Abstract
Anti-vascular endothelial growth factor (VEGF) treatment with intravitreal brolucizumab (IVBr) was launched as a novel treatment for neovascular age-related macular degeneration (AMD), but the incidence of intraocular inflammation (IOI) as a specific adverse effect of brolucizumab has been reported. We evaluated the dynamics of inflammatory factors in AMD in patients with or without IOI before and after anti-VEGF treatment with IVBr. We describe three patients who did not develop inflammation after three consecutive administrations of IVBr and three in whom inflammation occurred after the first IVBr treatment. The presence or absence of inflammation was determined by slit-lamp examination and a laser flare meter. Aqueous humor was obtained during anti-VEGF treatment with IVBr. Levels of VEGF, platelet-derived growth factor (PDGF)-AA, monocyte chemoattractant protein 1 (MCP-1), interleukin (IL)-6, IL-8, interferon-inducible 10 kDa protein (IP-10), Fms-related tyrosine kinase 3 ligands (Flt-3L), and fractalkine were measured. Vision worsened in one patient who developed IOI after initial IVBr, so IVBr was discontinued and the patient was switched to intravitreal aflibercept with sub-tenon injection of triamcinolone acetonide. IVBr was continued in the two other patients with IOI. VEGF decreased after IVBr in all patients with and without IOI. On the other hand, at 1 month IL-6, IL-8, MCP-1, IP-10, and Flt-3L were higher in the three patients with IOI compared with baseline and with the three patients without IOI. In two patients with IOI, not only flares but also IL-8, IP-10, and Flt-3L decreased from 1 to 2 months after IVBr despite continued IVBr. This case series might lead to a better understanding of the pathogenesis of IOI after IVBr.
1. Introduction
Anti-vascular endothelial growth factor (VEGF) treatment with the monoclonal antibody brolucizumab (Beovu, Novartis) was launched in the USA in October 2019 as a novel treatment for neovascular age-related macular degeneration (AMD) [1,2].
Brolucizumab has a low molecular weight, is administered at a high concentration, and easily penetrates the retina [1,2].
In terms of intraretinal fluid and subretinal fluid absorption (fluid control), brolucizumab has been reported to have superior efficacy to aflibercept (Eylea; Regeneron and Bayer HealthCare) [1,2]. Several real-world studies on brolucizumab demonstrated favorable visual and anatomical treatment outcomes in AMD [3,4]. Additionally, brolucizumab showed a robust anatomical response in cases resistant to other anti-VEGF treatments, such as ranibizumab and aflibercept, indicating the potential of brolucizumab as an effective alternative treatment for refractory AMD [5,6]. However, the incidence of intraocular inflammation (IOI) as a specific adverse effect of brolucizumab has been reported to be 11% to 22% [4,7,8,9]. The dynamics of inflammatory factors before and after treatment with ranibizumab (Lucentis; Genentech) and aflibercept in AMD have been reported, but there are few reports on brolucizumab. Therefore, we present a case series that illustrates the changes in inflammatory factors in patients with and without IOI after treatment with brolucizumab.
2. Materials and Methods
We examined six treatment-naïve patients with neovascular AMD who were treated by intravitreal brolucizumab (IVBr) at the Department of Ophthalmology, Hachioji Medical Center, Tokyo Medical University, Tokyo, Japan. All patients provided written informed consent to treatment by IVBr, and treatment was approved by the institutional review board of Tokyo Medical University Hachioji Medical Center (IRB No. T2023-0039). All patients were 45 years of age or older, and none had myopia of more than -6 diopters; a history of uveitis or vitrectomy; type 2 diabetes or diabetic retinopathy; advanced cataracts or other choroidal or retinal pathologies; or previous ocular treatment with laser photocoagulation, intravitreal injection of another anti-VEGF agents, or surgery. A thorough ophthalmologic examination was performed, including best-corrected visual acuity (BCVA), central macular thickness (CMT), and fundus examinations, and aqueous flare was assessed using a laser flare meter. Fluorescein angiography (FA) and indocyanine green angiography (ICGA) with spectral-domain optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany) were performed at baseline. AMD was classified into macular neovascularization type 1 (occult type), type 2 (classic type), type 3 (retinal angiomatous proliferation), and polypoidal choroidal vasculopathy. Polypoidal choroidal vasculopathy was diagnosed if ICGA showed polypoidal lesions. BCVA was converted to logarithm of the minimal angle of resolution (logMAR) units. CMT was measured manually from the superior border of the retinal pigment epithelium to the inner retinal layer border at the foveal center.
In three patients, three IVBr treatments could be performed as an induction phase without inflammation (patients without IOI), and in the other three, inflammation developed occurred after the first IVBr (patients with IOI). The presence or absence of inflammation was determined by slit-lamp examination and a laser flare meter (FC-600, Kowa Co., Ltd., Tokyo, Japan); i.e., in the slit-lamp examination, inflammation was defined as the presence of inflammatory cells in the anterior chamber, and in the laser flare meter examination, inflammation was defined as an aqueous flare value more than twice that of the pre-dose flare value.
For IVBr, topical anesthesia was administered and a 30 G needle was then used to obtain aqueous humor (mean, 0.1 mL); samples were stored in sterile plastic tubes at −80 °C until analysis. Then, brolucizumab was injected into the vitreous with a 30 G needle. For the injection, the needle was inserted through the pars plana in the superior temporal quadrant, 3.5 mm from the limbus. After IVBr, patients were instructed to apply antibiotic drops to the affected eye for 3 days.
Levels of VEGF, platelet-derived growth factor (PDGF)-AA, monocyte chemoattractant protein 1 (MCP-1), interleukin (IL)-6, IL-8, interferon-inducible 10 kDa protein (IP-10), Fms-related tyrosine kinase 3 ligands (Flt-3L), and fractalkine were measured in the aqueous humor samples by suspension array (xMAP; Luminex Corp., Austin, TX, USA) with the Milliplex kit (EMD Millipore, Burlington, MA, USA) [10,11,12,13,14,15]. A Human Cytokine/Chemokine kit (HCYTO-60K) was used for VEGF, PDGF-AA, MCP-1, IL-6, IL-8, IP-10, Flt-3L, and fractalkine. In accordance with the manufacturer’s instructions, fluorescence-labeled beads were mixed with standards and aqueous humor samples and incubated at 4 °C overnight. The next day, detection antibodies were added at room temperature for 1 h, followed by streptavidin-phycoerythrin at room temperature for 30 min. Readings were made with a Luminex 100/200 System (v5.1), and median fluorescent intensity was calculated with Milliplex Analyst software (v4.3). Concentrations were calculated by a 5-parameter logistic approach. All factors were present at detectable levels (minimum detectable concentrations: VEGF, 0.64 pg/mL; PDGF-AA, 0.64 pg/mL; MCP-1, 1.2 pg/mL; IL-6, 0.29 pg/mL; IL-8, 0.14 pg/mL; IP-10, 0.55 pg/mL; Flt-3L, 5.4 pg/mL; and fractalkine, 22.7 pg/mL).
We compared continuous variables with a paired t test and examined relationships among the variables by Pearson’s correlation analysis and linear regression analysis. Statistical significance was set as a p value of less than 0.05.
3. Results
The sociodemographic and clinical characteristics, BCVA, and CMT of the patients with and without IOI are presented in Table 1. VA fluctuation (logMAR) over the study period was 1.28, 0.18, and −0.08 in the IOI group and −0.20, 0, and −0.18 in the non-IOI group.

Table 1.
Demographic characteristics.
Cases 1 to 3 had IOI, and cases 4 to 6 did not. IVBr was discontinued in one patient (case 1) in whom vision worsened after the initial IVBr and IOI was confirmed; the patient was switched to intravitreal aflibercept and a sub-tenon injection of triamcinolone acetonide. In the other two cases of IOI, IVBr was continued because vision was not affected by the inflammation. The changes in the aqueous flare values are shown in Figure 1.
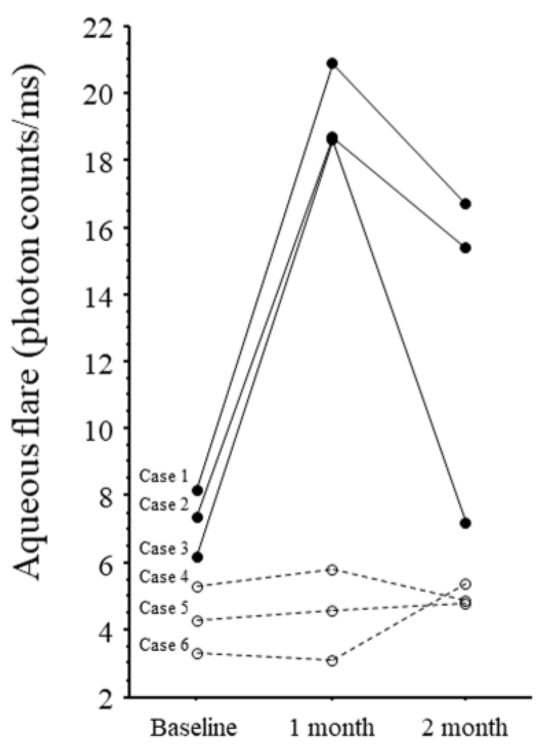
Figure 1.
Changes in aqueous flare values in six patients. Cases 1–3: Patients with intraocular inflammation (solid line). Cases 4–6: Patients with non-intraocular inflammation (dotted line).
The aqueous flare value was highly elevated in all three IOI patients at 1 month compared with baseline and with the non-IOI group, but it decreased from 1 to 2 months. In the two patients with IOI who continued treatment (cases 2 and 3), the aqueous flare value decreased from 1 to 2 months despite continued IVBr.
The dynamics of VEGF, PDGF-AA, MCP-1, IL-6, IL-8, IP-10, Flt-3L, and fractalkine are shown in Figure 2.
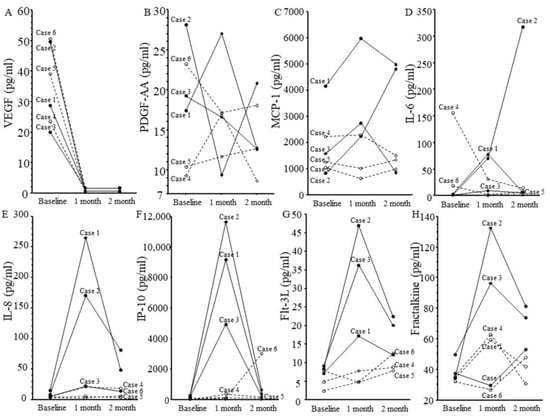
Figure 2.
Changes in levels of eight cytokines in six patients. Cases 1–3: Patients with intraocular inflammation (solid line). Cases 4–6: Patients with non-intraocular inflammation (dotted line). (A) Vascular endothelial growth factor, (B) platelet-derived growth factor AA, (C) monocyte chemoattractant protein 1, (D) interleukin (IL)-6, (E) IL-8, (F) interferon-inducible 10 kDa protein, (G) Fms-related tyrosine kinase 3 ligand, and (H) fractalkine.
In all patients, VEGF had significantly decreased at 1 month after IVBr (p = 0.001). IL-6, IL-8, MCP-1, IP-10, and Flt-3L were highly elevated in all three patients with IOI at 1 month compared with baseline and with the non-IOI group, but IL-8, IP-10, and Flt-3L decreased from 1 to 2 months. In the two patients with IOI who continued treatment (cases 2 and 3), IL-8, IP-10, and Flt-3L decreased from 1 to 2 months despite continued IVBr.
We found significant correlations between the aqueous flare value and aqueous humor level of IL-8 (y = 3.04 + 0.38x; r = 0.85, p = 0.03) but not between the aqueous flare value and aqueous humor level of the other factors (i.e., VEGF, PDGF-AA, MCP-1, IL-6, IP-10, fractalkine, and flt-3 ligand).
4. Discussion
This case series indicates that VEGF decreases after IVBr in patients with non-IOI and IOI. Changes in VEGF after anti-VEGF treatment were consistent with previous reports [8,16,17,18]. This result was reasonable because IVBr potently suppresses VEGF. On the other hand, IL-6, IL-8, MCP-1, IP-10, and Flt-3L were highly elevated in all three patients with IOI at 1 month compared with baseline and with non-IOI patients, suggesting an inflammatory response in the eye. IOI and inflammation after IVBr are assumed to be due to hypersensitivity because the vitreous humor of IOI patients contains inflammatory cells involved in hypersensitivity reactions (CD4+ T helper cells, CD8+ T-cytotoxic cells, CD3+ T cells, CD20+ B cells, and CD68+ histiocytes) [16].
Because IOI developed about 1 month after IVBr, the reaction is assumed to be delayed type IV hypersensitivity [19,20]. This type of reaction is subdivided into types IVa (the classic hypersensitivity reaction), IVb, IVc, and IVd and is due to CD4+ T helper-1 (Th1) cell responses to antigens presented by antigen-presenting cells (APCs) [21]. In type IVa reactions, APCs secrete interferon gamma, tumor necrosis factor alpha, chemokine ligand 2 (CCL2), and IL-18 and then activate macrophages; in type IVb reactions, APCs sensitize CD4+ T-helper 2 (Th2) cells, leading to the production of IL-4, IL-5, and IL-13, the survival and migration of eosinophils, and, subsequently, inflammation; type IVc reactions involve the secretion by cytotoxic CD8+ T cells of B perforin, granulysin, and granzyme; and type IVd reactions involve the secretion by neutrophils of pro-inflammatory cytokines after activation by T cells [22,23]. Various pro-inflammatory cytokines play a role in these hypersensitivity reactions. Studies have shown that IL-6 levels increase in hypersensitivity reactions that occur after administration of monoclonal antibodies such as brolucizumab, indicating that IL-6 may be useful as a biomarker [24,25].
In this case series, MCP-1, IP-10, and Flt-3L levels were higher in all patients with IOI at 1 month compared with both baseline and the three non-IOI patients. As regards MCP-1, animal studies have shown that MCP-1 causes macrophages to migrate into choroidal neovascularization lesions in AMD and is involved in the digestion of both Bruch’s membrane and the retinal pigment epithelium [26,27]. Compared with mice treated with vehicle, mice treated with the angiotensin II type 1 receptor blocker telmisartan had less intraocular expression of MCP-1 mRNA, fewer retinal adherent leukocytes, weaker responses of lymph node cells to human interphotoreceptor retinoid binding protein-derived peptide 1–20, and significantly fewer CD44 high CD4+ T cells [28].
IP-10 is a member of the CXC family of chemokines and considered to play an important role in inflammation because of its T-cell chemotactic and adhesion-promoting properties [29,30]. Both a Th1-like (interferon gamma, IP-10, CXC motif chemokine receptor 3) and a Th2-like (IL-5, IL-4 and STAT-6) pathway contribute to eosinophil recruitment in early delayed-type hypersensitivity [31].
Flt-3L is a cytokine known to be involved in the mobilization of hematopoietic stem cells and their differentiation to cells such as conventional dendritic cells (cDCs), which play important roles in T-cell immune responses [32,33]. A congenital deficiency of cDCs leads to abnormalities in the immune system, such as changes in the composition and function of granulocytes, group 2 innate lymphoid cells, and B cells, which may be due to a breakdown of the Flt-3L-mediated homeostatic feedback loop [34]. The above findings suggest that IOI involves the inflammatory cytokines that drive type IV hypersensitivity and the immune responses described above.
In this study, all three patients with IOI had high flare values before treatment compared with non-IOI patients (Figure 1). This finding suggests that patients with high pre-treatment flare values = already have high levels of inflammation and, consequently, are more prone to IOI. If we used an aqueous flare cut-off value of 6, aqueous flare values were all above 6 in the IOI group, indicating that a baseline aqueous flare value above 6 may be associated with an IOI. VEGF induces production of nitric oxide, which causes blood vessels to dilate [35], so anti-VEGF therapy induces retinal arteriolar vasoconstriction [36,37,38]. Therefore, the elevated cytokine levels in the patients with IOI may be due not only to a delayed response, but also to the relative ischemia from vasoconstriction caused by IVBr because the ischemia may further increase inflammation and cause levels of these cytokines to rise. This hypothesis is supported by our previous finding that the aqueous flare value was significantly correlated with the size of the nonperfused area of the retina [39]. Taken together, findings suggest that patients with high pre-treatment flare values have a high inflammatory status and may be at risk of developing IOI, so care needs to be taken when administering IVBr in these patients. Further research on IVBr and IOI is needed.
Interestingly, in two of the three the patients with IOI (cases 2 and 3), not only aqueous flare values but also IL-8, IP-10, and Flt-3L levels decreased from 1 to 2 months despite continued IVBr. These changes suggest that inflammation was subsiding. IL-8 is secreted by cells with Toll-like receptors and is involved in the innate immune response [40]. IP-10 is a chemoattractant for macrophages, dendritic cells, and T cells, is involved in type 1 immune responses mediated by Th-1 and Th2 cells, and generally activates cell-based immunity [29,30]. Higher expression levels of Flt-3L have been linked to autoimmune and chronic inflammatory responses in the lung, central nervous system, and gastrointestinal tract [41]. These findings indicate that inflammation may have decreased because of the effects of various immune mechanisms. Further studies are needed to determine whether and, if so, why inflammation decreases over time in patients with IOI.
The innate immune system involves complex intrinsic interactions between cytokines in which cytokines interact to drive multicellular responses, including vascular and neuronal inflammation [42]. Previously, we reported significant correlations between aqueous flare value and aqueous humor levels of MCP-1, IL-6, IL-8, and IP-10 [14]. Recently, significant correlations were found among CCL2, CXC motif chemokine ligand 1, IL-6, IL-8, IL-10, granulocyte colony stimulating factor, granulocyte-macrophage colony-stimulating factor, intercellular adhesion molecule 1, E-selectin, and VEGF in IOI patients [43]. These results indicate that interactions among inflammatory cytokines are involved in the development of IOI. In addition, in this series, the IL-8 concentration was significantly correlated with the aqueous flare value, suggesting that IL-8 is a key factor in increasing intraocular inflammation after IVBr. However, the small number of patients means that further investigation is needed to clarify the relationship between IOI and inflammatory factors before and after IVBr in patients with AMD.
The study has some limitations, including the small sample size (n = 6), limited information on patient background, and short study period (1–2 months). Furthermore, in case 1, the fractalkine level decreased despite the presence of IOI after injection. The available data on this case do not explain why this change occurred, so the effects of IVBr on fractalkine require further study.
5. Conclusions
In conclusion, this case series might lead to a better understanding of the pathogenesis of IOI after IVBr.
Author Contributions
Conceptualization, H.N. and M.S.; methodology, H.N. and M.S.; data curation: M.A., K.Y. and H.N.; formal analysis, M.A. and H.N.; writing—original draft preparation, M.A. and H.N.; writing—review and editing, H.G. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hidetaka Noma, grant number 22K09821.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Treatment was approved by the institutional review board of Tokyo Medical University Hachioji Medical Center (IRB No. T2023-0039; 31 May 2023).
Informed Consent Statement
Informed written consent was obtained from the patients for publication of this article (including the publication of images).
Data Availability Statement
The datasets used and/or analyzed during the current study are included in this article.
Acknowledgments
We thank Katsunori Shimada (Department of Biostatistics, STATZ Corpora tion, Tokyo) for assistance in preparing the figures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMD | age-related macular degeneration |
| BCVA | best-corrected visual acuity |
| CMT | central macular thickness |
| FA | fluorescein angiography |
| Flt-3L | Fms-related tyrosine kinase 3 ligand |
| ICGA | indocyanine green angiography |
| IL | interleukin |
| IOI | intraocular inflammation |
| IP-10 | interferon-inducible 10 kDa protein |
| IVBr | intravitreal brolucizumab |
| LogMAR | logarithm of the minimum angle of resolution |
| MCP-1 | monocyte chemoattractant protein 1 |
| MNV | macular neovascularization |
| OCT | optical coherence tomography |
| PCV | polypoidal choroidal vasculopathy |
| PDGF | platelet-derived growth factor |
| SD-OCT | spectral-domain OCT |
| SRD | serous retinal detachment |
References
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Singh, R.P.; Koh, A.; Ogura, Y.; Weissgerber, G.; Gedif, K.; Jaffe, G.J.; Tadayoni, R.; Schmidt-Erfurth, U.; Holz, F.G. HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, A.; Kodjikian, L.; March de Ribot, F.; Vasavada, V.; Gonzalez-Cortes, J.H.; Abukashabah, A.; Sudhalkar, A.; Mathis, T. Real-World Experience with Brolucizumab in Wet Age-Related Macular Degeneration: The REBA Study. J. Clin. Med. 2021, 10, 2758. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Maruyama-Inoue, M.; Kitajima, Y.; Ikeda, S.; Inoue, T.; Kadonosono, K. One-year outcomes of intravitreal brolucizumab injections in patients with polypoidal choroidal vasculopathy. Sci. Rep. 2022, 12, 7987. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Brown, D.M.; Jaffe, G.J.; Wykoff, C.C.; Adiguzel, E.; Wong, R.; Meng, X.; Heier, J.S. MERLIN: Phase 3a, Multicenter, Randomized, Double-Masked Trial of Brolucizumab in Participants with Neovascular Age-Related Macular Degeneration and Persistent Retinal Fluid. Ophthalmology 2022, 129, 974–985. [Google Scholar] [CrossRef]
- Khanani, A.M.; Zarbin, M.A.; Barakat, M.R.; Albini, T.A.; Kaiser, P.K.; Guruprasad, B.; Agashivala, N.; Justin, S.Y.; Wykoff, C.C.; MacCumber, M.W. Safety Outcomes of Brolucizumab in Neovascular Age-Related Macular Degeneration: Results From the IRIS Registry and Komodo Healthcare Map. JAMA Ophthalmol. 2022, 140, 20–28. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Akiyama, H. One-year results of treat-and-extend regimen with intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 macular neovascularization. Sci. Rep. 2022, 12, 8195. [Google Scholar] [CrossRef]
- Tanaka, K.; Koizumi, H.; Tamashiro, T.; Itagaki, K.; Nakayama, M.; Maruko, I.; Wakugawa, S.; Terao, N.; Onoe, H.; Wakatsuki, Y.; et al. Short-term results for brolucizumab in treatment-naïve neovascular age-related macular degeneration: A Japanese multicenter study. Jpn. J. Ophthalmol. 2022, 66, 379–385. [Google Scholar] [CrossRef]
- Pakravan, P.; Patel, V.; Lai, J.; Shaheen, A.; Kalahasty, K.; Reyes-Capo, D.P.; Chau, V.; Rosenfeld, P.J.; Haddock, L.J.; Schwartz, S.G.; et al. Intraocular Inflammation Incidence after Intravitreal Brolucizumab Injection for Exudative Age-Related Macular Degeneration. Retina 2023, 43, 1717–1722. [Google Scholar]
- Noma, H.; Mimura, T.; Yasuda, K.; Motohashi, R.; Kotake, O.; Shimura, M. Aqueous Humor Levels of Soluble Vascular Endothelial Growth Factor Receptor and Inflammatory Factors in Diabetic Macular Edema. Ophthalmologica 2017, 238, 81–88. [Google Scholar] [CrossRef]
- Motohashi, R.; Noma, H.; Yasuda, K.; Kotake, O.; Goto, H.; Shimura, M. Dynamics of Inflammatory Factors in Aqueous Humor during Ranibizumab or Aflibercept Treatment for Age-Related Macular Degeneration. Ophthalmic Res. 2017, 58, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, R.; Noma, H.; Yasuda, K.; Kotake, O.; Goto, H.; Shimura, M. Dynamics of soluble vascular endothelial growth factor receptors and their ligands in aqueous humour during ranibizumab for age-related macular degeneration. J. Inflamm. 2018, 15, 26. [Google Scholar] [CrossRef]
- Mastropasqua, R.; D’Aloisio, R.; Di Nicola, M.; Di Martino, G.; Lamolinara, A.; Di Antonio, L.; Tognetto, D.; Toto, L. Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Sci. Rep. 2018, 8, 16548. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, R.; Noma, H.; Yasuda, K.; Kasezawa, Y.; Goto, H.; Shimura, M. Aqueous Flare, Functional-Morphological Parameters, and Cytokines in Age-Related Macular Degeneration after Anti-VEGF Treatment. Open Ophthalmol. J. 2021, 15, 209–215. [Google Scholar] [CrossRef]
- Yasuda, K.; Noma, H.; Mimura, T.; Nonaka, R.; Sasaki, S.; Ofusa, A.; Shimura, M. Role of Novel Inflammatory Factors in Central Retinal Vein Occlusion with Macular Edema. Medicina 2023, 60, 4. [Google Scholar] [CrossRef]
- Fauser, S.; Schwabecker, V.; Muether, P.S. Suppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 532–536. [Google Scholar] [CrossRef]
- Wang, X.; Sawada, T.; Kakinoki, M.; Miyake, T.; Kawamura, H.; Saishin, Y.; Liu, P.; Ohji, M. Aqueous vascular endothelial growth factor and ranibizumab concentrations after monthly and bimonthly intravitreal injections of ranibizumab for age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1033–1039. [Google Scholar] [CrossRef]
- Sato, T.; Takeuchi, M.; Karasawa, Y.; Enoki, T.; Ito, M. Intraocular inflammatory cytokines in patients with neovascular age-related macular degeneration before and after initiation of intravitreal injection of anti-VEGF inhibitor. Sci. Rep. 2018, 8, 1098. [Google Scholar] [CrossRef]
- Iyer, P.G.; Peden, M.C.; Suñer, I.J.; Patel, N.; Dubovy, S.R.; Albini, T.A. Brolucizumab-related retinal vasculitis with exacerbation following ranibizumab retreatment: A clinicopathologic case study. Am. J. Ophthalmol. Case Rep. 2020, 20, 100989. [Google Scholar] [CrossRef]
- Haug, S.J.; Hien, D.L.; Uludag, G.; Ngoc, T.T.T.; Lajevardi, S.; Halim, M.S.; Sepah, Y.J.; Do, D.V.; Khanani, A.M. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am. J. Ophthalmol. Case Rep. 2020, 18, 100680. [Google Scholar] [CrossRef]
- Wilkerson, R.G. Drug Hypersensitivity Reactions. Emerg. Med. Clin. N. Am. 2022, 40, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.J.; Bigliardi, P.; Bircher, A.J.; Broyles, A.; Chang, Y.S.; Chung, W.H.; Lehloenya, R.; Mockenhaupt, M.; Peter, J.; Pirmohamed, M.; et al. Controversies in drug allergy: Testing for delayed reactions. J. Allergy Clin. Immunol. 2019, 143, 66–73. [Google Scholar] [CrossRef]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Isabwe, G.A.C.; Garcia Neuer, M.; de Las Vecillas Sanchez, L.; Lynch, D.M.; Marquis, K.; Castells, M. Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J. Allergy Clin. Immunol. 2018, 142, 159–170. [Google Scholar] [CrossRef]
- Morgenstern-Kaplan, D.; Vasquez-Echeverri, E.; Carrillo-Martin, I.; Chiarella, S.E.; Gonzalez-Estrada, A. Cytokine-release hypersensitivity reaction after the first dose of benralizumab for severe eosinophilic asthma. Ann. Allergy Asthma Immunol. 2021, 127, 701–702. [Google Scholar] [CrossRef]
- Yamada, K.; Sakurai, E.; Itaya, M.; Yamasaki, S.; Ogura, Y. Inhibition of laser-induced choroidal neovascularization by atorvastatin by downregulation of monocyte chemotactic protein-1 synthesis in mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1839–1843. [Google Scholar] [CrossRef]
- Austin, B.A.; Liu, B.; Li, Z.; Nussenblatt, R.B. Biologically active fibronectin fragments stimulate release of MCP-1 and catabolic cytokines from murine retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2896–2902. [Google Scholar] [CrossRef]
- Okunuki, Y.; Usui, Y.; Nagai, N.; Kezuka, T.; Ishida, S.; Takeuchi, M.; Goto, H. Suppression of experimental autoimmune uveitis by angiotensin II type 1 receptor blocker telmisartan. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2255–2261. [Google Scholar] [CrossRef]
- Feldman, E.D.; Weinreich, D.M.; Carroll, N.M.; Burness, M.L.; Feldman, A.L.; Turner, E.; Xu, H.; Alexander, H.R., Jr. Interferon gamma-inducible protein 10 selectively inhibits proliferation and induces apoptosis in endothelial cells. Ann. Surg. Oncol. 2006, 13, 125–133. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef]
- Akahira-Azuma, M.; Szczepanik, M.; Tsuji, R.F.; Campos, R.A.; Itakura, A.; Mobini, N.; McNiff, J.; Kawikova, I.; Lu, B.; Gerard, C.; et al. Early delayed-type hypersensitivity eosinophil infiltrates depend on T helper 2 cytokines and interferon-gamma via CXCR3 chemokines. Immunology 2004, 111, 306–317. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, J.; Gu, J.; Xu, B.; Sun, X.; Zhang, S. FMS-Related Tyrosine Kinase 3 Ligand Promotes Radioresistance in Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 2021, 12, 659735. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, P.; Chevallier, P.; Knapper, S.; Collin, M. FLT3 ligand in acute myeloid leukemia: A simple test with deep implications. Leuk. Lymphoma 2021, 62, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Fukaya, T.; Fukui, T.; Uto, T.; Takagi, H.; Nasu, J.; Miyanaga, N.; Riethmacher, D.; Choijookhuu, N.; Hishikawa, Y.; et al. Congenital Deficiency of Conventional Dendritic Cells Promotes the Development of Atopic Dermatitis-Like Inflammation. Front. Immunol. 2021, 12, 712676. [Google Scholar] [CrossRef] [PubMed]
- Tilton, R.G.; Chang, K.C.; LeJeune, W.S.; Stephan, C.C.; Brock, T.A.; Williamson, J.R. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Investig. Ophthalmol. Vis. Sci. 1999, 40, 689–696. [Google Scholar]
- Papadopoulou, D.N.; Mendrinos, E.; Mangioris, G.; Donati, G.; Pournaras, C.J. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology 2009, 116, 1755–1761. [Google Scholar] [CrossRef]
- Sacu, S.; Pemp, B.; Weigert, G.; Matt, G.; Garhofer, G.; Pruente, C.; Schmetterer, L.; Schmidt-Erfurth, U. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3046–3050. [Google Scholar] [CrossRef]
- Fukami, M.; Iwase, T.; Yamamoto, K.; Kaneko, H.; Yasuda, S.; Terasaki, H. Changes in Retinal Microcirculation After Intravitreal Ranibizumab Injection in Eyes With Macular Edema Secondary to Branch Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1246–1255. [Google Scholar] [CrossRef]
- Noma, H.; Mimura, T.; Shimada, K. Role of inflammation in previously untreated macular edema with branch retinal vein occlusion. BMC Ophthalmol. 2014, 14, 67. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 2015, 20, 196–209. [Google Scholar] [CrossRef]
- Ueland, H.O.; Ueland, G.; Løvås, K.; Breivk, L.E.; Thrane, A.S.; Meling Stokland, A.E.; Rødahl, E.; Husebye, E.S. Novel inflammatory biomarkers in thyroid eye disease. Eur. J. Endocrinol. 2022, 187, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Obata, R.; Okubo, A.; Aoki, S.; Azuma, K.; Ahmed, T.; Inoda, S.; Hashimoto, Y.; Takahashi, R.; Yoshida, H.; et al. Cytokine profiles in the aqueous humor following brolucizumab administration for exudative age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).