Abstract
ROMP is an effective method for preparing functional polymers due to its having characteristics of “living” polymerization and rapid development of catalysts. In the present work, poly(norbornene-methylamine), a mimic of chitosan, was synthesized via ROMP reaction. The amino-protected product, 5-norbornene-2-(N-methyl)-phthalimide, was prepared by a reaction of 5-norbornene-2-methylamine with phthalic anhydride, which was then subjected to the ROMP reaction in the presence of Hoveyda-Grubbs 2nd catalyst to afford poly(norbornene-(N-methyl)-phthalimide). The target product, poly(norbornene-methylamine), was obtained by deprotection reaction of poly(norbornene-(N-methyl)-phthalimide). The products in each step were characterized by FTIR and 1H-NMR, and their thermal stabilities were determined by TG analysis. The effects of molar ratio between monomer ([M]/[I]) and catalyst on the average relative molecular weight () and molecular weight distribution of the produced polymer products were determined by gel permeation chromatography (GPC). It was found that the of poly(norbornene-(N-methyl)-phthalimide) was controllable and exhibited a narrow polydispersity index (PDI) (~1.10). The synthesis condition of 5-norbornene-2-(N-methyl)-phthalimide was optimized by determining the yields at different reaction temperatures and reaction times. The highest yield was obtained at a reaction temperature of 130 °C and a reaction time of 20 min. Our work provides a new strategy to synthesize polymers with controllable structures and free –NH2 groups via ROMP.
1. Introduction
ROMP has been an effective method for preparing functional polymers due to its having the characteristics of living polymerization [1,2] and rapid development of catalysts [3,4,5,6]. The mechanism of ROMP is substantially different from other polymerization reactions, such as radical polymerization and anionic polymerization. During an ROMP reaction, the double bond translocates continuously, the chain gradually grows, and the double bond of monomer molecules remains in the generated polymer molecules as shown in Scheme 1 [7,8]. The resultant polymer of ROMP can inherit the single bond, double bond and even the ring structure of a double-ring monomer. Therefore, ROMP can be used to prepare completely alternating copolymer [9,10]. In addition, its advantages of mild reaction conditions, high reaction rate, absence of chain transfer and termination reaction in most cases provide the polymers a narrow molecular weight distribution. Over the last few decades, many new controllable polymers, such as new homopolymers, telechelic copolymers [11,12,13], block copolymers [14,15,16], graft copolymers [17,18,19], liquid crystal polymers [20], and so on, have been synthesized by ROMP. These novel polymers have been extensively employed as conductive materials, porous network materials, self-healing materials and biomedical materials.
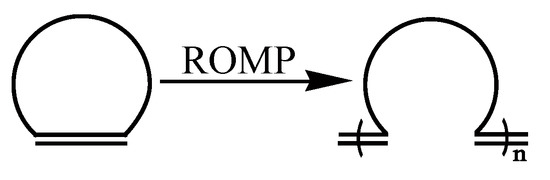
Scheme 1.
The reaction mechanism of ROMP.
Norbornene (NBE) and its derivatives are highly reactive due to their special cyclic structure, and thus are prone to a variety of polymerization reactions, such as radical polymerization, anionic polymerization, vinyl addition polymerization [21], and ROMP, in the presence of catalysts. Wherein, ROMP is one of the most commonly used polymerization methods due to its special reaction mechanism and mild reaction conditions. The ROMP of boron-, fluorine-, chlorine-, nitrile-, silicon-, silicon-, oxygen-, amides- and acid anhydride- derivatives of NBE has been well studied [22,23,24,25,26,27]. However, the ROMP of amino NBE has rarely been reported. Similar to chitosan, the cyclic polymers bearing –NH2 usually possess excellent hydrophilicity, high reactivity and excellent complexing abilities with metal ions [28], and thus they can be applied in a variety of fields. In addition, the –NH2 can be converted to –NH3+ in acidic conditions, which provides a pH sensitivity to the polymers. Therefore, these polymers are promising candidate materials for gene transfection vectors and drug release.
In the present work, poly(norbornene-(N-methyl)-phthalimide) was synthesized by the ROMP of 5-norbornene-2-(N-methyl)-phthalimide, an amino-protected product of 5-norbornene-2-methylamine. Its anhydride group was then deprotected to afford poly(norbornene-methylamine). The synthesis route is shown in Scheme 2. Our work provides a new strategy for the synthesis of polymers bearing –NH2 groups with controllable structures by ROMP. The intermediate product, poly(norbornene-(N-methyl)-phthalimide), can be used to prepare block copolymers with other unsaturated compounds. It also can be considered as a mimetic of natural chitosan with appropriate modifications. The produced polymer can be potentially applied in a variety of fields, such as gene transfection vectors, controlled-release carriers for functional drugs, and so on.
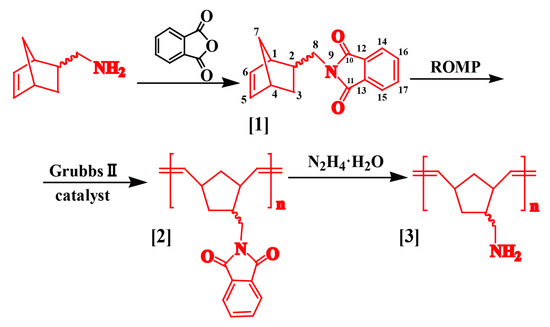
Scheme 2.
The reaction route for preparing poly(norbornene-methylamine) by ROMP.
2. Results and Discussion
2.1. FTIR Characterization
A comparison between the FTIR spectra of phthalic anhydride and 5-norbornene-2-(N-methyl)-phthalimide (product 1) indicates that the symmetric and asymmetric stretching vibration absorption peaks of C=O groups shifted from 1762 cm−1 and 1855 cm−1 (phthalic anhydride) to 1709 cm−1 and 1768 cm−1 (5-norbornene-2-(N-ethyl)-phthalimide), respectively, due to the formation of a lactam group between the –NH2 group and C–O–C group (Figure 1). The N-H stretch vibration absorption peaks of the –NH2 in 5-norbornene-2-methylamine at 1573 cm−1, 1483 cm−1 and 3330 cm−1 disappeared after the reaction with phthalic anhydride, indicating the formation of phthalimide group. The peak at 3000 cm−1 was attributed to the stretching vibration of –CH2– groups of product 1. The peaks at 2860 cm−1 and 3062 cm−1 were due to the stretching vibration of the unsaturated =CH group.
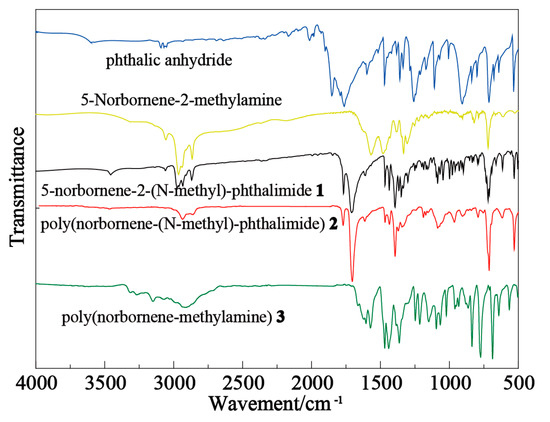
Figure 1.
FTIR spectra of raw materials and synthesized products.
Poly(norbornene-(N-methyl)-phthalimide) (Product 2) exhibited a similar FTIR spectrum to that of product 1, except that the stretching vibration peak of =CH at 3060 cm−1 disappeared due to the ring-opening reaction.
As product 2 was converted to poly(norbornene-methylamine) (Product 3), the peak at 1716 cm−1 that was attributed to the C=O group of phthalic anhydride disappeared, and new bands appeared at 3324 cm−1, 3276 cm−1, 1608 cm−1, 1573 cm−1 and 762 cm−1. The bands at 3324 cm−1 and 3276 cm−1 can be assigned to the symmetric and antisymmetric stretching vibrations of –NH2 groups, respectively. The bands at 1608 cm−1 and 1573 cm−1 were due to the in-plane bending vibrations of N-H group and that at 762 cm−1 was ascribed to the out-plane wagging vibrations of N-H group. These results indicate that target compounds were successfully synthesized and exhibited the expected structures.
2.2. 1H-NMR Characterization
The 1H-NMR spectra of the products in each step are shown in Figure 2. The chemical shift at 7.9 ppm of product 1 was ascribed to the 14H, 15H, 16H and 17H on the phenyl ring and the peak at 6.1–6.3 ppm was caused by the 5H and 6H on the NBE ring. The peaks at 3.5–3.6 ppm and 3.2–3.3 ppm were due to the 8H on -(N-methyl)-phthalimide, and the peak at 2.7–2.9 ppm was assigned to the 7H on the NBE ring bridge. The peaks at 2.4–2.5 ppm and 1.7–1.9 ppm were attributed to the 1H and 4H on the NBE ring, respectively. The 3H and 2H on the NBE ring caused the chemical shifts at 1.2–1.4 ppm and 0.5–0.6 ppm, respectively.
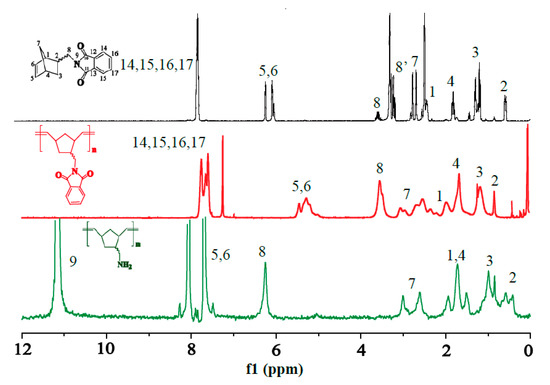
Figure 2.
1H-NMR spectra of products 1, 2 and 3.
No significant differences between the 1H-NMR spectra of products 1 and 2 were observed, except that the peak at 6.1–6.3 ppm of product 1 shifted to 5.1–5.6 ppm in the spectrum of product 2. The shift might be attributed to the transformation of H on the ring double bond into the H of double bond on the main chain during the ring-opening reaction of the NBE derivative. The chemical shift at 7.5–7.9 ppm of product 2 was ascribed to the 14H, 15H, 16H and 17H on its phenyl ring. The peaks at 3.3–3.7 ppm was due to the 8H on -(N-methyl)-phthalimide and the peak at 2.4–3.2 ppm could be assigned to the 7H on NBE ring bridge. The peaks at 1.8–2.3 ppm were ascribed to the 1H on the NBE ring and those at 1.6–1.8 ppm were attributed to the 4H on the NBE ring and the H of D2O in the CDCl3 solvent. The chemical shifts at 1.1–1.4 ppm and 0.8–0.9 ppm could be assigned to the 3H and 2H on the NBE ring.
Product 3 exhibited a significantly different 1H-NMR spectrum from product 2 due to the deprotection of phthalic anhydride. The –NH2 group obtained from the removal of phthalic anhydride exhibited an H peak at 11.0–11.1 ppm. The peak at 5.1–5.6 ppm of product 2 shifted to 7.6–8.3 ppm in the spectrum of product 3, possibly because the free –NH2 group is attached to a double bond on the main chain. The peaks at 6.2–6.4 ppm and 2.6–3.1 ppm were assigned to the 8H on -(N-methyl)-phthalimide and the 7H on the NBE ring bridge, respectively. The chemical shift at 1.4–2.1 ppm was attributed to the 1H and 4H on the NBE ring. The peaks at 0.7–1.2 ppm and 0.4–0.7 ppm were ascribed to the 3H and 2H on the NBE ring, respectively.
These 1H-NMR data indicate that the target products were successfully synthesized.
2.3. TG Characterization
The TG and DTG curves of products 2 and 3 are shown in Figure 3a,b, respectively.
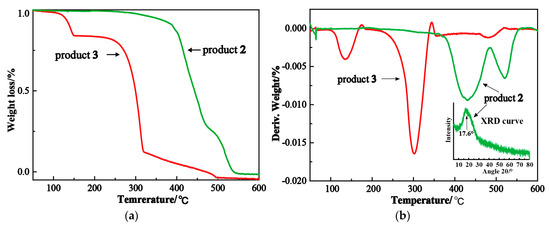
Figure 3.
TG characterization of products 2 and 3. (a) TG curves of products 2 and 3. (b) DTG curves of products 2 and 3.
The weight losses of poly(norbornene-(N-methyl)-phthalimide) occurred in the temperature ranges of 365–484 °C and 490–552 °C, respectively. Product 2 exhibited a sharp diffraction peak at 2θ = 17.6 ° (inset in Figure 3b), indicating its crystalline property. Therefore, the first weight loss might be attributed to the decomposition of the disordered region of poly(norbornene-(N-methyl)-phthalimide), and the other weight loss at the higher temperature was due to the decomposition of its crystalline region. The first weight loss temperature of 365–484 °C is higher than that of poly(norbornene-methylamine), possibly because the benzene ring on the side chain of poly(norbornene-(N-methyl)-phthalimide) is more stable than the amino group of poly(norbornene-methylamine).
The first weight loss of poly(norbornene-methylamine) occurred in the temperature range of 97–174 °C, due to its highly active amino group that usually reduces the stability of polymer. The active –NH2 group in poly(norbornene-methylamine) might be attached to the double bond in the main chain to form bulky alkyl group by intramolecular cyclization. The interaction between –NH2 and the double bond was reported by Zvonimir et al. [29]. They found that the gas phase basicity of iminocyclopropene could be enhanced by the NH2 groups attached to the C=C double bond and subsequent substitution(s) by bulky alkyl group(s). The NH2 groups could release some of the lone pair electron density distribution, enabling a uniform distribution of the positive charge over the whole molecule. The intramolecular cyclization decreased the first weight loss temperature of poly(norbornene-methylamine) from 365–484 °C to 97–174 °C. Lv reported similar phenomenon in a thermally degradable aliphatic polyester bearing 2-aminoethanethiol -groups (P1) with polyester and amino-BOC (P1-BOC) [30,31]. They found the first weight loss of P1-BOC was initiated at 198.2 °C, due to the fact that the pyrolysis of the BOC group and P1 was unstable even at room temperature, because of the nucleophilicity of the pendent amino groups with a five-membered lactam formed by the intramolecular cyclization. Therefore, they concluded that polyesters bearing amino groups that caused larger steric hindrance and weaker nucleophilicity were more thermostable. Similarly, the phthalimide in poly(norbornene-(N-methyl)-phthalimide) can reduce the effect of amino groups on double bonds. The main chain of poly(norbornene-methylamine) was decomposed at 224–337 °C, resulting in a second weight loss.
These TG results of product 2 and [3] indicate that poly(norbornene-(N-methyl)-phthalimide) was successfully deprotected to afford the target product.
2.4. Optimization of Synthesise Condition for Product 1
The amino group of 5-norbornene-2-methylamine can be oxidized by other functional groups during the late reaction stage due to its strong reactivity, which may affect the activity of Grubbs catalyst. Therefore, it is necessary to protect amino groups before the ROMP reaction. To determine the optimal condition for the protection of amino groups, the yields of product 1 at different reaction times and temperatures were investigated.
Figure 4a shows the variation in the yield of product 1 with reaction temperature at the reaction time of 20 min. The yield increased with the increase of reaction temperature, reached the maximum at 130 °C, and decreased as the reaction temperature further increased. This can be explained by the fact that the phthalic anhydride was melted at 130 °C, and thus completely reacted with 5-norbornene -2-methylamine, resulting in the highest yield. 5-norbornene-2-methylamine was evaporated at higher temperatures, which thus reduced the yield.
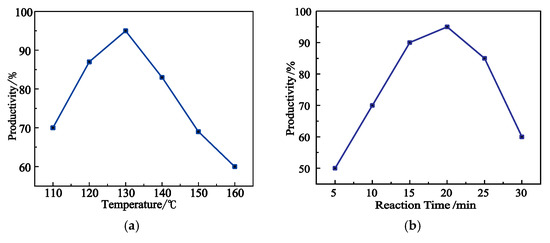
Figure 4.
Yields of product 1 under different reaction conditions. (a) Effects of reaction temperature on the yield of product 1. (b) Effects of reaction time on the yield of product 1 at 130 °C.
Figure 4b shows the yield changes of product 1 with reaction time at 130 °C. The yield increased with the prolongation of reaction time, reached the maximum at a reaction time of 20 min, and subsequently decreased, with the product becoming a brownish red due to the side reaction as the reaction time further prolonged.
In summary, the synthesis condition of product 1 was optimized as: a reaction time of 20 min and a reaction temperature of 130 °C. The yield of product 1 reached 94.5% under the optimal synthesis conditions.
2.5. Molecular Weight and Polydispersity Index
Figure 5 shows the effects of the molar ratio of monomer ([M]/[I]) on the molecular weight () and polydispersity index (PDI) of poly(norbornene-(N-methyl)-phthalimide) deduced from the GPC results. The increased linearly with [M]/[I] and exhibited a narrow PDI between 1.18 and 1.25. Therefore, the of poly(norbornene-(N-methyl)-phthalimide) can be tuned by adjusting the [M]/[I] ratio.
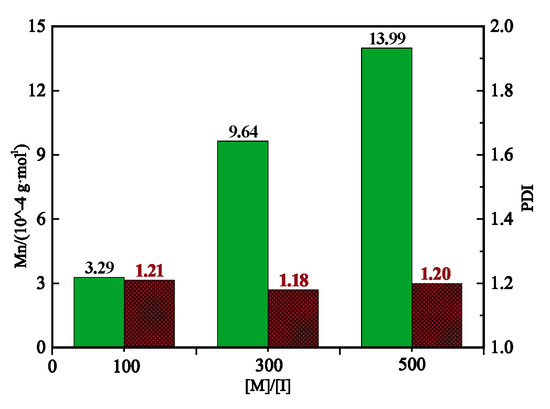
Figure 5.
and PDI of poly(norbornene-(N-methyl)-phthalimide) prepared by a 2 h ROMP at room temperature with different [M]/[I] ratios.
3. Materials and Methods
3.1. Materials
5-norbornene-2-methylamine (≥98%) was purchased from TCI Development Co., Ltd. (Shanghai, China). Di-tert-butyl dicarbonate and 2,6-di-tert-butyl paracresol were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Phthalic anhydride was provided by Tianjin Xingfu Technology Development Co., Ltd. (Tianjing, China). Ethyl vinyl ether and the 2nd generation Hoveyda-Grubbs catalyst (≥97%) were purchased from Aladdin Industrial Co. (Shanghai, China). Hydrazine hydrate (80%) was purchased from Beijing Tongguang Fine Chemicals Company (Beijing, China). CH2Cl2 was dried with CaH2 and freshly distilled prior to use. 5-norbornene-2-methylamine was of gas chromatography grade and spectroscopy grade. Other reagents were of chemical grade.
3.2. Synthesis of 5-Norbornene-2-(N-methyl)-phthalimide (Product 1)
5-norbornene-2-methylamine (0.246 g) was added to 0.291 g phthalic anhydride and stirred under nitrogen at 130 °C for 20 min. The mixture was then added to 15 mL n-hexane, heated to dissolve, cool-crystallized, and dried to afford product 1.
3.3. Synthesis of Poly(norbornene-(N-methyl)-phthalimide) (Product 2)
One milligram Grubbs II catalyst was dissolved in 1 mL CH2Cl2, vacuum aerated with nitrogen for 3 times, and stirred under nitrogen for 15 min. The catalyst solution was added to 3 mL product 1 (0.1 g) solution and stirred under nitrogen at room temperature for 2 h. To terminate the polymerization reaction, ethyl vinyl ether was added to the reaction solution and stirred for an additional 20 min. The final solution was poured into 400 mL of methanol to precipitate the polymer that was then dried to afford product 2.
3.4. Synthesis of Poly(norbornene-methylamine) (Product 3)
Product 2 (0.2 g) was dissolved in 10 mL ethanol and refluxed with 2 mL of hydrazine hydrate and a certain amount of 2, 6-Di-tert-butyl-4-methylphenol under nitrogen at 100 °C for 8 h. The reaction solution was cooled to room temperature and precipitated with CH2Cl2. The precipitate was dried to afford product 3.
3.5. Characterization
IR spectra were recorded on a Nicolet8700 FT-IR spectrometer that manufactured by Thermo Nicolet Co., (Madison, WI, USA) in the range of 4000–500 cm−1 at a resolution of 4 cm−1 using KBr pellets.
1H-NMR spectra were measured with a Bruker 500 MHz NMR spectrometer using TMS in DMSO, CDCl3 and CF3COOD as the internal standards for 5-norbornene-2-(N-methyl)-phthalimide, poly(norbornene-(N-methyl)-phthalimide) and poly(norbornene-methylamine), respectively.
Gel permeation chromatography (GPC) was conducted on a system containing a GPC detector (Waters Breeze HPLC, Waters Company, Framingham, MA, USA) and PSS Waters HR-3, HR-4 and HR-6 columns at 35 °C. Tetrahydrofuran (THF) was used as the mobile phase.
Thermogravimetric analysis (TG) was performed on a DTG-60 TGA/DTA Analyzer (Shimadzu, Japan). The sample was heated from 30 °C to 600 °C at 10 °C/min under 50 mL/min nitrogen flow.
4. Conclusions
In the current study, poly(norbornene-methylamine) was prepared by ROMP for the first time. 5-norbornene-2-(N-methyl)-phthalimide was first prepared by the reaction between 5-norbornene-2-methylamine and phthalic anhydride with the highest yield of 94.5% achieved at 130 °C for 20 min. The ROMP reaction of 5-norbornene-2-(N-methyl)-phthalimide proceeded in the presence of Hoveyda-Grubbs 2nd catalyst to form poly(norbornene-(N-methyl)-phthalimide). The molecular weight () of poly(norbornene-(N-methyl)-phthalimide) increased linearly with the increase of [M]/[I] ratio and exhibited a narrow PDI range of 1.18–1.25. Poly(norbornene-(N-methyl)-phthalimide was deprotected to afford the target polymer, poly(norbornene-methylamine). The products of each step were characterized with FTIR, 1H-NMR and TG. Our study provides a strategy for synthesizing chitosan like polymers bearing free –NH2 groups with controllable structures via ROMP.
Acknowledgments
The authors gratefully acknowledge the financial support of the Combination Project of Guangdong Province and the “Yangfan” Innovative Research Team Project of Guangdong Province.
Author Contributions
Yu Chen, Na Li and Huanhuan Wang conceived and designed the experiments; Huanhuan Wang performed the experiments; Yu Chen, Na Li and Huanhuan Wang analyzed the data; Yu Chen, Na Li and Xiaosai Qu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, X.; Mu, C.; Dai, D. Well-Defined Magnetic Responsive Polymers Containing Ammonium FeCl4 from ROMP. Macromol. Chem. Phys. 2016, 217, 2700–2707. [Google Scholar] [CrossRef]
- Nomura, K.; Abdellatif, M.M. Precise synthesis of polymers containing functional end groups by living ring-opening metathesis polymerization (ROMP): Efficient tools for synthesis of block/graft copolymers. Polymer 2010, 51, 1861–1881. [Google Scholar] [CrossRef]
- Ferrer, Í.; Rich, J.; Fontrodona, X. Ru(II) complexes containing dmso and pyrazolyl ligands as catalysts for nitrile hydration in environmentally friendly media. Dalton Trans. 2013, 42, 13461–13469. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R. Synthesis of Stereoregular Polymers through Ring-Opening Metathesis Polymerization. Acc. Chem. Res. 2014, 47, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Hyvl, J.; Autenrieth, B.; Schrock, R.R. Proof of Tacticity of Stereoregular ROMP Polymers through Post Polymerization Modification. Macromolecules 2015, 48, 3148–3152. [Google Scholar] [CrossRef]
- Autenrieth, B.; Jeong, H.; Forrest, W.P. Stereospecific Ring-Opening Metathesis Polymerization (ROMP) ofendo-Dicyclopentadiene by Molybdenum and Tungsten Catalysts. Macromolecules 2015, 48, 2480–2492. [Google Scholar] [CrossRef]
- Martinez, H.; Hillmyer, M.A. Ring-Opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 2014, 5, 3507–3532. [Google Scholar] [CrossRef]
- Parker, K.A.; Sampson, N.S. Precision Synthesis of Alternating Copolymers via Ring-Opening Polymerization of 1-Substituted Cyclobutenes. Acc. Chem. Res. 2016, 49, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ciganda, R.; Castel, P. Living ROMP Syntheses and Redox Properties of Triblock Metallocopolymer Redox Cascades. Macromolecules 2016, 49, 4763–4773. [Google Scholar] [CrossRef]
- Wang, Y.; Rapakousiou, A.; Astruc, D. ROMP Synthesis of Cobalticenium–Enamine Polyelectrolytes. Macromolecules 2014, 47, 3767–3774. [Google Scholar] [CrossRef]
- Martinez, H.; Hillmyer, M.A. Carboxy-Telechelic Polyolefins in Cross-Linked Elastomers. Macromolecules 2014, 47, 479–485. [Google Scholar] [CrossRef]
- Annunziata, L.; Fouquay, S.; Michaud, G. Mono- and di-cyclocarbonate telechelic polyolefins synthesized from ROMP using glycerol carbonate derivatives as chain-transfer agents. Polym. Chem. 2013, 4, 1313–1316. [Google Scholar] [CrossRef]
- Vanbiervliet, E.; Fouquay, S.; Jean-Francois, C. From Epoxide to Cyclodithiocarbonate Telechelic Polycyclooctene through Chain-Transfer Ring-Opening Metathesis Polymerization (ROMP): Precursors to Non-Isocyanate Polyurethanes (NIPUs). Macromolecules 2017, 50, 69–82. [Google Scholar] [CrossRef]
- Bingöl, B.; Kroeger, A.; Jannasch, P. Well-defined phosphonated homo- and copolymers via direct ring opening metathesis polymerization. Polymer 2013, 54, 6676–6688. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Fonseca, L.R.; Tomazetti, V. Copolymers from norbornene and norbornadiene with organized morphologies and high Tg values obtained via ROMP with a highly reactive [RuCl3(PCy3)2] complex. New J. Chem. 2016, 40, 9424–9431. [Google Scholar] [CrossRef]
- Suga, T.; Sakata, M.; Aoki, K. Synthesis of Pendant Radical- and Ion-Containing Block Copolymers via Ring-Opening Metathesis Polymerization for Organic Resistive Memory. ACS Macro Lett. 2014, 3, 703–707. [Google Scholar] [CrossRef]
- Leroux, F.; Montembault, V.; Piogé, S. High Molar Mass Poly(1,4-butadiene)-graft-poly(ε-caprolactone) Copolymers by ROMP: Synthesis via the Grafting-From Route and Self-Assembling Properties. Macromolecules 2016, 49, 4739–4745. [Google Scholar] [CrossRef]
- Xu, G.; Wang, D.; Buchmeiser, M.R. Functional Polyolefins: Poly(ethylene)-graft-Poly(tert-butyl acrylate) via Atom Transfer Radical Polymerization From a Polybrominated Alkane. Macromol. Rapid Commun. 2012, 33, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.M.; Khosravi, E. Comb-Like Graft Copolymers of Poly(oxa)norbornene: Efficient Synthesis Using a Combination of ROMP and Click Chemistry. Macromol. Chem. Phys. 2015, 216, 964–976. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Liao, P.L.; Shen, Z.H. Precise size control of sub-10 nm structures of cholesteryl-containing mesogen-jacketed liquid crystalline polymers. Polymer 2016. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, Y.F.; Zhu, F.M. Homo- and copolymerizaton of norbornene and norbornene derivative with Ni- and Pd-based β-ketoiminato complexes and MAO. Eur. Polym. J. 2006, 42, 322–327. [Google Scholar] [CrossRef]
- Chung, T.C. Synthesis of functional polymers via borane monomers and metathesis catalysts. J. Mol. Catal. 1992, 76, 15–31. [Google Scholar] [CrossRef]
- Santiago, A.A.; Cruz-Morales, J.A.; Vargas, J.; Tlenkopatchev, M.A.; Gavino, R.; Malkanduev, Y.A.; Sivov, N.A. Synthesis of New Polymer Ionomers via Ring-Opening Metathesis Polymerization. Open J. Org. Polym. Mater. 2014, 4, 84–91. [Google Scholar] [CrossRef][Green Version]
- Pawar, G.M.; Weckesser, J.; Blechert, S.; Buchmeiser, M.R. Ring opening metathesis polymerization-derived block copolymers bearing chelating ligands: Synthesis, metal immobilization and use in hydroformylation under micellar conditions. Beilstein J. Org. Chem. 2010, 6, 28. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Bai, X. Preparation of cationic cobaltoceniumpolymers and block copolymers by “living” ring-opening metathesispolymerization. Chem. Sci. 2012, 3, 580–583. [Google Scholar] [CrossRef]
- Ashok Kothapalli, V.; Shetty, M.; de Los Santos, C. Thio-bromo “Click,” post-polymerization strategy for functionalizing ring opening metathesis polymerization (ROMP)-derived materials. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 179–185. [Google Scholar] [CrossRef]
- Alfred, S.F.; Lienkamp, K.; Madkour, A.E. Water-soluble ROMP polymers from amine-functionalized norbornenes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6672–6676. [Google Scholar] [CrossRef]
- Li, P.; Zhao, J.; Chen, Y. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr. Polym. 2017, 157, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Zvonimir, B.M.; Borislav, K. Spatial and electronic structure of highly basic organic molecules: Cyclopropeneimines and some related systems. J. Phys. Chem. A 1999, 103, 6678–6684. [Google Scholar]
- Lv, A.; Cui, Y.; Du, F.-S.; Li, Z.-C. Thermally Degradable Polyesters with Tunable Degradation Temperatures via Postpolymerization Modification and Intramolecular Cyclization. Macromolecules 2016, 49, 8449–8458. [Google Scholar] [CrossRef]
- Lv, A.; Li, Z.-L.; Du, F.-S.; Li, Z.C. Synthesis, Functionalization, and Controlled Degradation of High Molecular Weight Polyester from Itaconic Acid via ADMET Polymerization. Macromolecules 2014, 47, 7707–7716. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).