Excavatolide-B Enhances Contextual Memory Retrieval via Repressing the Delayed Rectifier Potassium Current in the Hippocampus
Abstract
1. Introduction
2. Results
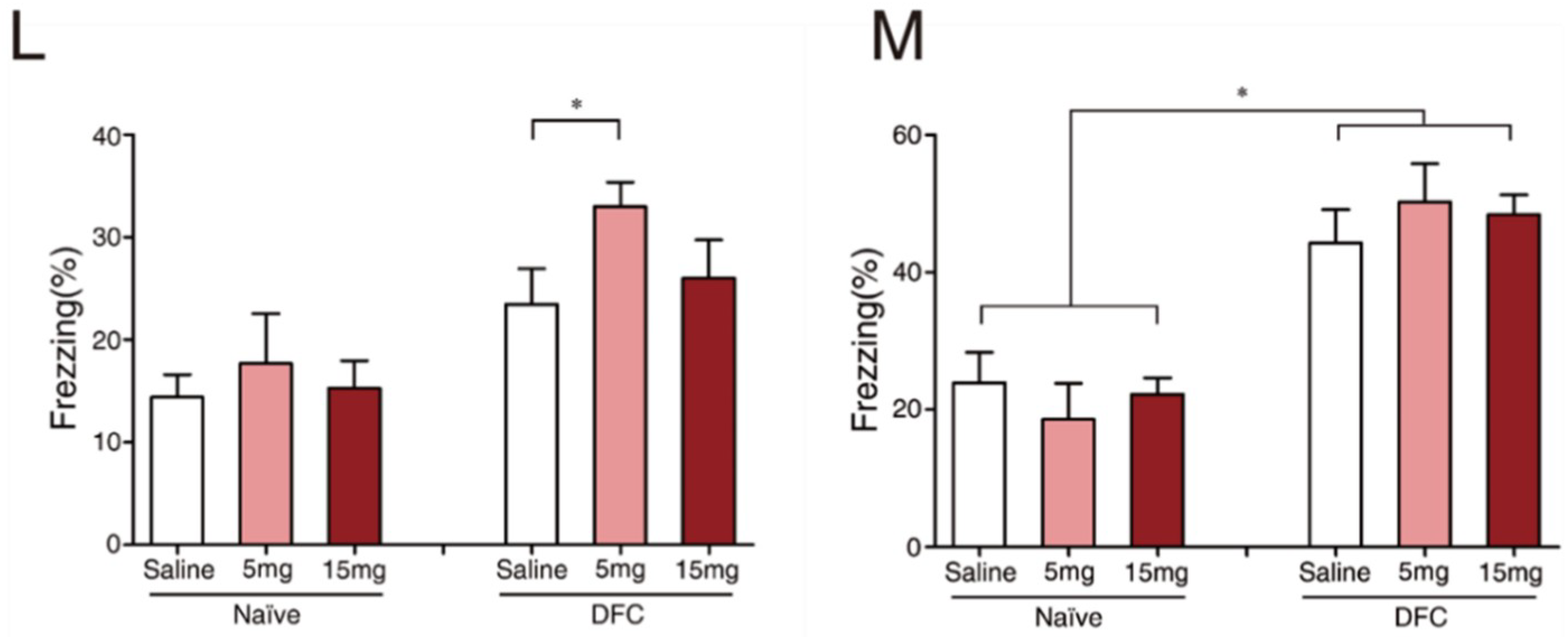
2.1. Exc-B Repressed Consolidation, Enhanced Retrieval of Contextual Memory, and Did Not Affect Tone Memory
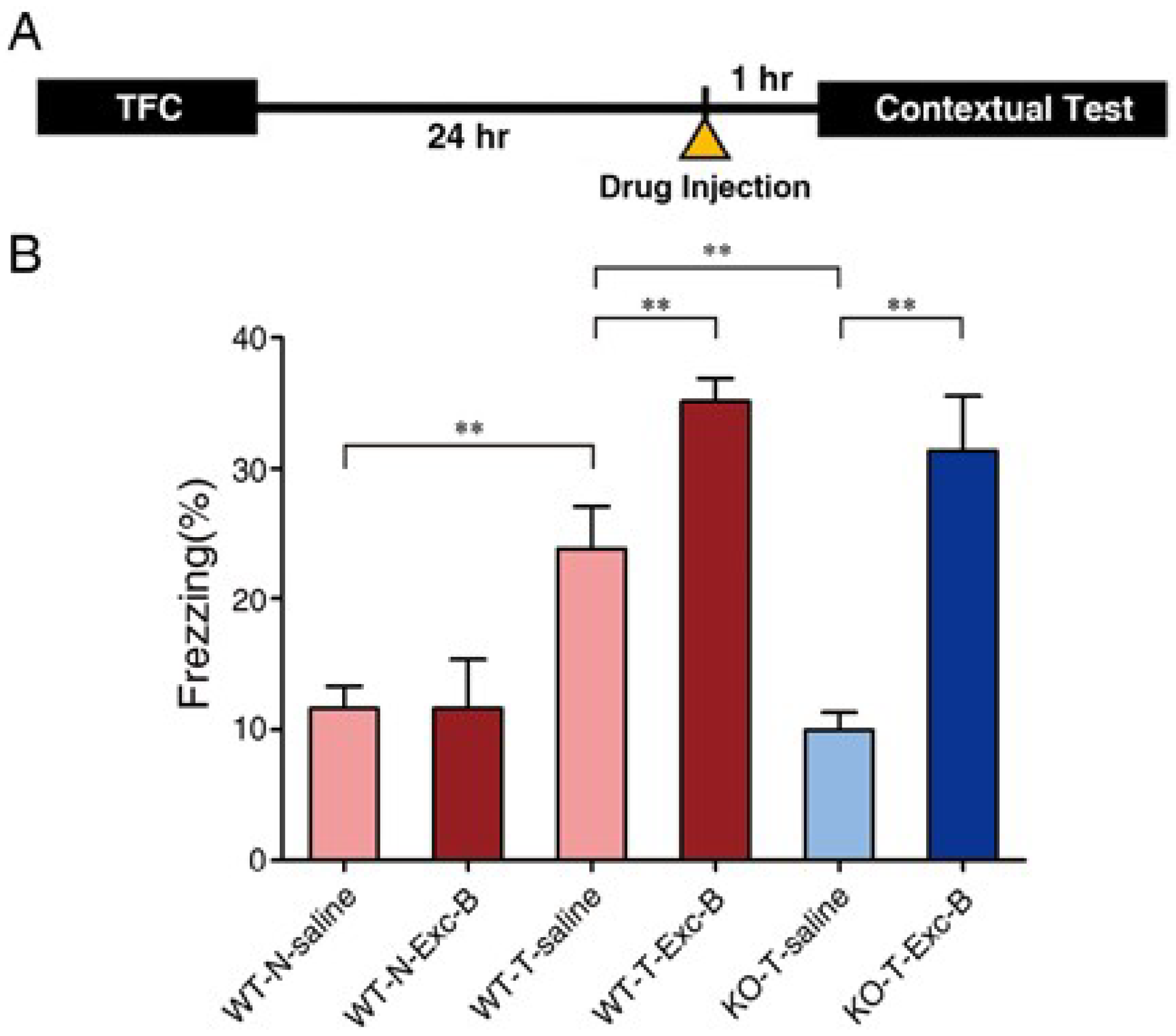
2.2. Exc-B Injection Reversed Impaired Memory Retrieval to the Context in Cav3.2−/− Mice
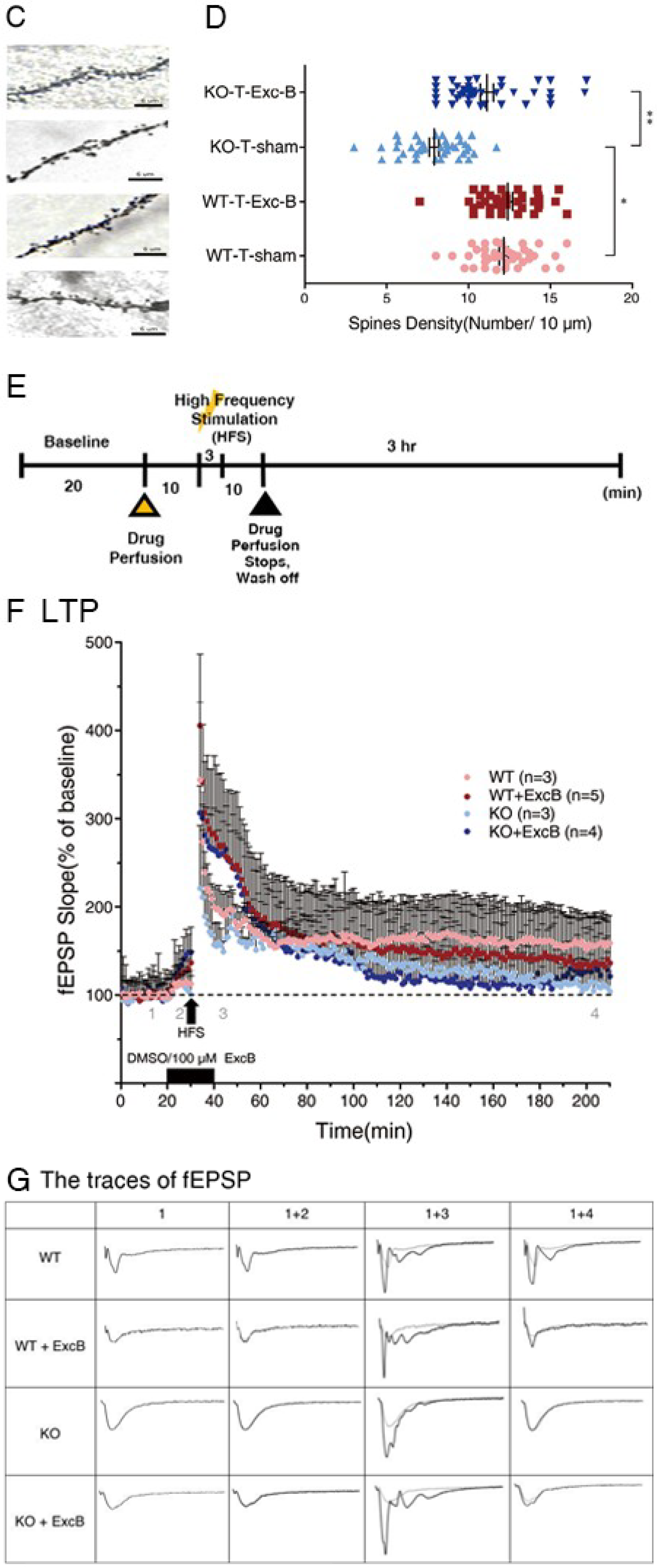
2.3. Exc-B Increased Dendritic Spine Density in Cav3.2−/− Mice
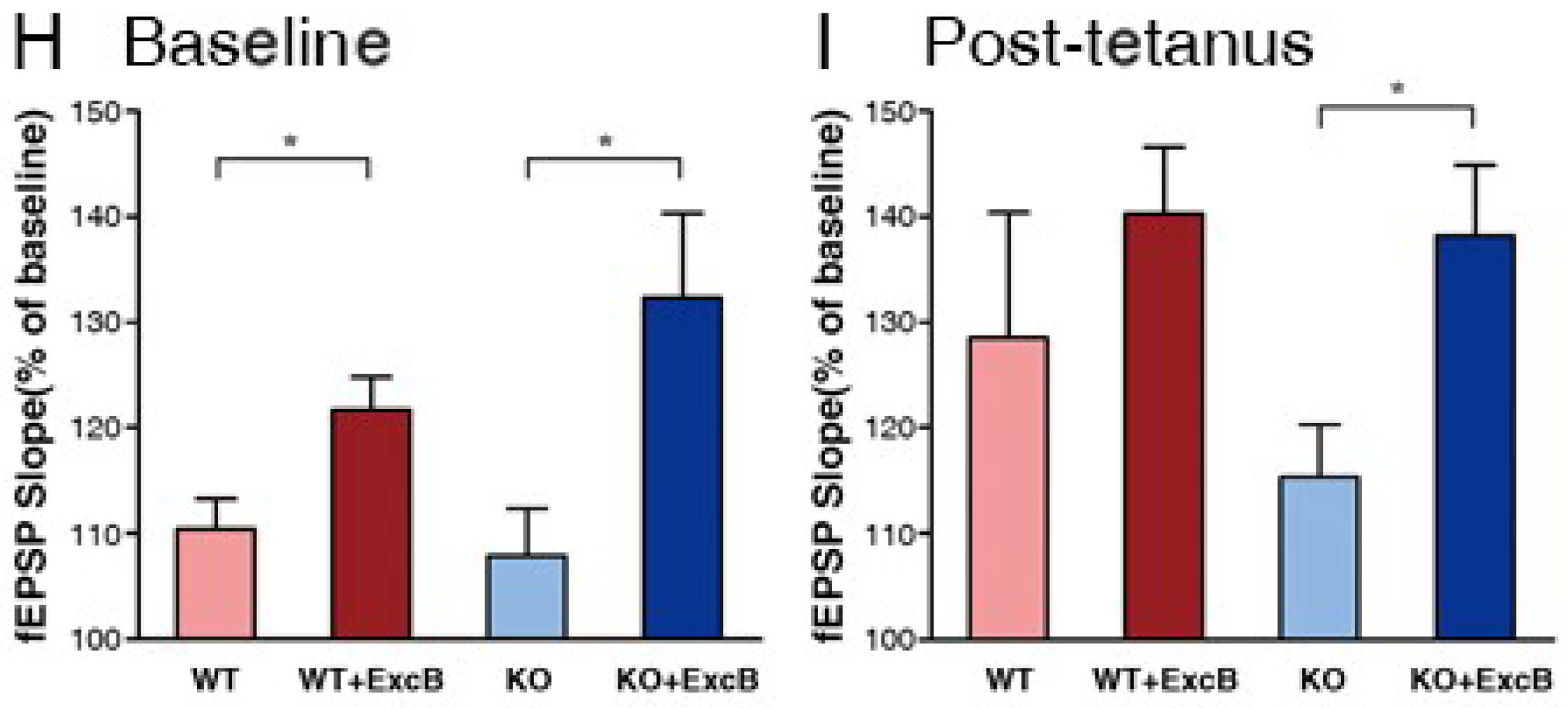
2.4. Early Long-Term Potentiation (LTP) Was Enhanced with Exc-B Injection
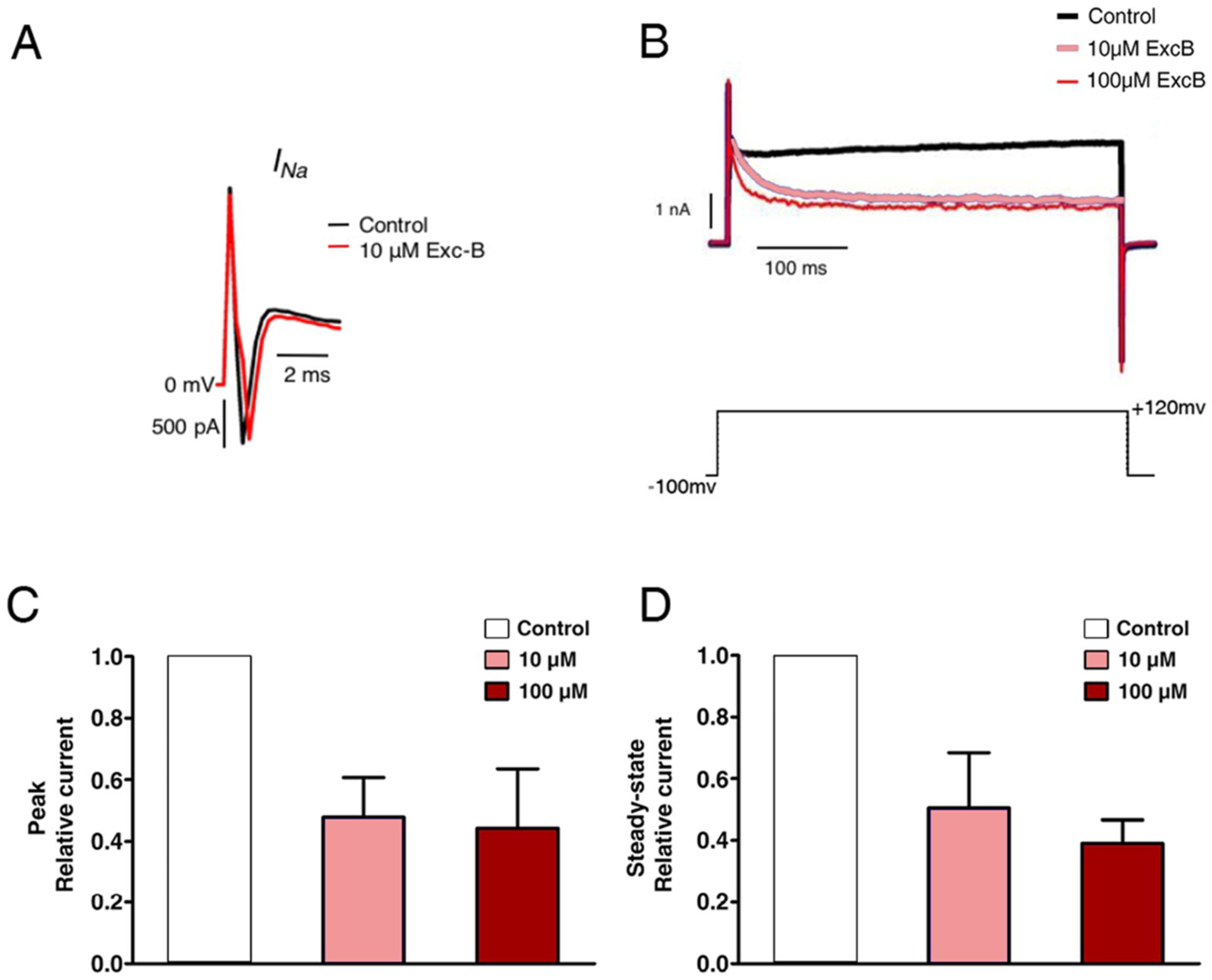
2.5. Exc-B Inhibited the Potassium Current Amplitude in Hippocampal Neurons
2.6. Exc-B Reduced the Increased Potassium Current in the Hippocampal Neurons of the Cav3.2−/− Mice
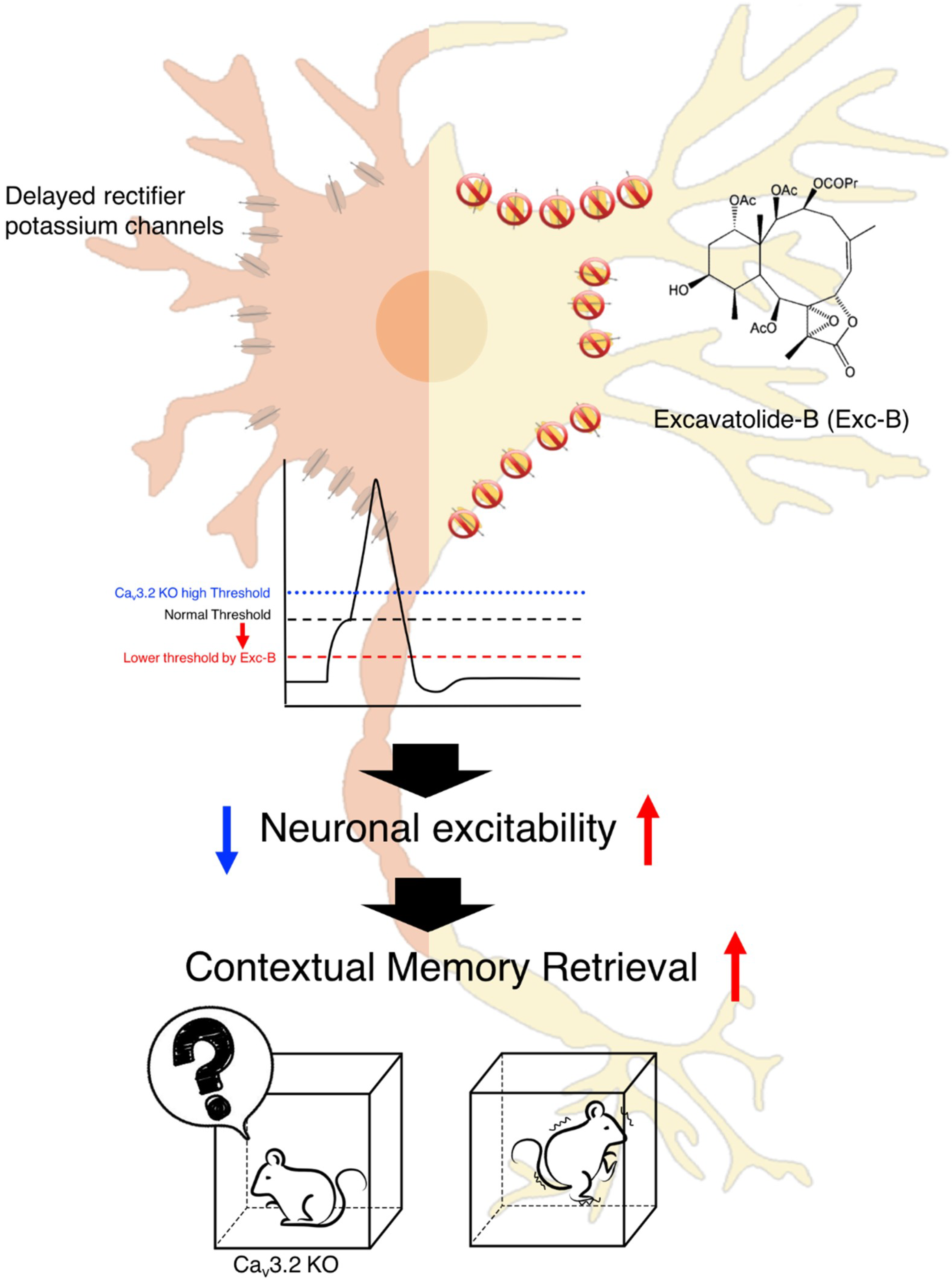
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Drug Administration
4.4. Locomotor and Social Behavior Test
4.4.1. Open Field Test
4.4.2. Fear Conditioning (FC)
4.5. Golgi Staining and Quantification of Dendritic Spine Density
4.6. Primary Neuronal Cell Culture
4.7. Whole-Cell Patch Recording
4.8. LTP Recording
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jafarian, M.; Karimzadeh, F.; Alipour, F.; Attari, F.; Lotfinia, A.A.; Speckmann, E.J.; Zarrindast, M.R.; Gorji, A. Cognitive impairments and neuronal injury in different brain regions of a genetic rat model of absence epilepsy. Neuroscience 2015, 298, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Yoo, D.S.; Stotz, S.C.; Cherry, A.; Clapham, D.E.; Keating, M.T. CACNA1H mutations in autism spectrum disorders. J. Boil. Chem. 2006, 281, 22085–22091. [Google Scholar] [CrossRef] [PubMed]
- Gangarossa, G.; Laffray, S.; Bourinet, E.; Valjent, E. T-type calcium channel Ca(v)3.2 deficient mice show elevated anxiety, impaired memory and reduced sensitivity to psychostimulants. Front. Behav. Neurosci. 2014, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, L.; Kwapil, T.R. Episodic memory retrieval is impaired in negative schizotypy under fast response deadline. Schizophr. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kissler, J.; Bauml, K.H. Memory retrieval in schizophrenia: Evidence from part-list cuing. J. Int. Neuropsychol. Soc. 2005, 11, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.S.; Arons, A.; Mitchell, T.I.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016, 531, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and Safety of Donepezil, Galantamine, and Rivastigmine for the Treatment of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar] [PubMed]
- Lockhart, I.A.; Mitchell, S.A.; Kelly, S. Safety and tolerability of donepezil, rivastigmine and galantamine for patients with Alzheimer’s disease: Systematic review of the ‘real-world’ evidence. Dement. Geriatr. Cogn. Disord. 2009, 28, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M. Natural products as a resource for new drugs. Pharm. Res. 1996, 13, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Peixe, L.; Gomes, N.C.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.H.; Sung, P.J.; Cheng, M.C.; Liu, H.Y.; Fang, L.S.; Duh, C.Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A-E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.R.; Tai, B.Y.; Cheng, P.Y.; Chen, P.N.; Sung, P.J.; Wen, Z.H.; Hsu, C.H. Excavatolide B Modulates the Electrophysiological Characteristics and Calcium Homeostasis of Atrial Myocytes. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Shen, J.W.; Chung, N.C.; Min, M.Y.; Cheng, S.J.; Liu, I.Y. Retrieval of context-associated memory is dependent on the Ca(v)3.2 T-type calcium channel. PLoS ONE 2012, 7, e29384. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.B. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: News from the world of knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R227–R237. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.; Henseler, C.; Lundt, A.; Wormuth, C.; Soos, J.; Broich, K.; Ehninger, D.; Weiergraber, M. Gender specific hippocampal whole genome transcriptome data from mice lacking the Cav2.3 R-type or Cav3.2 T-type voltage-gated calcium channel. Data Brief 2017, 12, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.L.; Cain, S.M.; Ng, C.; Sirdesai, S.; David, L.S.; Kyi, M.; Garcia, E.; Tyson, J.R.; Reid, C.A.; Bahlo, M.; et al. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Sheng, M. Dendritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Swant, J.; Chirwa, S.; Stanwood, G.; Khoshbouei, H. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS ONE 2010, 5, e11382. [Google Scholar] [CrossRef] [PubMed]
- Nicola, S.M.; Kombian, S.B.; Malenka, R.C. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 1591–1604. [Google Scholar] [CrossRef]
- Szapiro, G.; Galante, J.M.; Barros, D.M.; Levi de Stein, M.; Vianna, M.R.; Izquierdo, L.A.; Izquierdo, I.; Medina, J.H. Molecular mechanisms of memory retrieval. Neurochem. Res 2002, 27, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ye, M.; Tian, C.; Yang, M.; Wang, Y.; Shu, Y. Dopaminergic modulation of axonal potassium channels and action potential waveform in pyramidal neurons of prefrontal cortex. J. Physiol. 2013, 591, 3233–3251. [Google Scholar] [CrossRef] [PubMed]
- Martel, P.; Leo, D.; Fulton, S.; Berard, M.; Trudeau, L.E. Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS ONE 2011, 6, e20402. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhu, J.; Zhong, Q.; Shi, C.; Dang, Y.; Han, W.; Liu, X.; Xu, M.; Chen, T. Distinct roles of methamphetamine in modulating spatial memory consolidation, retrieval, reconsolidation and the accompanying changes of ERK and CREB activation in hippocampus and prefrontal cortex. Neuropharmacology 2013, 67, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; Bartolini, A. Influence of potassium channel modulators on cognitive processes in mice. Br. J. Pharmacol. 1998, 123, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.B.; Pan, Y.P.; Tong, X.Y.; Xu, X.H.; Wang, X.L. Delayed rectifier potassium currents and Kv2.1 mRNA increase in hippocampal neurons of scopolamine-induced memory-deficient rats. Neurosci. Lett. 2005, 373, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.H.; Eom, K.; Lee, K.H.; Ho, W.K.; Lee, S.H. Activity-dependent downregulation of D-type K+ channel subunit Kv1.2 in rat hippocampal CA3 pyramidal neurons. J. Physiol. 2013, 591, 5525–5540. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Lakkadwala, S.; Modgil, A.; Singh, J. The Role of Cell-Penetrating Peptide and Transferrin on Enhanced Delivery of Drug to Brain. Int. J. Mol. Sci. 2016, 17, 806. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Lombard, C.; Lemogne, C.; Gierski, F.; Bera-Potelle, C.; Tran, E.; Portefaix, C.; Kaladjian, A.; Pierot, L.; Limosin, F. Neural basis of autobiographical memory retrieval in schizophrenia. Br. J. Psychiatry 2012, 201, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, N.L.; Sreenivasan, K.R.; Deshpande, G.; Reid, M.A.; Hadley, J.; White, D.M.; Ver Hoef, L.; Lahti, A.C. Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Hum. Brain Mapp. 2015, 36, 1442–1457. [Google Scholar] [CrossRef] [PubMed]
- Brezis, R.S.; Galili, T.; Wong, T.; Piggot, J.I. Impaired social processing in autism and its reflections in memory: A deeper view of encoding and retrieval processes. J. Autism Dev. Disord. 2014, 44, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Richter, F.R.; Bays, P.M.; Plaisted-Grant, K.C.; Baron-Cohen, S.; Simons, J.S. Reduced Hippocampal Functional Connectivity During Episodic Memory Retrieval in Autism. Cereb. Cortex 2017, 27, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.J.; Roy, D.S.; Pignatelli, M.; Arons, A.; Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 2015, 348, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, S.; Gearing, M.; Jin, L.W.; Levey, A. Potassium channel Kv1.3 is highly expressed by microglia in human Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2015, 44, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Pannaccione, A.; Boscia, F.; Scorziello, A.; Adornetto, A.; Castaldo, P.; Sirabella, R.; Taglialatela, M.; Di Renzo, G.F.; Annunziato, L. Up-regulation and increased activity of KV3.4 channels and their accessory subunit MinK-related peptide 2 induced by amyloid peptide are involved in apoptotic neuronal death. Mol. Pharmacol. 2007, 72, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, M.; Plant, L.D.; Webster, N.J.; Vaughan, P.F.; Henderson, Z.; Pearson, H.A. Differential effects of unaggregated and aggregated amyloid beta protein (1–40) on K(+) channel currents in primary cultures of rat cerebellar granule and cortical neurones. J. Neurochem. 2001, 79, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Farhangrazi, Z.S.; Ying, H.S.; Yeh, C.H.; Choi, D.W. Enhancement of outward potassium current may participate in beta-amyloid peptide-induced cortical neuronal death. Neurobiol. Dis. 1998, 5, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.P.; Xu, X.H.; Wang, X.L. Galantamine blocks delayed rectifier, but not transient outward potassium current in rat dissociated hippocampal pyramidal neurons. Neurosci. Lett. 2003, 336, 37–40. [Google Scholar] [CrossRef]
- Wen, R.J.; Huang, D.; Zhang, Y.; Liu, Y.W. Bis(3)-tacrine inhibits the sustained potassium current in cultured rat hippocampal neurons. Physiol. Res. 2017, 66, 539–544. [Google Scholar] [PubMed]
- Wilkinson, D.G. Galantamine: A new treatment for Alzheimer’s disease. Expert Rev. Neurother. 2001, 1, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lamping, K.G.; Nuno, D.W.; Barresi, R.; Prouty, S.J.; Lavoie, J.L.; Cribbs, L.L.; England, S.K.; Sigmund, C.D.; Weiss, R.M.; et al. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 2003, 302, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.; Kolb, B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J. Neurosci. Methods 1998, 79, 1–4. [Google Scholar] [CrossRef]
- Beaudoin, G.M., 3rd; Lee, S.H.; Singh, D.; Yuan, Y.; Ng, Y.G.; Reichardt, L.F.; Arikkath, J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, I.Y.; Hsu, Y.-L.; Chen, C.-C.; Chen, M.-F.; Wen, Z.-H.; Huang, H.-T.; Liu, I.Y. Excavatolide-B Enhances Contextual Memory Retrieval via Repressing the Delayed Rectifier Potassium Current in the Hippocampus. Mar. Drugs 2018, 16, 405. https://doi.org/10.3390/md16110405
Huang IY, Hsu Y-L, Chen C-C, Chen M-F, Wen Z-H, Huang H-T, Liu IY. Excavatolide-B Enhances Contextual Memory Retrieval via Repressing the Delayed Rectifier Potassium Current in the Hippocampus. Marine Drugs. 2018; 16(11):405. https://doi.org/10.3390/md16110405
Chicago/Turabian StyleHuang, Irene Y., Yu-Luan Hsu, Chien-Chang Chen, Mei-Fang Chen, Zhi-Hong Wen, Hsien-Ting Huang, and Ingrid Y. Liu. 2018. "Excavatolide-B Enhances Contextual Memory Retrieval via Repressing the Delayed Rectifier Potassium Current in the Hippocampus" Marine Drugs 16, no. 11: 405. https://doi.org/10.3390/md16110405
APA StyleHuang, I. Y., Hsu, Y.-L., Chen, C.-C., Chen, M.-F., Wen, Z.-H., Huang, H.-T., & Liu, I. Y. (2018). Excavatolide-B Enhances Contextual Memory Retrieval via Repressing the Delayed Rectifier Potassium Current in the Hippocampus. Marine Drugs, 16(11), 405. https://doi.org/10.3390/md16110405




