Abstract
Background and Objectives: Typically, the external carotid artery (ECA) sends off separate anterior branches: the superior thyroid, lingual, and facial arteries. These could, however, form common trunks: thyrolinguofacial, linguofacial (LFT), or thyrolingual. Although known, the LFT variant was poorly detailed previously, and most authors just counted the variant. We aimed to demonstrate the individual anatomical possibilities of the LFT on a case-by-case basis. Materials and Methods: 150 archived angioCT files were used. After applying inclusion and exclusion criteria, 147 files of 86 males and 61 females were kept for this study. Results: In 34/147 cases, LFTs were found (23.12%). Bilateral LFTs were found in 13/34 cases (38.24%) and unilateral LFTs in 21/34 (61.76%) cases. Forty-seven LFTs were thus identified and further studied for different variables. Regarding the vertical topography of LFT origin, type 1a (suprahyoid and infragonial) was found in 28 LFTs (59.57%), type 1b (suprahyoid and gonial) was found in eight LFTs (17.02%), type 3 (suprahyoid and supragonial) was found in two LFTs (4.25%), type 2 (hyoid level of origin) in eight LFTs (17.02%), and type 3 (infrahyoid origin) in just one LFT (2.12%). Types of the initial course of the LFT were determined: type I, ascending, was found in 22/47 LFTs; type II, descending, in 12/47 LFTs; and type III, transverse, in 13/47 LFTs. Regarding the orientation of the first loop of the LFT, 23/47 LFTs had no loop, 4/47 had anterior loops, 1/47 had a posterior loop, 5/47 had superior loops, 5/47 had inferior loops, and 9/47 had medial loops. The position of the LFT relative to the ECA was classified as medial, anterior, or antero-medial. An amount of 12/47 LFTs were anterior to the ECA, 22/47 were antero-medial, 10/47 were medial, 2/47 were inferior, and 1/47 was lateral. Regarding their general morphology, 23/47 LFTs had a rectilinear course, 22/47 had loops, and 2/47 were coiled. A case-by-case presentation of results further demonstrated the diversity of the LFT. Conclusions: In conclusion, the morphology and topography of the LFT are individually specific and unpredictable. It can be anticipated case-by-case by surgeons on CT or MR angiograms.
1. Introduction
In typical anatomy, the external carotid artery (ECA) gives off distinctive collateral branches: the superior thyroid (STA), lingual (LA), facial (FA), ascending pharyngeal (APA), occipital (OA) and posterior auricular (PAA) arteries [1]. Successive arteries could leave the ECA as common trunks, such as thyrolinguofacial, thyrolingual, thyrolinguolaryngeal, linguofacial, or occipitoauricular trunks [2,3,4]. Knowledge of the variations in the branching pattern of the ECA is essential for surgeons, as the planning and execution of surgery is purely based on the anatomical pattern of the structures in the neck [5].
The linguofacial trunk (LFT) is commonly regarded and reported as a unique anatomical variant [2,6,7,8,9,10,11,12,13]. This is true if one observes it just as a common trunk of origin of the LA and FA. However, the morphology and topography of the LFT should be individually specific. The morphometric characteristics of the LFT have been only rarely described [14].
This study was, therefore, aimed at demonstrating on a case-by-case basis the individual anatomical possibilities of the LFT. The incidence of the LFT and the heterogeneous morphological possibilities were established.
2. Materials and Methods
There were 150 archived angioCT files used. Inclusion criteria were the age of the subjects (>18 years), adequate quality of the scans, and no pathologic processes distorting the neck anatomy. Exclusion criteria were pathological processes distorting the arterial anatomy and degraded or incomplete scans. After applying inclusion and exclusion criteria, 147 files of 86 males and 61 females were kept for this study.
The first two authors documented the anatomical variants using the Horos 4.0.1 for Apple Silicon software (Horos Project), as in previous studies [15,16,17]. The research was conducted ethically following The Code of Ethics of the World Medical Association (Declaration of Helsinki). The subjects gave their informed consent for inclusion before participating in the study, and the responsible authorities (affiliation 2) approved the study (approval no.10540/16.02.2022).
Different variables of the LFT were tracked as follows: Types 1–3 of the vertical topography of LFT origin from the ECA: type 1, origin superior to the hyoid bone (subtype 1a—inferior to gonial angle, subtype 1b—gonial level, and type 1c—superior to gonial angle); type 2, origin of the LFT at the level of the hyoid bone; and type 3, infrahyoid origin of the LFT. Types I–III of the initial course of the LFT: type I—ascending, type II—descending, and type III—transverse. The orientation of the first loop of the LFT has been classified as follows: S—superior orientation, I—inferior orientation, M—medial orientation, A—anterior orientation, P—posterior orientation, and NL—no initial loop. The position of the LFT relative to the ECA was classified as medial, anterior, or antero-medial. The general LFT morphology was systematized: R—rectilinear LFT, L—general loop, and C—arterial coil.
3. Results
Of the 147 cases, in 34 cases, 16 males and 18 females were found to have LFTs (23.12%). There were 13 cases (38.24%) with bilateral LFTs: six males and seven females. A left LFT was found in 13 cases (seven males, six females) (38.24%). A right LFT was found in eight cases (three males, five females) (23.53%).
Forty-seven LFTs were thus identified and further studied for several other variables: the vertical topography of the LFT origin relative to the hyoid, the initial course of the LFT, the convexity of the first TLF loop or rectilinear course, the position relative to the ECA, and the overall morphology (loop/coil/rectilinear).
3.1. The Vertical Topography of the LFT
On the right side, 21 LFTs were found. Nine of these right LTFs were found in males and 12 In females. In males, the vertical topography of the LFT origin was of type 1a (origin between the hyoid and the gonial angle) in six cases and type 2 (origin at the level of the hyoid bone) in three cases. In females, type 1a was found in eight cases, type 1b (gonial level of the LFT origin) in two cases, and type 2 in two other cases.
On the left side, 26 LFTs were found. Thirteen of these were in males and thirteen in females. The vertical topography of those left LFTs was as follows. In males, type 1a was found in seven instances, type 1b in three cases, type 1c (supragonial origin of the LFT) in one case, and type 2 in two cases. In six female cases, the left LFT was of type 1a in six cases, type 1b in three instances, type 1c in two cases, type 2 in one case, and type 3 (infrahyoid origin of the LFT) in one case.
Therefore, in the general lot of the 47 LFTs that were found, there were 28 LFTs of type 1a (59.57%), eight LFTs of type 1b (17.02%), two LFTs of type 1c (4.25%), eight LFTs of type 2 (17.02%), and just one LFT of type 3 (2.12%).
3.2. The Initial Course of the LFT
We found the following distribution regarding the initial direction of the LFT (types I–III). In males, the nine right LFTs were of type I (initially ascending) in seven cases and of type III (initial transverse course) in two instances; no right LFT was of type II (initially descending). The 13 left LFTs found in males were of type I in eight cases, type II in three cases, and type III in two cases. The twelve right LFTs in females were of type I in four cases, type II in four cases, and type III in four cases. On the left side in females, three LFTs of type I, five LFTs of type II, and five LFTs of type III were found. Therefore, of 47 LFTs, 22 (46.8%) were of type I, 12 (25.53%) were of type II, and 13 (27.65%) were of type III.
3.3. The Orientation of the First Loop of the LFT
Regarding the orientation of the first loop of the LFT, nine right LFTs in males were found: one case with an anteriorly oriented loop, one with a posterior orientation of the loop, two cases with the loop oriented medially, and five cases without an initial loop. In the 12 female cases with right LFTs, one had an anterior orientation, two were oriented inferiorly, two were oriented medially, and in seven cases, no loop was found. On the left side, the 13 LFTs in males were oriented anteriorly in one case, superiorly in four instances, medially in three cases, and in five cases, there were no loops. In the 13 left LFTs in females, we found one oriented anteriorly, one oriented superiorly, three with inferior orientation, and two oriented medially, and there were no such loops in the remaining six cases. In males, on the right side, 5/9 LFTs showed no loops; and on the left, 5/13 LFTs showed no loops. In females on the right, 7/12 LFTs showed no loops; and on the left, 6/13 LFTs showed no loops. In both sexes, we found no right LFT with a superior loop and no left LFT with a posterior loop.
Therefore, 23/47 LFTs had no loop, 4/47 had anterior loops, 1/47 had a posterior loop, 5/47 had superior loops, 5/47 had inferior loops, and 9/47 had medial loops.
3.4. The Position of the LFT Relative to the ECA
Regarding the position of the LFT relative to the ECA in males, three right and four left LFTs were anterior to the ECA, five right and nine left LFTs were antero-medial to the ECA, and one right LFT was medial to the ECA. In males, no LFTs were found inferior or lateral to the ECA. In females, three right and two left LFTs were anterior to the ECA, four right and four left LFTs were antero-medial to the ECA, five right and four left LFTs were medial to the ECA, two left LFTs were inferior to the ECA, and one left LFT crossed the ECA laterally.
Therefore, 12/47 LFTs were anterior to the ECA, 22/47 were antero-medial, 10/47 were medial, 2/47 were inferior, and 1/47 was lateral.
3.5. The General LFT Morphology
Regarding the general morphology of the LFT in males, five right and five left LFTs were rectilinear, four right and seven left LFTs were looped, and one left LFT was coiled. In females, seven right and six left LFTs were rectilinear, four right and seven left LFTs were looped, and one right LFT was coiled. So, only in two cases, one female and one male, was the LFT coiled. So, 23/47 LFTs had a rectilinear course, 22/47 had loops, and 2/47 were coiled.
3.6. Unilateral Left Linguofacial Trunks
Thirteen unilateral left LFTs were found.
In case #1 (male) a left LFT left from the medial side of the ECA was found (Figure 1A) at 1.8 cm postero-superior to the tip of the greater horn of the hyoid bone, therefore in the interval between the hyoid and the gonial angle. That left LFT had an initial loop oriented superiorly and was dividing into the LA and FA at 0.66 cm medially to the ECA.
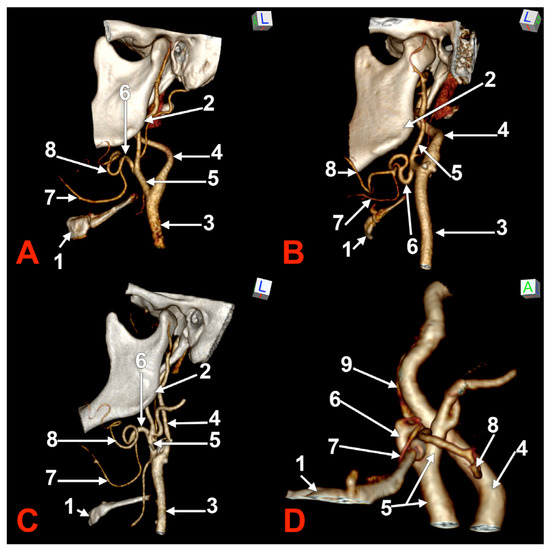
Figure 1.
Morphological variants of the left unilateral linguofacial trunk. Computed tomography angiograms. Three-dimensional volume renderings. Infero-lateral (A–C) and antero-lateral (D) views. 1. hyoid body; 2. gonial angle; 3. common carotid artery; 4. internal carotid artery; 5. external carotid artery; 6. linguofacial trunk; 7. lingual artery; 8. facial artery; and 9. ascending pharyngeal artery.
In case #2 (female), the LFT left from the left ECA was found at 0.37 cm inferior to the gonial angle and 1.29 cm postero-superior to the tip of the greater horn of the hyoid bone, therefore in the interval between the hyoid and the gonial angle. That LFT was initially descending, and the LFT had a general loop oriented inferiorly (Figure 1B).
In case #3 (male), a left LFT originated from the ECA at 3.54 cm postero-superior to the tip of the greater horn of the hyoid bone, in the interval between the hyoid and the gonial angle. It ascended medially to the ECA and described a superiorly convex loop (Figure 1C).
In case #4 (female), the LFT left originated from the medial side of the left ECA immediately inferior to the tip of the greater horn of the hyoid bone and further continued medially to it. Therefore, the tip of the greater horn was placed between the ECA on the outer side and the LFT on the inner side (Figure 1D). Although the LFT was initially ascending, it had an incomplete inferior loop posterior to the pharyngeal wall. Due to that course of the LFT, the LA and FA coursed transversally from medial to lateral and crossed anteriorly the superior thyroid artery and the ECA.
In case #5 (male), the origin of the LFT from the left ECA was at 1.19 cm postero-superior to the tip of the greater horn of the hyoid bone but at 2.29 cm deep and not inferior to the gonial angle (Figure 2A). That LFT divided into the FA and LA at 0.49 cm antero-medially to the ECA.
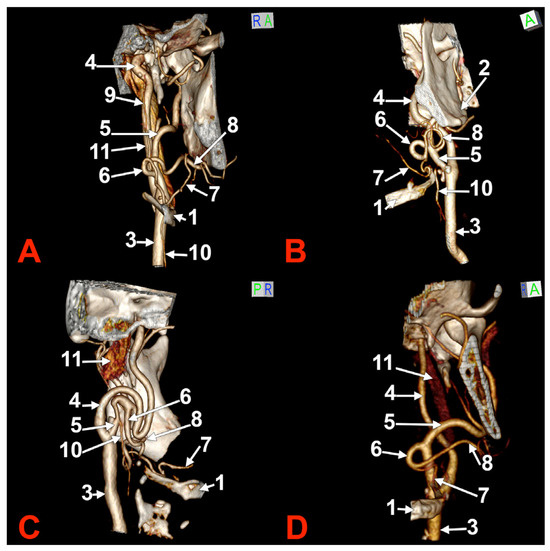
Figure 2.
Morphological variants of the left unilateral linguofacial trunk. Computed tomography angiograms. Three-dimensional volume renderings. Anterior (A,D), antero-lateral, (B) and antero-medial (C) views. 1. hyoid body; 2. gonial angle; 3. common carotid artery; 4. internal carotid artery; 5. external carotid artery; 6. linguofacial trunk; 7. lingual artery; 8. facial artery; 9. ascending pharyngeal artery; 10. superior thyroid artery; and 11. internal jugular vein.
In case #6 (male), a large and coiled left LFT (Figure 2B) was found that originated from the posterior side of the ECA distally to the occipital artery and at 1.15 cm postero-superior to the tip of the greater horn of the hyoid bone. While the caliber of the ECA was 0.44 cm, that of the LFT was 0.34 cm.
In case #7 (female), the carotid bifurcations were placed superior to the gonial angle, and both the ECA and the internal carotid artery (ICA) were looped deep to the gonial angles. Therefore, the ECA branches were longer than usual. A long left LFT descended at 1.11 cm deep to the gonial angle. The arterial morphology of the ECA, ICA, and LFT was that of an arterial labyrinth (Figure 2C). Due to the loop of the ECA, the LFT appeared to be an inferior branch of it. The origin of the LFT was 2.82 cm above the greater horn of the hyoid bone. The course of the LFT was rectilinear. At 1.34 cm, postero-supero-lateral to the tip of the greater horn, the LFT divided into the LA and FA.
In case #8 (male), the origin of the LFT from the left ECA was deep to the gonial angle, 1.67 cm postero-superior to the tip of the greater horn of the hyoid bone. It had a medially oriented loop (Figure 2D).
In case #9 (female), the left carotid bifurcation (CB) was immediately infero-lateral to the greater horn of the hyoid bone, and the initial segment of the ECA was applied on the lateral side of the greater horn (Figure 3A). The LFT originated from the left ECA at 0.22 cm above the tip of the greater horn. It was profound to the gonial angle. It was initially ascending and had a superior loop. It first gave off the LA, then the FA. Therefore, the LA crossed over the FA.
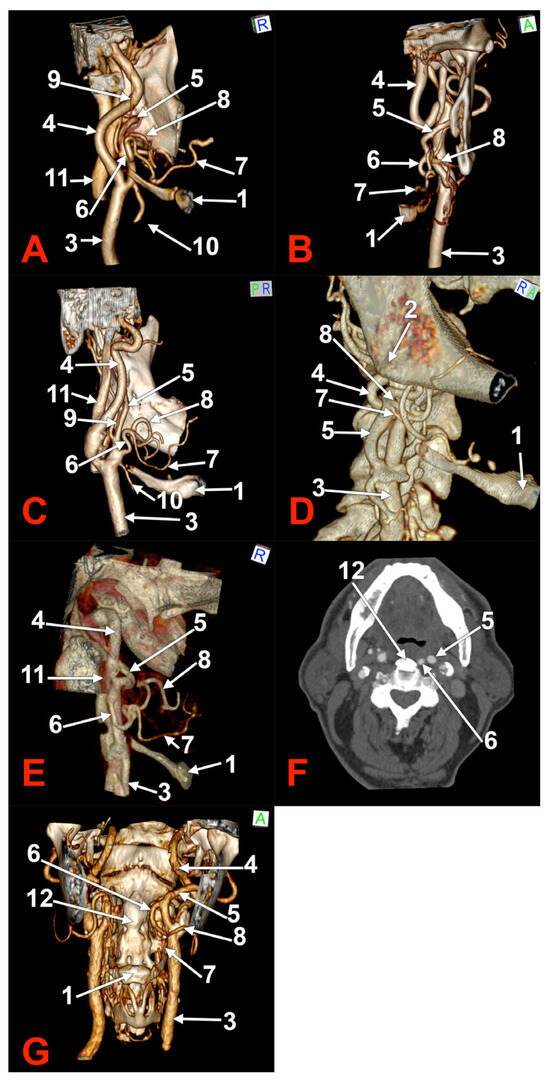
Figure 3.
Morphological variants of unilateral left linguofacial trunks. Computed tomography angiograms. Three-dimensional volume renderings. Anterior (B,C,G), right lateral (D), and medial (A,C,E) views. Axial slice (F). 1. hyoid body; 2. gonial angle; 3. common carotid artery; 4. internal carotid artery; 5. external carotid artery; 6. linguofacial trunk; 7. lingual artery; 8. facial artery; 9. ascending pharyngeal artery; 10. superior thyroid artery. 11. internal jugular vein; and 12. ossified anterior longitudinal ligament.
In case #10 (female), the left submandibular gland descended over the hyoid bone’s greater horn, and the ECA ascended deep to the gland. A 0.93 cm long LFT originated from the medial side of the left ECA, deep to the gonial angle (Figure 3B). At the same level, an occipitoauricular trunk formed from the lateral side of the ECA. The origin of the LFT was at 1.74 cm postero-superior to the tip of the greater horn of the hyoid bone. It had an initial descending course and looped inferiorly.
In case #11 (male), an LFT was found originating from the anterior side of the left ECA in the interval between the hyoid and the gonial angle, at 0.53 cm supero-lateral to the tip of the greater horn of the hyoid bone (Figure 3C). That LFT had a tortuous ascending course and reached the inferior margin of the mandible, where it divided into the LA and FA.
In case #12 (female) a left LFT was found, and, on the right side, adjacent origins of the LA and FA from the ECA (Figure 3D). The right LA and FA left the ECA in this order at 0.91 cm postero-superior to the tip of the right greater hyoid horn, therefore, in the interval between the hyoid and the gonial angle. The left LFT originated from the ECA at 1.59 cm postero-superior to the tip of the greater hyoid horn, deep to the gonial angle. It further descended and was applied on the medial side of the ECA to reach 0.27 cm superior to the tip of the greater hyoid horn. It looped inferiorly and ascended to further divide into the LA and FA at 1.01 cm superior to the greater horn (Figure 3E).
In case #13 (male), a unilateral LFT was found on the left side. It was retropharyngeal, at 2.44 mm lateral to an ossified anterior longitudinal ligament (Figure 3F). The origin of that LFT was at 2.88 cm supero-medial to the tip of the greater horn of the hyoid bone, deep to the gonial angle. It had a rectilinear descending retropharyngeal course at 0.37 cm medial to the ECA at the level of the third cervical vertebra (Figure 3G). The LFT divided into the LA and FA at the C3/C4 intervertebral disc level, those two arteries being medial to the CB and the superior thyroid artery.
3.7. Unilateral Right Linguofacial Trunks
Eight unilateral right LFTs were found.
In case #14 (male), the right ECA was applied on the lateral side of the greater hyoid horn. On that side, the lesser hyoid horn was 16.5 mm long, suggesting a distal ossification of the ceratohyal. A right LFT was found leaving from the ECA (Figure 4) at 0.83 cm superior to the lesser hyoid horn and 1.89 cm antero-superior to the tip of the greater hyoid horn. This latter was contacting the superior horn of the thyroid cartilage (absent lateral thyrohyoid ligament). The LFT coursed initially transversally and described a medial loop crossing anteriorly to the ICA. Anterior to the ICA’s inner side, the LFT divided into the LA and FA. The right LA further crossed the LFT anteriorly to reach the greater hyoid horn. Therefore, the LFT coursed between the ICA posteriorly and the LA anteriorly.
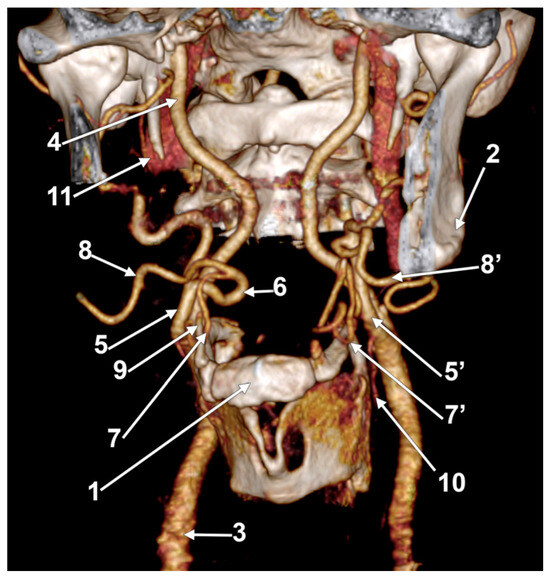
Figure 4.
Right linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Antero-inferior view. 1. hyoid body; 2. gonial angle; 3. right common carotid artery; 4. right internal carotid artery; 5, 5’. external carotid arteries; 6. linguofacial trunk; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9. ossified ceratohyal; 10. superior thyroid arteryș and 11. internal jugular vein.
In case #15 (female), a right LFT originated from the ECA deep to the gonial angle, at 1.34 cm postero-superior to the tip of the greater horn of the hyoid bone. It coursed transversally medially and described a complete coil on the medial side of the ECA. The coil divided into the LA and FA that crossed the origin of the LFT anteriorly (Figure 5A).
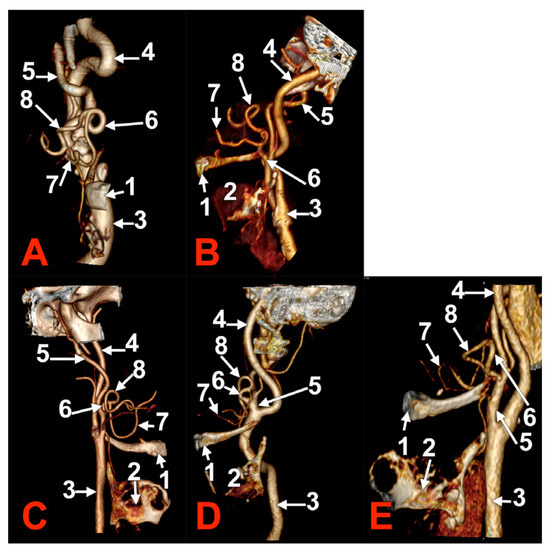
Figure 5.
Morphological variants of right unilateral linguofacial trunks. Computed tomography angiograms. Three-dimensional volume renderings. Anterior (A), antero-medial (B,D,E), and medial (C) views. 1. hyoid body; 2. thyroid cartilage; 3. common carotid artery; 4. internal carotid artery; 5. external carotid artery; 6. linguofacial trunk; 7. lingual artery; and 8. facial artery.
In case #16 (female), the right LFT origin from the ECA was in the interval between the hyoid and the gonial angle. It had a short descending rectilinear course and was divided into the LA and FA at 0.76 cm superior to the tip of the greater horn.
In female case #17, the CBs were located inferiorly at the level of the cricoid cartilage. Therefore, the right LFT originated from the anterior side of the ECA at 0.84 cm postero-inferior to the tip of the greater horn of the hyoid bone (Figure 5B). On the outer side of the LFT origin was an anterior loop of the ICA. That LFT ascended 0.92 cm anteriorly to the ECA, crossing the greater horn of the hyoid bone laterally.
In case #18 (male), a right LFT originated from the ECA at 1.45 cm superior to the greater hyoid horn in the interval between the hyoid and the gonial angle (Figure 5C). It ascended on the medial side of the ECA and looped posteriorly.
In case #19 (female), the origin of the right LFT was at 1.01 cm superior to the tip of the greater hyoid horn. It described an anterior loop (Figure 5D).
In case #20 (male), the right LFT originated from the ECA at the level of the hyoid bone, at 1.8 mm postero-lateral to its tip (Figure 5E). It ascended and crossed the ECA anteriorly.
In case #21 (female), the right LFT originated from the ECA at 2.22 cm postero-supero-lateral to the greater horn of the hyoid bone but inferior to the gonial angle (Figure 6). It had a transverse rectilinear course. It divided into the LA and FA at 1.91 cm superior to the greater horn of the hyoid bone.
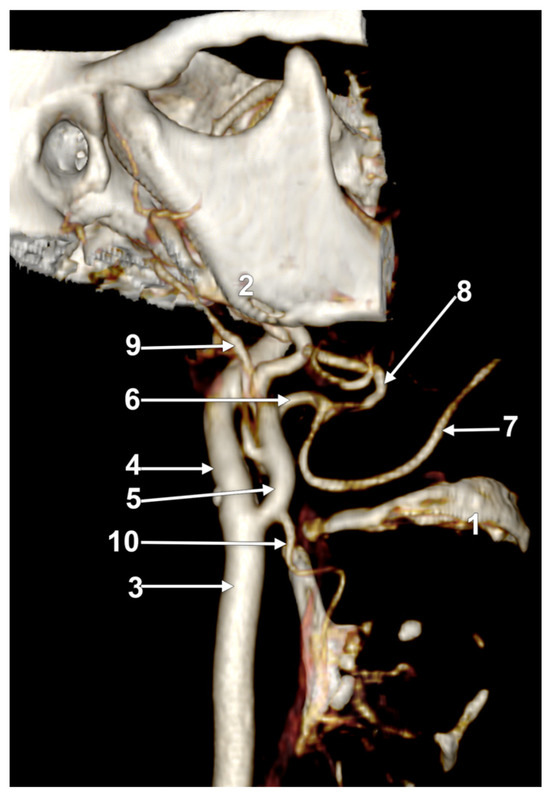
Figure 6.
Right linguofacial trunk, CT angiogram. Three-dimensional volume rendering. Right lateral view. 1. hyoid body; 2. gonial angle; 3. common carotid artery; 4. internal carotid artery; 5. external carotid artery; 6. linguofacial trunk; 7. lingual artery; 8. facial artery; 9. occipital arteryș and 10. superior thyroid artery.
3.8. Bilateral Linguofacial Trunks
In thirteen cases, bilateral linguofacial trunks were found.
In case #22 (female), the LFTs were morphologically and topographically bilaterally asymmetrical (Figure 7). The right LFT left the anterior side of the ECA in the interval between the hyoid and gonial angle, at 1.4 cm postero-superior to the tip of the greater horn. It coursed anteriorly and reached 0.52 cm superior to the tip of the greater horn to divide into the LA and FA. The left LFT origin was on the medial side of the ECA, at 1.18 cm postero-supero-lateral to the greater hyoid horn, and had a medial rectilinear course to divide into the LA and FA at 1.28 cm postero-superior to the tip of the greater horn of the hyoid bone.
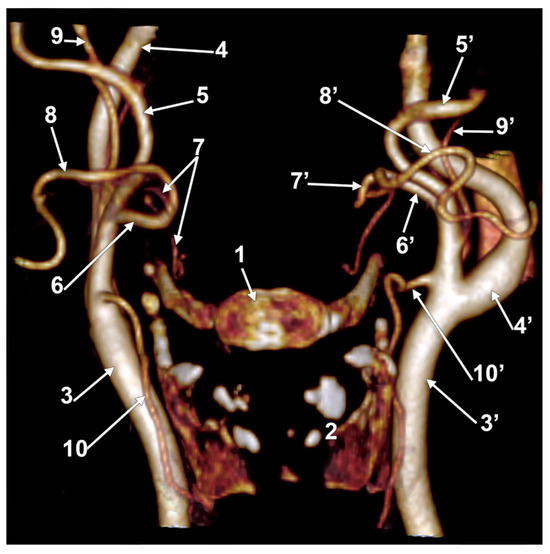
Figure 7.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Anterior view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9, 9’. occipital arteries; and 10, 10’. superior thyroid arteries.
In case #23 (female), there was a degree of bilateral symmetry of the LFTs (Figure 8). The CBs, origins of the superior thyroid arteries, and the LFTs were located beneath the angle of the mandible on each side. The right LFT left the ECA at 1.48 cm postero-supero-lateral to the tip of the greater horn of the hyoid bone and at 0.67 cm superior to the origin of the superior thyroid artery. It coursed antero-inferiorly, without a loop, and divided into the LA and FA deep to the submandibular gland, at 1.49 cm supero-lateral to the greater horn of the hyoid bone. The left LFT origin from the ECA was 1.85 cm postero-supero-lateral to the tip of the greater hyoid horn. It initially coursed antero-inferiorly and divided into the LA and FA at 1.24 cm supero-lateral to the greater horn.
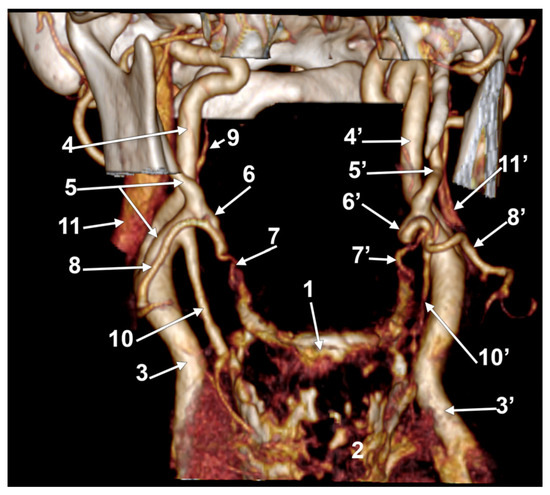
Figure 8.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Anterior view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9. ascending pharyngeal artery; 10, 10’. superior thyroid arteries; and 11, 11’. internal jugular veins.
Case #24 (female) had bilateral LFTs originating each from the ECA in the interval between the hyoid bone and the gonial angle (Figure 9). The right LFT origin was at 0.79 cm postero-supero-lateral to the tip of the greater horn of the hyoid bone. It had a rectilinear antero-superior course. It was divided into the LA and FA deep to the submandibular gland at 0.58 cm, superior to the tip of the greater hyoid horn. The left LFT origin was 0.63 cm postero-superior to the tip of the greater horn. It had a rectilinear anterior course and was divided into the LA and FA at 0.67 cm superior to the greater horn’s tip.
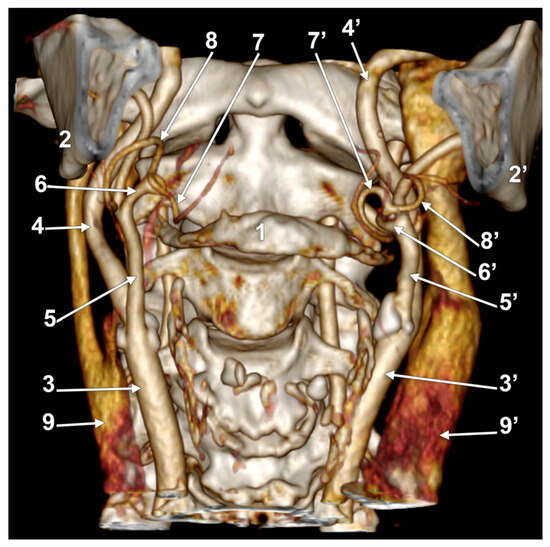
Figure 9.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Anterior view. 1. hyoid body; 2, 2’. gonial angles; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; and 9, 9’. internal jugular veins.
In case #25 (female), the LFTs were relatively bilaterally symmetrical. The right LFT (Figure 10A) left the ECA at 1.29 cm postero-supero-lateral to the tip of the greater horn of the hyoid bone, deep to the gonial angle. It had a rectilinear antero-inferior course and, at 1.08 cm superior to the tip of the greater horn, divided into the LA and FA. The left LFT origin was at 1.69 cm postero-supero-lateral to the tip of the greater hyoid horn, deep to the gonial angle (Figure 10B). It descended antero-inferiorly and at 0.87 cm superior to the greater horn’s tip, it divided into the LA and FA.
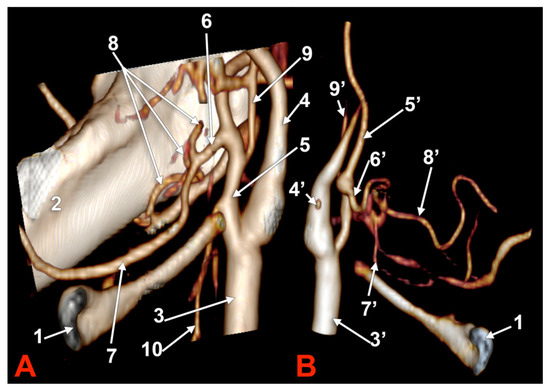
Figure 10.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Antero-medial views, right side (A) and left side (B). 1. hyoid body; 2. mandible; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9, 9’. occipital arteries; and 10. superior thyroid artery.
In case #26 (female), the CBs were located at the level of the superior margin of the thyroid cartilage, but the right one was sagittal, while the left one was coronal. Therefore, the course of the bilateral LFTs differed. Both LFTs had a hyoid level of origin (Figure 11). The right one originated from the anterior side of the ECA, at 1.2 cm postero-superior to the tip of the greater hyoid’s horn. It coursed anteriorly, without a loop, reaching 0.49 cm superior to the tip of the greater horn, deep to the submandibular gland, where it divided. The left LFT origin was from the medial side of the ECA, at 0.54 cm postero-lateral to the tip of the greater horn. It ascended supero-medially and divided into the LA and FA at 0.87 cm postero-superior to the tip of the greater horn.
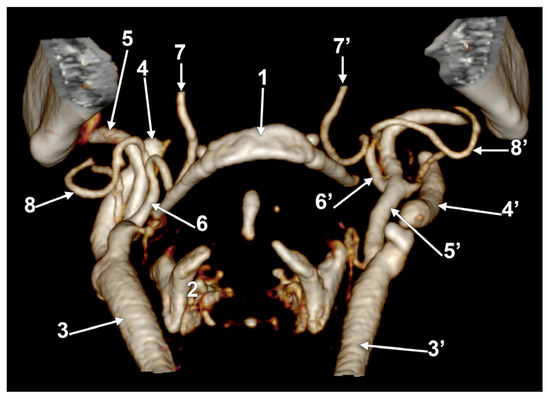
Figure 11.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Antero-inferior view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; and 8, 8’. facial arteries.
In case #27 (male), there were bilateral LFTs with low origins (Figure 12). The right one’s origin from the ECA was immediately postero-lateral (0.15 cm) to the tip of the greater hyoid’s horn, therefore a hyoid level of origin. It had a rectilinear antero-superior course reaching 0.74 cm superior to the greater horn, which gave off the LA and FA. The origin of the left LFT from the ECA was at 0.35 cm postero-infero-lateral to the tip of the greater horn. It ascended supero-medially and divided at 0.6 cm postero-superior to the tip of the greater horn.
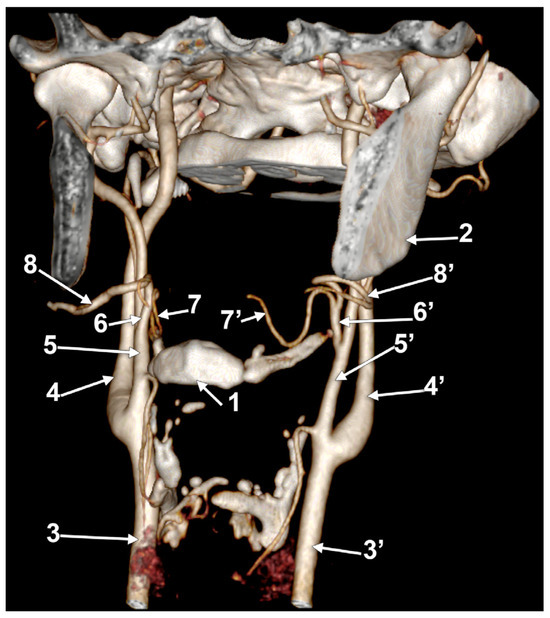
Figure 12.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Antero-inferior view. 1. hyoid body; 2. gonial angle; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; and 8, 8’. facial arteries.
In case #28 (male), the LFTs were asymmetrical (Figure 13). The right LFT originated at the hyoid bone level at 1.59 cm postero-lateral to the tip of its greater horn. It divided at 1.78 cm postero-supero-lateral to the greater horn into the LA and FA. The left LFT originated from the ECA in the interval between the hyoid and the gonial angle, at 1.25 cm postero-supero-lateral to the tip of the greater horn. It had a medial loop and divided at 1.8 cm postero-supero-lateral to the tip of the greater horn into the LA and FA.
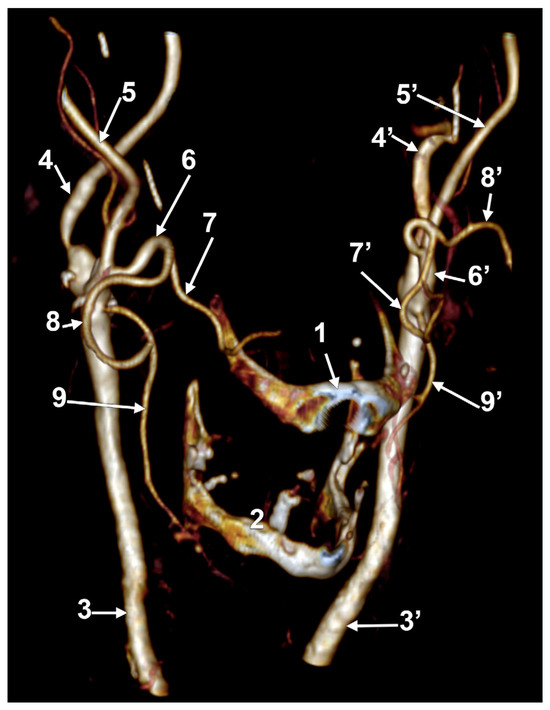
Figure 13.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Right antero-inferior view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; and 9, 9’. superior thyroid arteries.
In case #29 (female), the right LFT left from the anterior side of a lateralized ECA between the hyoid and the gonial angle (Figure 14). Its origin was at 1.93 cm postero-supero-lateral to the tip of the greater hyoid horn. The proximal part of the right LFT looped anteriorly in front of the ECA, and its distal part looped posteriorly and reached 0.51 cm in front of the ICA. The right LFT divided into the LA and FA at 0.75 cm superior to the tip of the hyoid’s greater horn. The left ECA had a typical course, and the left LFT origin was from its anterior side, at 0.81 cm superior to the tip of the greater horn and inferior to the gonial angle. The left LFT had an anterior rectilinear course above the greater horn to divide into the LA and FA.
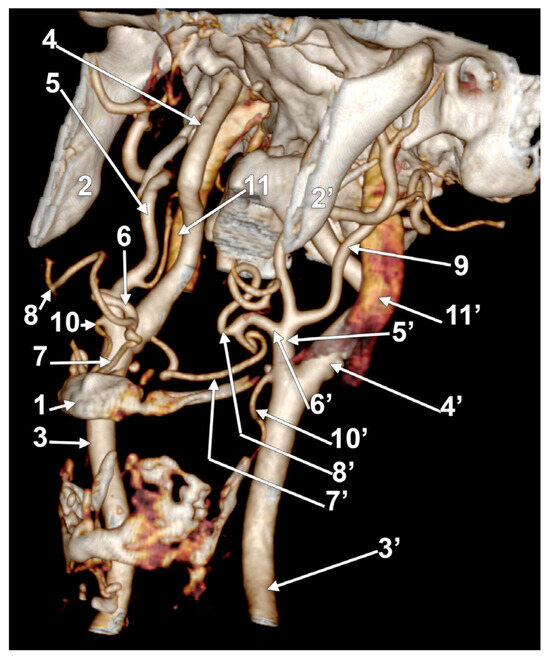
Figure 14.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Left inferior view. 1. hyoid body; 2, 2’. gonial angles; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9. left occipital artery; 10, 10’. superior thyroid arteries; and 11, 11’. internal jugular veins.
In case #30 (female), the right LFT left the ECA at 0.51 cm postero-superior to the tip of the greater horn, ascended supero-medially on the inner side of the ECA, and at 0.89 cm, supero-medial to the tip of the greater horn, divided into the LA and FA. The left LFT originated from the ECA at 0.98 cm supero-lateral to the tip of the greater hyoid horn; it was short (0.45 cm) and had a rectilinear course (Figure 15).
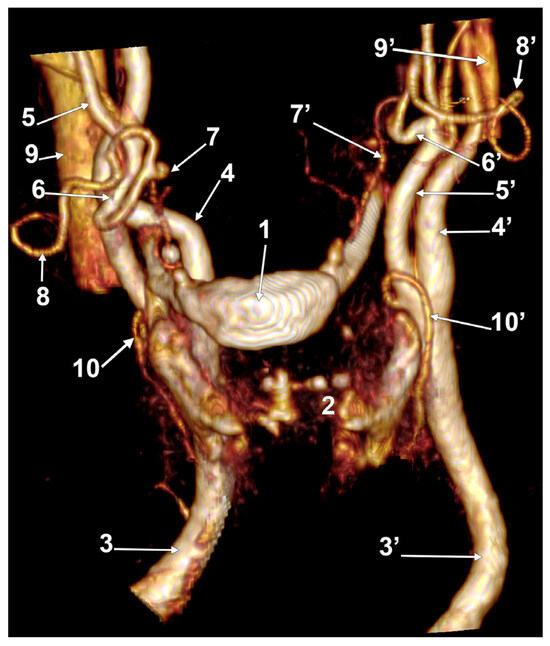
Figure 15.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Left antero-lateral view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9, 9’. internal jugular veins; and 10, 10’. superior thyroid arteries.
In case #31 (male), the left and right LFTs were short: the right was 0.77 cm, and the left was 0.26 cm. They were both rectilinear and had no loops (Figure 16).
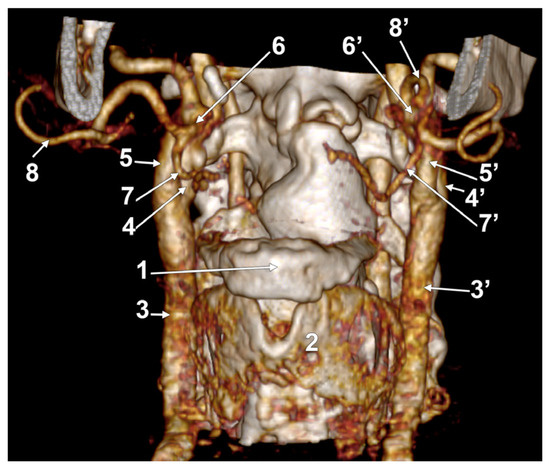
Figure 16.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Left antero-lateral view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; and 8, 8’. facial arteries.
Case #32 was a male one. Bilateral LFTs were found (Figure 17). On both sides, the LFTs originated in the interval between the hyoid bone and the gonial angle. On the right side, the LFT origin was at 0.29 cm superior to the greater horn’s tip, which, in turn, had an ossified triticeal cartilage medially. The rectilinear right LFT divided into the LA and FA at 0.95 cm superior to the greater horn. The left LFT origin was 0.46 cm superior to the hyoid’s greater horn and 0.85 cm antero-supero-lateral to its tip. At 1.15 cm, superior to the greater horn, it divided into the FA and LA.
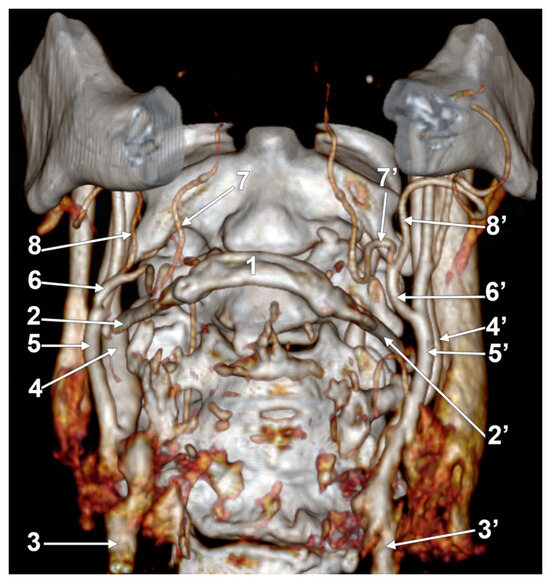
Figure 17.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Antero-inferior view. 1. hyoid body; 2, 2’. greater horns of the hyoid bone; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; and 8, 8’. facial arteries.
Another male case (#33) had bilateral LFTs. Peculiarly, it had on the left side a stylohyoid chain that was almost entirely ossified (Figure 18, Video S1). On the right side, it had just a 0.75 cm long and 0.16 cm thick ceratohyal bone embedded within the stylohyoid ligament. The right LFT originated at 0.95 cm superior to the tip of the greater hyoid’s horn. It had an inferior loop approaching 0.93 cm above the greater horn. At 1.8 cm above it, the LFT divided into the LA and FA. Interestingly, the right LA had an initial posterior course, then redirected anteriorly and crossed the origin of the right LFT medially to reach above the greater horn.
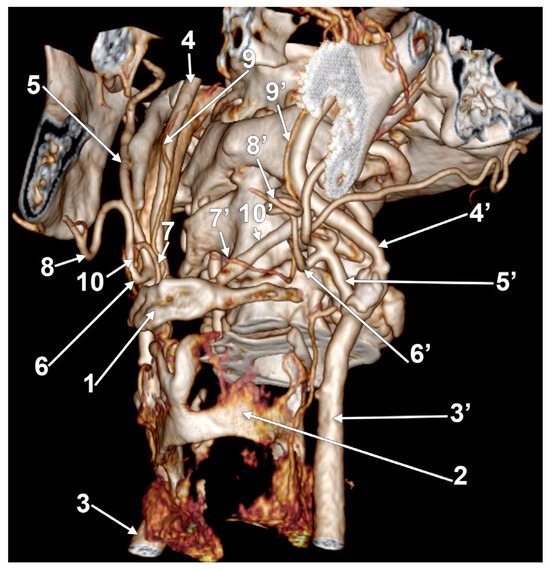
Figure 18.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Left infero-lateral view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; 8, 8’. facial arteries; 9, 9’. ascending pharyngeal arteries; and 10, 10’. ossified ceratohyals.
On the opposite side, the stylohyoid chain was almost entirely ossified, but the inclinations of the stylohyal and ceratohyal differed. The left ceratohyal was laterally displacing three arteries (Figure 18). These were the occipital artery, the ECA, and the FA in a posterior-to-anterior sequence. The left occipital artery and LFT origins from the ECA were at the same level, resulting in a trident of the ECA. The left LFT had an inferior loop reaching 0.3 cm superior to the tip of the greater horn. At 0.92 cm above the greater horn, the LFT was divided into the LA and FA immediately inferior to the ossified ceratohyal. The resulting left LA looped medially inferior to the ceratohyal and continued above the greater horn but was on the lateral side of the arthrosis between the ceratohyal and hypohyal. The left FA ascended on the lateral side of the ceratohyal.
The last case we present here, case #34, was a male case with bilateral LFTs (Figure 19). The right one’s origin was at 0.33 cm supero-lateral to the tip of the greater horn; it ascended supero-medially, with no loop. It divided into the LA and FA at 1.15 cm superior to the greater hyoid’s horn. Immediately posterior to the termination of the right LFT coursed the ascending pharyngeal artery. The left LFT origin from the ECA was at 0.48 cm supero-lateral to the greater hyoid horn; it coursed directly postero-supero-medially and divided into the LA and FA at 0.96 cm superior to the tip of the greater horn.
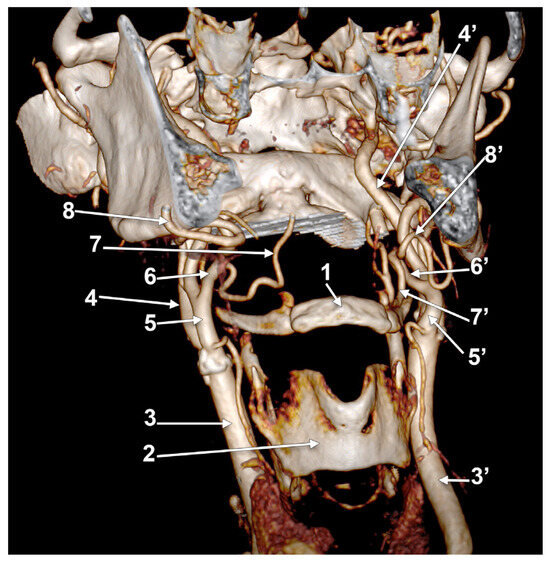
Figure 19.
Bilateral linguofacial trunk. Computed tomography angiogram. Three-dimensional volume rendering. Right antero-infero-lateral view. 1. hyoid body; 2. thyroid cartilage; 3, 3’. common carotid arteries; 4, 4’. internal carotid arteries; 5, 5’. external carotid arteries; 6, 6’. linguofacial trunks; 7, 7’. lingual arteries; and 8, 8’. facial arteries.
4. Discussion
Lippert and Pabst (1985) indicated an 18% prevalence of the LFT in their publication on arterial variations in man [2]. However, one could not find the size of the lots documented for that prevalence. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation mentions only that the FA and LA could have a common trunk of origin—the LFT [18]. However, the Illustrated Encyclopedia of Human Anatomic Variation by Bergman, Afifi, and Miyauchi mentions that the LA arises from a common trunk with the FA, the LFT, in 10–20% of cases [19]. The bilateral LFT is considered a rare variation [14]. Different dissection studies did not find bilateral LFTs [20,21]. A dissection study on 52 sides found LFTs only on the left side [22]. After a 30-cadaver study, no LFTs were detected [23]. In some previous studies, the LFT was termed the “faciolingual trunk” [24,25,26] or “lingual-facial trunk” [14,27].
The incidence of the LFT was variably reported, the unilateral cases being or not being distinguished from the bilateral ones (Table 1). Of these studies, a computed tomography study on 100 cases found a 15% prevalence of LFTs [28]. A different angioCT study on 76 cases found a prevalence of the LFT of 15.5%, but the uni- and bilateral cases were not distinguished [29]. The present CT study used 147 cases and found a 22.44% prevalence of the LFT.

Table 1.
Previous studies and reports of the linguofacial trunk (LFT) are listed chronologically [6,7,8,9,11,12,14,20,21,22,24,25,26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. D: dissection. CTA: computed tomography angiography. MRA: magnetic resonance angiography. A: angiography. Cor.: corrosion casts. CR: case report. CB: carotid bifurcation.
A histological study revealed a thinner wall and a wider inner diameter of ECA, LA, and FA that originated from an LFT compared to those that originated separately [51].
Cappabianca et al. (2012) found different possibilities for the origin of the LFT: from the ECA (7%), from the CB (2%), or from the common carotid artery (1%) [7]. In a different study, only one from 34 LFTs left the CB, the others arising from the ECA [33]. In the present study, only ECA origins of the LFT were found. LFT crosses and obscures the ICA when it leaves a lateralized ECA [54]. A dissection study in 79 cadavers found more LFTs on the right side [62], but we couldn’t distinguish whether or not some of the reported LFTs were bilateral [62]. We found 47 LFTs, 13 unilateral left, eight unilateral right, and bilateral in the other 13 cases.
The insertion of an LFT into the ECA is also variable. In 56 dissected carotid systems, the sequence of ECA’s branch origins was the superior thyroid, LFT, occipital, and maxillary arteries in 21.4% and the superior thyroid, occipital, LFT, and maxillary arteries in 10.7% [26]. These patterns were considered to be due to a shift in the occipital artery branching site [26].
A recent dissection study in 35 cadavers was focused on the LA [63]. It was observed that the LFT tended to originate at a more superior level than a solitary LA [63]. The distance between the LFT and the greater horn of the hyoid bone was 1.59 + 0.52 cm on the right side and 1.67 + 0.61 on the left side [63]. Other authors determined the levels of origin for the solitary LA and, respectively, LFTs, as referred to the CBs [27]. They found no significant differences even though there was a tendency for the LFT to have a higher origin [27]. In 7/200 sides of fetuses, the only suprahyoid variant of the LA and FA origin from the ECA was the LFT [44].
A dissection study found the length of the LFT to be < 1 cm in 81.3% of sides and <1 cm in 18.7% of sides [26]. A CTA study found the mean length of the LFT to be 0.923 cm [29]. In a report of a unilateral LFT in a 103-year-old cadaver, the LFT was found to have a length of 0.88 cm [40]. Another study found the LFT to have an average length of 0.94 ± 0.17 cm on the right and 0.76 ± 0.13 cm on the left, with no difference between sides [27]. The present results do not support such a bilateral symmetry.
Recently, a short LFT further divided into the LA and FA was reported as an aberrant origin of the FA from the LA because the latter continued the course of the LFT [48]. An origin of the LA from the FA was also described (23.1% in 80 CTAs and 20% in 10 dissected cadavers) [64]. However, in our view, a common arterial trunk is just a common trunk, no matter the topography of its resulting branches.
Both the LFT and LA have individually variable loops. In 84% of cases, the LA forms a first superior loop with a variable height; in 16% of cases, the LA without the first superior loop runs forward obliquely; and in 8%, the LA forms an inferior loop [53]. Two subtypes of the LA’s superior loop were found: a double hump and an anticlockwise loop [53].
In case #9, the LA resulted from an LFT crossed over the FA. When the two arteries leave the ECA separately, such arterial crossing cannot occur. However, this possibility should not be ignored by surgeons because it can occur in cases with an LFT.
In case #12, an LFT was applied on the medial side of the ECA. When the ECA ligation is intended to spare the LA and FA territories, such an LFT should be distinguished and isolated.
The CB has different anatomical variables, such as the axial spin, the vertical location, and the hyoid–carotid topographical relations [9,65,66,67]. These modify the topography of the ECA’s branches [15]. Therefore, the anatomical variability of the morphology and topography of the LFT should be expected. As it was observed in this case-by-case study, the variables of the LFT are the ECA’s side of the origin of the LFT, the vertical height of the LFT origin (infrahyoid, hyoid, suprahyoid, subgonial), the morphology of the LFT (rectilinear, looped, coiled), and the length of the LFT. The potential loops of an LFT are diverse and thus unpredictable.
In case #10, a submandibular gland was displaced inferiorly over the hyoid bone and the ECA. Such submandibular gland ptosis and glandular enlargement obscure the ECA’s branches and complicate the identification of potential common arterial trunks. The submandibular gland ptosis is a significant contributor to age-related submandibular fullness [68].
Different reports of retropharyngeal carotid arteries are available. Most of these present retropharyngeal ICAs [69,70,71,72,73]. However, all carotid arteries could course medially to the greater horn of the hyoid bone [66], and are therefore posterior to the pyriform sinus. As it results from the present study (case #15), the LFT could also be retropharyngeal.
The stylohyoid chain derives from the second branchial arch. It is a bone–ligament complex consisting of the styloid process, the stylohyoid ligament, and the lesser horn of the hyoid bone [74]. It consists of four structures: the tympanohyal, forming the root of the styloid process; the stylohyal, forming the body of the styloid process; the ceratohyal, becoming the stylohyoid ligament; and the hypohyal, forming the lesser horn of the hyoid bone [74].
In case #33, we found asymmetrical bilateral ossifications of the stylohyoid chain. On one side, the ceratohyal was displacing the occipital artery, the ECA, and the FA laterally. Conversely, the ossified ceratohyal interfered with the LFT and the resulting FA. Therefore, different patterns of ossification of the stylohyoid chain could alter the normal course of different arteries and, eventually, determine an Eagle’s syndrome. These individual modifications could be easily documented on CTAs.
Arteries beneath the gonial angle are exposed and at risk during tonsillectomies. As we presented, the TLF could ascend and approach the tonsillar bed. Pseudoaneurysms may arise after a localized arterial laceration caused by blunt or penetrating trauma, including the traction and thermal damage produced by electrocautery [75]. Post-tonsillectomy hemorrhages from pseudoaneurysms of the LA, FA, ICA, and LFT were reported [75].
Although pseudoaneurysms of the LFT after tonsillectomy are rare, prompt diagnosis and timely management are mandatory to avoid profuse bleeding and death [76]. It can present intraoperatively and hours or days after tonsillectomy, either with massive bleeding or cervical swelling [76].
Superselective intra-arterial chemotherapy for oral cancer delivers a good concentration of anticancer agents into a tumor-feeding artery [77]. Such distribution through an LFT was investigated using computational fluid dynamics [77]. It was found that the agent’s behavior in the LFT is determined by the blood flow field, which depends on the topography of the vessels in each patient [77]. The latter was demonstrated here as an individual variable; therefore, it should be investigated before injections into the LFT. The catheter tip position should be changed according to the vessel topography to deliver anticancer agents into the tumor-feeding artery [77], the LA, or the FA. Nevertheless, vascular tortuosity, such as a loop or coil of an LFT, is expected to cause difficulties in catheter insertion [26].
5. Conclusions
The morphology and topography of the LFT are individually specific and unpredictable. It should be documented case-by-case by surgeons on CT or MR angiograms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina60020291/s1, Video S1: Almost complete ossification of the left stylohyoid chain in Case #33.
Author Contributions
Conceptualization, M.C.R. and A.D.V.; methodology, C.C.D.; software M.C.R. and C.C.D.; validation, M.C.R. and A.D.V.; formal analysis, C.C.D.; investigation, C.C.D.; resources, M.C.R., A.D.V. and C.C.D.; data curation, A.D.V.; writing—original draft preparation, M.C.R. and C.C.D.; writing—review and editing, A.D.V.; visualization, C.C.D.; supervision, M.C.R.; project administration, M.C.R. and A.D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of University Emergency Hospital Bucharest, Romania (protocol code 10540/16.02.2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gray, H.; Standring, S.; Anand, N.; Birch, R.; Collins, P.; Crossman, A.; Gleeson, M.; Jawaheer, G.; Smith, A.L.; Spratt, J.D.; et al. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier: London, UK, 2016. [Google Scholar]
- Lippert, H.; Pabst, R. Arterial Variations in Man: Classification and Frequency; J.P. Bergmann Verlag: Munich, Germany, 1985. [Google Scholar]
- Seki, S.; Sumida, K.; Yamashita, K.; Baba, O.; Kitamura, S. Gross anatomical classification of the courses of the human lingual artery. Surg. Radiol. Anat. 2017, 39, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bozhikova, E.; Novakov, S.; Uzunov, N. A rare variation in the origin of the lingual artery: Thyro-linguo-laryngeal trunk. Folia Morphol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Baxla, M.; Kumari, C.; Kaler, S. Bilateral thyrolinguofacial trunk: Unusual and rare branching pattern of external carotid artery. Anat. Cell Biol. 2018, 51, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Jianu, A.M.; Monea, M.D.; Ilie, A.C. Two cases of combined anatomical variations: Maxillofacial trunk, vertebral, posterior communicating and anterior cerebral atresia, linguofacial and labiomental trunks. Folia Morphol. 2022, 81, 237–246. [Google Scholar] [CrossRef]
- Cappabianca, S.; Scuotto, A.; Iaselli, F.; Pignatelli di Spinazzola, N.; Urraro, F.; Sarti, G.; Montemarano, M.; Grassi, R.; Rotondo, A. Computed tomography and magnetic resonance angiography in the evaluation of aberrant origin of the external carotid artery branches. Surg. Radiol. Anat. 2012, 34, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, Z.; Govsa, F.; Ozgur, T. Assessment of origin characteristics of the front branches of the external carotid artery. J. Craniofac. Surg. 2008, 19, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Anangwe, D.; Saidi, H.; Ogeng’o, J.; Awori, K.O. Anatomical variations of the carotid arteries in adult Kenyans. East Afr. Med. J. 2008, 85, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Natsis, K.; Raikos, A.; Foundos, I.; Noussios, G.; Lazaridis, N.; Njau, S.N. Superior thyroid artery origin in Caucasian Greeks: A new classification proposal and review of the literature. Clin. Anat. 2011, 24, 699–705. [Google Scholar] [CrossRef]
- Zumre, O.; Salbacak, A.; Cicekcibasi, A.E.; Tuncer, I.; Seker, M. Investigation of the bifurcation level of the common carotid artery and variations of the branches of the external carotid artery in human fetuses. Ann. Anat. 2005, 187, 361–369. [Google Scholar] [CrossRef]
- Devadas, D.; Pillay, M.; Sukumaran, T.T. A cadaveric study on variations in branching pattern of external carotid artery. Anat. Cell Biol. 2018, 51, 225–231. [Google Scholar] [CrossRef]
- Lucev, N.; Bobinac, D.; Maric, I.; Drescik, I. Variations of the great arteries in the carotid triangle. Otolaryngol. Head Neck Surg. 2000, 122, 590–591. [Google Scholar] [PubMed]
- Troupis, T.; Michalinos, A.; Kakisis, J.; Natsis, K.; Sofidis, G.; Skandalakis, P. Bilateral lingual-facial trunk: Anatomic and clinical implications. Folia Morphol. 2015, 74, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.C.; Hostiuc, S.; Vrapciu, A.D.; Rusu, M.C. Vertical Levels of the Occipital Artery Origin. Medicina 2023, 59, 317. [Google Scholar] [CrossRef] [PubMed]
- Minca, D.I.; Rusu, M.C.; Radoi, P.M.; Hostiuc, S.; Toader, C. A New Classification of the Anatomical Variations of Labbe’s Inferior Anastomotic Vein. Tomography 2022, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Minca, D.I.; Rusu, M.C.; Radoi, P.M.; Vrapciu, A.D.; Hostiuc, S.; Toader, C. The Infraoptic or Infrachiasmatic Course of the Anterior Cerebral Artery Emerging an Elongated Internal Carotid Artery. Tomography 2022, 8, 188. [Google Scholar] [CrossRef]
- Bergman, R.A.; Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bergman, R.; Afifi, A.; Miyauchi, R. Illustrated Encyclopedia of Human Anatomic Variation: Opus II: Cardiovascular System: Arteries: Head, Neck, and Thorax. Maxillary Artery. 2013. Available online: https://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Text/Arteries/Maxillary.shtml (accessed on 10 December 2023).
- Ovhal, A.G.; Ansari, M.M.; Rajgopal, L.; Ovhal, A.G. A cross sectional study of variations in the external carotid artery in cadavers. Ind. J. Clin. Anat. Physiol. 2016, 3, 282–286. [Google Scholar] [CrossRef]
- Gupta, V.; Agarwal, R. Anomalous branching pattern of the external carotid artery in cadavers. Int. J. Sci. Study 2014, 2, 28–31. [Google Scholar]
- Esakkiammal, N.; Renu, C.; Rakhee, S. Implications of variable origin of external carotid artery branches and high level bifurcation of common carotid artery. Int. J. Anat. Res. 2017, 5, 3958–3963. [Google Scholar]
- Tippireddy, S.; Manikanta Reddy, V.; Sofia, P.; Lakshmi Devi, C. A Study on the Branching Pattern of External Carotid Artery. IOSR J. Dent. Med. Sci. 2019, 18, 19–22. [Google Scholar]
- Shintani, S.; Terakado, N.; Alcalde, R.E.; Tomizawa, K.; Nakayama, S.; Ueyama, Y.; Ichikawa, H.; Sugimoto, T.; Matsumura, T. An anatomical study of the arteries for intraarterial chemotherapy of head and neck cancer. Int. J. Clin. Oncol. 1999, 4, 327–330. [Google Scholar] [CrossRef]
- Kısaoğlu, A.; Bilge, D.; Aydın, M.; Özdemir, B.; Köse, E.; Anatomy, C. Facial artery and lingual artery originating from a common trunk: A case report. Int. J. Exp. Clin. Anat. 2022, 16, 542. [Google Scholar]
- Yonenaga, K.; Tohnai, I.; Mitsudo, K.; Mori, Y.; Saijo, H.; Iwai, T.; Yonehara, Y.; Ota, Y.; Torigoe, K.; Takato, T. Anatomical study of the external carotid artery and its branches for administration of superselective intra-arterial chemotherapy via the superficial temporal artery. Int. J. Clin. Oncol. 2011, 16, 654–659. [Google Scholar] [CrossRef]
- Fazan, V.P.; da Silva, J.H.; Borges, C.T.; Ribeiro, R.A.; Caetano, A.G.; Filho, O.A. An anatomical study on the lingual-facial trunk. Surg. Radiol. Anat. 2009, 31, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, N.; Vijayalakshmi, S.; Raghunath, G.; Karunakaran, B.; Nithya, S.; Ks, P.D.; Kumaresan, M.; Gurusamy, K.; Francis, Y.M.; Dharshini, P. Morphometric Study and Branching Patterns of External Carotid Artery Using Computed Tomography Angiography among the South Indian Population: A Retrospective Study. Cureus 2023, 15, e35624. [Google Scholar] [CrossRef]
- Herrera-Nunez, M.; Menchaca-Gutierrez, J.L.; Pinales-Razo, R.; Elizondo-Riojas, G.; Quiroga-Garza, A.; Fernandez-Rodarte, B.A.; Elizondo-Omana, R.E.; Guzman-Lopez, S. Origin variations of the superior thyroid, lingual, and facial arteries: A computed tomography angiography study. Surg. Radiol. Anat. 2020, 42, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.; Biswas, S.; Sharma, S.; Rathia, D.S.; Chakraborty, S. Bilateral linguofacial trunk in a cadaver: A case report. J. Anat. Soc. India 2017, 66, S8–S9. [Google Scholar] [CrossRef]
- Sanjeev, I.; Anita, H.; Ashwini, M.; Mahesh, U.; Rairam, G. Branching pattern of external carotid artery in human cadavers. J. Clin. Diagn. Res. 2010, 4, 3128–3133. [Google Scholar]
- Mata, J.R.; Mata, F.R.; Souza, M.C.; Nishijo, H.; Ferreira, T.A. Arrangement and prevalence of branches in the external carotid artery in humans. Ital. J. Anat. Embryol. 2012, 117, 65–74. [Google Scholar]
- Cobiella, R.; Quinones, S.; Aragones, P.; Leon, X.; Abramovic, A.; Vazquez, T.; Ramon Sanudo, J.; Maranillo, E.; Olewnik, L.; Simon de Blas, C.; et al. Anatomic mapping of the collateral branches of the external carotid artery with regard to daily clinical practice. Ann. Anat. 2021, 238, 151789. [Google Scholar] [CrossRef]
- Dnyanesh, S.; Bhimalli, S.; Dnyanesh, D.; Dixit, D.; More, M.; Amgain, K. Unilateral Linguofacial Trunk: A Rare Case Report. Int. J. Appl. Sci. Biotechnol. 2013, 1, 154–157. [Google Scholar] [CrossRef]
- Ogeng’o, J.A.; Misiani, M.K.; Loyal, P.; Ongeti, K.W.; Gimongo, J.; Inyimili, M.I.; Murunga, A.K. Variations in branching pattern of external carotid artery in a black Kenyan population. Anat. J. Afr. 2015, 4, 584–590. [Google Scholar]
- Sarna, K.; Sonigra, K.J.; Amuti, T.; Kamau, M.; Ngeow, W.C.; Mandela Idenya, P. The Journey of the Lingual Artery from the Neck to the Oral Cavity: A Cadaveric Study. Craniomaxillofacial Trauma Reconstr. 2022, 15, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, M.; Dixit, A. Research Article A Research on The Incidence of Linguofacial Trunk Arising From External Carotid Artery in Adult Cadavers. Sch. Acad. J. Biosci. 2012, 8, 590–591. [Google Scholar]
- Kumar, A.; Kumar, V. Abnormal Branching Pattern of Lingual and Facial Arteries–A. IOSR-J. Dent. Med. Sci. 2019, 18, 31–33. [Google Scholar]
- Troupis, T.G.; Dimitroulis, D.; Paraschos, A.; Michalinos, A.; Protogerou, V.; Vlasis, K.; Troupis, G.; Skandalakis, P. Lingual and facial arteries arising from the external carotid artery in a common trunk. Am. Surg. 2011, 77, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, G.C.; Coronado, G.C.; Aravena, T.P.; Suazo, G.I. Lingual-facial trunk arising from the external carotid artery: A case report. Int. J. Morphol. 2014, 32, 1108–1110. [Google Scholar] [CrossRef][Green Version]
- Shreevastava, A.K.; Das, R.S.; Maheshwari, T.P.; Damodhar, B.K. Bilateral High Trifurcation of the Common Carotid Artery and Variable Emergence of the Lower Branches of the External Carotid Artery: A Cadaveric Case Report. Cureus 2022, 14, e27657. [Google Scholar] [CrossRef]
- Nirmaladevi, M.; Sruthi, G. Linguo-Facial Trunk—A case report. Anat. Karnataka 2010, 4, 54–56. [Google Scholar]
- Diwan, R.K.; Rani, A.; Chopra, J.; Kumar, N. Thyro-linguo-facial trunk of external carotid artery: A rare variation. J. Anat. 2017, 25, 54–55. [Google Scholar]
- Kala, M.; Prakash, N.J.; Kumar, B.I.N.; Sailaja, T.K.; Jayasree, N. Study of variations in Lingual and Facial arteries. MRIMS J. Health Sci. 2016, 4, 100–102. [Google Scholar] [CrossRef]
- Kishve, P.S.; Kishve, S.P.; Joshi, M.; Aarif, S.M.; Kalakoti, P. An unusual branching pattern of common and external carotid artery in a human cadaver: A case report. Australas Med. J. 2011, 4, 180–182. [Google Scholar] [CrossRef]
- Vinnakota, S.; Nandagiri, B.; Neelee, J. A rare association of curving and looping of internal carotid artery and variation in the branching pattern of external carotid artery—A case report. Int. J. Biol. Med. Res. 2011, 2, 822–823. [Google Scholar]
- Desai, S.D.; Nuchhi, A.B.; Karjagi, S.B. Bilateral linguofacial trunk-a case report. Nat. J. Clin. Anat. 2012, 1, 193–195. [Google Scholar] [CrossRef]
- Piagkou, M.; Triantafyllou, G.; Nikolopoulou, E.; Karampelias, V.; Tsakotos, G. Lingual and Facial Artery Fusion: A Cadaveric Report With Clinical Significance. Cureus 2023, 15, e43495. [Google Scholar] [CrossRef]
- Sirasanagandla, S.R.; Nayak, S.; Potu, B.K.; Bhat, K.M. Origin of the facial artery from the lingual-facial trunk and its course through the submandibular salivary gland: A case report. Rev. Arg. Anat. Clin. 2012, 4, 20–24. [Google Scholar] [CrossRef]
- Paramasivam, K. Linguo-facial trunk (right side)—A case report. Univ. J. Pre Paraclin. Sci. 2019, 5, 4. [Google Scholar]
- Ahmed, R.; Al-Shaarawy, S.; Atteya, M.; Al-Fayez, M.; Aldahmash, A.; Mohal, S.; Aziz, R. Anatomical variations of lingual and facial arteries in male cadavers and histological study of their structure. Int. J. Integr. Biol. 2012, 13, 36–39. [Google Scholar]
- Lohan, D.G.; Barkhordarian, F.; Saleh, R.; Krishnam, M.; Salamon, N.; Ruehm, S.G.; Finn, J.P. MR angiography at 3 T for assessment of the external carotid artery system. AJR Am. J. Roentgenol. 2007, 189, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Shangkuan, H.; Xinghai, W.; Zengxing, W.; Shizhen, Z.; Shiying, J.; Yishi, C. Anatomic bases of tongue flaps. Surg. Radiol. Anat. 1998, 20, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, A. Lateral external carotid artery and linguofacial trunk: A rare anatomic variant. MOJ Anat. Physiol. 2015, 1, 1–3. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Alikunju, M.; Raveendran, V.L.; Govindapillai, U.K. Variations in the Branching Pattern of External Carotid Artery in South Kerala Population—A Cadaveric Study. J. Evid. Based Med. Healthc. 2021, 8, 1780–1785. [Google Scholar] [CrossRef]
- Thwin, S.S.; Soe, M.M.; Myint, M.; Than, M.; Lwin, S. Variations of the origin and branches of the external carotid artery in a human cadaver. Singap. Med. J. 2010, 51, e40–e42. [Google Scholar]
- Anu, V.R.; Pai, M.M.; Rajalakshmi, R.; Latha, V.P.; Rajanigandha, V.; D’Costa, S. Clinically-relevant variations of the carotid arterial system. Singap. Med. J. 2007, 48, 566–569. [Google Scholar]
- Charles, S.; Rabi, S.; Jain, A.; Rana, P.K. Origin and branching pattern of external carotid artery-a cadaveric study. Eur. J. Anat. 2021, 25, 187–196. [Google Scholar]
- Gwunireama, I.; Bob-Manuel, I.; Collins, G. A Study of Morphological Variations of Carotid Bifurcation and Branching Pattern of External Carotid Artery in Adult Nigerian Cadavers. Arch. Curr. Res. Int. 2023, 23, 40–48. [Google Scholar] [CrossRef]
- Homze, E.J.; Harn, S.D.; Bavitz, B.J. Extraoral ligation of the lingual artery: An anatomic study. Oral. Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, M.; Chitra, S. A study of the common origin of lingual and facial artery from the external carotid artery. Int. J. Anat. Res. 2017, 5, 3656–3658. [Google Scholar] [CrossRef][Green Version]
- Heltzel, S.; Jelinek, L.; Jaynes, D. Variation in the caudal branches of the external carotid artery: Comparison of sex and side. Med. Res. Arch. 2015. Available online: https://esmed.org/MRA/mra/article/view/21 (accessed on 7 December 2023). [CrossRef][Green Version]
- Sarna, K.; Kamau, M.; Sonigra, K.J.; Amuti, T. Anatomical Variations in the Origin of the Lingual Artery in the Kenyan Population. Craniomaxillofacial Trauma Reconstr. 2022, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liu, Y.; Tao, Y.; Wu, J.; Wang, S.; Zhao, H.; Liu, B.; Xu, S. Clinical anatomy of the lingual artery. Chin. Arch. Otolaryngol. Head Neck Surg. 2006. Available online: https://search.bvsalud.org/gim/resource/es/wpr-529160 (accessed on 7 December 2023).
- Manta, M.D.; Rusu, M.C.; Hostiuc, S.; Vrapciu, A.D.; Manta, B.A.; Jianu, A.M. The Axial Spin of the Carotid Bifurcation. Diagnostics 2023, 13, 3122. [Google Scholar] [CrossRef]
- Manta, M.D.; Rusu, M.C.; Hostiuc, S.; Vrapciu, A.D.; Manta, B.A.; Jianu, A.M. The Carotid-Hyoid Topography Is Variable. Medicina 2023, 59, 1494. [Google Scholar] [CrossRef]
- Hayashi, N.; Hori, E.; Ohtani, Y.; Ohtani, O.; Kuwayama, N.; Endo, S. Surgical anatomy of the cervical carotid artery for carotid endarterectomy. Neurol. Med. Chir. 2005, 45, 25–29. [Google Scholar] [CrossRef]
- McCleary, S.P.; Moghadam, S.; Le, C.; Perez, K.; Sim, M.S.; Roostaeian, J. Age-Related Changes in the Submandibular Gland: An Imaging Study of Gland Ptosis Versus Volume. Aesthet. Surg. J. 2022, 42, 1222–1235. [Google Scholar] [CrossRef]
- Nayak, S.B.; Shetty, S.D. Surgical and embryological perspective of a big loop of internal carotid artery extending laterally beyond internal jugular vein. Surg. Radiol. Anat. 2021, 43, 413–416. [Google Scholar] [CrossRef]
- De Stercke, A.; Dequanter, D.; Rodriguez, A. Having a carotid in the throat: A rare case of internal carotid medialization-induced dysphonia. Clin. Case Rep. 2023, 11, e7997. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, F.; Tillmann, B.; Christofides, C.; Richter, W.; Koebke, J. Curving and looping of the internal carotid artery in relation to the pharynx: Frequency, embryology and clinical implications. J. Anat. 2000, 197 Pt 3, 373–381. [Google Scholar] [CrossRef]
- Moraru, L.; Rusu, M.C.; Popescu, S.A. True terminal pentafurcation of the external carotid artery and terminal trifurcation of the contralateral one, occipitoauricular trunk, retropharyngeal internal carotid artery. Surg. Radiol. Anat. 2021, 43, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Lukins, D.E.; Pilati, S.; Escott, E.J. The Moving Carotid Artery: A Retrospective Review of the Retropharyngeal Carotid Artery and the Incidence of Positional Changes on Serial Studies. AJNR Am. J. Neuroradiol. 2016, 37, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.U.; Gokharman, D.; Tuncbilek, I.; Kacar, M.; Kosar, P.; Kosar, U. Assessment of the stylohoid chain by 3D-CT. Surg. Radiol. Anat. 2007, 29, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Manzato, L.; Trivelato, F.P.; Alvarenga, A.Y.; Rezende, M.T.; Ulhoa, A.C. Endovascular treatment of a linguofacial trunk pseudoaneurysm after tonsillectomy. Braz. J. Otorhinolaryngol. 2013, 79, 524. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, S.; Belet, U.; Baris, S. Post-tonsillectomy pseudoaneurysm of the linguofacial trunk: An ENT surgeon’s nightmare. Int. J. Ped. Otorhinolaryngol. Extra 2012, 7, 12–14. [Google Scholar] [CrossRef]
- Kitajima, H.; Iwai, T.; Yajima, Y.; Mitsudo, K. Computational Fluid Dynamics Study of Superselective Intra-arterial Chemotherapy for Oral Cancer: Flow Simulation of Anticancer Agent in the Linguofacial Trunk. Appl. Sci. 2020, 10, 7496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).