Abstract
We performed an oral administration study of fucoidan in 396 Japanese volunteers and investigated significant factors concerning the absorption of fucoidan. Urine samples were collected at 0, 3, 6, and 9 h after ingestion of 3 g of fucoidan. Fucoidan was detected in urine after ingestion in 385 out of 396 subjects. The maximum value (mean ± standard deviation (SD)) of urinary fucoidan was 332.3 ± 357.6 μg/gCr in subjects living in Okinawa prefecture, compared with 240.1 ± 302.4 μg/gCr in subjects living outside Okinawa. Compared with the estimated urinary excretion of fucoidan by place of residence, those of subjects living in Okinawa prefecture were significantly higher than those living outside Okinawa prefecture (p < 0.01). In addition, subjects living in Okinawa prefecture consumed significantly greater amounts of mozuku compared with those living outside Okinawa prefecture (p < 0.01). Multiple regression analysis showed that having Okinawa prefecture as a place of residence was a significant factor (p < 0.01) contributing to the estimated urinary excretion of fucoidan. Because the habit of eating mozuku was significantly higher (p < 0.01) in subjects living in Okinawa prefecture than in those living outside Okinawa prefecture, the habit of eating mozuku was speculated to be a factor in the absorption of fucoidan.
1. Introduction
According to the National Nutrition Survey in Japan, Japanese people eat about 14.3 g of seaweed per adult person daily [1], which includes konbu (Laminaria japonica), wakame (Undaria pinnatifida), hijiki (Hizikia fusiforme), nori (Porphyra tenera), and mozuku (Nemacystus decipiens). Mozuku belongs to the brown seaweeds, and several kinds of mozuku such as ishimozuku (Sphaerotrichia divaricate), futomozuku (Tinocladia crassa), kuromo (Hydrilla verticillata), and Okinawa mozuku (Cladosiphon okamuranus Tokida) grow wild on the shores of Japan. Okinawa mozuku is a species endemic to the Ryukyu archipelago, which is located between the southern limit (24° N) of the Yaeyama Islands in Okinawa prefecture and the northern limit (29° N) of Amami Island in Kagoshima prefecture (Figure 1) [2]. Okinawa mozuku has been popular as a food among inhabitants of Okinawa prefecture for a long time [3]. Okinawa mozuku contains about 1% fucoidan, and its structure was determined by Nagaoka et al., in 1999 [4]. Mozuku fucoidan is mainly composed of fucose-enriched sulfated polysaccharide with a molecular weight of 28.8 kDa (Figure 2) [4]. Fucodan exhibits many different biological properties, including anti-inflammatory, anticoagulant, antithrombotic, antiadhesive, antiangiogenic, antiviral, antitumor and antioxidant activities [5,6,7,8,9,10]. Currently, the clinical trials of fucoidan have been reported in patients with osteoarthritis and malignant tumors [11,12,13,14] and in the immunocompromised elderly [15]. The biological properties of fucoidan vary depending on the species, molecular weight, structure, and route of intake [9,16,17,18].
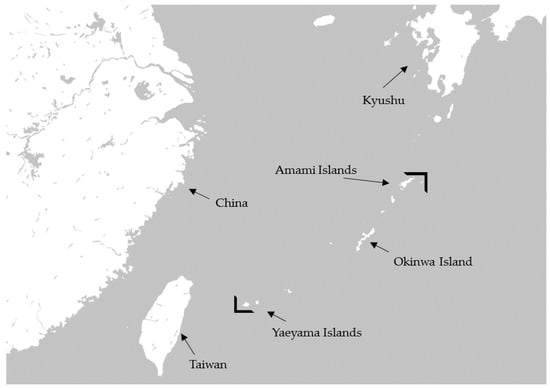
Figure 1.
Geographical distribution of Okinawa mozuku.
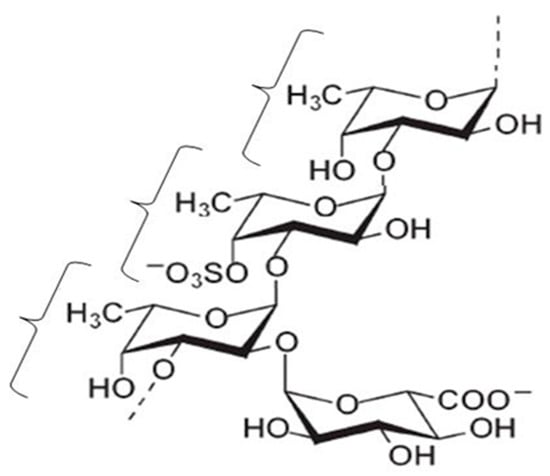
Figure 2.
Structure of Okinawa mozuku fucoidan adapted from [4], with permission from Nagaoka, 2012.
Previously, high molecular weight polysaccharides such as fucoidan were not considered to be absorbed orally in humans, because of the lack of a digestive enzyme [19,20]. On the other hand, several studies have reported its biological effects after oral ingestion, but it is unclear how these systemic effects occur. In 2005, Irhimeh et al. [21] reported intestinal absorption of fucoidan using volunteers for the first time. The volunteers took 3 g of Galactofucan (containing 10% and 75% fucoidan) per day for 12 days and showed a 0.6% absorption rate of fucoidan via the intestine. We raised a specific antibody for fucoidan extracted from Okinawa mozuku, and established a sandwich enzyme-linked immuno sorbent assay (ELISA) method for a fucoidan assay [22]. Using this antibody and the ELISA assay, we investigated intestinal absorption of mozuku fucoidan in rats, and showed that ingested fucoidan was absorbed across the intestinal tract and taken up by intestinal macrophages and hepatic Kupffer cells [23]. In addition, we assayed fucoidan concentrations in serum and urine after ingestion of 1 g of mozuku fucoidan using 10 Japanese volunteers. Fucodan was detected in the serum of 7 volunteers and in the urine of 10 volunteers. The rate of absorption through the small intestine was highly variable among the participants. Because it was a preliminary study using a small number of subjects, the mechanism of intestinal absorption of mozuku fucoidan was not elucidated.
In this study, we clarified the factors associated with mozuku fucoidan absorption using larger numbers of Japanese volunteers. Although fucoidan absorption in humans is extremely low, fucoidan concentrations after oral administration were about 10 times higher in urine than in serum. Therefore, urinary fucoidan concentrations were measured before and after an oral administration of mozuku fucoidan.
2. Results
2.1. Maximum Fucoidan Value in Urine
Urinary fucoidan was detected in 385 out of 396 subjects after a single oral intake of 3 g of fucoidan. Eleven subjects who did not exhibit urinary fucoidan consisted of 3 subjects living in Okinawa prefecture and 8 subjects living outside Okinawa prefecture. Because the maximum value of urinary fucoidan varied widely among subjects, the values were ranked into groups from A to E (Table 1). Fourteen out of 16 subjects in group E (>1200 μg/gCr), which had the highest fucoidan value, lived in Okinawa prefecture. Maximum values of urinary fucoidan (mean ± SD) were as follows: subjects living in Okinawa prefecture (332.3 ± 357.6 μg/gCr) and subjects living outside Okinawa prefecture (240.1 ± 302.4 μg/gCr); males (257.4 ± 310.4 μg/gCr) and females (352.4 ± 373.1 μg/gCr). By age brackets: 309.0 ± 337.4 μg/gCr for subjects in their 20s; 320.0 ± 384.5 μg/gCr for subjects in their 30s; 321.5 ± 392.8 μg/gCr for subjects in their 40s; 249.7 ± 217.6 μg/gCr for subjects in their 50s; 341.3 ± 357.4 μg/gCr for subjects in their 60s; and 153.7 ± 90.0 μg/gCr for subjects in their >70s.

Table 1.
Maximum value of urinary fucoidan (A: 0–149, B: 150–299, C: 300–699, D: 700–1199, E: >1200 (µg/gCr)).
2.2. Statistical Analysis
2.2.1. Estimated Urinary Excretion of Fucoidan
Comparing the estimated urinary excretion of fucoidan by place of residence, subjects living in Okinawa prefecture exhibited significantly higher values than those living outside Okinawa prefecture (p < 0.01) (Table 2). Gender, age, and habit of eating mozuku were not significant factors associated with the estimated urinary excretion of fucoidan.

Table 2.
Comparison of the estimated urinary excretion of fucoidan.
2.2.2. Factors Contributing to the Estimated Urinary Excretion of Fucoidan Evaluated by Multiple Regression Analysis
Place of residence of Okinawa prefecture was a significant factor contributing to the estimated urinary excretion of fucoidan (p < 0.01), but gender, age, habit of eating mozuku were not significant factors (Table 3). In addition, we evaluated the contributing factors to the estimated urinary excretion of fucoidan in subjects living in Okinawa prefecture and those living outside Okinawa prefecture by multiple regression analysis, respectively. As a result, gender, age, and custom of eating mozuku were not significant factors in both subjects living in Okinawa and living outside Okinawa prefecture (data not shown).

Table 3.
Multiple regression analysis.
2.3. Fucoidan Positive and Negative in Urine before Fucoidan Ingestion
Fucoidan was detected in the urine of 295 subjects (fucoidan positive) but not in 101 subjects (fucoidan negative) before fucoidan ingestion. The backgrounds of both groups are listed in Table 4.

Table 4.
Comparison between the fucoidan detectors and the fucoidan non-detectors in urine before ingestion.
The estimated urinary excretion of fucoidan was significantly higher (p < 0.05) in the fucoidan-positive subjects than in the fucoidan-negative subjects. Subjects living in Okinawa prefecture constituted the majority of fucoidan-positive subjects, and the habit of eating mozuku was significantly higher in the fucoidan-positive subjects than the fucoidan-negative subjects.
By multiple regression analysis, the factors relating to the estimated urinary excretion of fucoidan were evaluated in the fucoidan-negative subjects in order to avoid the influence of urinary fucoidan before ingestion on fucoidan absorption. As a result, a subject living in Okinawa prefecture was a significant factor contributing to the estimated urinary excretion of fucoidan (Table 5). However, gender, age, custom of eating mozuku were not significant factors.

Table 5.
Multiple regression analysis in fucoidan-positive (n = 295) versus fucoidan-negative subjects (n = 101).
3. Discussion
The physiological activity of fucoidan differs depending on its molecular weight, structure, and the route of administration. In order to apply fucoidan in a clinical setting, oral intake is more convenient than intravenous injection or intramuscular injection. Fucoidan was absorbed across the intestinal tract in rats and humans [22,23]; however, little is known about the mechanism of its absorption. The present study was conducted to clarify the factors associated with absorption of mozuku fucoidan in 396 Japanese volunteers.
Because 97% of subjects enrolled in this study exhibited urinary fucoidan excretion, intestinal absorption of mozuku fucoidan in humans was reconfirmed. Notably, the estimated urinary excretion of fucoidan was significantly higher in volunteers living in Okinawa prefecture than those living outside Okinawa prefecture (t-test). By multiple regression analysis, place of residence of Okinawa prefecture was a significant factor associated with the estimated urinary excretion of fucoidan. As a conceivable reason for this finding, this is a human study on fucoidan absorption using fucoidan derived from Okinawa mozuku. Approximately 90% of Okinawa mozuku is cultivated in Okinawa prefecture, and has been familiar as an edible seaweed among inhabitants of Okinawa prefecture for a long time. The habit of eating mozuku was significantly higher in volunteers living in Okinawa prefecture compared with those living outside Okinawa prefecture, which may reflect the dietary habits of eating mozuku in Okinawa prefecture. The findings suggested the habit of eating mozuku may be associated with fucoidan absorption.
If so, how does the habit of eating mozuku enhance fucoidan absorption? More recently, Tokita et al. reported that Okinawa mozuku was digested and that the fucoidan contained in it was absorbed in humans. The dietary habit of eating mozuku was assumed to be associated with the absorption of fucoidan contained in mozuku [24]. Recently, the transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota was reported by Hehemann et al. [25]. They showed that digestive enzymes for seaweed were detected frequently in Japanese individuals, whereas those enzymes were absent in North American individuals who did not eat seaweed. In addition, Song T. et al. reported that aga1 (an agarase) may have been transferred together with its surrounding genes, from marine bacteria to soil bacteria via human microbiota. They speculated that microbes from inland humans may degrade agar and that these microbes may have acquired seaweed associated genes because of increased seaweed in diets [26]. Considering these reports, the inhabitants of Okinawa prefecture may have acquired digestive enzymes from mozuku in their gut bacteria because Okinawa mozuku is extensively consumed within this area [3,27].
Of note, urinary fucoidan prior to fucoidan administration was detected in 295 subjects (fucoidan positive) but not in 101 subjects (fucoidan negative). The estimated urinary excretion of fucoidan was significantly higher in the fucoidan positive group than in the fucoidan negative group. In addition, subjects living in Okinawa prefecture and with a habit of eating mozuku were significantly more prevalent in the fucoidan positive group than in the fucoidan negative group. The findings may support the hypothesis that the habit of eating mozuku may be associated with the absorption of fucoidan. The following can be inferred as the reason why urinary fucoidan is detected prior to administration. After oral administration of fucoidan to rats, fucoidan was absorbed and was phagocytosed by hepatic Kupffer cells, and the hepatic fucoidan concentration was increased [24]. Heparin, a polysaccharide similar to fucoidan, was detected in urine 120 h after the oral administration of heparin. Heparin was absorbed through the intestinal tract and taken into the liver immediately, and was then eliminated gradually into the urine [28]. A rapid organ or cellular uptake of fucoidan followed by a slow decrease was noted by Deux et al. [29] although in this case, the fucoidan was delivered intravenously. Similar to the fate after intravenous administration, fucoidan contained in habitually ingested mozuku may be absorbed across the intestinal tract, accumulated in the liver, and then excreted slowly in the urine over a long period of time.
Our study has limitations. First, by multiple regression analysis, the habit of eating mozuku was not a significant factor affecting urinary fucoidan excretion. Second, urinary fucoidan was detected in subjects without the habit of eating mozuku. We speculate the following reason for this. We assayed urinary fucoidan value using polyclonal antibody for Okinawa mozuku fucoidan, which weakly cross-reacted with Fucus vesiculosus fucoidan [22]. Since brown seaweeds of konbu (Laminaria japonica) and wakame (Undariapinnatifida) are traditional foodstuffs in Japan, fucoidan contained in these seaweeds may cross-react with our fucoidan assay method. Further studies are necessary to elucidate the reference of the habit of eating mozuku to intestinal absorption of fucoidan by new ELISA assay using a monoclonal antibody.
In conclusion, mozuku fucoidan was absorbed after oral ingestion. The habit of eating mozuku was assumed to be associated with the absorption of mozuku fucoidan. The precise mechanism for fucoidan absorption across the intestinal tract may be elucidated in future studies.
4. Materials and Methods
4.1. Subjects
We published pamphlets describing the purpose, method, exclusion items, etc. of our research entitled ‘The human trial on intestinal absorption of mozuku fucoidan’ on the Internet and recruited volunteer participants (Supplementary Figure S1). Four hundreds and three Japanese people submitted applications from April 2014 to June 2017. They responded to questionnaires on gender, age, residence place, and habit of eating mozuku. We enrolled 396 volunteers who completed questionnaires and collected urine samples as planned.
Age groups were divided into subjects in their 20s, 30s, 40s, 50s, 60s, >70s. Habit of eating mozuku was divided into: (1) almost every day; (2) about 1–3 times a week; (3) about once every 2 weeks; (4) about once a month; (5) about once in 2–3 months; (6) about 1–2 times a year; (7) do not eat/do not like (Table 6).

Table 6.
Characteristics of the volunteers living in Okinawa prefecture (n = 272) and those living outside Okinawa prefecture (n = 124).
Residence place of the volunteers was divided into two categories of living in Okinawa prefecture and living outside Okinawa prefecture, because 68% of the participants lived in Okinawa prefecture and the others lived outside Okinawa prefecture. The habit of eating mozuku was significantly higher in volunteers living in Okinawa prefecture than those living outside Okinawa prefecture (Table 6).
This study was carried out in accordance with the Declaration of Helsinki. The protocol of the study was approved by the Ethics Committee of South Product Co., Ltd. (No.14-02). Following an explanation of the study and its aim, all subjects gave informed consent.
Subjects refrained from consuming marine algae or fucoidan supplements on the day before the test and on the day in order to avoid the effects of existing diet. Subjects took orally two fucoidan drinks (1500 mg/bottle) at 9:00 in the morning. Urine samples were collected four times, before, 3, 6, and 9 h after fucoidan administration. Urinary fucoidan concentration was measured using an ELISA method we developed [22]. In addition, we measured urinary creatinine concentration (ELISA method) and corrected urinary fucoidan concentration. In this study, the subjects took orally 3 g of mozuku fucoidan. This dose level was chosen because it was safe and urinary excretion of fucoidan showed dose dependency, which was increased more by administration of 3 g of fucoidan than by 1 or 2 g of fucoidan (Supplementary Figure S2).
Amount of urinary excretion of fucoidan was calculated as follows
[1]. Urinary excretion of fucoidan before fucoidan ingestion.
Urinary fucoidan value before fucoidan ingestion was corrected by urinary creatinine value (μg/gCr), which was matched with urinary excretion of fucoidan for 24 h. Three-eighths of the creatinine correction value was equivalent to the amount of urinary excretion of fucoidan for 9 h.
[2]. Total amount of urinary excretion of fucoidan after fucoidan ingestion.
The creatinine correction value of fucoidan at 3 h after fucoidan ingestion was calculated and one-eighth of it was the equivalent of the amount of urinary excretion of fucoidan for the first 3 h. The creatinine correction value of fucoidan at 6 h after fucoidan ingestion was calculated and one-eighth of it was the equivalent amount to the urinary excretion of fucoidan from 3 to 6 h. Similarly, one-eighth of the creatinine correction value of fucoidan at 9 h was equivalent to the amount of urinary excretion of fucoidan from 6 to 9 h. The total amount of urinary excretion of fucoidan at 3, 6, and 9 h served as the amount of urinary excretion of fucoidan for 9 h after fucoidan ingestion.
[3]. Estimated urinary excretion of fucoidan for 9 h after fucoidan ingestion.
The change in urinary fucoidan excretion before and after fucoidan ingestion [2]−[1] was taken as the estimated urinary excretion of fucoidan for 9 h after fucoidan ingestion.
Calculation formula of fucoidan excretion
- a: Urinary fucoidan value before fucoidan ingestion
- b: The creatinine correction value of fucoidan at 3 h after fucoidan ingestion
- c: The creatinine correction value of fucoidan at 6 h after fucoidan ingestion
- d: The creatinine correction value of fucoidan at 9 h after fucoidan ingestion
- h: hour
- [1]: Urinary excretion of fucoidan before fucoidan ingestion
- [2]: Total amount of urinary excretion of fucoidan after fucoidan ingestion
- [3]: Estimated urinary excretion of fucoidan for 9 h after fucoidan ingestion
- * If the amount of urinary excretion of fucoidan before ingestion was higher than the total amount of urinary excretion of fucoidan after fucoidan ingestion, the estimated urinary excretion of fucoidan was judged as 0 (zero).
4.2. Statistical Analysis
SAS version 9.4 (Statistical Analysis Software 9.4, SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses. Differences in gender and habit of eating mozuku between subjects living in Okinawa prefecture and those living outside Okinawa prefecture were carried out by means of χ2-test, and age distribution was compared by Wilcoxon rank sum test. Comparison between subjects who produced urinary fucoidan (fucoidan positive) and subjects who did not produce urinary fucoidan (fucoidan negative) prior to taking fucoidan was also carried out by the same statistical analysis.
Correlations between the estimated urinary excretion of fucoidan and gender, age, place of residence, and habit of eating mozuku were analyzed as follows. Gender and place of residence were examined using a two-sample t-test; age and habit of eating mozuku were examined using one-way analysis of variance (ANOVA).
In addition, multiple regression analysis was performed with a dependent variable as the estimated urinary excretion of fucoidan, and the independent variable as gender, age, place of residence, and habit of eating mozuku. For all tests, p < 0.05 was regarded as significant.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/8/254/s1, Figure S1: The study leaflet, Figure S2: Dose dependent study for fucoidan absorption.
Author Contributions
T.N. designed the composition of the experiment and the manuscript; K.K. performed the experiment; M.T. and M.I. reviewed the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Assistant Professor Ideno (Gunma University) for helpful advice on statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fukuda, S.; Saito, H.; Nakaji, S.; Yamada, M.; Ebine, N.; Tsushima, E.; Oka, E.; Kumeta, K.; Tsukamoto, T.; Tokunaga, S. Pattern of dietary fiber intake among the Japanese general population. Eur. J. Clin. Nutr. 2007, 61, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Toma, T. Geographical distribution of the brown alga Tinocladia crassa (Suringar) kylin in Ryukyu islands. Aquac. Sci. 1993, 41, 293–297. [Google Scholar]
- Sho, H. History and characteristics of Okinawan longevity food. Asia Pac. J. Clin. Nutr. 2001, 10, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Kimura, K.; Makino, T.; Aiyama, R.; Ueyama, S.; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus Tokida. Glycoconj. J. 1999, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, K.; Takada, H.; Iha, M.; Nagamine, T. Attenuation of N-nitrosodiethylamine-induced liver fibrosis by high-molecular-weight fucoidan derived from Cladosiphon okamuranus. J. Gastroenterol. Hepatol. 2010, 25, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 10, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.L.; Xavier, C.A.; Bezerra, M.B.; Paiva, A.O.; Carvalho, M.F.; Benevides, N.M.; Rocha, F.A.; Leite, E.L. Assessment of zymosan-induced leukocyte influx in a rat model using sulfated polysaccharides. Planta Med. 2010, 76, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. Consorzio interuniversitario nazionale per la bio-oncologia, Italy. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.P.; Mulder, A.M.; Baker, D.G.; Robinson, S.R.; Rolfe, M.I.; Brooks, L.; Fitton, J.H. Effects of fucoidan from Fucus vesiculosus in reducing symptoms of osteoarthritis: A randomized placebo-controlled trial. Biologics 2016, 26, 81–88. [Google Scholar]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An exploratory study on the anti-inflammatory effects of fucoidan in relation to quality of life in advanced cancer patients. Integr. Cancer Ther. 2018, 17, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The Effect of Undaria pinnatifida fucoidan on the pharmacokinetics of letrozole and tamoxifen in patients with breast cancer. Integr. Cancer Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of low-molecular weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of elderly japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Till, S.; Jiang, C.; Knappe, S.; Reutterer, S.; Scheiflinger, F.; Szabo, C.M.; Dockal, M. Structure-activity relationship of the pro- and anticoagulant effects of Fucus vesiculosus fucoidan. Thromb. Haemost. 2014, 111, 429–437. [Google Scholar] [PubMed]
- Michel, C.; Lahaye, M.; Bonnet, C.; Mabeau, S.; Barry, J.L. In vitro fermentation by human faecal bacteria of total and purified dietary fibers from brown seaweeds. Br. J. Nutr. 1996, 75, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J. Sci. Food Agric. 2010, 90, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M.; Kongtawelert, P. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar. Drugs 2015, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Hirayama, M.; Nakajima, K.; Tamaki, K.; Iha, M.; Nagamine, T. Detection of fucoidan in urine after oral intake of a traditional Japanese seaweed, Okinawa mozuku (Cladosiphon okamuranus Tokida). J. Nutr. Sci. Vitaminol. 2017, 63, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.H.; Gaelle, C.; Tristan, B.; William, H.; Mirjam, C.; Gurvan, M. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xu, H.; Wei, C.; Jiang, T.; Qin, S.; Zhang, W.; Cao, Y.; Hu, C.; Zhang, F.; Qiao, D.; Cao, Y. Horizontal transfer of a novel soil agarase gene from marine bacteria to soil bacteria via human microbiota. Sci. Rep. 2016, 6, 34103. [Google Scholar] [CrossRef] [PubMed]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136–137, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Deux, J.F.; Anne, M.P.; Alain, F.L.B.; Laurent, J.F.; Sylvia, C.J.; Françoise, B.; Frank, B.; Michel, J.B.; Didier, L. Low molecular weight fucoidan prevents neointimal hyperplasia in rabbit iliac artery in-stent restenosis model. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).