Diagnosis and Surgical Treatment Outcomes of Cardiac Myxoma: Twenty Years of Data at a Single Institution
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Clinical Presentations
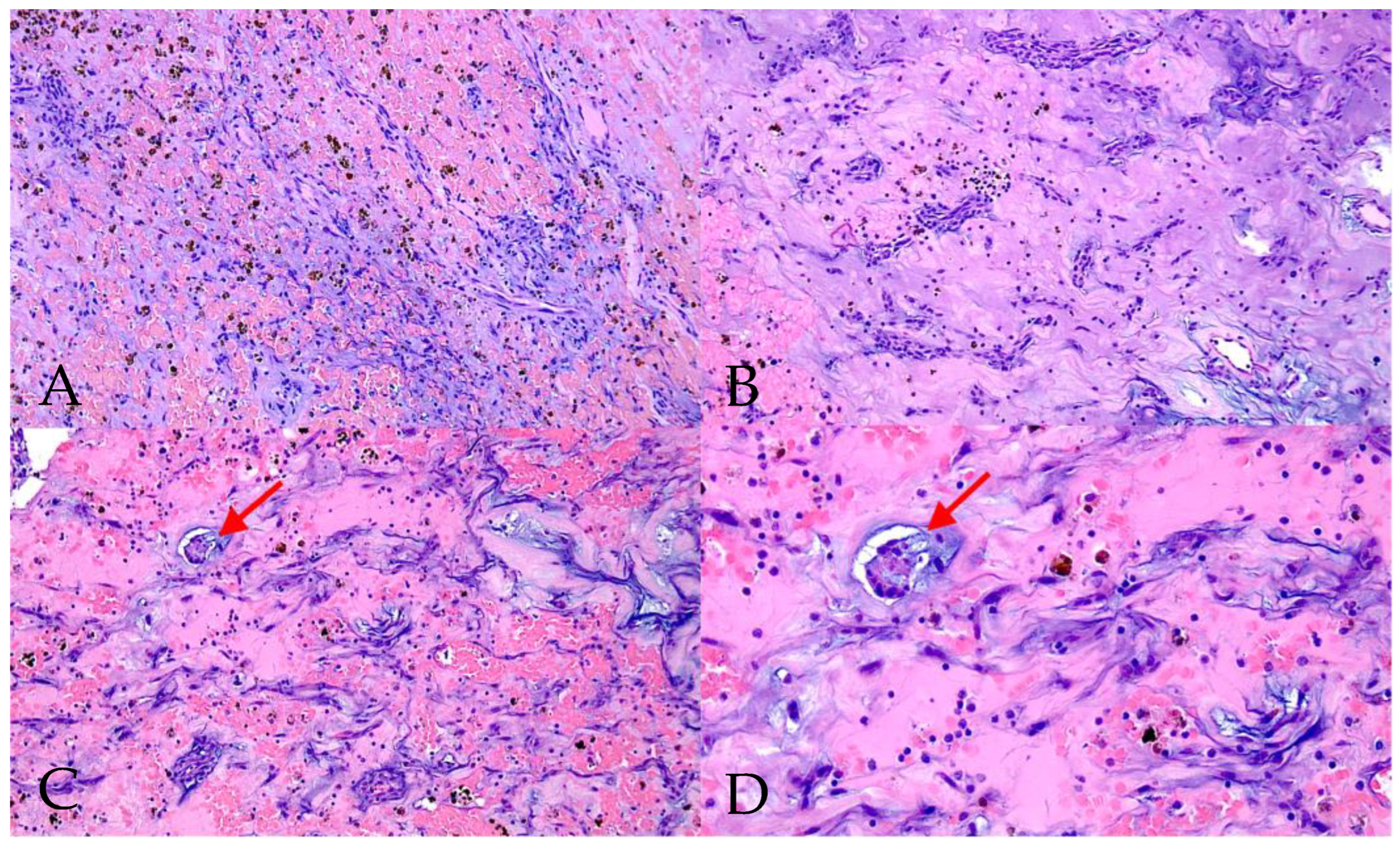
3.3. Diagnostic Features
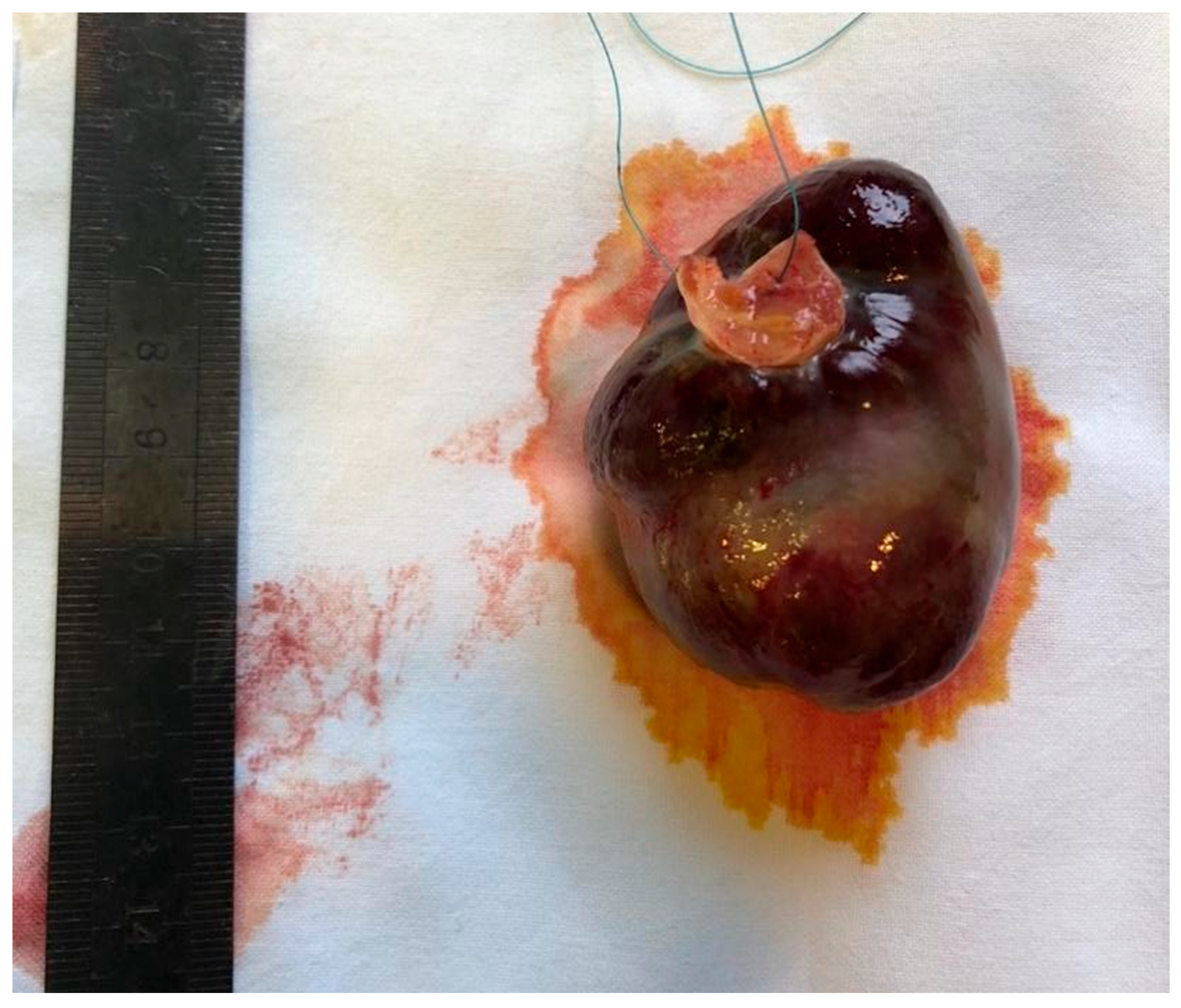
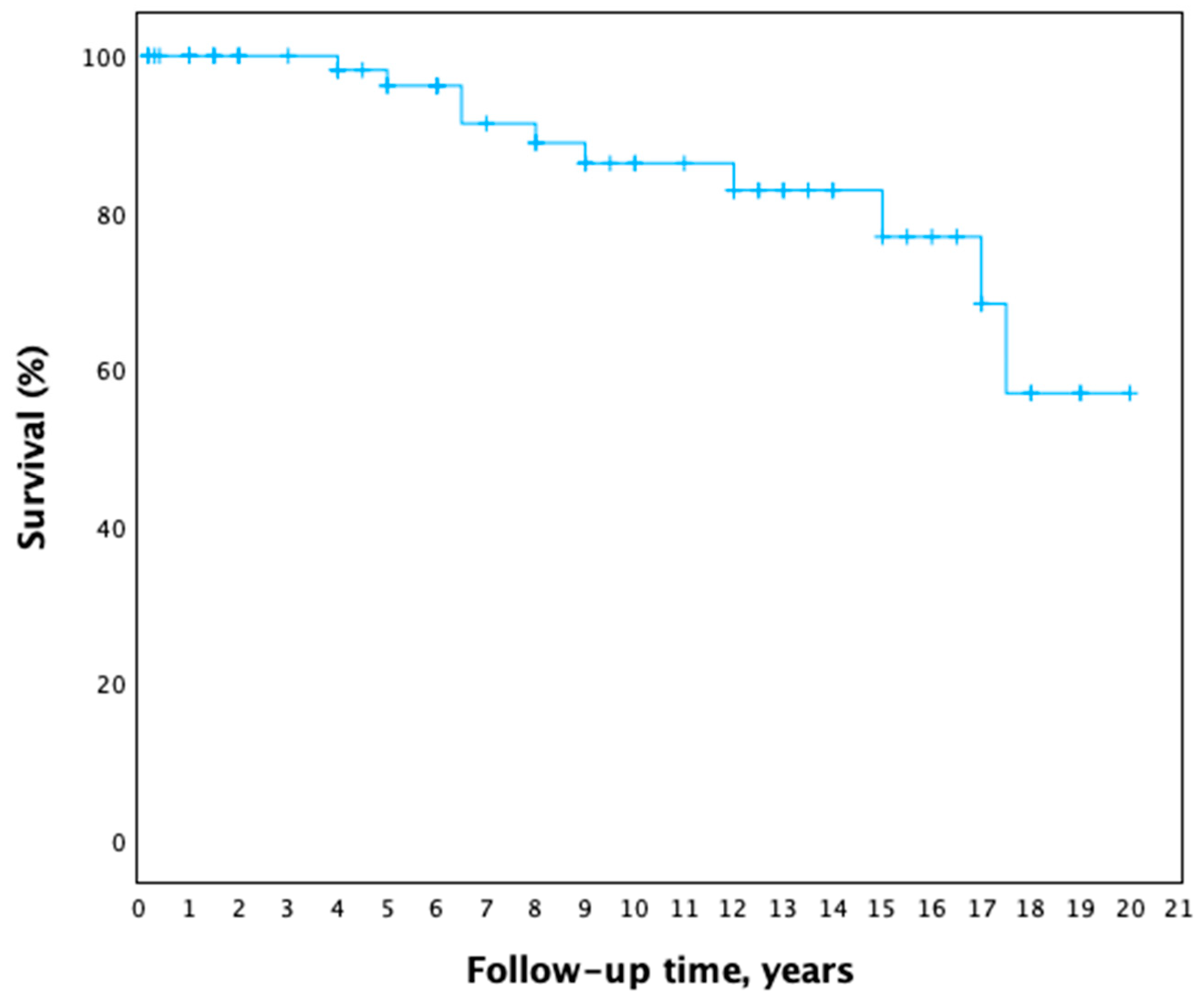
3.4. Surgical Treatment and Postoperative Period
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | coronary artery bypass graft |
| CM | cardiac myxoma |
| CODP | chronic obstructive pulmonary disease |
| CT | computed tomography |
| EF | ejection fraction |
| Gmean | mean gradient |
| LA | left atrium |
| LV | left ventricle |
| LVEDD | left ventricle end-diastolic diameter |
| MRI | magnetic resonance imaging |
| NYHA | New York Heart Association |
| PASP | pulmonary artery systolic pressure |
| PET | positron emissions tomography |
| SD | standard deviation |
| TEE | transoesophageal echocardiography |
| TTE | transthoracic echocardiography |
References
- Yanagawa, B.; Mazine, A.; Chan, E.Y.; Barker, C.M.; Gritti, M.; Reul, R.M.; Ravi, V.; Ibarra, S.; Shapira, O.M.; Cusimano, R.J.; et al. Surgery for Tumors of the Heart. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, T.J.; Kochav, J.; O’cOnnor, D.S.; Rosner, G.F. Cardiac Tumors: Clinical Presentation, Diagnosis, and Management. Curr. Treat. Options Oncol. 2019, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Tavora, F. The 2015 WHO Classification of Tumors of the Heart and Pericardium. J. Thorac. Oncol. 2016, 11, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Karabinis, A.; Samanidis, G.; Khoury, M.; Stavridis, G.; Perreas, K. Clinical presentation and treatment of cardiac myxoma in 153 patients. Medicine 2018, 97, e12397. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-X.; Wang, J.-G.; Qi, R.-D.; Wang, W.; Gao, L.-J.; Zhao, J.-H.; Zhang, C.-X.; Zhou, M.-C.; Tu, X.; Shang, M.-S.; et al. Long-term outcome of patients with atrial myxoma after surgical intervention: Analysis of 403 cases. J. Geriatr. Cardiol. 2019, 16, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Garatti, A.; Nano, G.; Canziani, A.; Gagliardotto, P.; Mossuto, E.; Frigiola, A.; Menicanti, L. Surgical Excision of Cardiac Myxomas: Twenty Years Experience at a Single Institution. Ann. Thorac. Surg. 2012, 93, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors. JACC CardioOncology 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Samanidis, G.; Khoury, M.; Balanika, M.; Perrea, D.N. Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiologia Polska 2020, 78, 269–277. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.J. Multi Modality Imaging Features of Cardiac Myxoma. J. Cardiovasc. Imaging 2020, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Durante, A.; Limite, L.R.; Cianflone, D. Postoperative Arrhythmias after Cardiac Surgery: Incidence, Risk Factors, and Therapeutic Management. Cardiol. Res. Pr. 2014, 2014, 615987. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.K.; Dearani, J.A.; Daly, R.C.; Suri, R.M.; Park, S.J.; Joyce, L.D.; Li, Z.; Schaff, H.V. Cardiac Myxomas: A 50-Year Experience with Resection and Analysis of Risk Factors for Recurrence. Ann. Thorac. Surg. 2015, 100, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Mahani, M.G.; Lu, J.C.; Dorfman, A.L.; Srinivasan, A.; Agarwal, P.P. Primary cardiac tumors associated with genetic syndromes: A comprehensive review. Pediatr. Radiol. 2017, 48, 156–164. [Google Scholar] [CrossRef] [PubMed]
| Comorbidities and Risk Factors | All Patients (n = 76) N (%) |
|---|---|
| Arterial hypertension | 57 (75.0) |
| History of myocardial infarction | 5 (6.6) |
| Cerebral stroke | 4 (5.3) |
| Diabetes mellitus | 6 (7.9) |
| Non-cardiac neoplastic diseases | 10 (13.2) |
| COPD | 8 (10.5) |
| Dyslipidaemia | 41 (53.9) |
| Obesity | 28 (36.8) |
| Symptoms | All Patients (n = 76) N (%) | |
|---|---|---|
| Generalized/nonspecific symptoms: | 23 (30.3) | |
| Fever | 4 (5.3) | |
| Weight loss | 5 (6.6) | |
| General weakness | 17 (22.4) | |
| Arthralgia, myalgia | 2 (2.6) | |
| Obstructive cardiac symptoms: | 67 (88.2) | |
| Dyspnoea | 49 (64.5) | |
| Functional classification according to the NYHA: | ||
| Class I | 11 (14.5) | |
| Class II | 43 (56.6) | |
| Class III | 15 (19.7) | |
| Class IV | 7 (9.2) | |
| Chest pain | 30 (39.5) | |
| Arrythmias | 27 (35.5) | |
| Syncope | 7 (9.2) | |
| Chronic heart failure symptoms | 5 (6.6) | |
| Peripheral embolisms: | 6 (7.9) | |
| Cerebral stroke | 5 (6.6) | |
| Peripheral (limb) emboli | 1 (1.6) | |
| Echocardiographic Characteristics | All Patients (n = 76) |
|---|---|
| Myxoma location | |
| Left atrium, n (%) | 68 (89.5%) |
| Right atrium, n (%) | 7 (9.2%) |
| Left ventricle, n (%) | 1 (1.3%) |
| Size of myxoma | |
| Dimension 1 (height), mm ± SD (range) | 37.8 ± 16.1 (8–80) |
| Dimension 2 (length), mm ± SD (range) | 26.4 ± 11.4 (7–55) |
| LV ejection fraction (EF): | |
| ≥50%, n (%) | 67 (88.2) |
| 30–49%, n (%) | 7 (9.2) |
| <30%, n (%) | 2 (2.6) |
| Mitral stenosis | 62 (81.6) |
| Mild (Gmean < 5 mmHg), n (%) | 52 (68.4) |
| Moderate (Gmean 5–10 mmHg), n (%) | 7 (9.2) |
| Severe (Gmean > 10 mmHg), n (%) | 3 (4.0) |
| Mitral regurgitation | 63 (82.9) |
| Mild-moderate (I–II degree), n (%) | 60 (78.9) |
| Moderate-severe (III–IV degree), n (%) | 3 (4.0) |
| Tricuspid regurgitation | 60 (78.9) |
| Mild-moderate (I–II degree), n (%) | 53 (69.7) |
| Moderate-severe (III–IV degree), n (%) | 7 (9.2) |
| Pulmonary hypertension (PASP > 40 mmHg), n (%) | 15 (19.7) |
| LVEDD, mm ± SD | 48.1 (±6.7) |
| LA size, mm ± SD | 42.1 (±5.9) |
| Malignant Tumours | Time After CM Surgery | Benign Neoplasms | Time After CM Surgery |
|---|---|---|---|
| Cervical cancer | 17 years | Intraductal papillary mucinous neoplasm | 11 years |
| Lung cancer (squamous-cell carcinoma) | 14 years | Low-grade colon dysplasia | 10 years |
| Facial basal-cell carcinoma | 5 years | Adrenal adenoma | 1 year |
| Lung cancer (adenocarcinoma) | 2 years | Uterine leiomyoma | 1 year |
| Liver haemangioma | 1 year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakuskaite, G.; Jakuska, P.; Benetis, R.; Vaskelyte, J.J.; Ereminiene, E. Diagnosis and Surgical Treatment Outcomes of Cardiac Myxoma: Twenty Years of Data at a Single Institution. Medicina 2025, 61, 2025. https://doi.org/10.3390/medicina61112025
Jakuskaite G, Jakuska P, Benetis R, Vaskelyte JJ, Ereminiene E. Diagnosis and Surgical Treatment Outcomes of Cardiac Myxoma: Twenty Years of Data at a Single Institution. Medicina. 2025; 61(11):2025. https://doi.org/10.3390/medicina61112025
Chicago/Turabian StyleJakuskaite, Gabriele, Povilas Jakuska, Rimantas Benetis, Jolanta Justina Vaskelyte, and Egle Ereminiene. 2025. "Diagnosis and Surgical Treatment Outcomes of Cardiac Myxoma: Twenty Years of Data at a Single Institution" Medicina 61, no. 11: 2025. https://doi.org/10.3390/medicina61112025
APA StyleJakuskaite, G., Jakuska, P., Benetis, R., Vaskelyte, J. J., & Ereminiene, E. (2025). Diagnosis and Surgical Treatment Outcomes of Cardiac Myxoma: Twenty Years of Data at a Single Institution. Medicina, 61(11), 2025. https://doi.org/10.3390/medicina61112025







