Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Identification of Isolated Marine Fungus
2.2. Total Phenolic Content
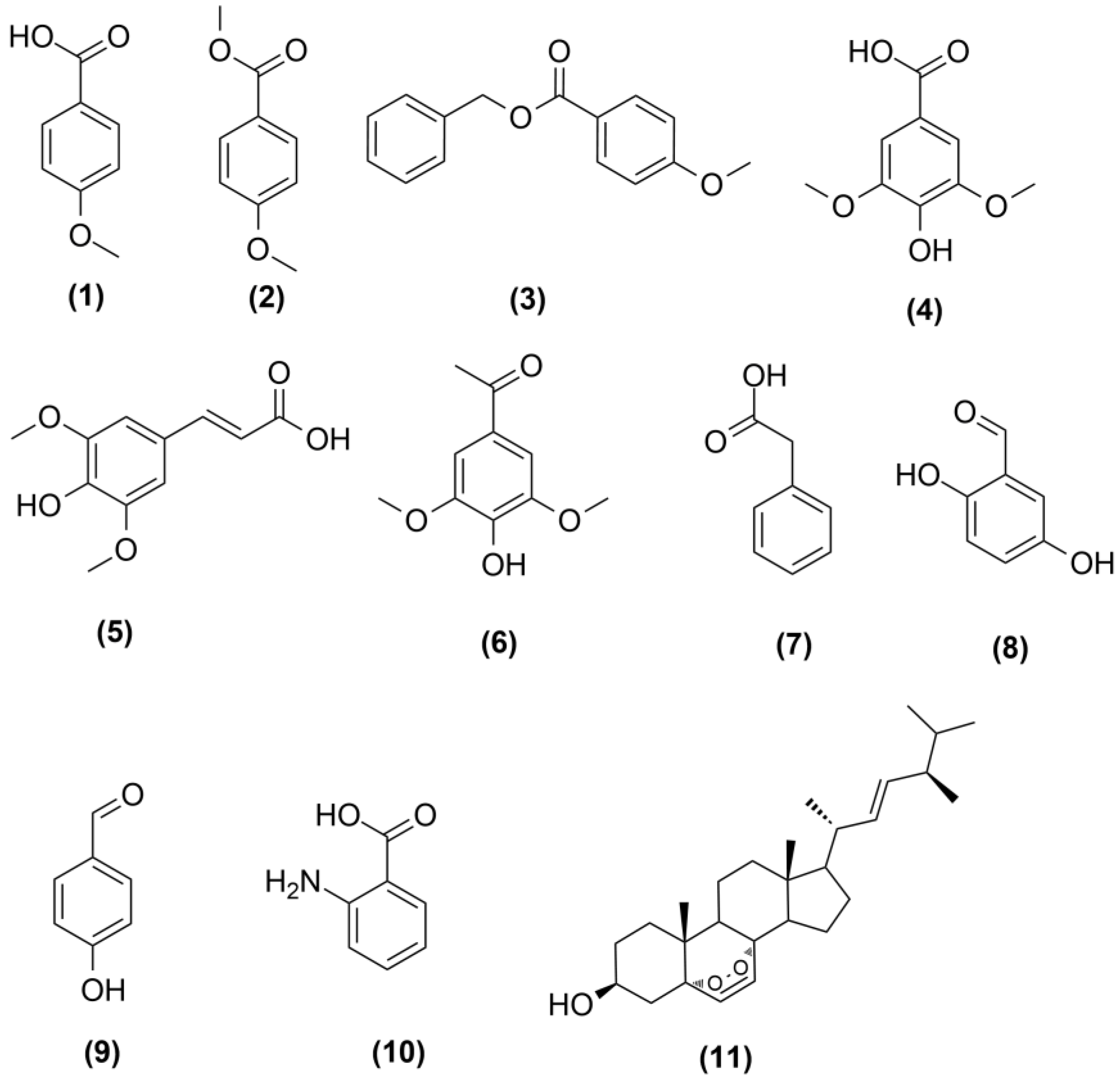
2.3. Isolation and Characterization of Induced Metabolites
2.4. Free Radical Scavenging Activity
2.5. Antiproliferative Activity against Human Liver Cancer (HepG2)
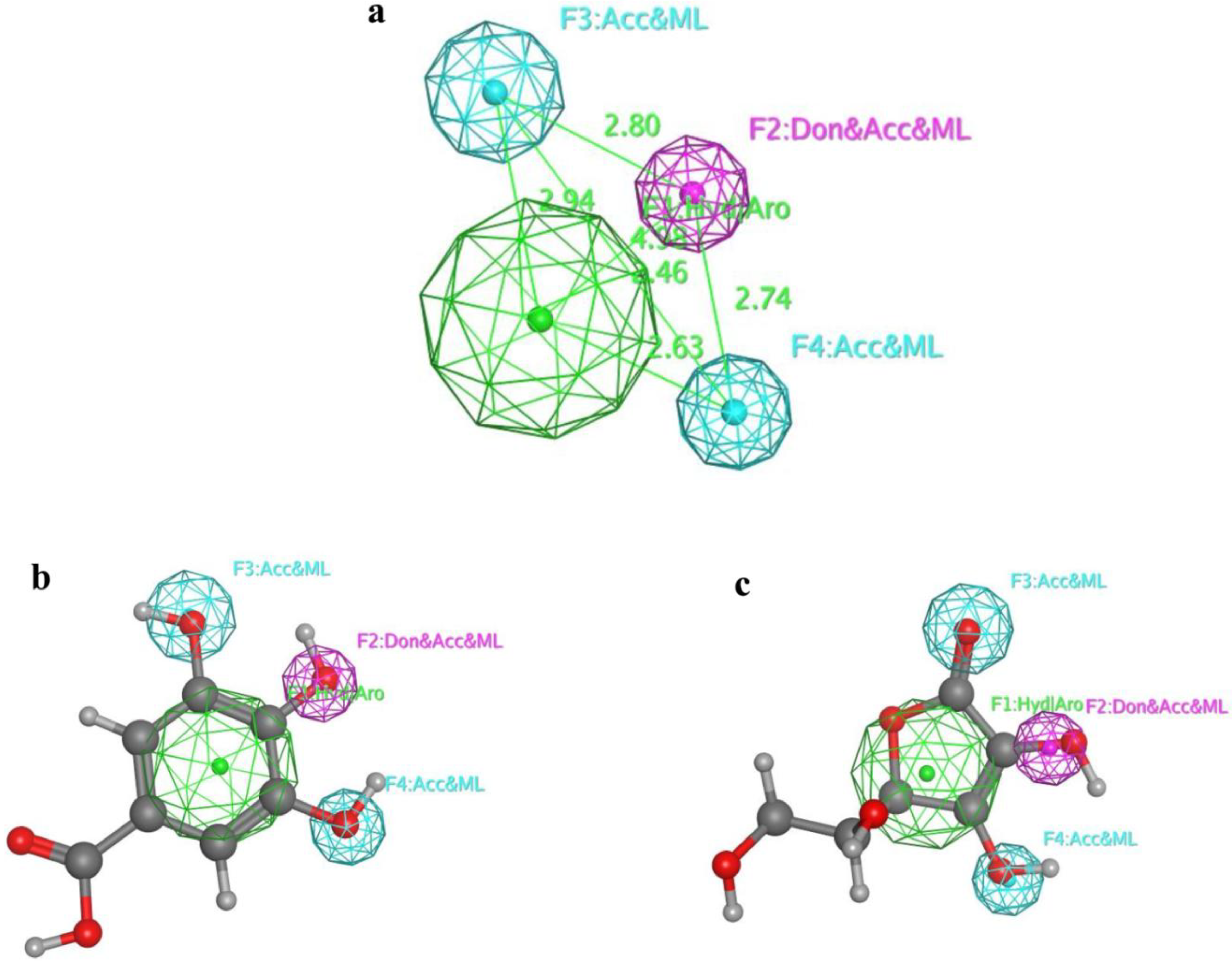
2.6. 3D Alignment and Pharmacophore Generation
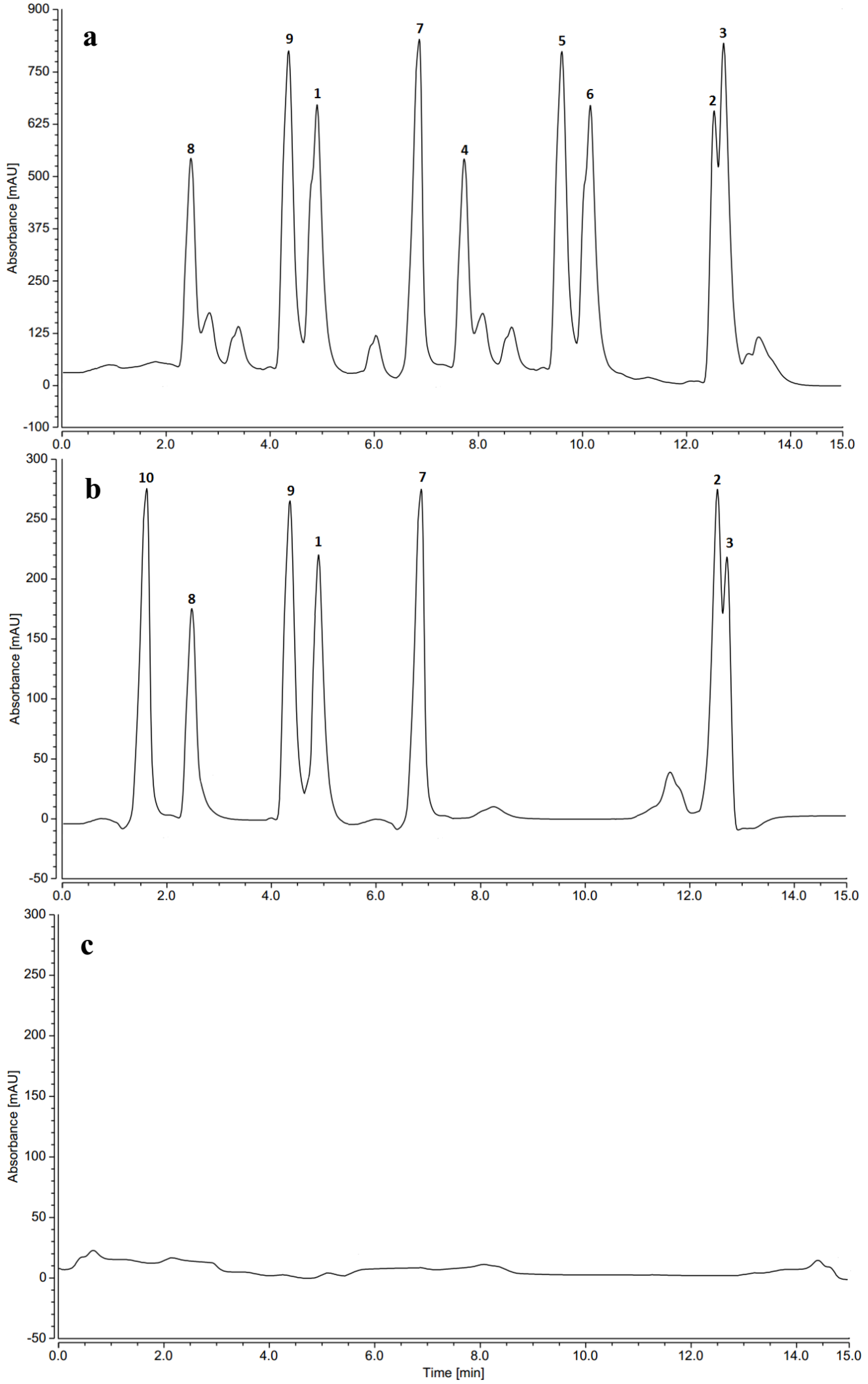
2.7. HPLC Profiles of Induced Phenolics
3. Experimental
3.1. Isolation of Marine Fungal Strain
3.2. Fungal Strain Identification
3.3. Set up Growing Condition and Crude Extract Production
3.4. Chemical Analysis and Instrumentation
3.5. Determination of Antioxidant Activity
3.6. Determination of TPC
3.7. Isolation and Identification of Induced Metabolites
3.8. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Cytotoxicity Assay
3.9. Pharmacophore Model Generation
3.10. High-Performance Liquid Chromatography (HPLC) Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food. Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The anti-oxidant and antitumor properties of plant polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Su, T.J.; Cai, S.M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var. wabuensis: Diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shao, C.L.; Chen, M.; Liu, Q.A.; Wang, C.Y. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron. Lett. 2014, 55, 4888–4891. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Harrison, W.T.; Deng, H.; Okoro, C.K.; Asenjo, J.A.; Andrews, B.A.; Bull, A.T.; Goodfellow, M.; Ebel, R. Diverse metabolic profiles of a streptomyces strain isolated from a hyper-arid environment. J. Nat. Prod. 2011, 74, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chem. Biochem. 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Shen, Y. Guanacastane-type diterpenoids from Coprinus plicatilis. Phytochem. Lett. 2014, 7, 161–164. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, D.Y.; Li, Y.C.; Hua, H.M.; Ma, E.L.; Li, Z.L. Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain-many compounds strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Dhalluin, C.; Carlson, J.E.; Zeng, L.; He, C.; Aggarwal, A.K.; Zhou, M.M. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999, 399, 491–496. [Google Scholar] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Henrikson, J.C.; Hoover, A.R.; Lee, A.E.; Cichewicz, R.H. Epigenetic remodeling of the fungal secondary metabolome. Org. Biomol. Chem. 2008, 6, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Brosch, G.; Loidl, P.; Graessle, S. Histone modifications and chromatin dynamics: A focus on filamentous fungi. Fems. Microbiol. Rev. 2008, 32, 409–439. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat. Prod. Rep. 2010, 27, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Morita, S.; Shirata, N.; Taniguchi, T.; Monde, K.; Sakurai, H.; Ozeki, T.; Oshima, Y. Structural diversity of new C13-polyketides produced by Chaetomium mollipilium cultivated in the presence of a NAD+-dependent histone deacetylase inhibitor. Org. Lett. 2012, 21, 5456–5459. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Chung, Y.M.; Sakurai, H.; Ozeki, T.; Chang, F.R.; Yamashita, K.; Oshima, Y. Tenuipyrone, a novel skeletal polyketide from the entomopathogenic fungus, Isaria tenuipes, cultivated in the presence of epigenetic modifiers. Org. Lett. 2011, 14, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Chung, Y.M.; Sakurai, H.; Ozeki, T.; Chang, F.R.; Wu, Y.C.; Oshima, Y. Highly oxidized ergosterols and isariotin analogs from an entomopathogenic fungus, Gibellula formosana, cultivated in the presence of epigenetic modifying agents. Tetrahedron 2012, 68, 5817–5823. [Google Scholar] [CrossRef]
- Asai, T.; Morita, S.; Taniguchi, T.; Monde, K.; Oshima, Y. Epigenetic stimulation of polyketide production in Chaetomium cancroideum by an NAD+-dependent HDAC inhibitor. Org. Biomol. Chem. 2016, 14, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Rančić, A.; Soković, M.; Karioti, A.; Vukojević, J.; Skaltsa, H. Isolation and structural elucidation of two secondary metabolites from the filamentous fungus Penicillium ochrochloron with antimicrobial activity. Environ. Toxicol. Pharmacol. 2006, 22, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Zutz, C.; Bandian, D.; Neumayer, B.; Speringer, F.; Gorfer, M.; Wagner, M.; Rychli, K. Fungi treated with small chemicals exhibit increased antimicrobial activity against facultative bacterial and yeast pathogens. BioMed. Res Inter. 2014, 2014, 540292. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.J.; Talia, Y.H.; Murthy, Z.V.P. Production of p-anisic acid by modified Williamson etherification reaction using design of experiments. Chem. Prod. Proc. Model. 2010, 5. [Google Scholar] [CrossRef]
- Kiran, I.C.; Lalwani, K.; Sudalai, A. N-Heterocyclic carbene catalyzed esterification of aromatic aldehydes with alcohols under aerobic conditions. RSC Adv. 2013, 3, 1695–1698. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Denisko, O.V.; Fang, Y.; Zhang, L.; Wang, Z. Unexpected reactions of organozinc reagents with N-acylbenzotriazoles. Arkivoc 2001, 11, 41–48. [Google Scholar]
- Jewison, T.; Neveu, V.; Lee, J.; Knox, C.; Liu, P.; Mandal, R.; Murthy, R.K.; Sinelnikov, I.; Guo, A.C.; Wilson, M.; et al. YMDB: The Yeast Metabolome Database. Nucleic Acids Res. 2012, 40, D815–D820. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Andayani, D.S.; Sukandar, E.; Sukandar, U.K.A.N.; Adnyana, I.K. Isolation, identification of phenyl acetic acid from Streptomyces galbus TP2 strain and its toxicity. Int. J. Pharm. Pharm. Sci. 2014, 6, 643–646. [Google Scholar]
- Malak, L.G.; Ibrahim, M.A.; Bishay, D.W.; Abdel-Baky, A.M.; Moharram, A.M.; Tekwani, B.; Cutler, S.J.; Ross, S.A. Antileishmanial metabolites from Geosmithia langdonii. J. Nat. Prod. 2014, 77, 1987–1991. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Rateb, M.E.; Bakeer, W.; AbouZid, S.F.; Mohammed, R. Secondary metabolites from fungal endophytes of Solanum nigrum. Nat. Prod. Res. 2017, 31, 2568–2571. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.H.; Mazzio, E.; Badisa, R.B.; Zhu, Z.P.; Agharahimi, M.; Oriaku, E.T.; Goodman, C.B. Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res. 2012, 32, 1595–1602. [Google Scholar] [PubMed]
- Mahmoud, A.M.; El-Shemy, H.A. Cytotoxic Profiling of Some Compounds of Natural Origin against HepG2 Liver Cancer Cell Line in-vitro. J. Arid. Land Stud. 2012, 22, 191–194. [Google Scholar]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Pimentel-Elardo, S.M.; Hanora, A.; Radwan, M.; Abou-El-Ela, S.H.; Ahmed, S.; Hentschel, U. Isolation, phylogenetic analysis and anti-infective activity screening of marine sponge-associated actinomycetes. Mar. Drugs 2010, 8, 399–412. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Mohammed, R.; AbouZid, S.F.; Bakeer, W.; Ebel, R.; Sayed, A.M.; Rateb, M.E. Solamargine production by a fungal endophyte of Solanum nigrum. J. Appl. Microbiol. 2016, 4, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Seifert, K.A.; Kuijpers, A.F.; Houbraken, J.A.M.P.; Frisvad, J.C. Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud. Mycol. 2004, 49, 175–200. [Google Scholar]
- Blois, M. Antioxidant determinations by the use of astable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benmerache, A.; Benteldjoune, M.; Alabdul Magid, A.; Abedini, A.; Berrehal, D.; Kabouche, A.; Gangloff, S.C.; Nazabadioko, L.V.; Kabouche, Z. Chemical composition, antioxidant and antibacterial activities of Tamarix balansae J. Gay aerial parts. Nat. Prod. Res. 2017, 24, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Andi, S.A.; Gholami, M.; Ford, C.M. The effect of methyl jasmonate and light irradiation treatments on the stilbenoid biosynthetic pathway in Vitis vinifera cell suspension cultures. Nat. Prod. Res. 2018, 32, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Määttä, K.R.; Kamal-Eldin, A.; Törrönen, A.R. High-performance liquid chromatography (HPLC) analysis of phenolic compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: Ribes species. J. Agric. Food. Chem. 2003, 51, 6736–6744. [Google Scholar] [CrossRef] [PubMed]
| Tested Extract | Total Phenolic Content ± S.D. (µg GAE/mg) a |
|---|---|
| Nicotinamide Ext. | 93.4 ± 0.12 |
| Butyrate Ext. | 19.6 ± 0.05 |
| Malt Ext. | 9.5 ± 0.05 |
| Malt + salt Ext. | 11.5 ± 0.05 |
| Sabouraud Ext. | 7.5 ± 0.02 |
| Czapek Dox Ext. | 8.7 ± 0.02 |
| Rice Ext. | 15.1 ± 0.05 |
| Fungal Metabolite | IC50 ± S.D. (µg/mL) |
|---|---|
| p-Anisic acid (1) | >200 |
| p-Anisic acid methyl ester (2) | >200 |
| Benzyl anisate (3) | >200 |
| Syringic acid (4) | 20 ± 0.09 |
| Sinapic acid (5) | 30 ± 0.05 |
| Acetosyringone (6) | 25 ± 0.08 |
| Phenyl acetic acid (7) | >200 |
| Gentisaldehyde (8) | 75 ± 0.02 |
| p-Hydroxy benzaldehyde (9) | 95 ± 0.08 |
| Anthranilic acid (10) | >200 |
| Ergosterol peroxide (11) | >200 |
| Gallic acid | 15 ± 0.05 |
| Ascorbic acid | 10 ± 0.05 |
| % Viability of Human HepG2 Cell Line | Concentration µM | IC50 | ||||
|---|---|---|---|---|---|---|
| 0.01 | 0. 1 | 1 | 10 | 100 | ||
| p-Anisic acid (1) | 107.8 ± 1.15 | 95.3 ± 1.14 | 86.2 ± 6.39 | 59.15 ± 2.8 | 34.84 ± 5.4 | 25.1 |
| p-Anisic acid methyl ester (2) | 121.3 ± 7.12 | 100.8 ± 6.33 | 133.3 ± 3.85 | 214.6 ± 6.73 | 201.8 ± 4.71 | >100 |
| Benzyl anisate (3) | 141.15 ± 5.49 | 100.41 ± 5.45 | 121.81 ± 4.25 | 79.83 ± 2.37 | 58.23 ± 0.74 | >100 |
| Syringic acid (4) | 108.02 ± 7.61 | 80.59 ± 2.29 | 54.45 ± 3.72 | 18.94 ± 3.07 | 1.99 ± 2.69 | 1.22 |
| Sinapic acid (5) | 108.43 ± 8.62 | 88.62 ± 4.94 | 60.66 ± 2.88 | 20.27 ± 3.39 | 3.29 ± 1.088 | 1.71 |
| Acetosyringone (6) | 102.51 ± 2.57 | 79.21 ± 6.19 | 53.55 ± 2.08 | 19.92 ± 1.34 | 2.88 ± 0.20 | 1.14 |
| Phenyl acetic acid (7) | 120.57 ± 1.91 | 88.27 ± 3.75 | 108.02 + 6.26 | 110.08 ± 0.82 | 93.20 ± 9.88 | >100 |
| Gentisaldehyde (8) | 139.71 ± 3.14 | 109.46 ± 2.26 | 118.93 ± 2.96 | 149.17 ± 6.1 | 118.93 ± 4.51 | >100 |
| p-Hydroxy benzaldehyde (9) | 96.70 ± 6.98 | 99.17 ± 4.83 | 73.45 ± 6.79 | 52.46 ± 9.14 | 60.08 ± 2.3 | >100 |
| Anthranilic acid (10) | 86.00 ± 6.26 | 51.44 ± 2.37 | 61.11 ± 2.33 | 91.15 ± 4.73 | 94.23 ± 3.311 | >100 |
| Ergosterol peroxide (11) | 98.71 ± 3.19 | 88.72 ± 2.52 | 54.45 ± 1.34 | 30.65 ± 2.26 | 3.70 ± 0.35 | 1.84 |
| Gallic acid | 106.79 ± 4.54 | 86.60 ± 1.96 | 59.60 ± 4.2 | 17.20 ± 1.28 | 2.08 ± 0.54 | 1.53 |
| Doxorubicin | 91.52 ± 1.40 | 80.82 ± 0.46 | 41.92 ± 1.77 | 33.45 ± 0.77 | 8.02 + 0.708 | 0.7 |
| Fungal Metabolite | Concentration ± S.D. (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| Nicotinamide Ext. | Butyrate Ext. | Malt Ext. | Malt + Salt Ext. | Sabouraud Ext. | Czapek Dox Ext. | Rice Ext. | |
| p-Anisic acid (1) | 25.6 ± 1.6 | 5.6 ± 0.5 | - | - | - | - | - |
| p-Anisic acid methyl ester (2) | 12.1 ± 0.7 | 2.3 ± 0.9 | - | - | - | - | - |
| Benzyl anisate (3) | 15.7 ± 1.3 | 1.8 ± 1.4 | - | - | - | - | - |
| Syringic acid (4) | 19.9 ± 0.2 | - | - | - | - | - | - |
| Sinapic acid (5) | 9.8 ± 0.8 | - | - | - | - | - | - |
| Acetosyringone (6) | 8.1 ± 1.1 | - | - | - | - | - | - |
| Phenyl acetic acid (7) | 14.4 ± 0.3 | 1.7 ± 0.7 | - | - | - | - | - |
| Gentisaldehyde (8) | 5.3 ± 0.6 | 2.7 ± 1.4 | - | - | - | - | - |
| p-Hydroxy benzaldehyde (9) | 7.1 ± 1.2 | 2.1 ± 0.8 | - | - | - | - | - |
| Anthranilic acid (10) | - | 9.8 ± 0.1 | - | - | 0.6 ± 0.2 | 0.2 ± 0.4 | 3.2 ± 0.2 |
| Ergosterol peroxide (11) | - | 11.2 ± 0.3 | - | - | 0.9 ± 0.6 | - | 2.7 ± 0.8 |
| Fungal Metabolite | Nicotinamide Concentrations (µM) | Sodium Butyrate Concentrations (M) | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 500 | 0.005 | 0.01 | 0.015 | 0.02 | |
| p-Anisic acid (1) | 4.4 ± 0.4 | 16.8 ± 0.8 | 25.6 ± 1.6 | 21.9 ± 0.9 | 3.2 ± 0.2 | 5.6 ± 0.5 | 4.5 ± 0.8 | 4.2 ± 0.3 |
| p-Anisic acid methyl ester (2) | 2.3 ± 0.2 | 4.8 ± 1.3 | 12.1 ± 0.7 | 13.1 ± 0.6 | 0.3 ± 0.1 | 2.3 ± 0.9 | 2.1 ± 1.2 | 1.9 ± 0.8 |
| Benzyl anisate (3) | 1.8 ± 0.6 | 6.9 ± 0.9 | 15.7 ± 1.3 | 10.9 ± 0.9 | 0.2 ± 0.1 | 1.8 ± 0.4 | 2.2 ± 0.4 | 1.9 ± 0.9 |
| Syringic acid (4) | 3.1 ± 1.1 | 13.8 ± 0.5 | 19.9 ± 0.2 | 18.3 ± 0.3 | - | - | - | |
| Sinapic acid (5) | 0.9 ± 1.3 | 3.1 ± 0.4 | 9.8 ± 0.8 | 8.5 ± 0.6 | - | - | - | |
| Acetosyringone (6) | 0.2 ± 0.2 | 2.7 ± 0.6 | 8.1 ± 1.1 | 5.5 ± 0.2 | - | - | - | |
| Phenyl acetic acid (7) | 2.5 ± 0.7 | 3.7 ± 0.8 | 14.4 ± 0.3 | 12.4 ± 0.7 | 0.3 ± 1.3 | 1.7 ± 0.7 | 1.4 ± 0.5 | 1.3 ± 0.6 |
| Gentisaldehyde (8) | 0.7 ± 0.6 | 1.1 ± 0.1 | 5.3 ± 0.6 | 3.8 ± 0.8 | 1.6 ± 0.8 | 2.7 ± 1.4 | 2.4 ± 0.3 | 3.1 ± 0.2 |
| p-Hydroxy benzaldehyde (9) | 3.2 ± 0.6 | 1.4 ± 0.6 | 7.1 ± 1.2 | 6.6 ± 0.7 | 0.2 ± 0.2 | 2.1 ± 0.8 | 1.1 ± 0.6 | 1.2 ± 0.5 |
| Anthranilic acid (10) | - | - | - | - | 1.7 ± 0.7 | 9.8 ± 0.1 | 7.7 ± 0.3 | 8.1 ± 0.4 |
| Ergosterol peroxide (11) | - | - | - | - | 7.9 ± 0.6 | 11.2 ± 0.3 | 16.5 ± 0.7 | 15.2 ± 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdelmohsen, U.R. Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. https://doi.org/10.3390/md16080253
El-Hawary SS, Sayed AM, Mohammed R, Hassan HM, Zaki MA, Rateb ME, Mohammed TA, Amin E, Abdelmohsen UR. Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum. Marine Drugs. 2018; 16(8):253. https://doi.org/10.3390/md16080253
Chicago/Turabian StyleEl-Hawary, Seham S., Ahmed M. Sayed, Rabab Mohammed, Hossam M. Hassan, Mohamed A. Zaki, Mostafa E. Rateb, Tarek A. Mohammed, Elham Amin, and Usama Ramadan Abdelmohsen. 2018. "Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum" Marine Drugs 16, no. 8: 253. https://doi.org/10.3390/md16080253
APA StyleEl-Hawary, S. S., Sayed, A. M., Mohammed, R., Hassan, H. M., Zaki, M. A., Rateb, M. E., Mohammed, T. A., Amin, E., & Abdelmohsen, U. R. (2018). Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum. Marine Drugs, 16(8), 253. https://doi.org/10.3390/md16080253








