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. Alpha-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Natural bromophenols from the marine red alga Polysiphonia urceolata (Rhodomelaceae): structural elucidation and DPPH radical-scavenging activity. Bioorg. Med. Chem. 2007, 15, 6627–6631. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Bromophenols from the marine red alga Polysiphonia urceolata with DPPH radical scavenging activity. J. Nat. Prod. 2008, 71, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An overview of odoriferous marine seaweeds of the Dictyopteris genus: insights into their chemical diversity, biological potential and ecological roles. Braz. J. Pharmacog. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- AlgaebBase. Available online: http://www.algaebase.org (accessed on 7 October 2019).
- Feng, M.T.; Wang, T.; Liu, A.H.; Li, J.; Yao, L.G.; Wang, B.; Guo, Y.W.; Mao, S.C. PTP1B inhibitory and cytotoxic C-24 epimers of Δ28-24-hydroxy stigmastane-type steroids from the brown alga Dictyopteris undulata Holmes. Phytochemistry 2018, 146, 25–35. [Google Scholar] [CrossRef]
- Segawa, M.; Yamano, K.; Shirahama, H. A germacrane-type sesquiterpene from the brown alga Dictyopteris divaricata. Phytochemistry 1990, 29, 973–974. [Google Scholar] [CrossRef]
- El Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of various extraction methods for identification and determination of volatile metabolites from the brown alga Dictyopteris membranacea. J. Chromatogr. A. 2007, 1143, 1–7. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, L.W.; Feng, M.T.; Liu, A.H.; Li, J.; Zhao, T.S.; Lai, X.P.; Wang, B.; Guo, Y.W.; Mao, S.C. Dictyoptesterols A–C, ∆ 22-24-oxo cholestane-type sterols with potent PTP1B inhibitory activity from the brown alga Dictyopteris undulata Holmes. Fitoterapia 2018, 130, 241–246. [Google Scholar] [CrossRef]
- Ji, N.Y.; Song, Y.P.; Miao, F.P.; Liang, X.R. Three cadinane derivatives from the marine brown alga Dictyopteris divaricata. Magn. Reson. Chem. 2016, 54, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.; Kang, Q.; Lu, J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int. J. Biol. Macromol. 2018, 117, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dimou, M.; Ioannou, E.; Daskalaki, M.G.; Tziveleka, L.A.; Kampranis, S.C.; Roussis, V. Disulfides with anti-inflammatory activity from the brown alga Dictyopteris membranacea. J. Nat. Prod. 2016, 79, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Fan, X.; Xu, X.; Zhao, J.; Yang, Y.; Shi, J. Cadinane sesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2004, 67, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Wen, W.; Li, X.M.; Xue, Q.Z.; Xiao, H.L.; Wang, B.G. Brominated selinane sesquiterpenes from the marine brown alga Dictyopteris divaricata. Mar. Drugs 2009, 7, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Khan, A.; Al-Broumi, M.; Al-Harrasi, R.; Al-Kharusi, L.; Hussain, J.; Al-Harrasi, A. New enzyme-inhibitory triterpenoid from marine macro brown alga Padina boergesenii Allender & Kraft. Mar. Drugs 2017, 15, E19. [Google Scholar] [PubMed]
- Ali, L.; Khan, A.; Al-Kharusi, L.; Hussain, J.; Al-Harrasi, A. New α-glucosidase inhibitory triterpenic acid from marine macro green alga Codium dwarkense Boergs. Mar. Drugs 2015, 13, 4344–4356. [Google Scholar] [CrossRef]
- Ali, L.; Al-Kharusi, L.; Al-Harrasi, A. Two new sulfonoglycolipids from green alga Codium dwarkense. Nat. Prod. Commun. 2017, 12, 583–585. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Fan, W.; Li, B.; Liu, G.; Liu, X.; Zhao, X. Structural diversity, luminescence and photocatalytic properties of six coordination polymers based on designed bifunctional 2-(imidazol-1-yl) terephthalic acid. CrystEngComm 2016, 18, 6914–6925. [Google Scholar] [CrossRef]
- Huang, X.; Yang, L.; Emanuelsson, R.; Bergquist, J.; Strømme, M.; Sjödin, M.; Gogoll, A. A versatile route to polythiophenes with functional pendant groups using alkyne chemistry. Beilstein J. Org. Chem. 2016, 12, 2682–2688. [Google Scholar] [CrossRef]
- Fast, C.D.; Woods, J.; Lentchner, J.; Makal, T.A. Stabilizing defects in metal–organic frameworks: pendant Lewis basic sites as capping agents in UiO-66-type MOFs toward highly stable and defective porous materials. Dalton Trans. 2019, 48, 14696–14704. [Google Scholar] [CrossRef] [PubMed]
- Aboutabl, E.A.; Zeid, A.H.A.; Sleem, A.A.; El Rafi, H.M. Secondary metabolites and certain bioactivities of Pterocladia capillacea (S. Gmelin) Bornet and Dictyopteris membranacea (Stackhouse) Batters. Med. Aromat. Plant Sci. Biotechnol. 2010, 4, 41–48. [Google Scholar]
- Nyemb, J.N.; Magnibou, L.M.; Talla, E.; Tchinda, A.T.; Tchuenguem, R.T.; Henoumont, C.; Laurent, S.; Mbafor, J.T. Lipids constituents from gardenia aqualla stapf & hutch. Open Chem. 2018, 16, 371–376. [Google Scholar]
- Hwang, S.H.; Jang, J.M.; Lim, S.S. Isolation of fucosterol from Pelvetia siliquosa by high-speed countercurrent chromatography. Fish. Aquat. Sci. 2012, 15, 191–195. [Google Scholar] [CrossRef]
- Kim, I.K.; Park, S.K.; Park, S.H.; Jhang, S.K. Lipids Constituents of the Korean marine sponges. J. Korean Chem. Soc. 1991, 35, 85–89. [Google Scholar]
- Ajoku, G.A.; Okwute, S.K.; Okogun, J.I. Isolation of hexadecanoic acid methyl ester and 1, 1, 2-ethanetricarboxylic acid-1-hydroxy-1, 1-dimethyl ester from the calyx of green Hibiscus sabdariffa (Linn). Nat. Prod. Chem. Res. 2015, 3, 169–174. [Google Scholar]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. SpringerPlus 2016, 5, 1210. [Google Scholar] [CrossRef]
- Ming, Y.; Russell, L.M. Predicted hygroscopic growth of sea salt aerosol. J. Geophys. Res. Atmos. 2001, 106, 28259–28274. [Google Scholar] [CrossRef]
- De Rosaa, S.; Seizova, K.; Kamenarska, Z.; Petrova, A.; Iodicea, C.; Stefanov, K.; Popov, S. Sterol and lipid composition of three adriatic sea sponges. Z. Naturforsch. C. J. Biosci. 2006, 61, 129–134. [Google Scholar] [CrossRef][Green Version]
- Sumitra, S.; Reddu, S.K.; Sharma, S.K.; Mohammad, A. New unsaturated fatty acid from roots of Bougainvillea spectabilis Willd. Asian J. Chem. 2009, 21, 4744–4748. [Google Scholar]
- Ahmad, M.Z.; Ali, M.; Mir, S.R. New phytoconstituents from the roots of Ocimum sanctum L. J. Pharm. Res. 2012, 5, 548–550. [Google Scholar]
- Chang, H.S.; Chen, Y.S.; Cheng, M.J.; Wu, H.C.; Chan, H.Y.; Hsieh, S.Y.; Yang, S.S.; Chen, I.S.; Chen, J.J. Chemical constituents of the fungus Mycoleptodiscus sp. 09F0149. Chem. Nat. Compd. 2018, 54, 396–398. [Google Scholar] [CrossRef]
- Al-Saif, S.S.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of human lysosomal acid α-glucosidase—a guide for the treatment of Pompe disease. Nat. Comm. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pipo, J.R.; Feng, J.H.; Yamamoto, T.; Ohsaki, Y.; Nanba, E.; Tsujino, S.; Sakuragawa, N.; Martiniuk, F.; Ninomiya, H.; Oka, A.; et al. New GAA mutations in Japanese patients with GSDII (Pompe disease). Pediatr. Neurol. 2003, 29, 284–287. [Google Scholar] [CrossRef]
- Hermans, M.M.; Leenen, D.V.; Kroos, M.A.; Beesley, C.E.; Van der Ploeg, A.T.; Sakuraba, H.; Wevers, R.; Kleijer, W.; Michelakakis, H.; Kirk, E.P.; et al. Twenty-two novel mutations in the lysosomal α-glucosidase gene (GAA) underscore the genotype–phenotype correlation in glycogen storage disease type II. Hum. Mutat. 2004, 23, 47–56. [Google Scholar] [CrossRef]
- MOE (Molecular Operating Environment). Chemical Computing Group ULC, 1010 Sherbooke St. West, Suite 910, Montreal, QC, Canada. 2014. [Google Scholar]
- Rizvi, T.S.; Hussain, I.; Ali, L.; Mabood, F.; Khan, A.L.; Shujah, S.; Rehman, N.U.; Al-Harrasi, A.; Hussain, J.; Khan, A.; et al. New gorgonane sesquiterpenoid from Teucrium mascatense Boiss, as α-glucosidase inhibitor. S. Afr. J. Bot. 2019, 124, 218–222. [Google Scholar] [CrossRef]
| Compounds | IC50 (μM) |
|---|---|
| 1 | 522.0 ± 0.51 |
| 2 | 234.2 ± 4.18 |
| 3 | 289.4 ± 4.91 |
| 4 | NA |
| 5 | ND |
| 6 | 659.78 ± 2.15 |
| 7 | 30.5 ± 0.41 |
| 8 | 480.1 ± 2.11 |
| Acarbose | 942 ± 0.74 |
| S. cerevisiae α-glucosidase | ||||||
| Compounds | IC50 (μM) | Docking score | Protein–ligand interactions | |||
| Ligand | Receptor | Interactions | Distances (Å) | |||
| 1 | 522.0 | −7.87 | O12 | NE2-HIS111 | HBA | 2.43 |
| 2 | 234.2 | −8.47 | O21 O12 | NH1-ARG439 O-WAT1174 | HBA HBA | 1.95 1.97 |
| 3 | 289.4 | −8.23 | O69 O69 O69 | OD1-ASP214 OD2-ASP214 NE2-HIS111 | HBD HBD HBA | 2.15 1.83 2.30 |
| 6 | 659.78 | −5.33 | O1 O7 | O-PHE157 N-TYR313 | HBD HBA | 1.88 2.33 |
| 7 | 30.5 | −10.62 | O8 O8 | OD1-ASP214 NE2-HIS111 | HBD HBA | 2.62 2.14 |
| 8 | 480.1 | −8.18 | O22 O24 | NH2-ARG212 O-WAT1026 | HBA HBA | 2.09 1.96 |
| Acarbose | 942 | −1.24 | O17 O19 O21 O40 O42 O87 O19 O21 O21 O40 O44 | OE1-GLU276 OD1-ASP214 OD1-ASP349 O-HOH1228 OD1-ASP408 OE2-GLU304 NH2-ARG212 NE2-HIS348 O-HOH1026 O-HOH1056 O-HOH1174 | HBD HBD HBD HBD HBD HBD HBA HBA HBA HBA HBA | 2.43 2.79 3.27 2.53 2.99 2.96 3.47 3.02 2.71 2.42 2.56 |
| H. Sapiens α-glucosidase | ||||||
| Compounds | IC50 (μM) | Docking score | Protein–ligand interactions | |||
| Ligand | Receptor | Interactions | Distances (Å) | |||
| 1 | ND | −6.41 | O12 O21 O23 | NH1-ARG600 NE2-HIS674 NE2-HIS674 | HBA HBA HBA | 1.95 2.44 1.91 |
| 2 | ND | −6.44 | O12 O21 | NH1-ARG600 NE2-HIS674 | HBA HBA | 2.03 1.95 |
| 3 | ND | −7.07 | O69 O69 O69 | OD1-ASP616 OD2-ASP616 NH1-ARG600 | HBD HBD HBA | 1.94 2.22 2.84 |
| 6 | ND | −6.26 | O1 | OD2-ASP518 | HBD | 1.85 |
| 7 | ND | −9.08 | O8 O10 | OD1-ASP404 O-Wat1106 | HBD HBA | 2.00 3.01 |
| 8 | ND | −7.23 | O22 O24 | OD2-ASP518 NE2-HIS674 | HBD HBA | 1.94 3.14 |
| Acarbose | ND | −9.65 | O15 O17 O19 O21 N37 O40 O42 O42 O15 O19 O42 O64 O87 N37 N37 | OD1-ASP616 O-HOH1164 OD2-ASP404 OD1-ASP404 OD2-ASP616 OD1-ASP282 OD2-ASP282 SD-MET519 NH1-ARG600 NE2-HIS674 NH1-ARG600 N-ALA284 O-HOH1276 OD1-ASP616 OD2-ASP616 | HBD HBD HBD HBD HBD HBD HBD HBD HBA HBA HBA HBA HBA IONIC IONIC | 2.60 2.84 2.90 2.86 2.75 3.12 2.78 3.21 3.05 3.38 3.08 3.06 3.14 3.36 2.75 |
| Compounds | Pharmacokinetic Properties |
|---|---|
| 1 | HIA = High, Caco-2 = Good, BBB = Yes, HOB = Yes, P-glycoprotein inh/subs = No, Carcinogenicity = No, Ames mutagenesis = No, Hepatotoxicity = No, Acute Oral Toxicity = III (2.28 kg/mol), Biodegradation = Yes, Log Kp = −6.14 cm/s, CYP3A4 subs = No, CYP2C9 subs = No, CYP2D6 subs = No, CYP3A4 inh = No, CYP2C9 inh = No, CYP2C19 inh = Yes, CYP2D6 inh = Yes, CYP1A2 inh = Yes, CYP inhibitory promiscuity = No |
| 2 | HIA = High, Caco-2 = Good, BBB = Yes, HOB = Yes, P-glycoprotein inh/subs = No, Carcinogenicity = No, Ames mutagenesis = No, Hepatotoxicity = No, Acute Oral Toxicity = III (1.695 kg/mol), Biodegradation = No, Log Kp = −5.97 cm/s, CYP3A4 subs = No, CYP2C9 subs = No, CYP2D6 subs = No, CYP3A4 inh = No, CYP2C9 inh = No, CYP2C19 inh = No, CYP2D6 inh = No, CYP1A2 inh = Yes, CYP inhibitory promiscuity = No |
| 3 | HIA = Low, Caco-2 = Good, BBB = No, HOB = No, P-glycoprotein inh/subs = No, Carcinogenicity = No, Ames mutagenesis = No, Hepatotoxicity = No, Acute Oral Toxicity = I (3.471 kg/mol), Biodegradation = No, Log Kp = −2.53 cm/s, CYP3A4 subs = Yes, CYP2C9 subs = No, CYP2D6 subs = No, CYP3A4 inh = No, CYP2C9 inh = No, CYP2C19 inh = No, CYP2D6 inh = No, CYP1A2 inh = No, CYP inhibitory promiscuity = No |
| 6 | HIA = Low, Caco-2 = No, BBB = No, HOB = No, P-glycoprotein inh/subs = No, Carcinogenicity = No, Ames mutagenesis = No, Hepatotoxicity = No, Acute Oral Toxicity = IV (1.499kg/mol), Biodegradation = Yes, Log Kp = −0.39cm/s, CYP3A4 subs = Yes, CYP2C9 subs = No, CYP2D6 subs = No, CYP3A4 inh = No, CYP2C9 inh = No, CYP2C19 inh = No, CYP2D6 inh = No, CYP1A2 inh = No, CYP inhibitory promiscuity = No |
| 7 | HIA = Low, Caco-2 = No, BBB = No, HOB = No, P-glycoprotein inh/subs = No, Carcinogenicity = No, Ames mutagenesis = No, Hepatotoxicity = No, Acute Oral Toxicity = IV (1.078kg/mol), Biodegradation = Yes, Log Kp = −0.11cm/s, CYP3A4 subs = No, CYP2C9 subs = Yes, CYP2D6 subs = No, CYP3A4 inh = No, CYP2C9 inh = No, CYP2C19 inh = No, CYP2D6 inh = No, CYP1A2 inh = Yes, CYP inhibitory promiscuity = No |
| 8 | HIA = High, Caco-2 = Good, BBB = Yes, HOB = No, P-glycoprotein inh/subs = No, Carcinogenicity = No, Ames mutagenesis = No, Hepatotoxicity = No, Acute Oral Toxicity = IV (1.699kg/mol), Biodegradation = Yes, Log Kp = −2.90cm/s, CYP3A4 subs = No, CYP2C9 subs = Yes, CYP2D6 subs = No, CYP3A4 inh = No, CYP2C9 inh = No, CYP2C19 inh = No, CYP2D6 inh = No, CYP1A2 inh = Yes, CYP inhibitory promiscuity = No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ur Rehman, N.; Rafiq, K.; Khan, A.; Ahsan Halim, S.; Ali, L.; Al-Saady, N.; Hilal Al-Balushi, A.; Al-Busaidi, H.K.; Al-Harrasi, A. α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii. Mar. Drugs 2019, 17, 666. https://doi.org/10.3390/md17120666
Ur Rehman N, Rafiq K, Khan A, Ahsan Halim S, Ali L, Al-Saady N, Hilal Al-Balushi A, Al-Busaidi HK, Al-Harrasi A. α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii. Marine Drugs. 2019; 17(12):666. https://doi.org/10.3390/md17120666
Chicago/Turabian StyleUr Rehman, Najeeb, Kashif Rafiq, Ajmal Khan, Sobia Ahsan Halim, Liaqat Ali, Nadiya Al-Saady, Abdullah Hilal Al-Balushi, Haitham Khamis Al-Busaidi, and Ahmed Al-Harrasi. 2019. "α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii" Marine Drugs 17, no. 12: 666. https://doi.org/10.3390/md17120666
APA StyleUr Rehman, N., Rafiq, K., Khan, A., Ahsan Halim, S., Ali, L., Al-Saady, N., Hilal Al-Balushi, A., Al-Busaidi, H. K., & Al-Harrasi, A. (2019). α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii. Marine Drugs, 17(12), 666. https://doi.org/10.3390/md17120666