Abstract
The potent antimicrobial extract of a culture of the marine derived actinomycete Streptomyces cyaneofuscatus M-169 was fractionated by reversed phase flash chromatography and preparative HPLC to yield the new Gram-positive antibiotic, anthracimycin B (1), together with its congener, anthracimycin (2). The structure of the new compound was established by analysis of its ESI-TOF MS and 1D and 2D NMR spectra, and comparison with data published for anthracimycin and anthracimycin BII-2619 (3). Notably, anthracimycin seemed to be the major and almost unique component of the extract detected by HPLC-UV-MS, making our S. cyanofuscatus strain an excellent candidate for further biosynthetic studies of this potent antibiotic.
1. Introduction
Deep sea actinomycetes are the source of a wide range of new natural products with antibiotic and anti-cancer properties [1]. The macrolide anthracimycin (2) was first isolated from cultures of a sediment derived Streptomyces sp. CNH365 collected in the Pacific Ocean, off the shore of Santa Barbara (California), and its structure determined by NMR analysis and X-ray crystallography [2]. Since its recent discovery in 2013, anthracimycin became the subject of active research given its extraordinary potent antibiotic activities against Bacillus anthracis [3], whose spores have been used as bioterrorism weapons. Anthracimycin is also active against methicillin-resistant Staphylococcus aureus [4], and novel activities against human hepatocarcinoma have also been reported [5]. Its biosynthetic pathway, involving a type I polyketide synthase (PKS), was deciphered in different studies [6,7,8]. Very recently an isomer of anthracimycin, designated as anthracimycin BII-2619 (3), was identified from a different actinomycete, Nocardiopsis kusanensis [9].
As part of our program aimed at the characterization of actinomycete strains isolated from deep-sea water and invertebrates (1500–4700 m depth) collected in the Cantabrian Sea [10], we focused our attention in extracts of the strain M-169 of Streptomyces cyaneofuscatus, isolated from a gorgonian coral (Order Gorgonacea) collected at 1500 m depth in the Avilés submarine Canyon. New natural products with antibiotic and cytotoxic activities were previously identified from actinomycetes collected in this canyon [11,12,13]. Very recently, the strain M-157 of S. cyaneofuscatus was reported by our groups to produce 3-hydroxyquinaldic acid derivatives that might be biosynthetic precursors of chromodepsipeptides with antimicrobial or cytotoxic properties [14]. Herein, the finding of a new compound with potent antimicrobial activities in samples collected from this extreme environment is reported. LC-UV-HRMS dereplication [15] of the antimicrobial ethyl acetate extract of strain M-169 revealed the presence in the chromatographic UV trace (Figure 1) of only two peaks with molecular formulae of C24H30O4 (P1) and C25H32O4 (P2). Dereplication using the latter molecular formula, the UV spectrum, and the Chapman and Hall Dictionary of Natural Products identified anthracimycin (2) [2] or its isomer, anthracimycin BII-2619 [9], as the only possible hits. Given the potent and interesting antibiotic activities described for these molecules, including activity against B. anthracis, we decided to isolate the two peaks present in the extract to confirm the identity of the major compound (P2) by HRMS and NMR, and to establish the structure of the second component of the extract (P1), whose molecular formula and UV spectrum suggested it to be an analogue of anthracimycin. Fractionation of the extract using reversed phase flash chromatography and preparative HPLC resulted in the isolation of the new natural product, anthracimycin B (1), and the confirmation of the identity of the major component of the extract as anthracimycin (2).
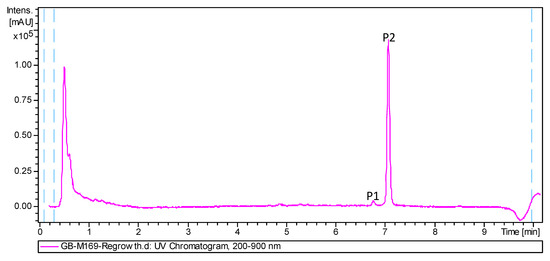
Figure 1.
HPLC trace of the ethyl acetate extract of Streptomyces cyaneofuscatus M-169.
2. Results
2.1. Taxonomy and Phylogenetic Analysis of the Strain M-157
The 16S rDNA of strain M-169 was amplified by polymerase chain reaction (PCR) and sequenced [10]. After Basic Logic Alignment Search Tool (BLAST) sequence comparison, strain M-169 showed 100% identity to S. cyaneofuscatus (Accession number NR_115383); thus, this strain was designated as S. cyaneofuscatus M-169 (EMBL Sequence number LN824211). The phylogenetic tree generated by the neighbour-joining and maximum likelihood method based on the 16S rDNA sequence clearly revealed the evolutionary relationship of strain M-169 with a group of known Streptomyces species (Figure 2).
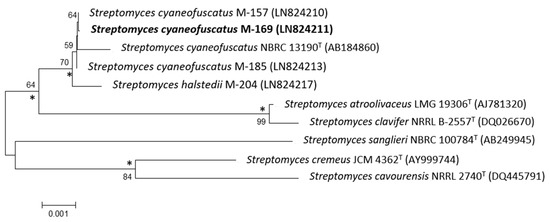
Figure 2.
Neighbour-joining phylogenetic tree obtained by distance matrix analysis of 16S rDNA sequences, showing Streptomyces cyaneofuscatus M-169 position and most closely related phylogenetic neighbours. Numbers on branch nodes are bootstrap values (1000 resamplings; only values >50% are given). Asterisks indicate that the corresponding nodes were also recovered in the maximum likelihood tree. Bar indicates 0.1% sequence divergence.
2.2. Isolation and Structural Elucidation of Compounds 1 and 2
A culture of S. cyaneofuscatus M-169 in solid R5A medium was extracted with acidified ethyl acetate. Fractionation of this extract using reversed phase C18 column chromatography and preparative HPLC yielded the new compound, anthracimycin B (1), together with anthramycin (2) (Figure 3), whose NMR spectra were identical to those described in the literature [2].
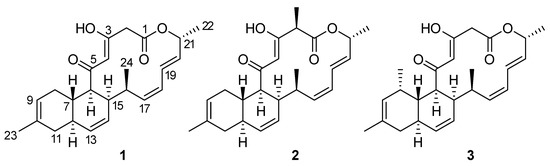
Figure 3.
Structures of compounds 1–3.
Anthracimycin B (1) was isolated as a white amorphous solid, whose molecular formula, C24H30O4, was deduced from the presence of a protonated ion at m/z 383.2220 in its ESI-TOF mass spectrum (calc. for C24H31O4+, 383.2217). This molecular formula together with UV absorption maxima at 236 and 288 nm were compatible with an anthramycin-like structure for the molecule. Chemical shifts measured in its 1H and 13C NMR spectra (Table 1) corroborated this structural similarity. Comparing the 1D and 2D NMR spectra with those published for anthracimycin [2], the major differences were found in the region around carbons, C-1 to C-3, where the methyl group attached to C-2 in 2 was absent in the structure of 1. Analysis of the 1H NMR and HSQC spectra revealed the presence of two doublet methylene protons (δH = 3.50 y 3.22 ppm), which correlated in the HMBC spectrum to the signals of the lactone carbonyl C-1 and the oxygenated enol carbon C-3. Additionally, signals corresponding to H-23 and C-23 of anthracimycin were not observed in the spectra of 1. Similar NMR chemical shifts reported for 3 [9], having the same substructure as 1 around the C-1 to C-3 region, confirmed our structural proposal. These evidences, together with correlations observed in the COSY and HMBC spectra of 1 (Figure 4), confirmed the planar structure of anthracimycin B as depicted in Figure 3. The similarity in magnitude and negative value of the specific rotations of 1 and 2 ( −338.9 (1), −333.0 (2) [2]), revealed that the same absolute configuration could be proposed for these two molecules. The absolute configuration of anthracimycin had previously been determined by X-ray diffraction [3]. It is also worth mentioning that during the structural elucidation of anthracimycin B, a mistake was detected in the NMR data of anthracimycin published by Jang et al. [3], where protons attached to C-15 and C-16 seemed to be exchanged based on COSY correlations analysis. The proton correlating to the signal of the methyl group H-24 in the COSY spectrum has necessarily to be H-16, so the signal at 2.60 ppm belongs to H-16, and, therefore, the signal at 1.93 ppm to proton H-15.

Table 1.
NMR spectroscopic data (CDCl3, 500 MHz) for compound 1.
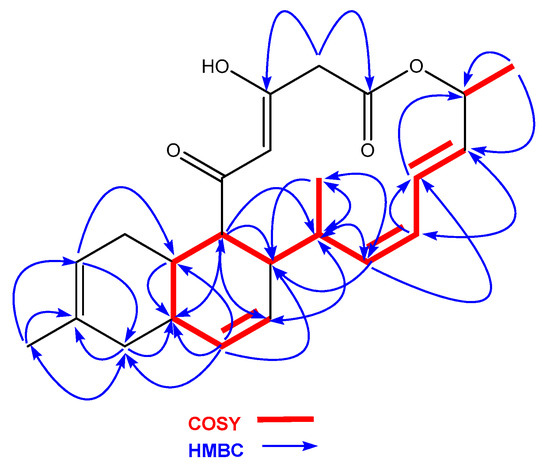
Figure 4.
COSY and HMBC correlations observed in the structure of 1.
Compounds 1 and 2 were tested against a panel of pathogenic bacteria consisting of four Gram-positive (methicillin sensitive and methicillin resistant S. aureus (MSSA and MRSA), vancomycin sensitive Enterococcus faecium, and vancomycin sensitive Enterococcus faecalis), and two Gram-negative (Escherichia coli and Klebsiella pneumoniae). Additionally, the anti-tubercular activity against Mycobacterium tuberculosis was also evaluated. Anthracimycin confirmed the potent activity against Gram-positive bacteria previously reported [2], with MICs in all cases below the lowest concentration tested of 0.03 μg/mL. Although less potent, anthracimycin B also displayed potent antibacterial activities with minimum inhibitory concentrations (MICs) in the submicromolar range against most of the pathogens tested (Table 2). Similar levels of activity were recently reported for compound 3 [9], perhaps indicating that, although the target and mode of action of the compound are still unknown, the presence of the methyl group at C-2 in anthracimycin is essential for its potent antimicrobial activity. Unfortunately, due to the safety level of our facilities, B. anthracis was not among the bacterial strains tested, but based on the previously reported activity for 2 against that pathogen, perhaps a significant level of activity for compound 1 can also be expected. Interestingly, anthracimycin also displayed activity in our study against M. tuberculosis, expanding the panel of antimicrobial properties of this structurally unique drug.

Table 2.
MIC values of compounds 1 and 2 against bacterial pathogens.
3. Discussion
Anthracimycin B, a potent antibiotic against Gram-positive pathogens, was obtained from a marine-derived actinomycete isolated from a cold-water coral collected in the Avilés Canyon (Cantabrian Sea, Northeast Atlantic Ocean). This unique coral reef ecosystem is becoming a hot spot for the discovery of novel natural products with antibiotic and cytotoxic activities. Anthracimycin B can be considered the biosynthetic precursor of anthracimycin. Its isolation from cultures of S. cyaneofuscatus M-169 would point out to the existence in the biosynthetic pathway of anthracimycin of a post-PKS tailoring reaction catalyzed by a free-standing S-adenosylmethionine (SAM)-dependent methyltransferase similar to CtoF in the biosynthesis of chlorotonils A and B [6]. This finding would be somehow in contradiction with the current biosynthetic proposal for anthracimycin, where the final methylation step at C-2 seems to be encoded by a methyltransferase incorporated to module 10 of the atcF biosynthetic gene [6,7]. Alternatively, the presence of 2 in our extracts might be the result of a malfunctioning of the methyltransferase in module 10 of the atcF gene, leading to the biosynthesis of a demethylated version of the drug in low quantities. Furthermore, our findings are another example of the significance of the genus, Streptomyces, particularly bioactive S. cyaneofuscatus strains from oceanic environments, for the discovery of novel natural products of medical interest. The relevance of the strain M-169 of S. cyaneofuscatus, in addition to the production of a new member of the anthramycin structural class with implications in the current biosynthetic pathway proposals, resides in its ability to produce high quantities (17.7 mg/L in our study) of the final compound, anthracimycin, highlighting this strain as an ideal candidate for further biosynthetic studies of this interesting antibacterial drug. Additionally, novel biological activities against M. tuberculosis were also detected for anthracimycin, expanding the antimicrobial potential of the molecule. Finally, although very preliminary, our study also suggests that the methyl group at C-2 present in anthracimycin plays a key role in its potent antibacterial activity.
4. Materials and Methods
4.1. General Experimental Procedures
Optical rotations were measured using a Jasco P-2000 polarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were obtained with an Agilent 1100 DAD (Agilent Technologies, Santa Clara, CA, USA). IR spectra were recorded on a JASCO FT/IR-4100 spectrometer (JASCO Corporation, Tokyo, Japan) equipped with a PIKE MIRacleTM single reflection ATR accessory (PIKE Technologies Inc., Madison, WI, USA). NMR spectra were recorded on a Bruker Avance III spectrometer (500 and 125 MHz for 1H and 13C NMR, respectively) equipped with a 1.7 mm TCI MicroCryoProbeTM (Bruker Biospin, Falländen, Switzerland). Chemical shifts were reported in ppm using the signals of the residual solvent as internal reference (δH 7.26 and δC 77.0 for CDCl3). LC–MS and LC–HRMS analyses were performed as described previously [16].
4.2. Taxonomic Identification of the Producing Microorganism
The strain, S. cyaneofuscatus M-169, was subjected to phylogenetic analysis based on 16S rDNA sequence analysis [10]. Phylogenetic analysis was performed using MEGA version 6.0 [17] after multiple alignment of data by Clustal Omega [18]. Distances (distance options according to the Kimura two-parameter model [19]) and clustering with the neighbour-joining [20] and maximum likelihood [21] methods were evaluated using bootstrap values based on 1000 replications [22].
4.3. Fermentation of the Producing Microorganism
35 petri dishes with R5A solid medium [23] (20 mL each) were inoculated with spores of strain M-169. After 6 days at 28 °C, plates were extracted with ethyl acetate. The extract was evaporated to dryness, resuspended in tert-butanol: water (1:1), and freeze dried.
4.4. Extraction and Isolation
The ethyl acetate extract obtained as described above was subjected to reversed phase column chromatography (Redisep Rf Reversed Phase C-18 (Teledyne Isco, Lincoln, NE, USA), 43 g, 0.040–0.063 mm). The column was eluted with H2O: MeOH using isocratic elution 75:25 for 10 min followed by a gradient to 100% MeOH in 35 min, a flow of 18 mL/min and UV detection at 236 and 288 nm. Fractions 13–17 containing the compounds of interest, identified by LC-MS, were pooled and subjected to semipreparative HPLC (Waters™ XBridge® C18 (Waters Corporation, Milford, MA, USA), 10 × 150 mm, 5 µm), using a gradient H2O: CH3CN from 75 to 100% CH3CN in 45 min, a flow of 3.6 mL/min, and UV detection at 210 and 236 nm. Pure compounds 1 (1.0 mg, tR 17.0 min) and 2 (12.4 mg, tR 20.4 min) were obtained under these conditions.
Anthracimycin B (1): White amorphous solid; −338.9 (c 0.053, CHCl3); UV (DAD) λmax 236, 288 nm; IR (ATR) νmax 3014, 2959, 2925, 2873, 2855, 2832, 1740, 1715, 1606, 1456, 1376, 1280, 1250, 1215 cm−1; for 1H and 13C NMR data see Table 1; (+)-ESI-TOFMS m/z 383.220 [M + H]+ (calcd. for C24H31O4+, 383.2217), 400.2487 [M + NH4]+ (calcd. for C24H34NO4+, 400.2482).
4.5. Antibacterial Activity Assays
The antibacterial activities of the compounds were evaluated using sequential 2-fold serial dilutions of each compound in DMSO to provide 10 concentrations starting at 16 µg/mL for all the assays. Activity was measured against the strains included in Table 2 as previously reported [24,25].
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/11/406/s1, Figure S1: HPLC-UV trace, UV and ESI-TOF spectra of compound 1; Figure S2: 1H NMR spectrum (CDCl3, 500 MHz) of compound 1; Figure S3: 1H NMR (CDCl3, 500 MHz) of compound 1 (expansion from 13 to 17 ppm). Figure S4: 13C NMR spectrum (CDCl3, 125 MHz) of compound 1; Figure S5: COSY spectrum of compound 1; Figure S6: HSQC spectrum of compound 1; Figure S7: HMBC spectrum of compound 1; Figure S8: HPLC-UV trace, UV and ESI-TOF spectra of compound 2; Figure S9: 1H NMR spectrum (CDCl3, 500 MHz) of compound 2; Figure S10: 1H NMR (CDCl3, 500 MHz) of compound 1 (expansion from 13 to 17 ppm).
Author Contributions
A.S.-V. and G.B. carried out the fermentation, extraction and taxonomic identification of the strain. V.R. performed the purification of the compounds. V.R., J.M and F.R. performed the structural elucidation of the compounds; M.d.l.C. performed the antibacterial assays; L.A.G, G.B., J.M. and F.R. conceived and supervised the work; F.R. and G.B. wrote the paper, which was revised and approved by all the authors.
Funding
This study was financially supported by the Universidad de Oviedo (UNOV-11-MA-02), Gobierno del Principado de Asturias (SV-PA-13-ECOEMP-62) and Fundación MEDINA. The polarimeter, IR, and NMR equipment used in this work were acquired via grants for scientific and technological infrastructures from the Ministerio de Ciencia e Innovación (Grants No. PCT-010000-2010-4 (NMR), INP-2011-0016-PCT-010000 ACT6 (polarimeter and IR)).
Acknowledgments
The authors are grateful to José Luis Acuña for his invitation to the BIOCANT3 oceanographic expedition (DOSMARES) where the strain M-169 of Streptomyces cyaneofuscatus was isolated. Ignacio Pérez-Victoria helped with the NMR analyses. This is a contribution of the Asturias Marine Observatory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamjam, M.; Sivalingam, P.; Deng, Z.; Hong, K. Deep Sea Actinomycetes and Their Secondary Metabolites. Front. Microbiol. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Nam, S.J.; Locke, J.B.; Kauffman, C.A.; Beatty, D.S.; Paul, L.A.; Fenical, W. Anthracimycin, a Potent Anthrax Antibiotic from a Marine-Derived Actinomycete. Angew. Chem. Int. Ed. 2013, 52, 7822–7824. [Google Scholar] [CrossRef] [PubMed]
- Head, B.M.; Rubinstein, E.; Meyers, A.F.A. Alternative pre-approved and novel therapies for the treatment of anthrax. BMC Infect. Dis. 2016, 16, 621. [Google Scholar] [CrossRef] [PubMed]
- Hensler, M.E.; Jang, K.H.; Thienphrapa, W.; Vuong, L.; Tran, D.N.; Soubih, E.; Lin, L.; Haste, N.M.; Cunningham, M.L.; Kwan, B.P.; et al. Anthracimycin activity against contemporary methicillin-resistant Staphylococcus aureus. J. Antibiot. 2014, 67, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamashita, T.; Okada, H.; Oishi, N.; Sunagozaka, H.; Nio, K.; Hayashi, T.; Hara, Y.; Asahina, Y.; Yoshida, M.; et al. A Novel mTOR Inhibitor; Anthracimycin for the Treatment of Human Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, K.; Jansen, R.; Gerth, K.; Huch, V.; Krug, D.; Fenical, W.; Müller, R. Two of a Kind—The Biosynthetic Pathways of Chlorotonil and Anthracimycin. ACS Chem. Biol. 2015, 10, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Alt, S.; Wilkinson, B. Biosynthesis of the Novel Macrolide Antibiotic Anthracimycin. ACS Chem. Biol. 2015, 10, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Harunari, E.; Komaki, H.; Igarashi, Y. Biosynthetic origin of anthracimycin: A tricyclic macrolide from Streptomyces sp. J. Antibiot. 2015, 69, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Sirota, F.L.; Goh, F.; Low, K.N.; Yang, L.K.; Crasta, S.C.; Eisenhaber, B.; Eisenhaber, F.; Kanagasundaram, Y.; Ng, S.B. Isolation and Identification of an Anthracimycin Analogue from Nocardiopsis kunsanensis, a Halophile from a Saltern, by Genomic Mining Strategy. J. Genom. 2018, 6, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; González, V.; Braña, A.F.; Palacios, J.J.; Otero, L.; Fernández, J.; Molina, A.; Kulik, A.; Vázquez, F.; Acuña, J.L.; et al. Pharmacological Potential of Phylogenetically Diverse Actinobacteria Isolated from Deep-Sea Coral Ecosystems of the Submarine Avilés Canyon in the Cantabrian Sea. Microb. Ecol. 2017, 73, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Braña, A.; Sarmiento-Vizcaíno, A.; Osset, M.; Pérez-Victoria, I.; Martín, J.; de Pedro, N.; de la Cruz, M.; Díaz, C.; Vicente, F.; Reyes, F.; et al. Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa. Mar. Drugs 2017, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Braña, A.F.; Sarmiento-Vizcaíno, A.; Pérez-Victoria, I.; Otero, L.; Fernández, J.; Palacios, J.J.; Martín, J.; de la Cruz, M.; Díaz, C.; Vicente, F.; et al. Branimycins B and C, Antibiotics Produced by the Abyssal Actinobacterium Pseudonocardia carboxydivorans M-227. J. Nat. Prod. 2017, 80, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; Braña, A.; Pérez-Victoria, I.; Martín, J.; de Pedro, N.; Cruz, M.; Díaz, C.; Vicente, F.; Acuña, J.; Reyes, F.; et al. Paulomycin G, a New Natural Product with Cytotoxic Activity against Tumor Cell Lines Produced by Deep-Sea Sediment Derived Micromonospora matsumotoense M-412 from the Avilés Canyon in the Cantabrian Sea. Mar. Drugs 2017, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-López, F.J.; Alcalde, E.; Sarmiento-Vizcaíno, A.; Díaz, C.; Cautain, B.; García, L.A.; Blanco, G.; Reyes, F. New 3-Hydroxyquinaldic Acid Derivatives from Cultures of the Marine Derived Actinomycete Streptomyces cyaneofuscatus M-157. Mar. Drugs 2018, 16, 371. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Victoria, I.; Martín, J.; Reyes, F. Combined LC/UV/MS and NMR Strategies for the Dereplication of Marine Natural Products. Planta Med. 2016, 82, 857–871. [Google Scholar] [CrossRef]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Vicente, F.; Genilloud, O.; et al. MDN-0104, an Antiplasmodial Betaine Lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Weissbach, U.; Reillo, C.S.; Braña, A.F.; Méndez, C.; Rohr, J.; Salas, J.A. Identification of two genes from Streptomyces argillaceus encoding two glycosyltransferases involved in the transfer of a disaccharide during the biosynthesis of the antitrumor drug mithramycin. J. Bacteriol. 1998, 180, 4929–4937. [Google Scholar] [PubMed]
- Audoin, C.; Bonhomme, D.; Ivanisevic, J.; Cruz, M.; Cautain, B.; Monteiro, M.; Reyes, F.; Rios, L.; Perez, T.; Thomas, O. Balibalosides, an Original Family of Glucosylated Sesterterpenes Produced by the Mediterranean Sponge Oscarella balibaloi. Mar. Drugs 2013, 11, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Martín, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First identification of marine diatoms with anti-tuberculosis activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).