ADAS-Cog Trajectories Differ from Expected Decline in Dementia Following Repeated Non-Invasive Interventions over 3 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Treatment Protocols
2.4. Inclusion and Exclusion Criteria
2.5. Outcome Measures
2.6. Estimating ADAS-Cog from WMS Data
2.7. Cognitive Trajectories Analysis
2.8. Medication Analysis
- AD medications: cholinesterase inhibitors (donepezil, galantamine, rivastigmine) or memantine
- Antidepressants: selective serotonin reuptake inhibitors (SSRIs) (paroxetine, citalopram, escitalopram, fluoxetine, sertraline) or other types (bupropion, trazodone)
- Hypertension medications: valsartan, telmisartan, ramipril, enalapril, candesartan, irbesartan, perindopril, fosinopril, hydrochlorothiazide, nifedipine, amlodipine, bisoprolol, atenolol, metoprolol
- Cholesterol medications: atorvastatin, rosuvastatin, simvastatin, ezetimibe
2.9. No-Treatment Interval Analysis
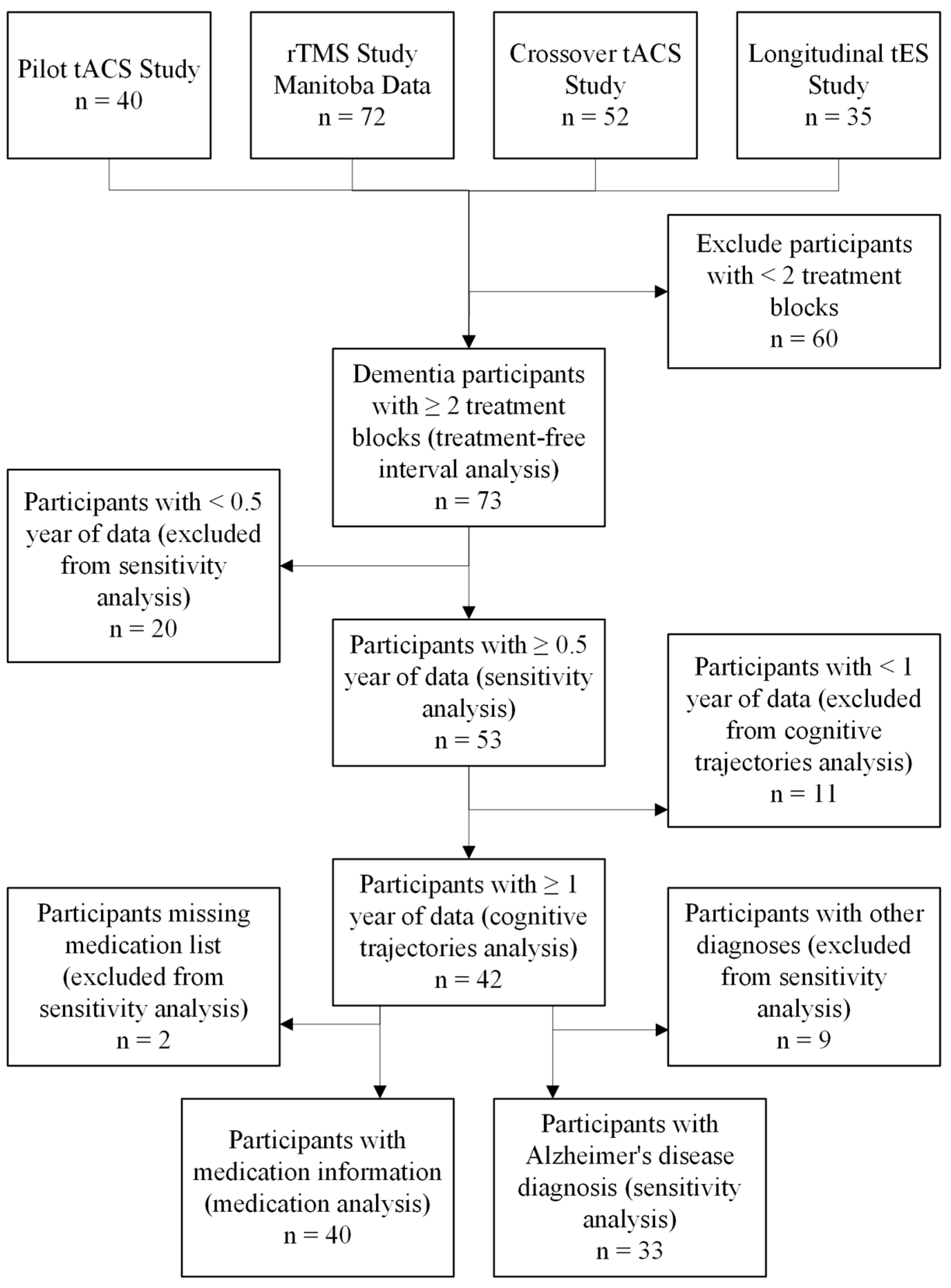
3. Results
3.1. Estimating ADAS-Cog from WMS Data
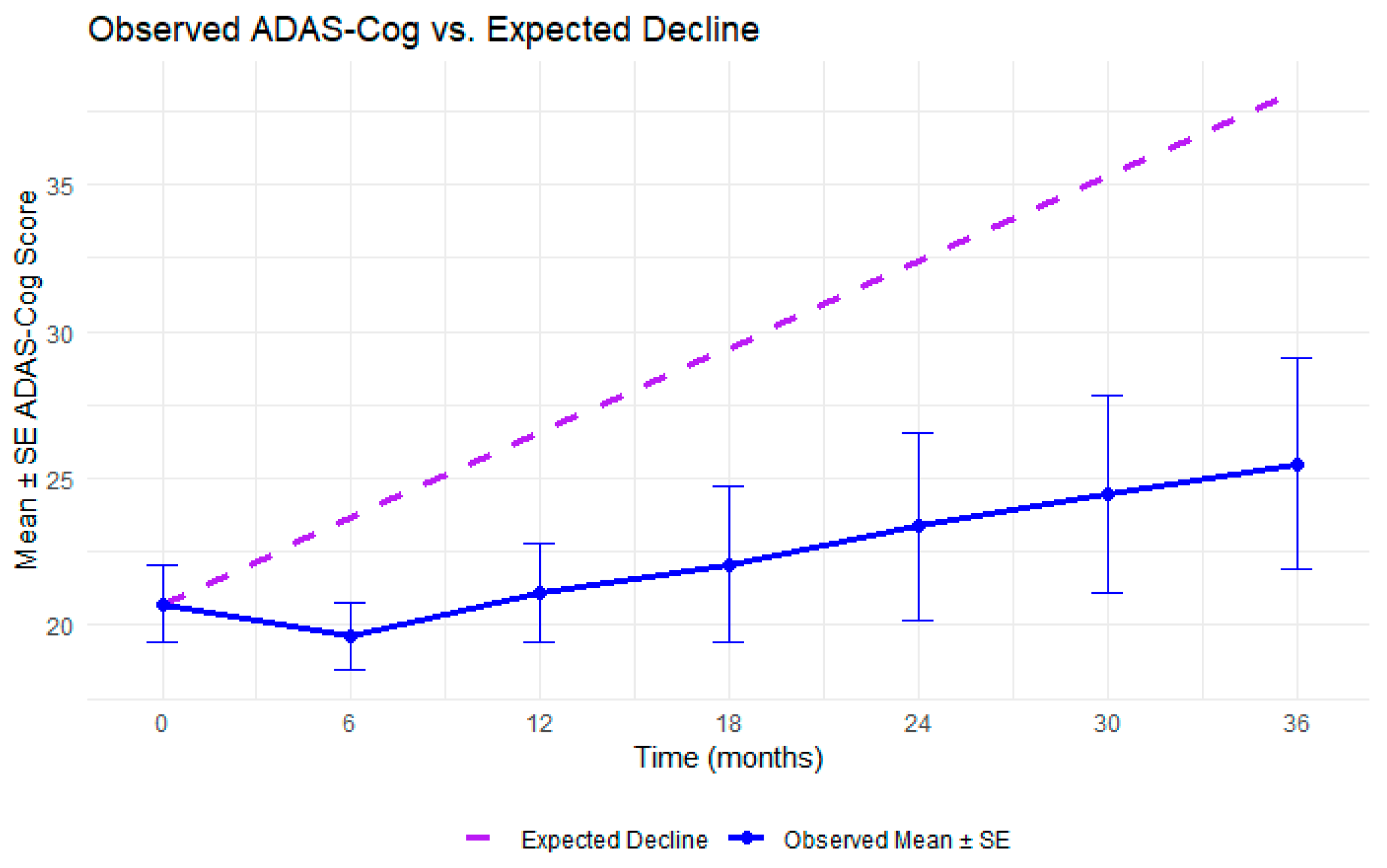
3.2. Cognitive Trajectories Analysis
3.3. Medication Analysis
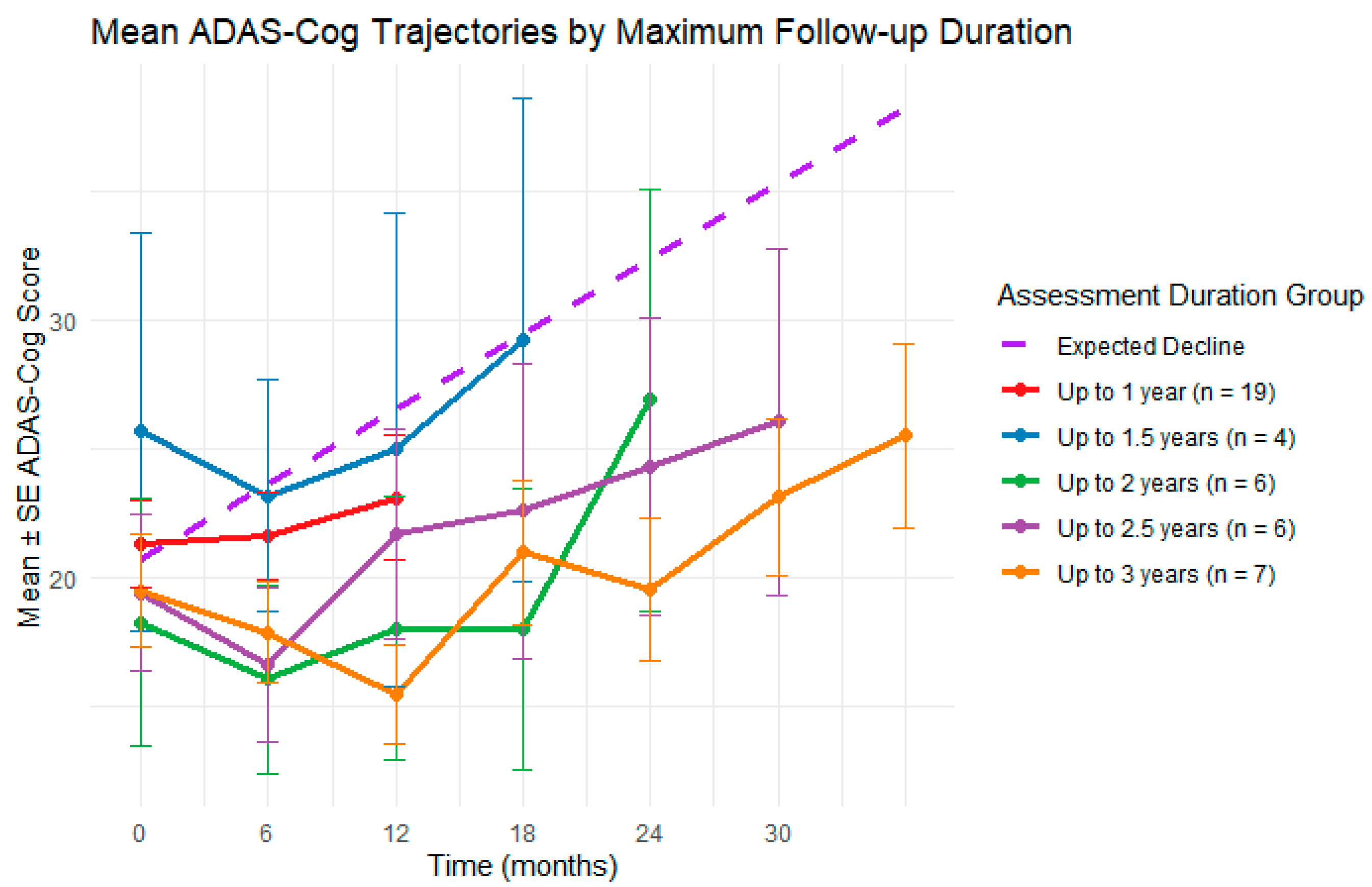
3.4. No-Treatment Interval Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ADAS-Cog | Alzheimer’s Disease Assessment Scale–Cognitive Subscale |
| CT | Cognitive Training |
| DLPFC | Dorsolateral Prefrontal Cortex |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| MADRS | Montgomery–Asberg Depression Rating Scale |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| NIBS | Non-Invasive Brain Stimulation |
| rTMS | Repetitive Transcranial Magnetic Stimulation |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| tACS | Transcranial Alternating Current Stimulation |
| tDCS | Transcranial Direct Current Stimulation |
| tES | Transcranial Electrical Stimulation |
| WMS | Wechsler Memory Scale |
Appendix A
| Variable | Coefficient (β) | Robust SE | t Value | p Value |
|---|---|---|---|---|
| Intercept | 55.032 | 10.212 | 5.389 | 1.29 × 10−7 |
| Brief Cognitive Status Exam Total Raw Score | −0.222 | 0.033 | −6.716 | 7.38 × 10−11 |
| Logical Memory I | −0.659 | 0.155 | −4.251 | 2.72 × 10−5 |
| Logical Memory II | 0.251 | 0.145 | 1.733 | 0.084 |
| Verbal Paired Associates I | −0.682 | 0.190 | −3.599 | 3.65 × 10−4 |
| Verbal Paired Associates II | 0.692 | 0.491 | 1.410 | 0.159 |
| Visual Reproduction I | −0.152 | 0.144 | −1.053 | 0.293 |
| Visual Reproduction II | 0.461 | 0.176 | 2.616 | 0.009 |
| Symbol Span | −0.081 | 0.062 | −1.312 | 0.190 |
| Logical Memory II Recognition | 0.116 | 0.089 | 1.308 | 0.192 |
| Verbal Paired Associates II Recognition | −0.174 | 0.063 | −2.750 | 0.006 |
| Verbal Paired Associates II Recall | 0.079 | 0.096 | 0.820 | 0.413 |
| Visual Reproduction II Recognition | 0.010 | 0.233 | 0.042 | 0.967 |
| Auditory Memory Sum of Scaled Scores | −0.562 | 0.677 | −0.831 | 0.407 |
| Auditory Memory Index | −0.766 | 0.341 | −2.245 | 0.025 |
| Visual Memory Sum of Scaled Scores | −1.760 | 0.794 | −2.217 | 0.027 |
| Visual Memory Index | −0.283 | 0.142 | −2.001 | 0.046 |
| Immediate Memory Sum of Scaled Scores | 2.920 | 0.866 | 3.372 | 8.28 × 10−4 |
| Immediate Memory Index | 0.040 | 0.042 | 0.950 | 0.343 |
| Delayed Memory Sum of Scaled Scores | 0.614 | 0.386 | 1.591 | 0.112 |
Appendix B
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 41.249 | 7.254 | 38.085 | 5.686 | 1.52 × 10−6 |
| Time (months) | 0.227 | 0.045 | 163.040 | 5.089 | 9.82 × 10−7 |
| Baseline Age (years) | 0.056 | 0.095 | 39.322 | 0.591 | 0.558 |
| Sex (male) | −1.050 | 1.689 | 38.126 | −0.622 | 0.538 |
| Baseline MoCA Score | −1.478 | 0.165 | 38.406 | −8.966 | 5.89 × 10−11 |
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 44.349 | 7.052 | 45.125 | 6.289 | 1.15 × 10−7 |
| Time (months) | 0.191 | 0.037 | 199.660 | 5.126 | 6.97 × 10−7 |
| Baseline Age (years) | 0.053 | 0.092 | 46.063 | 0.571 | 0.571 |
| Sex (male) | −0.031 | 1.656 | 44.257 | −0.019 | 0.985 |
| Baseline MoCA Score | −1.610 | 0.150 | 47.695 | −10.723 | 2.66 × 10−14 |
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 43.970 | 8.227 | 31.607 | 5.345 | 7.55 × 10−6 |
| Time (months) | 0.200 | 0.043 | 156.744 | 4.595 | 8.84 × 10−6 |
| Baseline Age (years) | 0.040 | 0.110 | 32.643 | 0.360 | 0.721 |
| Sex (male) | −2.306 | 1.918 | 30.601 | −1.203 | 0.238 |
| Baseline MoCA Score | −1.447 | 0.186 | 32.472 | −7.761 | 6.80 × 10−9 |
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 35.191 | 7.416 | 36.133 | 4.745 | 3.24 × 10−5 |
| Time (months) | 0.181 | 0.066 | 155.117 | 2.754 | 6.58 × 10−3 |
| AD Med. (yes) | 3.075 | 1.963 | 63.229 | 1.566 | 0.122 |
| Baseline Age (years) | 0.111 | 0.093 | 36.665 | 1.189 | 0.242 |
| Sex (male) | −0.266 | 1.692 | 35.729 | −0.157 | 0.876 |
| Baseline MoCA Score | −1.480 | 0.159 | 35.823 | −9.335 | 3.96 × 10−11 |
| Time: AD Med. (yes) | 0.094 | 0.091 | 158.269 | 1.034 | 0.303 |
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 40.238 | 7.186 | 35.326 | 5.599 | 2.53 × 10−6 |
| Time (months) | 0.230 | 0.051 | 158.638 | 4.527 | 1.17 × 10−5 |
| Antidepressant (yes) | 4.152 | 2.541 | 64.352 | 1.6234 | 0.107 |
| Baseline Age (years) | 0.064 | 0.094 | 36.514 | 0.681 | 0.500 |
| Sex (male) | −1.211 | 1.700 | 35.024 | −0.712 | 0.481 |
| Baseline MoCA Score | −1.480 | 0.164 | 35.894 | −9.052 | 8.53 × 10−11 |
| Time: Antidepressant (yes) | −0.032 | 0.115 | 154.353 | −0.277 | 0.782 |
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 42.345 | 7.369 | 35.935 | 5.746 | 1.53 × 10−6 |
| Time (months) | 0.272 | 0.068 | 155.957 | 4.022 | 8.97 × 10−5 |
| Hypertension Med. (yes) | −1.875 | 1.998 | 63.188 | −0.939 | 0.351 |
| Baseline Age (years) | 0.048 | 0.096 | 37.342 | 0.503 | 0.618 |
| Sex (male) | −1.553 | 1.747 | 35.363 | −0.889 | 0.380 |
| Baseline MoCA Score | −1.441 | 0.167 | 36.349 | −8.612 | 2.65 × 10−10 |
| Time: Hypertension Med. (yes) | −0.078 | 0.091 | 155.828 | −0.849 | 0.397 |
| Estimate | SE | df | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 40.599 | 7.531 | 35.015 | 5.391 | 4.90 × 10−6 |
| Time (months) | 0.274 | 0.061 | 157.476 | 4.518 | 1.22 × 10−5 |
| Cholesterol Med. (yes) | 0.442 | 2.064 | 60.516 | 0.214 | 0.831 |
| Baseline Age (years) | 0.066 | 0.101 | 36.036 | 0.657 | 0.515 |
| Sex (male) | −1.361 | 1.777 | 35.74 | −0.766 | 0.449 |
| Baseline MoCA Score | −1.473 | 0.170 | 35.457 | −8.643 | 2.99 × 10−10 |
| Time: Cholesterol Med. (yes) | −0.111 | 0.092 | 156.304 | −1.212 | 0.227 |
References
- Rektorová, I.; Pupíková, M.; Fleury, L.; Brabenec, L.; Hummel, F.C. Non-Invasive Brain Stimulation: Current and Future Applications in Neurology. Nat. Rev. Neurol. 2025, 1–18. [Google Scholar] [CrossRef]
- Saxena, V.; Pal, A. Role of Transcranial Direct Current Stimulation in the Management of Alzheimer’s Disease: A Meta-Analysis of Effects, Adherence and Adverse Effects. Clin. Psychopharmacol. Neurosci. 2021, 19, 589–599. [Google Scholar] [CrossRef]
- Prathum, T.; Chantanachai, T.; Vimolratana, O.; Laksanaphuk, C.; Apiworajirawit, I.; Aneksan, B.; Latthirun, K.; Yang, C.T.; Klomjai, W. A Systematic Review and Meta-Analysis of the Impact of Transcranial Direct Current Stimulation on Cognitive Function in Older Adults with Cognitive Impairments: The Influence of Dosage Parameters. Alzheimer’s Res. Ther. 2025, 17, 37. [Google Scholar] [CrossRef]
- Majdi, A.; van Boekholdt, L.; Sadigh-Eteghad, S.; Mc Laughlin, M. A Systematic Review and Meta-Analysis of Transcranial Direct-Current Stimulation Effects on Cognitive Function in Patients with Alzheimer’s Disease. Mol. Psychiatry 2022, 27, 2000–2009. [Google Scholar] [CrossRef]
- Kraft, J.D.; Hampstead, B.M. A Systematic Review of TACS Effects on Cognitive Functioning in Older Adults Across the Healthy to Dementia Spectrum. Neuropsychol. Rev. 2024, 34, 1165–1190. [Google Scholar] [CrossRef]
- Nissim, N.R.; Pham, D.V.H.; Poddar, T.; Blutt, E.; Hamilton, R.H. The Impact of Gamma Transcranial Alternating Current Stimulation (TACS) on Cognitive and Memory Processes in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Literature Review. Brain Stimul. 2023, 16, 748–755. [Google Scholar] [CrossRef]
- Zhang, T.; Sui, Y.; Lu, Q.; Xu, X.; Zhu, Y.; Dai, W.; Shen, Y.; Wang, T. Effects of RTMS Treatment on Global Cognitive Function in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 984708. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Song, W.; Wang, X.; Teng, L.; Li, J.; Zhang, J.; Li, X.; Yu, D.; Jia, H.; Wang, B.; et al. Efficacy of Non-Invasive Brain Stimulation Interventions on Cognitive Impairment: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. J. Neuroeng. Rehabil. 2025, 22, 22. [Google Scholar] [CrossRef]
- Yang, T.; Liu, W.; He, J.; Gui, C.; Meng, L.; Xu, L.; Jia, C. The Cognitive Effect of Non-Invasive Brain Stimulation Combined with Cognitive Training in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Alzheimer’s Res. Ther. 2024, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive Training and Cognitive Rehabilitation for Persons with Mild to Moderate Dementia of the Alzheimer’s or Vascular Type: A Review. Alzheimers Res. Ther. 2013, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.A.; Kalia, S.; Campuzano, M.G.; Jafari-Jozani, M.; Lithgow, B.; Moussavi, Z. Cognitive and Neuropsychiatric Effects of 40 Hz TACS Simultaneously with Cognitive Exercises for Dementia: A Randomized, Crossover, Double-Blind, Sham-Controlled Study. Medicina 2025, 61, 757. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Casula, E.P.; Bonnì, S.; Borghi, I.; Assogna, M.; Di Lorenzo, F.; Esposito, R.; Maiella, M.; D’Acunto, A.; Ferraresi, M.; et al. Effects of 52 Weeks of Precuneus RTMS in Alzheimer’s Disease Patients: A Randomized Trial. Alzheimers Res. Ther. 2025, 17, 69. [Google Scholar] [CrossRef]
- Moussavi, Z.; Uehara, M.; Rutherford, G.; Lithgow, B.; Millikin, C.; Wang, X.; Saha, C.; Mansouri, B.; Omelan, C.; Fellows, L.; et al. Repetitive Transcranial Magnetic Stimulation as a Treatment for Alzheimer’s Disease: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Neurotherapeutics 2024, 21, 100438. [Google Scholar] [CrossRef]
- Bergamaschi, S.; Arcara, G.; Calza, A.; Villani, D.; Orgeta, V.; Mondini, S. One-Year Repeated Cycles of Cognitive Training (CT) for Alzheimer’s Disease. Aging Clin. Exp. Res. 2013, 25, 421–426. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mrudula, K.; Sreepada, S.S.; Sathyaprabha, T.N.; Pal, P.K.; Chen, R.; Udupa, K. An Overview of Noninvasive Brain Stimulation: Basic Principles and Clinical Applications. Can. J. Neurol. Sci. 2022, 49, 479–492. [Google Scholar] [CrossRef]
- Koch, G.; Casula, E.P.; Bonnì, S.; Borghi, I.; Assogna, M.; Minei, M.; Pellicciari, M.C.; Motta, C.; D’Acunto, A.; Porrazzini, F.; et al. Precuneus Magnetic Stimulation for Alzheimer’s Disease: A Randomized, Sham-Controlled Trial. Brain 2022, 145, 3776–3786. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Y.; Hu, P.; Wang, L.; Wu, Y.; Wu, P.; Geng, Z.; Xiao, G.; Zhou, S.; Ji, G.; et al. Effects of a Periodic Intermittent Theta Burst Stimulation in Alzheimer’s Disease. Gen. Psychiatry 2024, 37, e101106. [Google Scholar] [CrossRef]
- Kehler, L.; Francisco, C.O.; Uehara, M.A.; Moussavi, Z. The Effect of Transcranial Alternating Current Stimulation (TACS) on Cognitive Function in Older Adults with Dementia. In Proceedings of the Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 3649–3653. [Google Scholar]
- Moussavi, Z.; Kimura, K.; Kehler, L.; de Oliveira Francisco, C.; Lithgow, B. A Novel Program to Improve Cognitive Function in Individuals With Dementia Using Transcranial Alternating Current Stimulation (TACS) and Tutored Cognitive Exercises. Front. Aging 2021, 2, 632545. [Google Scholar] [CrossRef]
- Bretecher, C.A.; Verot, A.; Teschuk, J.M.; Uehara, M.A.; Fitzgerald, P.B.; Koski, L.; Lithgow, B.J.; Moussavi, Z. Quantitative Analysis of Factors of Attrition in a Double-Blind RTMS Study for Alzheimer Treatment. Alzheimer Dis. Assoc. Disord. 2024, 38, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.A.; Moussavi, Z. Investigating Different Frequencies of Transcranial Electrical Stimulations in Dementia Population. Available online: https://clinicaltrials.gov/study/NCT06547021 (accessed on 10 August 2025).
- Moussavi, Z.; Rutherford, G.; Lithgow, B.; Millikin, C.; Modirrousta, M.; Mansouri, B.; Wang, X.; Omelan, C.; Fellows, L.; Fitzgerald, P.; et al. Repeated Transcranial Magnetic Stimulation for Improving Cognition in Patients with Alzheimer Disease: Protocol for a Randomized, Double-Blind, Placebo-Controlled Trial. JMIR Res. Protoc. 2021, 10, e25144. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.; Lithgow, B.; Jafari Jozani, M.; Moussavi, Z. The Effect of Transcranial Alternating Current Stimulation With Cognitive Training on Executive Brain Function in Individuals With Dementia: Protocol for a Crossover Randomized Controlled Trial. JMIR Res. Protoc. 2022, 11, e37282. [Google Scholar] [CrossRef]
- Rosen, W.G.; Mohs, R.C.; Davis, K.L. A New Rating Scale for Alzheimer’s Disease. Am. J. Psychiatry 1984, 141, 1356–1364. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale. PsycTESTS Dataset 1945. [Google Scholar] [CrossRef]
- Li, X.; Jiao, J.; Shimizu, S.; Jibiki, I.; Watanabe, K.I.; Kubota, T. Correlations between Atrophy of the Entorhinal Cortex and Cognitive Function in Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Psychiatry Clin. Neurosci. 2012, 66, 587–593. [Google Scholar] [CrossRef]
- Lee, S.C.; Chien, T.H.; Chu, C.P.; Lee, Y.; Chiu, E.C. Practice Effect and Test–Retest Reliability of the Wechsler Memory Scale-Fourth Edition in People with Dementia. BMC Geriatr. 2023, 23, 209. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2025. [Google Scholar]
- Zhang, N.; Zheng, X.; Liu, H.; Zheng, Q.; Li, L. Testing Whether the Progression of Alzheimer’s Disease Changes with the Year of Publication, Additional Design, and Geographical Area: A Modeling Analysis of Literature Aggregate Data. Alzheimers Res. Ther. 2020, 12, 64. [Google Scholar] [CrossRef]
- Zeileis, A.; Köll, S.; Graham, N. Various Versatile Variances: An Object-Oriented Implementation of Clustered Covariances in r. J. Stat. Softw. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Liang, K.Y.; Zeger, S.L. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Rodríguez-Mora, Á.; Cordón, J.R.; de la Torre, G.G.; Mestre, J.M. The Impact of a Twelve-Month Comprehensive Program of Cognitive Training for Alzheimer Patients: A Pilot Study. Psychiatry Int. 2020, 1, 83–97. [Google Scholar] [CrossRef]
- Rainer, M.; Mucke, H.A.M.; Krüger-Rainer, C.; Kraxberger, E.; Haushofer, M.; Jellinger, K.A. Cognitive Relapse after Discontinuation of Drug Therapy in Alzheimer’s Disease: Cholinesterase Inhibitors versus Nootropics. J. Neural Transm. 2001, 108, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M.; Potkin, S.; Koumaras, B.; Veach, J.; Mirski, D. Analysis of Outcome in Retrieved Dropout Patients in a Rivastigmine vs. Placebo, 26-Week, Alzheimer Disease Trial. Arch. Neurol. 2003, 60, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; Stengl, H.; Grittner, U.; Kosiolek, R.; Ölschläger, A.; Weidemann, A.; Floël, A. Effects of Anodal Transcranial Direct Current Stimulation and Serotonergic Enhancement on Memory Performance in Young and Older Adults. Neuropsychopharmacology 2017, 42, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Cornea, M.; Vintilă, B.I.; Bucuța, M.; Ștef, L.; Anghel, C.E.; Grama, A.M.; Lomnasan, A.; Stetiu, A.A.; Boicean, A.; Sava, M.; et al. Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2025, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.J.; Taylor, J.P. Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation: Treatments for Cognitive and Neuropsychiatric Symptoms in the Neurodegenerative Dementias? Alzheimers Res. Ther. 2014, 6, 74. [Google Scholar] [CrossRef]
- McLaren, M.E.; Nissim, N.R.; Woods, A.J. The Effects of Medication Use in Transcranial Direct Current Stimulation: A Brief Review. Brain Stimul. 2018, 11, 52–58. [Google Scholar] [CrossRef]
| Study | Total Enrolled | Completed | Withdrawn | Used in Analysis |
|---|---|---|---|---|
| [18,19] | n = 40 | n = 31 | n = 9 | n = 26 |
| [13] | n = 72 | n = 61 | n = 11 | n = 33 |
| [11] | n = 52 | n = 42 | n = 10 | n = 42 |
| [21] | n = 35 | n = 8 | n = 6 | n = 28 |
| Study | Study Design | Assessments | Treatment Schedule | Stimulation Parameters |
|---|---|---|---|---|
| [18,19] | 3 groups, no randomization, no blinding:
| Baseline, post-treatment, 1-month follow-up:
| 5 days/week, 4 weeks OR 3 days/week, 8 weeks | Two 30 min sessions/day with a 30-min break in between, 40 Hz tACS, 0.75 mA, left DLPFC |
| [13,22] | 3 groups, stratified randomization, double-blind:
| Baseline (week 0), week 3, 5, 12, 20 and 28:
| 5 days/week for 2 or 4 weeks | 20 Hz, 1.5 s trains with 10 s intertrain interval, 25 trains bilaterally, 90–100% resting motor threshold, left and right DLPFC |
| [11] | Crossover, stratified randomization, double-blind:
| Baseline, post-treatment:
| Two 30 min sessions/day, 5 days/week for 4 weeks, with average washout period of 11 weeks | Two 30 min sessions/day with a 30-min break in between, 40 Hz tACS, 0.75 mA, left DLPFC |
| [21] | Single-arm, simple randomization, double-blind, longitudinal, each participant receives all tES protocols + CT in randomized order for 4 weeks with a 2–5-month washout period | Baseline, post-treatment, 1-month follow-up:
| Two 30 min sessions/day, 5 days/week for 4 weeks, with washout period between treatment blocks of 2–5 months | Two 30 min sessions/day with a 30 min break in between, left DLPFC
|
| Characteristic | Categories | Cognitive Trajectories Analysis | No-Treatment Interval Analysis | ||
|---|---|---|---|---|---|
| Count (n) | Percent (%) | Count (n) | Percent (%) | ||
| Sex | Female | 17 | 40.5 | 26 | 35.6 |
| Male | 25 | 59.5 | 47 | 64.4 | |
| Education | Primary education | 8 | 19 | 12 | 16.4 |
| Secondary education | 10 | 23.8 | 18 | 24.7 | |
| Some post-secondary education | 3 | 7.1 | 6 | 8.2 | |
| College Diploma | 2 | 4.8 | 3 | 4.1 | |
| Bachelor’s degree | 11 | 26.2 | 20 | 27.4 | |
| Master’s degree | 5 | 11.9 | 8 | 11.0 | |
| Doctorate degree | 3 | 7.1 | 6 | 8.2 | |
| Handedness | Right | 38 | 90.5 | 67 | 91.8 |
| Left | 3 | 7.1 | 5 | 6.8 | |
| Ambidextrous | 1 | 2.4 | 1 | 1.4 | |
| Diagnosis | Alzheimer’s disease | 32 | 78 | 46 | 67.7 |
| Dementia | 7 | 14.7 | 12 | 16.4 | |
| Vascular Dementia | 2 | 4.9 | 7 | 9.6 | |
| Mild Cognitive Impairment | 1 | 2.4 | 5 | 6.8 | |
| Posterior Cortical Atrophy | 0 | 0 | 1 | 1.4 | |
| Lewy Body Dementia | 0 | 0 | 1 | 1.4 | |
| Frontotemporal Dementia | 0 | 0 | 1 | 1.4 | |
| Medication | Alzheimer’s Disease | 23 | 57.5 | ||
| Antidepressants | 7 | 17.5 | |||
| Hypertension | 17 | 55 | |||
| Cholesterol | 18 | 45 | |||
| Mean | SD | Mean | SD | ||
| Age (years) | 72.1 | 8.9 | 73.6 | 8.6 | |
| MoCA | 17.1 | 5.1 | 17.1 | 5.3 | |
| MADRS | 6.1 | 8.1 | 5.3 | 6.6 | |
| ADAS-Cog | 20.7 | 8.6 | 21.7 | 9.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehara, M.A.; Kalia, S.; Garcia Campuzano, M.; Moussavi, Z. ADAS-Cog Trajectories Differ from Expected Decline in Dementia Following Repeated Non-Invasive Interventions over 3 Years. Medicina 2025, 61, 1994. https://doi.org/10.3390/medicina61111994
Uehara MA, Kalia S, Garcia Campuzano M, Moussavi Z. ADAS-Cog Trajectories Differ from Expected Decline in Dementia Following Repeated Non-Invasive Interventions over 3 Years. Medicina. 2025; 61(11):1994. https://doi.org/10.3390/medicina61111994
Chicago/Turabian StyleUehara, Maria Anabel, Sumeet Kalia, Mari Garcia Campuzano, and Zahra Moussavi. 2025. "ADAS-Cog Trajectories Differ from Expected Decline in Dementia Following Repeated Non-Invasive Interventions over 3 Years" Medicina 61, no. 11: 1994. https://doi.org/10.3390/medicina61111994
APA StyleUehara, M. A., Kalia, S., Garcia Campuzano, M., & Moussavi, Z. (2025). ADAS-Cog Trajectories Differ from Expected Decline in Dementia Following Repeated Non-Invasive Interventions over 3 Years. Medicina, 61(11), 1994. https://doi.org/10.3390/medicina61111994







