Rapidly Growing and Ruptured Great Saphenous Vein Aneurysm in a Liver Transplant Patient
Abstract
1. Introduction
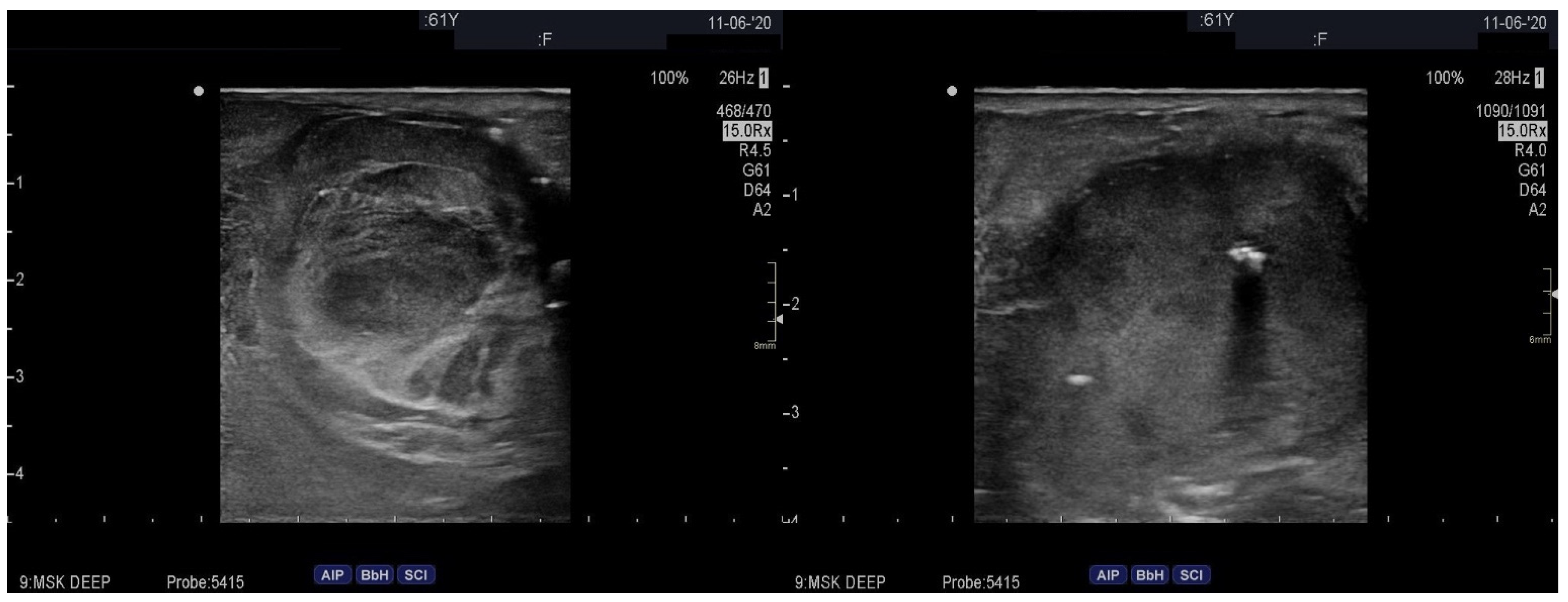
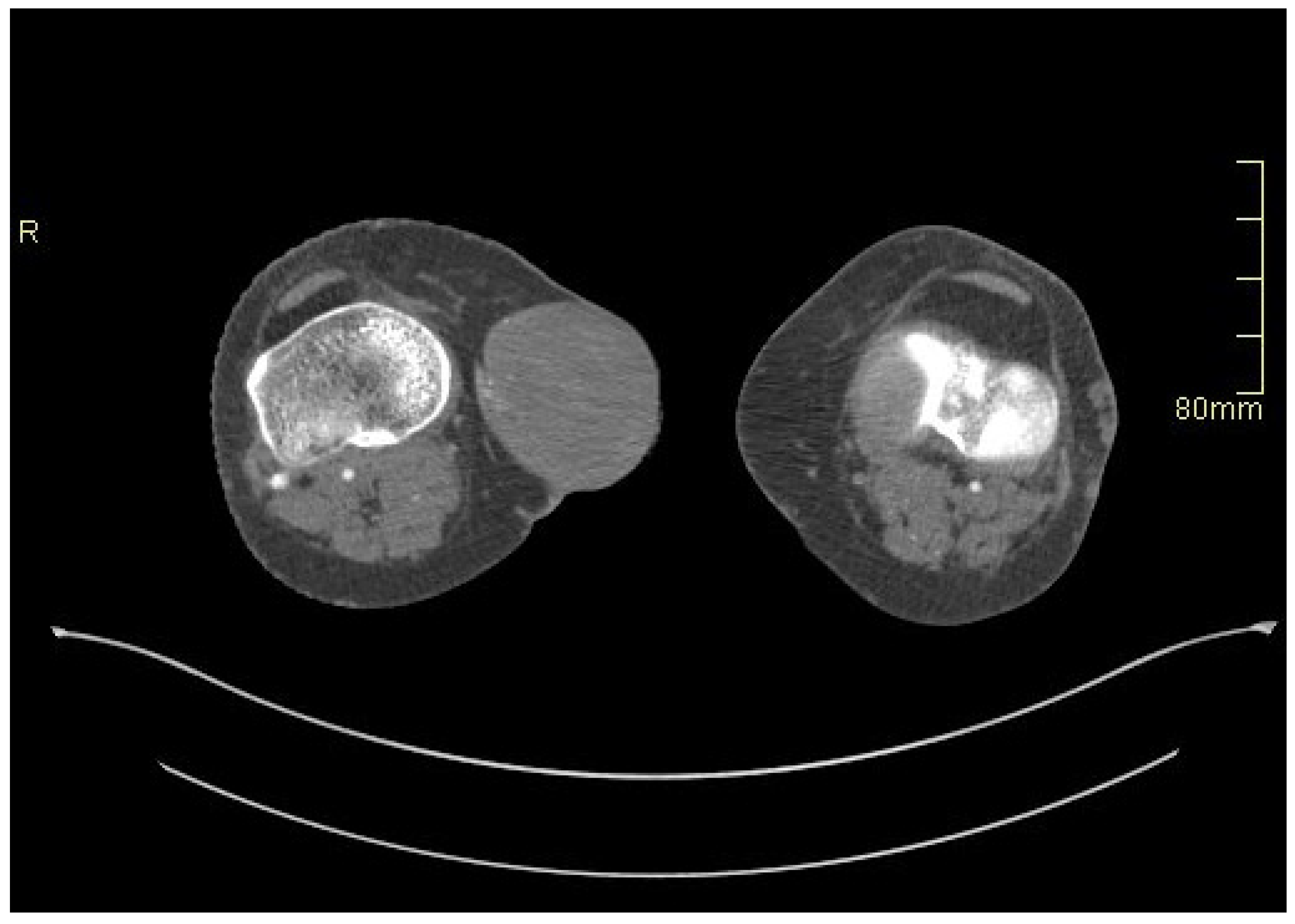
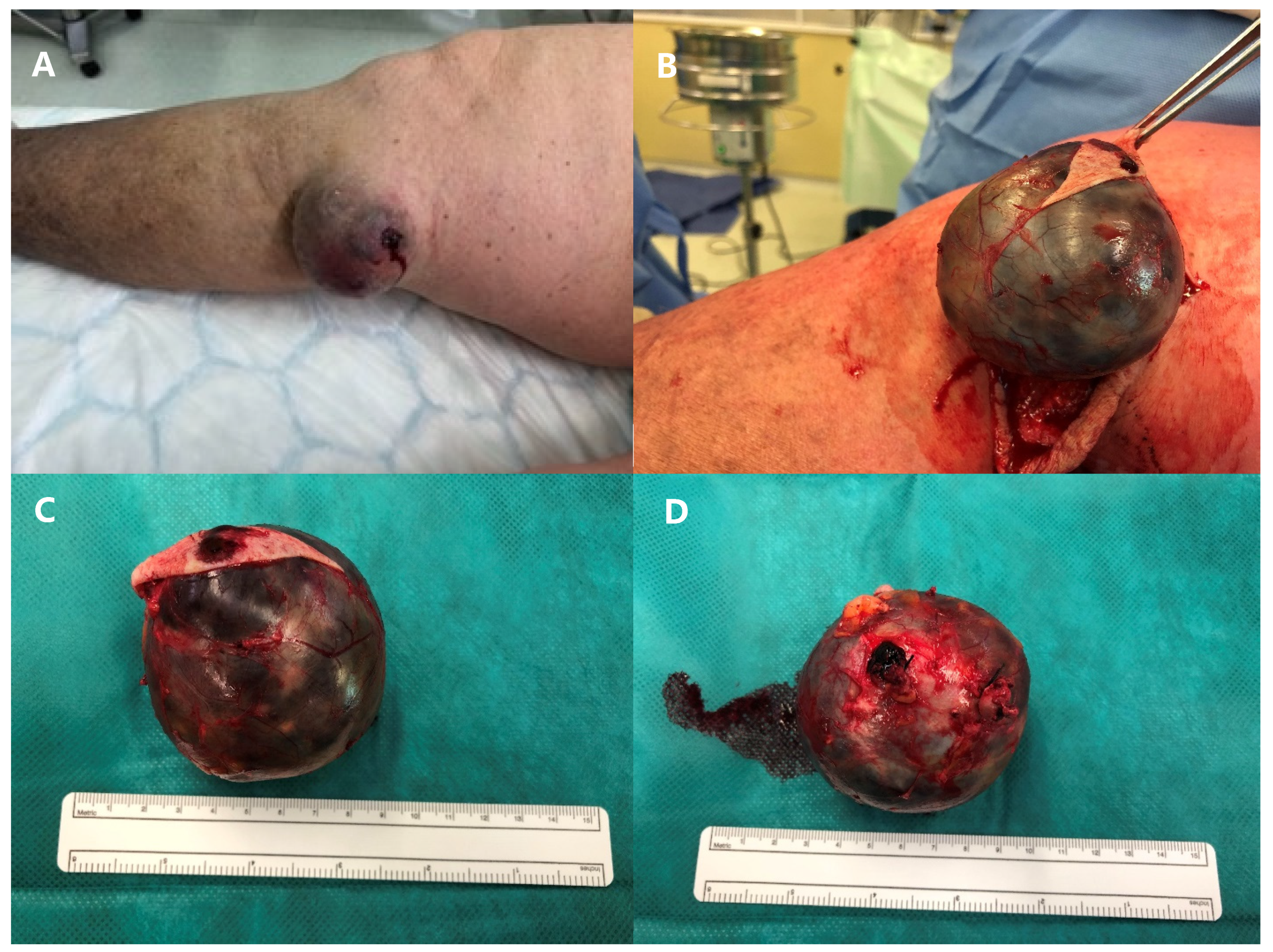
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillespie, D.L.; Villavicencio, J.L.; Gallagher, C.; Chang, A.; Hamelink, J.K.; Fiala, L.A.; O’Donnell, S.D.; Jackson, M.R.; Pikoulis, E.; Rich, N.M. Presentation and management of venous aneurysms. J. Vasc. Surg. 1997, 26, 845–852. [Google Scholar] [CrossRef]
- Teter, K.A.; Maldonado, T.M.; Adelman, M.A. A systematic review of venous aneurysms by anatomic location. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 408–413. [Google Scholar] [CrossRef]
- Weiske, N.; Gebauer, T.; Burger, T. Klinische Bedeutung und Therapie venoser Aneurysmen der unteren Extremitaten. Zentralbl. Chir. 2015, 140, 530–534. [Google Scholar] [CrossRef]
- Syed, A.; Bhagat, T.; Bradpiece, H. Primary venous aneurysm of the long saphenous vein: An uncommon case. Br. J. Hosp. Med. (Lond.) 2010, 71, 354. [Google Scholar] [CrossRef]
- Vermeer, N.C.; Elshof, J.W.; Vriens, P.W. Clinical presentation, diagnosis, and treatment of venous aneurysms. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 349–353.e3. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef] [PubMed]
- McKesey, J.; Cohen, P.R. Spontaneous Venous Aneurysm: Report of a Non-traumatic Superficial Venous Aneurysm on the Distal Arm. Cureus 2018, 10, e2641. [Google Scholar] [CrossRef] [PubMed]
- Schatz, I.J.; Fine, G. Venous aneurysms. N. Engl. J. Med. 1962, 266, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Osler, W.S. An arteriovenous aneurysm of the axillary vessels of 30 years duratio. Lancet 1913, 182, 1248–1249. [Google Scholar] [CrossRef]
- Kota, A.A.; Stephen, E.; Samuel, V.; Premkumar, P.; Selvaraj, D.; Agarwal, S. Primary great saphenous vein aneurysm in a child. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 765. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Biondi, A.; Brancato, G.; Donati, A.; Basile, F. Venous aneurysms of saphena magna: Is this really a rare disease? Comment to: A challenging hernia: Primary venous aneurysm of the proximal saphenous vein. Hernia 2013, 17, 115–117. [Google Scholar] [CrossRef]
- Irwin, C.; Synn, A.; Kraiss, L.; Zhang, Q.; Griffen, M.M.; Hunter, G.C. Metalloproteinase expression in venous aneurysms. J. Vasc. Surg. 2008, 48, 1278–1285. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Tang, P.C.; Coady, M.A.; Lovoulos, C.; Dardik, A.; Aslan, M.; Elefteriades, J.A.; Tellides, G. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation 2005, 112, 1098–1105. [Google Scholar] [CrossRef]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Wu, A.H.; Clowes, A.W.; Zierler, R.E. The prevalence and natural history of aortic aneurysms in heart and abdominal organ transplant patients. J. Vasc. Surg. 2003, 37, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Sun, J.; Han, X.; Liu, H.; Li, J.; Du, J.; Feng, W.; Liu, B.; Cui, J.; Guo, J.; et al. Immunolocalization of MMP 2, 9 and 13 in prednisolone induced osteoporosis in mice. Histol. Histopathol. 2016, 31, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Surowka, A.; Wilk, A.; Szumilas, K.; Kedzierska-Kapuza, K. The Effect of Immunosuppressive Drugs on MMPs Activity in The Walls of Blood Vessels—A Systematic Review. Int. J. Med. Sci. 2021, 18, 1502–1509. [Google Scholar] [CrossRef]
- Tepperman, E.; Ramzy, D.; Prodger, J.; Sheshgiri, R.; Badiwala, M.; Ross, H.; Raoa, V. Surgical biology for the clinician: Vascular effects of immunosuppression. Can. J. Surg. 2010, 53, 57–63. [Google Scholar]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef]
- Sedki, N.; Zrihni, Y.; Jiber, H.; Zaghloul, R.; Bouarhroum, A. Primary great saphenous vein aneurysm. Dermatol. Surg. 2011, 37, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Nicolini, P.; Perrin, M.; Farah, I.; Magne, J.L.; Guidicelli, H. Management of symptomatic and asymptomatic popliteal venous aneurysms: A retrospective analysis of 25 patients and review of the literature. J. Vasc. Surg. 2000, 32, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Halandras, P.; Hershberger, R.; Aulivola, B.; Crisostomo, P. Giant Spontaneous Greater Saphenous Vein Aneurysm. Ann. Vasc. Surg. 2017, 42, 302.e11–302.e14. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Okada, M. Primary venous aneurysms—Case reports. Angiology 1999, 50, 239–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racman, M.; Kafol, J.; Jug, B.; Stankovic, M.; Piljic, D.; Ksela, J. Rapidly Growing and Ruptured Great Saphenous Vein Aneurysm in a Liver Transplant Patient. Medicina 2024, 60, 290. https://doi.org/10.3390/medicina60020290
Racman M, Kafol J, Jug B, Stankovic M, Piljic D, Ksela J. Rapidly Growing and Ruptured Great Saphenous Vein Aneurysm in a Liver Transplant Patient. Medicina. 2024; 60(2):290. https://doi.org/10.3390/medicina60020290
Chicago/Turabian StyleRacman, Mark, Jan Kafol, Borut Jug, Milenko Stankovic, Dragan Piljic, and Jus Ksela. 2024. "Rapidly Growing and Ruptured Great Saphenous Vein Aneurysm in a Liver Transplant Patient" Medicina 60, no. 2: 290. https://doi.org/10.3390/medicina60020290
APA StyleRacman, M., Kafol, J., Jug, B., Stankovic, M., Piljic, D., & Ksela, J. (2024). Rapidly Growing and Ruptured Great Saphenous Vein Aneurysm in a Liver Transplant Patient. Medicina, 60(2), 290. https://doi.org/10.3390/medicina60020290





