Temporal Trends of Dengue Surveillance in Sardinia, Italy: Implications of Climate Change on Human and Entomological Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Human and Entomological Surveillance System
2.2. Study Setting
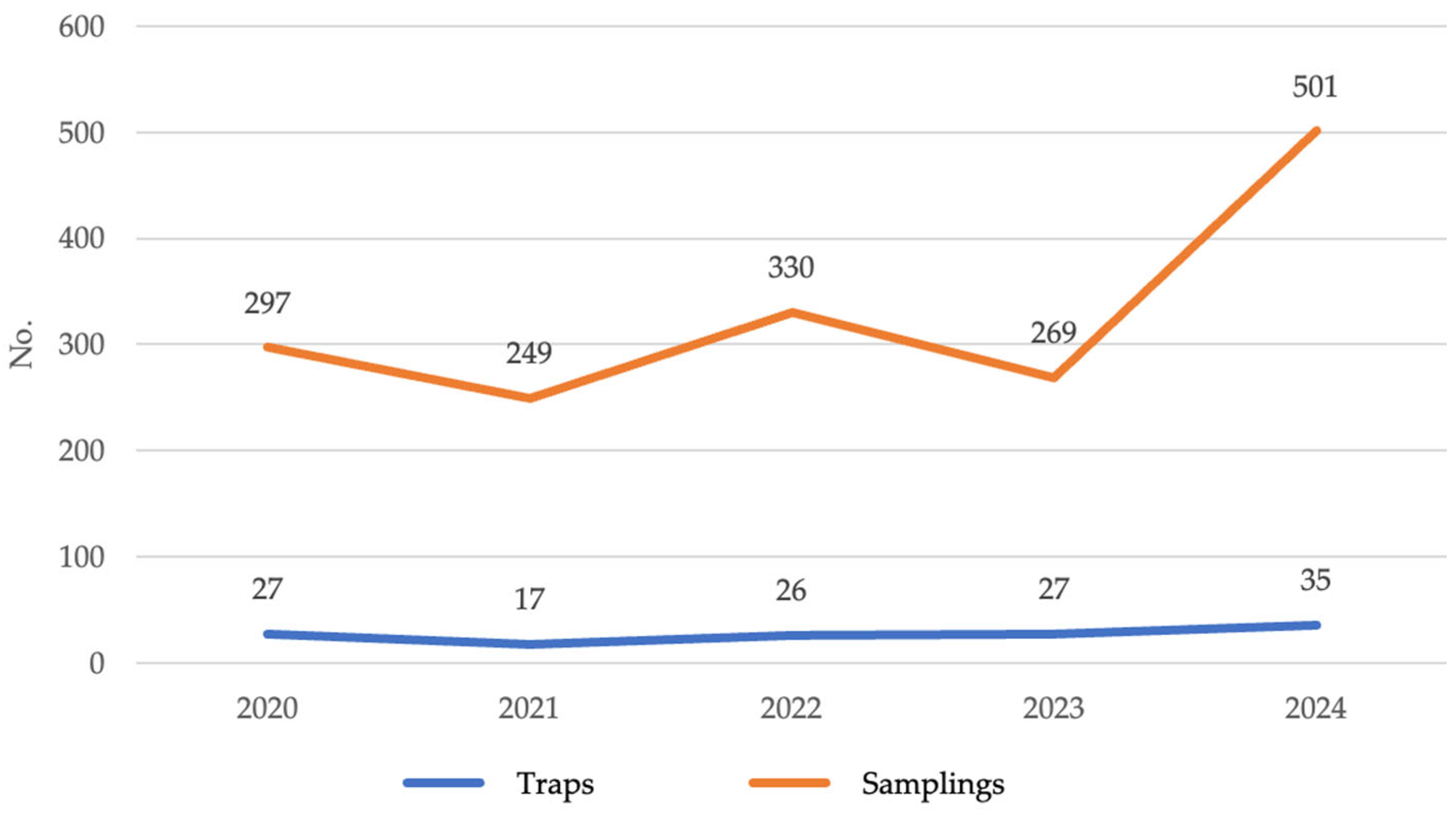
2.3. Trap Distribution
2.4. Sample Handling
2.5. Data Sources and Analysis
3. Results
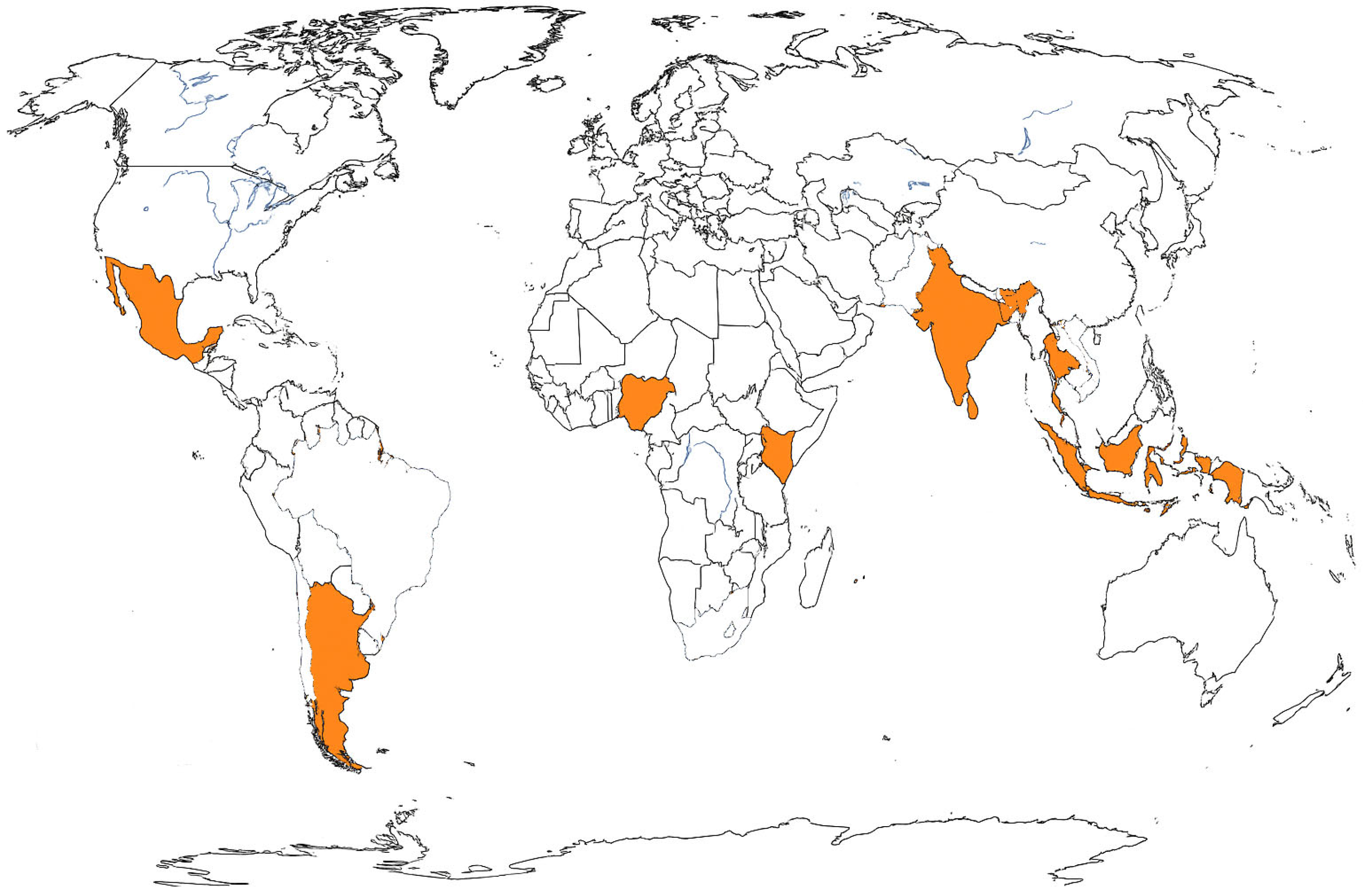
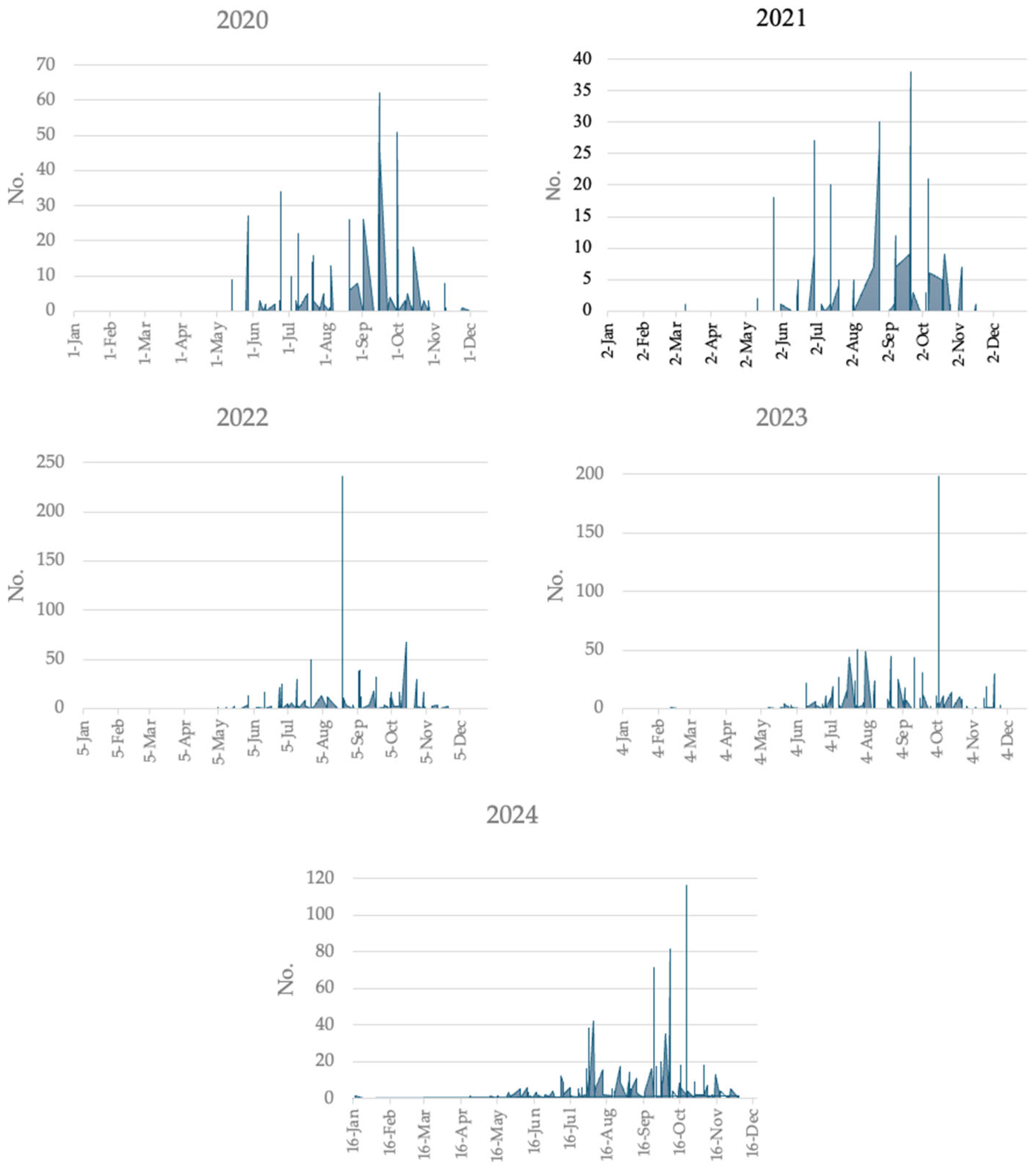
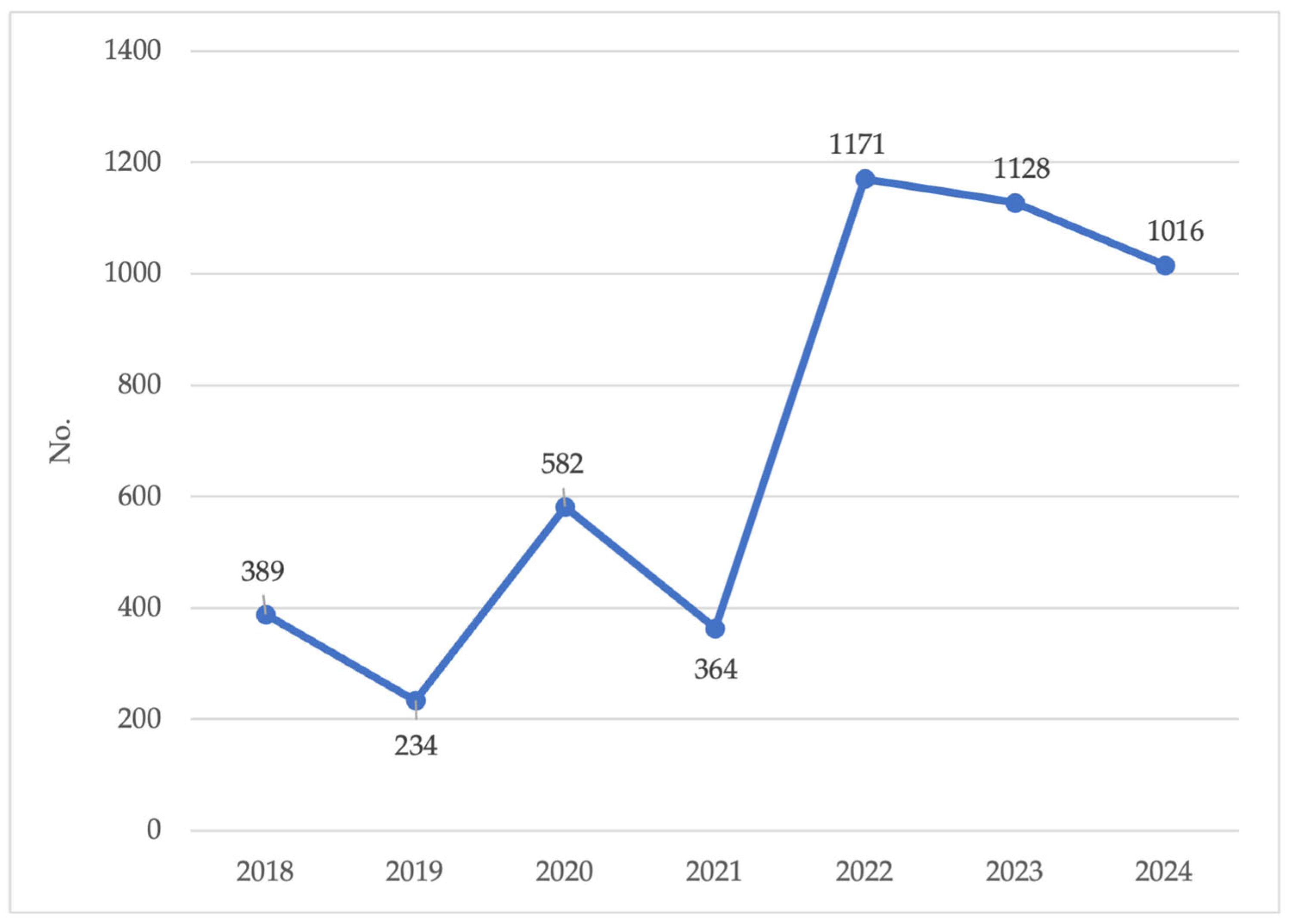
3.1. Human Surveillance
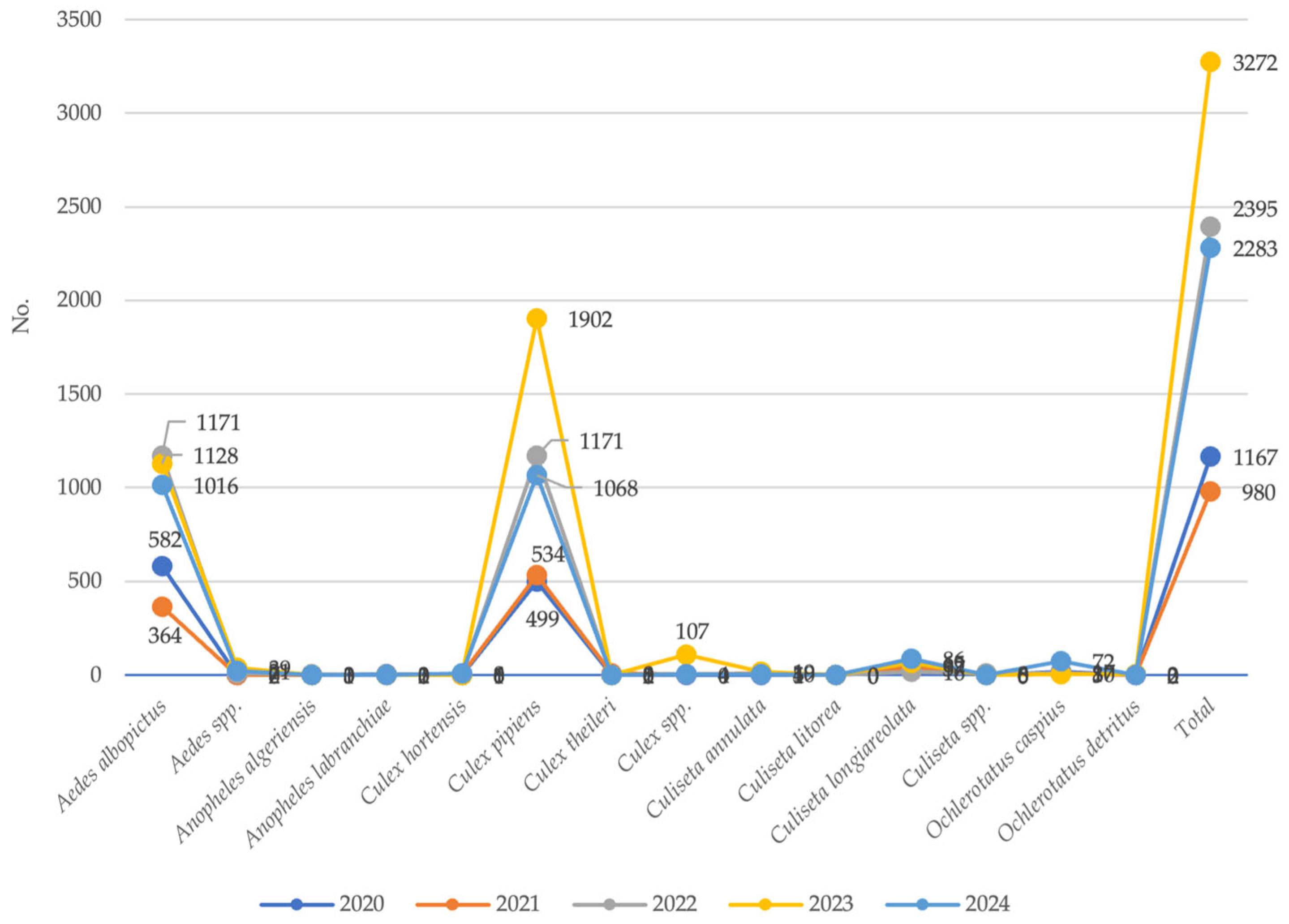
3.2. Entomological Surveillance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LHA | Local Health Authorities |
| EZS | Experimental Zooprophylactic Institutes |
References
- Adepoju, O.A.; Afinowi, O.A.; Tauheed, A.M.; Danazumi, A.U.; Dibba, L.B.S.; Balogun, J.B.; Flore, G.; Saidu, U.; Ibrahim, B.; Balogun, O.O.; et al. Multisectoral Perspectives on Global Warming and Vector-borne Diseases: A Focus on Southern Europe. Curr. Trop. Med. Rep. 2023, 10, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef]
- Fouque, F.; Reeder, J.C. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: A look at the evidence. Infect. Dis. Poverty 2019, 8, 51. [Google Scholar] [CrossRef]
- Bartlow, A.W.; Manore, C.; Xu, C.; Kaufeld, K.A.; Del Valle, S.; Ziemann, A.; Fairchild, G.; Fair, J.M. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet. Sci. 2019, 6, 40. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Vector-Borne Diseases. 2024. Available online: https://www.cdc.gov/climate-health/php/effects/vectors.html (accessed on 5 November 2025).
- Zhang, Y.; Wang, M.; Huang, M.; Zhao, J. Innovative strategies and challenges mosquito-borne disease control amidst climate change. Front. Microbiol. 2024, 15, 1488106. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Dengue Worldwide Overview. 2025. Available online: https://www.ecdc.europa.eu/en/dengue-monthly (accessed on 5 November 2025).
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef]
- Barcellos, C.; Matos, V.; Lana, R.M.; Lowe, R. Climate change, thermal anomalies, and the recent progression of dengue in Brazil. Sci. Rep. 2024, 14, 5948, Erratum in Sci. Rep. 2024, 14, 7428. https://doi.org/10.1038/s41598-024-58202-8. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Lindsay, S.W.; Scott, T.W.; Ooi, E.E.; Gubler, D.J.; Das, P. The Lancet Commission on dengue and other Aedes-transmitted viral diseases. Lancet 2020, 395, 1890–1891. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 5 November 2025).
- De Santis, O.; Bouscaren, N.; Flahault, A. Asymptomatic dengue infection rate: A systematic literature review. Heliyon 2023, 9, e20069. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Sjö, P.; Singh, K.; Piedagnel, J.M.; Mowbray, C.; Estani, S.; Lim, S.C.L.; Siquierra, A.M.; Ogg, G.S.; Fraisse, L.; et al. Facing the escalating burden of dengue: Challenges and perspectives. PLoS Glob. Public Health 2023, 3, e0002598. [Google Scholar] [CrossRef] [PubMed]
- Lühken, R.; Brattig, N.; Becker, N. Introduction of invasive mosquito species into Europe and prospects for arbovirus transmission and vector control in an era of globalization. Infect. Dis. Poverty 2023, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Fernández de Marco, M.; Giovannini, A.; Ippoliti, C.; Danzetta, M.L.; Svartz, G.; Erster, O.; Groschup, M.H.; Ziegler, U.; Mirazimi, A.; et al. Emerging Mosquito-Borne Threats and the Response from European and Eastern Mediterranean Countries. Int. J. Environ. Res. Public Health 2018, 15, 2775. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863, Erratum in Nat. Microbiol. 2019, 4, 900. https://doi.org/10.1038/s41564-019-0429-2. Erratum in Nat. Microbiol. 2019, 4, 901. https://doi.org/10.1038/s41564-019-0440-7. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Barreca, F.; Benvenuto, D.; Braccialarghe, N.; Campogiani, L.; Lodi, A.; Aguglia, C.; Cavasio, R.A.; Giacalone, M.L.; Kontogiannis, D.; et al. Human Arboviral Infections in Italy: Past, Current, and Future Challenges. Viruses 2023, 15, 368. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Local Transmission of Dengue Virus in Mainland EU/EEA, 2010–2024. 2025. Available online: https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea-previous-years (accessed on 5 November 2025).
- Regional Agency for Environmental Protection of Sardinia. Agrometeorological Analysis 2023–2024. Available online: https://www.sardegnaambiente.it/index.php?xsl=612&s=462630&v=2&c=4581&idsito=21 (accessed on 5 November 2025).
- Pedrotta, T.; Gobet, E.; Schwörer, C.; Beffa, G.; Butz, C.; Henne, P.D.; Morales-Molino, C.; Pasta, S.; van Leeuwen, J.F.N.; Vogel, H.; et al. 8000 years of climate, vegetation, fire and land-use dynamics in the thermo-mediterranean vegetation belt of northern Sardinia (Italy). Veg. Hist. Archaeobotany 2021, 30, 789–813. [Google Scholar] [CrossRef]
- National Plan for the Prevention, Surveillance and Response to Arboviral Diseases 2020–2025. 2019. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2947_allegato.pdf (accessed on 5 November 2025).
- Decree of 7 March 2022. Revision of the Infectious Disease Reporting System (PREMAL). Official Journal of the Italian Republic, General Series no. 82 of 7 April 2022. Available online: https://www.gazzettaufficiale.it/eli/id/2022/04/07/22A02179/SG (accessed on 5 November 2025).
- Italian National Institute of Health. Arboviral Diseases. 2022. Available online: https://www.epicentro.iss.it/arbovirosi/ (accessed on 5 November 2025).
- Italian National Institute of Health. Epidemiology and Public Health. ISTISAN Reports 22/22. Mosquito Surveillance in Italy; Italian National Institute of Health: Rome, Italy, 2022. [Google Scholar]
- Logiudice, J.; Alberti, M.; Ciccarone, A.; Rossi, B.; Tiecco, G.; De Francesco, M.A.; Quiros-Roldan, E. Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health. Pathogens 2025, 14, 63. [Google Scholar] [CrossRef]
- Menegale, F.; Manica, M.; Del Manso, M.; Bella, A.; Zardini, A.; Gobbi, A.; Mignuoli, A.D.; Mattei, G.; Vairo, F.; Vezzosi, L.; et al. Risk assessment and perspectives of local transmission of chikungunya and dengue in Italy, a European forerunner. Nat. Commun. 2025, 16, 6237. [Google Scholar] [CrossRef]
- Vita, S.; Lalle, E.; Caputi, P.; Faraglia, F.; D’Abramo, A.; Bordi, L.; De Carli, G.; Sberna, G.; Giancola, M.L.; Maffongelli, G.; et al. Dengue fever as autochthonous infectious disease in Italy: Epidemiological, clinical and virological characteristics. Travel Med. Infect. Dis. 2024, 62, 102762. [Google Scholar] [CrossRef]
- Rovida, F.; Faccini, M.; Molina Granè, C.; Cassaniti, I.; Senatore, S.; Rossetti, E.; Scardina, G.; Piazza, M.; Campanini, G.; Lilleri, D.; et al. The 2023 dengue outbreak in Lombardy, Italy: A one-health perspective. Travel Med. Infect. Dis. 2025, 64, 102795. [Google Scholar] [CrossRef]
- Sacco, C.; Liverani, A.; Venturi, G.; Gavaudan, S.; Riccardo, F.; Salvoni, G.; Fortuna, C.; Marinelli, K.; Marsili, G.; Pesaresi, A.; et al. Autochthonous dengue outbreak in Marche Region, Central Italy, August to October 2024. Eurosurveillance 2024, 29, 2400713. [Google Scholar] [CrossRef]
- Fortuna, C.; Severini, F.; Marsili, G.; Toma, L.; Amendola, A.; Venturi, G.; Argentini, C.; Casale, F.; Bernardini, I.; Boccolini, D.; et al. Assessing the Risk of Dengue Virus Local Transmission: Study on Vector Competence of Italian Aedes albopictus. Viruses 2024, 16, 176. [Google Scholar] [CrossRef]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors 2014, 7, 338. [Google Scholar] [CrossRef]
- Anh, D.D.; Recker, M.; The, N.T.; Krishna, S.; Kremsner, P.G.; Song, L.H.; Velavan, T.P. Risk Stratification of Dengue Cases Requiring Hospitalization. J. Med. Virol. 2025, 97, e70511. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, K.; Ravi Kumar, Y.S.; Roy, R.; Phadnis, S.; Meena, V.; Bhattacharyya, S.; Verma, B. Dengue virus pathogenesis and host molecular machineries. J. Biomed. Sci. 2024, 31, 43. [Google Scholar] [CrossRef]
- World Health Organization. Vaccines and Immunization: Dengue. 2025. Available online: https://www.who.int/news-room/questions-and-answers/item/dengue-vaccines (accessed on 5 November 2025).

| Species | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|
| M | F | Total | M | F | Total | |
| Aedes albopictus | 85 | 304 | 389 | 78 | 156 | 234 |
| Aedes spp. | 2 | 81 | 83 | 7 | 68 | 75 |
| Aedes vexans | 0 | 192 | 192 | 0 | 8 | 8 |
| Anopheles algeriensis | 0 | 93 | 93 | 1 | 15 | 16 |
| Anopheles labranchiae | 3 | 200 | 203 | 1 | 56 | 57 |
| Anopheles spp. | 0 | 3 | 3 | 0 | 6 | 6 |
| Coquillettidia buxtoni | 0 | 1 | 1 | 0 | 7 | 7 |
| Coquillettidia richiardii | 0 | 1 | 1 | 0 | 0 | 0 |
| Culex hortensis | 2 | 7 | 9 | 10 | 21 | 31 |
| Culex mimeticus | 0 | 1 | 1 | 0 | 0 | 0 |
| Culex pipiens | 214 | 3818 | 4032 | 230 | 1034 | 1264 |
| Culex spp. | 12 | 78 | 90 | 24 | 47 | 71 |
| Culex theileri | 13 | 988 | 1001 | 0 | 62 | 62 |
| Culiseta annulata | 39 | 1019 | 1058 | 6 | 160 | 166 |
| Culiseta longiareolata | 50 | 95 | 145 | 39 | 36 | 75 |
| Culiseta spp. | 1 | 10 | 11 | 1 | 11 | 12 |
| Culiseta subochrea | 0 | 9 | 9 | 0 | 2 | 2 |
| Ochlerotatus berlandi | 0 | 2 | 2 | 0 | 0 | 0 |
| Ochlerotatus caspius | 64 | 4683 | 4747 | 12 | 442 | 454 |
| Ochlerotatus detritus | 35 | 984 | 1019 | 2 | 116 | 118 |
| Ochlerotatus mariae | 0 | 0 | 0 | 0 | 5 | 5 |
| Uranotaenia unguiculata | 0 | 39 | 39 | 0 | 0 | 0 |
| Total | 520 | 12,608 | 13,128 | 411 | 2252 | 2663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deiana, G.; Figoni, I.; Arghittu, A.; Campus, G.; Satta, G.; Foxi, C.; Piana, A.; Castiglia, P.; Dettori, M. Temporal Trends of Dengue Surveillance in Sardinia, Italy: Implications of Climate Change on Human and Entomological Monitoring. Medicina 2025, 61, 2024. https://doi.org/10.3390/medicina61112024
Deiana G, Figoni I, Arghittu A, Campus G, Satta G, Foxi C, Piana A, Castiglia P, Dettori M. Temporal Trends of Dengue Surveillance in Sardinia, Italy: Implications of Climate Change on Human and Entomological Monitoring. Medicina. 2025; 61(11):2024. https://doi.org/10.3390/medicina61112024
Chicago/Turabian StyleDeiana, Giovanna, Isabella Figoni, Antonella Arghittu, Guglielmo Campus, Giuseppe Satta, Cipriano Foxi, Andrea Piana, Paolo Castiglia, and Marco Dettori. 2025. "Temporal Trends of Dengue Surveillance in Sardinia, Italy: Implications of Climate Change on Human and Entomological Monitoring" Medicina 61, no. 11: 2024. https://doi.org/10.3390/medicina61112024
APA StyleDeiana, G., Figoni, I., Arghittu, A., Campus, G., Satta, G., Foxi, C., Piana, A., Castiglia, P., & Dettori, M. (2025). Temporal Trends of Dengue Surveillance in Sardinia, Italy: Implications of Climate Change on Human and Entomological Monitoring. Medicina, 61(11), 2024. https://doi.org/10.3390/medicina61112024









