Abstract
Background and Objectives: This review paper highlights the key alternatives to the blue dye/radioisotope method of sentinel lymph node biopsy (SLNB). It analyses the research available on these alternative methods and their outcomes compared to the traditional techniques. Materials and Methods: This review focused on fifteen articles, of which five used indocyanine green (ICG) as a tracer, four used magnetic tracers, one used one-step nucleic acid amplification (OSNA) and Metasin (quantitative reverse transcriptase-polymerase chain reaction), one used the photosensitiser talaporfin sodium, one used sulphur hexafluoride gas microbubbles, one used CT-guided lymphography and two focused on general SLNB technique reviews. Results: Of the 15 papers analysed, the sentinel node detection rates were 69–100% for indocyanine green, 91.67–100% for magnetic tracers, 81% for talaporfin sodium, 9.3–55.2% for sulphur hexafluoride gas microbubbles, 90.5% for CTLG and 82.7–100% for one-step nucleic acid amplification. Conclusions: Indocyanine green fluorescence (ICG) and magnetic tracers have been proven non-inferior to traditional blue dye and isotope regarding SLNB localisation. Further studies are needed to investigate the use of these techniques in conjunction with each other and the possible use of language learning models. Dedicated studies are required to assess cost efficacy and longer-term outcomes.
1. Introduction
Breast cancer is the most common cancer in the United Kingdom, with one woman diagnosed every 10 min [1]. Axillary node management has long been a controversial question for breast surgeons nationwide, with a shift in strategy from the most aggressive but tolerable treatment to the minimal effective treatment. Sentinel lymph node biopsy (SLNB) was proven non-inferior to axillary node dissection [2], and SLNB has been the gold standard for axillary staging of breast cancer since its first use in 1994 [3].
The two most commonly used methods for SLNB are blue dye and radioisotope (RI) labelling, with a combination of blue dye/RI giving the most accurate results. However, these methods come with significant disadvantages, including cost, risk of an allergic reaction, the need for a nuclear medicine department and scheduling of isotope injections preoperatively. In some countries, such as Japan, the lack of nuclear medicine facilities [4] has led to the development of alternative methods of SLNB. This review analyses the alternatives available, including their localisation outcomes, advantages and disadvantages.
2. Materials and Methods
We searched the PubMed database for articles published between 1 January 2013 and 30 June 2023 using the following search term: ‘alternatives sentinel lymph node biopsy breast cancer’. This yielded 548 results. Inclusion criteria filters were applied, including:
- Date of publication 1 January 2013 to 30 June 2023, narrowing the list to 242 results.
- Language of publication English only, further narrowing the list to 229 results.
- Article types filtered to only include clinical trials, randomised controlled trials, reviews and systematic reviews, narrowing the list to 59 results.
- Finally, a ‘full text’ filter was applied, which yielded a final 58 articles.
Fifty-eight article abstracts were screened. Forty-one articles were excluded, as they did not focus on our parameters of interest—specifically techniques used to identify the sentinel node in breast cancer, SLNB outcomes and the advantages and disadvantages of these methods. Irrelevant articles were excluded, such as those focusing on non-breast cancers, sentinel node biopsy vs axillary node dissection, or wireless vs. guided localisations. Thus, seventeen final articles were screened, of which ten were excluded as they had a small sample size (less than 100 patients), yielding seven final articles. Subsearching these articles yielded eight more articles that were deemed relevant to this paper (focusing on sentinel node biopsy techniques, advantages and disadvantages) and of a sufficient sample size (n > 100) to draw accurate conclusions. Thus, fifteen articles were finally included in this review paper, consisting of five on indocyanine green (ICG) fluorescence, four on magnetic tracers, four on other methods (photosensitiser, contrast ultrasound gas microbubbles, nucleic acid amplification and CT lymphography) and, finally, two review papers on SLNB techniques. The PRISMA diagram in Figure 1 describes the literature search process.
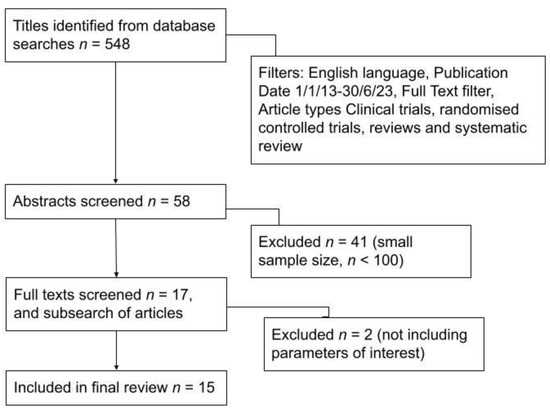
Figure 1.
PRISMA diagram showing literature search process.
The strengths of our paper include being one of the first review papers to compare all of the alternatives to the sentinel lymph node biopsy in breast cancer treatment, as a search on PubMed with the term ‘Alternative sentinel lymph node biopsy breast cancer’ revealed that there are currently no review papers which do this. The limitations of our study are that we did not undertake a statistical pooled analysis to formally compare the sentinel node outcomes across each paper. Furthermore, there are limited data available on the long-term effects of these new technologies (e.g., regional recurrence and overall survival rate) and the conclusions we can draw about long-term safety, efficacy and future consequences are thus limited. There is also a control bias as methods were not tested in isolation—e.g., most papers assessed the new localisation technology with radioisotope and/or blue dye as concurrent controls.
3. Results
The various methods analysed in this review are described below.
3.1. Indocyanine Green (ICG) Fluorescence
ICG is a low-molecular-weight, non-toxic, non-radioactive substance with a short serum half-life [5]. It is injected into the subdermal/peritumoral region just before surgery, which enables visualisation of the lymphatic drainage to the axilla using a fluorescence probe.
SLNB outcomes for ICG are shown in Table 1 below.

Table 1.
SLNB outcomes for Indocyanine green (ICG) fluorescence.
3.2. Magnetic Tracers, Most Commonly SPIO (Superparamagnetic Iron Oxide)
A magnetic tracer is injected into the breast, and the sentinel node is stained brown and identified with a handheld magnetometer. The magnetic tracers studied in this review [10] include ferumoxytol, magnetite/maghemite and ferumoxide.
SLNB outcomes for magnetic tracers are shown in Table 2 below.

Table 2.
SLNB outcomes for magnetic tracers.
3.3. Photosensitiser Talaporfin Sodium
Talaporfin is injected at the subareolar region just before the operation, and the sentinel node is identified as ‘pink’ by using fluorescence with xenon light [4].
3.4. Contrast-Enhanced Microbubbles
An ultrasound contrast agent (sulphur hexafluoride gas microbubbles) is injected at the periareolar region [14], followed by ultrasound imaging of the breast, with immediate visualisation of the lymphatic channels towards the sentinel node, which is then marked with a clip/wire.
3.5. One-Step Nucleic Acid Amplification (OSNA)/Reverse Transcriptase Gene Therapy (Metasin)
The OSNA test analyses genetic material from solubilised SLNB biopsy samples [15] and detects the presence of the cytokeratin-19 (CK19) gene, a marker associated with breast cancer—and will provide a result within a short time, enabling surgeons to determine whether other lymph nodes should be removed at the same time. The Metasin test uses the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) to detect CK19 and mammaglobin (two markers of metastases).
3.6. Computed Tomography Lymphography (CTLG)
CT-guided lymphography (CTLG) involves the injection of iopamidol (CT dye) into the subareolar region [16], followed by contiguous CT imaging to identify the sentinel node (the node that enhances with the dye). The location of the sentinel node can be mapped on the skin by marking the crossing point of the lines of the laser beam of the CT.
SLNB outcomes for these four methods are displayed below in Table 3.

Table 3.
SLNB outcomes for other new techniques.
As Table 1, Table 2 and Table 3 demonstrate, the SLNB detection rates for ICG fluorescence and magnetic tracers are generally higher than those of the other methods.
Table 4 below illustrates each method’s main advantages and disadvantages, as taken from each paper.

Table 4.
Comparison of the alternative techniques.
4. Discussion
4.1. Discussion of Techniques Evaluated in this Paper
Accurate sentinel lymph node assessment is vital in managing breast cancer. As the Z11 trial [2] has shown, sentinel lymph node biopsy (SLNB) has shown to be non-inferior to axillary node dissection. It offers the added advantage of reduced morbidity and reduced side effects such as axillary lymphedema, numbness and reduced range of motion. The standard method of SLNB has thus far been dual: radioisotope and blue dye injection [18]. However, many countries worldwide do not have nuclear medicine facilities [4], and the need for preoperative radioisotope injections creates scheduling difficulties and the need for safe handling and disposal of these materials. Blue dye has been used for many years as a localisation technique, but it can cause skin staining for months to years and poses a potential allergy risk to patients. There are many up-and-coming alternative techniques for SLNB, and this paper reviewed the most common methods.
Indocyanine green (ICG) fluorescence is one of the most well-known alternative methods for SLNB localisation. As Table 1; Table 4 demonstrate, ICG fluorescence offers a comparable SLNB detection rate to radioisotope/blue dye [8], and it has not shown any allergic reactions in the studied patient samples as compared to blue dye. ICG is a non-toxic substance with a low molecular weight, allowing it to move freely through vessels damaged by neoadjuvant chemotherapy, surgery or radiotherapy-related fibrosis. The evidence suggests SLNB via ICG fluorescence is up to five times less expensive than that of a radioisotope [8], and ICG-guided SLNB does not increase operative time compared to traditional methods. However, ICG has difficulty penetrating deeper tissues (beyond 10–20 mm), giving it the same difficulties as blue dye/radioisotope in patients with a high BMI [7]. ICG leakage is one of its main drawbacks, as it can be challenging to localise damaged nodes if ICG leakage has caused vessel rupture and contamination of the surroundings with ICG. As with many new SLNB methods, ICG fluorescence also increases the number of lymph nodes that are removed. However, there is no clear analysis of whether this leads to increased patient morbidity; thus, more detailed follow-up studies are needed. Furthermore, there are not many widespread data on the cost efficacy of ICG.
Magnetic tracers offer a tangible alternative to SLNB detection methods. The size of the magnetic particles used hugely influences the outcome, with the medium-sized tracer (59 mm) demonstrated to be the most accurate at SLNB localisation [10]. More data have been published on the relative costs of magnetic tracers in SLNB localisation when compared to other new techniques. The mean cost for superparamagnetic iron oxide (SPIO) was EUR 225, compared with EUR 252 for the radioisotope [11]. The preoperative administration of SPIO saved at least 20 min of operating theatre time, saving an additional EUR 352.70 per procedure. The smallest tracer size (32 mm) is disadvantageous as it passes through the sentinel node and up to higher-level nodes, increasing the number of nodes removed, with subsequent possible morbidity [10]. However, magnetic residue was found to last in tissue up to at least 42 months postoperatively, causing artefacts on MRI scans in 40% of cases [17]. Further work has been carried out on SPIO magnetic tracers, including use of an ultra-low dose of 0.1 mL SPIO injections (compared to the usual 1–2 mL dose), achieving a 100% SLN detection rate with reduced skin staining [19]. However, further studies are needed to prove the long-term efficacy of this ultra-low dose, with follow-up imaging to prove a reduction in artefacts on MRI scans.
Other methods of SLNB detection include a photosensitiser (talaporfin), which was able to identify sentinel nodes of all patients [4] but did not show a consistent correlation with fluorescence and pathological node metastases. A more novel SLNB technique includes a contrast-enhanced ultrasound (CEUS), with hexafluoride gas microbubbles. This method caused less trauma to the lymph nodes [14] and was able to localise the SLN accurately, with an overall detection rate of 92.8% in a recent meta-analysis [18]. However, the false negative rate of CEUS is higher than that of radio tracer alone, though lower than that of blue dye [18]. The contrast used for CEUS is not specifically designed for SLNB and the size of the microbubble particle can cause problems with the accuracy of the method. Moreover, there is a minimal number of single-centre studies assessing this technique; thus more data are needed to assess its efficacy and safety.
One-step nucleic acid amplification (OSNA) has also been trialled in SLNB, screening for CK19 expression from breast tumour cells [15]. The test provides results within 30 min, enabling intra-operative decisions about further lymph node removal. OSNA has also been trialled in the treatment of other cancers, and has been shown to detect a higher percentage of micrometastatic lymph nodes in patients with non-small-cell lung cancer (NSCLC) and colorectal carcinoma (CRC) [20]. However, the higher sensitivity of OSNA does not correlate with disease progression when compared to traditional haematoxylin and eosin (H&E) staining. The data have also shown that OSNA is not cost-effective [15] for SLNB compared to histopathology as a standard method of cell analysis, and some tumour cells do not express CK19 MRA and thus will not be detected [15]. Finally, CT lymphography (CTLG) offers a novel way of localising the sentinel node [16], with possible combination with other techniques (e.g., ICG fluorescence) allowing for a non-invasive, highly accurate localisation process when compared to the radioisotope/blue dye method.
4.2. Future Directions for Sentinel Node Biopsy in Breast Cancer
The current standard of care in breast cancer is for patients with positive sentinel lymph nodes to progress to completion of axillary node dissection—a procedure with a high morbidity risk. The mandate for future breast surgery techniques is to provide the most clinically effective care with the least invasive/harmful methods. This is paralleled in other specialties, such as in the management of ovarian cancer, where ongoing research is being conducted into sentinel node staging [21] to enable minimally invasive surgery and staging of nodal status, as an alternative to open laparotomy with total hysterectomy, bilateral salpingo-oophorectomy, lymphadenectomy and omentectomy.
The current practice in SLNB diagnosis involves the biopsy samples being sent for histopathological assessment, which is a time-consuming process with less-than-ideal sensitivity and specificity. New studies have looked at the possible use of artificial intelligence algorithms (AI) in reducing the workload of pathologists, enabling faster and more accurate pathological outcomes. Holten-Rossing et al. [22] conducted a 135-patient digital image analysis study where sentinel nodes were assessed by pathologists conventionally, and simultaneously the stained sections were digitally analysed by an algorithm (which was trained to assess for cytokeratin antibodies and staining intensity). The digital algorithm had an SLNB sensitivity of 100% with no false negative results, and across the three centres it could have decreased the workload by 58.2% on average [22]. The study also concluded that pathologists spend an average of 6.88 min per sentinel node, and if 58.2% of these samples are excluded from conventional microscopy because of digital algorithm pre-screening, this could save up to five working days of time (based on an average of 580 patients annually) for pathologists [22]. However, this small time study only focused on 12 patients and assumed that the time taken to assess positive and negative nodes would be the same. The same idea has also been explored in radiology. Ha et al. [23] evaluated the role of the conventional neural network (CNN) in examining breast MRIs and predicting lymph node metastasis. In this study, with 133 metastatic axillary nodes and 142 negative control nodes, the CNN was able to identify metastatic nodes with an accuracy of 84.3% [23], showing that with further training on larger datasets, these CNN models may eventually be able to remove the need for core needle or sentinel lymph node biopsies.
Using language learning models in conjunction with new SLNB techniques could offer a promising future for breast cancer surgery, driving a decrease in invasive axillary dissection surgeries. In light of the need for less invasive procedures, there is a keen interest in studies that analyse which breast cancer patients with positive sentinel nodes can safely have axillary node dissection omitted. Wu et al. [24] highlight an interesting point about the possible future use of language learning models in breast cancer management and SLNB. They use a predictive language learning model, focusing on four key factors (number of positive SLNs, total number of SLNs harvested, absence of hilar lymph node, and lympho-vascular invasion) to generate a predictive nomogram to identify patients that could safely avoid axillary node dissection [24].
The future of sentinel node biopsy in breast cancer is moving towards a hybrid approach with multimodal techniques [18] and concurrent real-time visualisation of the lymphatic drainage pathways. The current gold standard for SLNB is a combined radioisotope and blue dye method to improve detection rates and reduce false negative rates. Mokhtar et al. [25] analysed the use of a new triple protocol, consisting of preoperative CT lymphography, intraoperative SLNB with ICG fluorescence and intraoperative one-step nucleic acid amplification (OSNA) to detect positive nodes. OSNA when compared to the traditional histopathological analysis showed a concordance of 90% [25], thus allowing the completion of axillary surgery in one combined procedure, rather than needing a second procedure, leading to reduced costs and better patient outcomes [25].
5. Conclusions
Indocyanine green fluorescence (ICG) and magnetic tracers have been proven non-inferior to traditional blue dye and isotope regarding SLNB localisation. Further work is needed to assess the long-term effects of these techniques, particularly the use of magnetic tracers and postoperative MRI scans. More recent techniques include nucleic acid amplification, which has not yet been tested on a large human population. These therapies offer promising SLNB localisation techniques, particularly in conjunction with new language learning models and digital algorithm screening, for the possible omittance of axillary dissection surgery. As with any new methods, there would be a retraining period where surgeons would have to acclimate to the new technique. However, many of these methods are similar to the radioisotope/blue dye technique, using slightly different materials. Further research is needed to assess the longer-term effects of these new techniques in isolation (rather than combined studies using new techniques with blue dye/isotope as controls), and dedicated studies into cost savings are required.
Author Contributions
Conceptualisation, S.S., S.C. and R.V.; methodology, S.S. and S.C.; writing—original draft preparation, S.S.; writing—review and editing, S.S., S.C. and R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Patient consent was waived as nil patients identifiable from this study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Breast Cancer Now: Breast Cancer Facts and Statistics. 2022. Available online: https://breastcancernow.org/about-us/why-we-do-it/breast-cancer-facts-and-statistics/ (accessed on 19 September 2023).
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef]
- Yamada, K.; Ogata, A.; Kaise, H.; Oda, M.; Kimura, F.; Komatsu, S.; Nakamura, Y.; Hosonaga, M.; Matsumura, M.; Kawate, T.; et al. Accuracy and validity of sentinel lymph node biopsy for breast cancer using a photosensitizer: 8-year follow-up. Lasers Surg. Med. 2013, 45, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Vaz, T.; Costa, S.; Peleteiro, B. Fluorescence-Guided: Sentinel Lymph Node Biopsy in Breast Cancer: Detection Rate and Diagnostic Accuracy. Acta Med. Port. 2018, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Maeda, N.; Yoshimura, K.; Oka, M. Intraoperative detection of sentinel lymph nodes in breast cancer patients using ultrasonography-guided direct indocyanine green dye-marking by real-time virtual sonography constructed with three-dimensional computed tomography-lymphography. Breast 2013, 22, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Bargon, C.A.; Huibers, A.; Young-Afat, D.A.; Jansen, B.A.; Borel-Rinkes, I.H.; Lavalaye, J.; van Slooten, H.-J.; Verkooijen, H.M.; van Swol, C.F.; Doeksen, A. Sentinel Lymph Node Mapping in Breast Cancer Patients Through Fluorescent Imaging Using Indocyanine Green. Ann. Surg. 2022, 276, 913–920. [Google Scholar] [CrossRef]
- Thongvitokomarn, S.; Polchai, N. Indocyanine Green Fluorescence Versus Blue Dye or Radioisotope Regarding Detection Rate of Sentinel Lymph Node Biopsy and Nodes Removed in Breast Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2020, 21, 1187–1195. [Google Scholar] [CrossRef]
- Goonawardena, J.; Yong, C.; Law, M. Use of indocyanine green fluorescence compared to radioisotope for sentinel lymph node biopsy in early-stage breast cancer: Systematic review and meta-analysis. Am. J. Surg. 2020, 220, 665–676. [Google Scholar] [CrossRef]
- Pouw, J.J.; Ahmed, M.; Anninga, B.; Schuurman, K.; Pinder, S.; Van Hemelrijck, M.; Pankhurst, Q.; Douek, M.; Haken, B.T. Comparison of three magnetic nanoparticle tracers for sentinel lymph node biopsy in an in vivo porcine model. Int. J. Nanomed. 2015, 10, 1235–1243. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Daskalakis, K.; Stålberg, P.; Olofsson, H.; Andersson, Y.; Eriksson, S.; Bergkvist, L.; Wärnberg, F. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br. J. Surg. 2017, 104, 1675–1685. [Google Scholar] [CrossRef]
- Man, V.; Wong, T.T.; Co, M.; Suen, D.; Kwong, A. Sentinel Lymph Node Biopsy in Early Breast Cancer: Magnetic Tracer as the Only Localizing Agent. Mol. Med. 2019, 43, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Argáez, C. Magnetic Localization System for Sentinel Lymph Node Biopsy: A Review of the Diagnostic Accuracy, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- Gkegkes, I.D.; Lavazzo, C. Contrast Enhanced Ultrasound (CEU) Using Microbubbles for Sentinel Lymph Node Biopsy in Breast Cancer: A Systematic Review. Acta Chir. Belg. 2015, 115, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Huxley, N.; Jones-Hughes, T.; Coelho, H.; Snowsill, T.; Cooper, C.; Meng, Y.; Hyde, C.; Mújica-Mota, R. A systematic review and economic evaluation of intraoperative tests [RD-100i one-step nucleic acid amplification (OSNA) system and Metasin test] for detecting sentinel lymph node metastases in breast cancer. Health Technol. Assess. 2015, 19, 1–215. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Farouk, O.; El-Badrawy, A.; Denewer, A.; Setit, A. Sentinel lymph node biopsy in breast cancer guided by CT lymphography; History, evolution and current applications. Breast Dis. 2021, 40, 219–225. [Google Scholar] [CrossRef]
- Krischer, B.; Forte, S.; Niemann, T.; Kubik-Huch, R.A.; Leo, C. Feasibility of breast MRI after sentinel procedure for breast cancer with superparamagnetic tracers. Eur. J. Surg. Oncol. 2018, 44, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Cykowska, A.; Marano, L.; D’Ignazio, A.; Marrelli, D.; Swierblewski, M.; Jaskiewicz, J.; Roviello, F.; Polom, K. New technologies in breast cancer sentinel lymph node biopsy; from the current gold standard to artificial intelligence. Surg. Oncol. 2020, 34, 324–335. [Google Scholar] [CrossRef]
- Mirzaei, N.; Wärnberg, F.; Zaar, P.; Leonhardt, H.; Bagge, R.O. Ultra-Low Dose of Superparamagnetic Iron Oxide Nanoparticles for Sentinel Lymph Node Detection in Patients with Breast Cancer. Ann. Surg. Oncol. 2023, 30, 5685–5689. [Google Scholar] [CrossRef]
- Vodicka, J.; Pesta, M.; Kulda, V.; Houfkova, K.; Vankova, B.; Sebek, J.; Skala, M.; Fichtl, J.; Prochazkova, K.; Topolcan, O. Prognostic Significance of Lymph Node Examination by the OSNA Method in Lung Cancer Patients—Comparison with the Standard Histopathological Procedure. Cells 2020, 9, 2611. [Google Scholar] [CrossRef]
- Ronsini, C.; Pasanisi, F.; Molitierno, R.; Iavarone, I.; Vastarella, M.G.; De Franciscis, P.; Conte, C. Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3831. [Google Scholar] [CrossRef]
- Holten-Rossing, H.; Talman, M.M.; Jylling, A.M.B.; Lænkholm, A.; Kristensson, M.; Vainer, B. Application of automated image analysis reduces the workload of manual screening of sentinel lymph node biopsies in breast cancer. Histopathology 2017, 71, 866–873. [Google Scholar] [CrossRef]
- Ha, R.; Chang, P.; Karcich, J.; Mutasa, S.; Fardanesh, R.; Wynn, R.T.; Liu, M.Z.; Jambawalikar, S. Axillary Lymph Node Evaluation Utilizing Convolutional Neural Networks Using MRI Dataset. J. Digit. Imaging 2018, 31, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Deng, L.; Jiang, Y.; Zhang, H. Application of the Machine-Learning Model to Improve Prediction of Non- Sentinel Lymph Node Metastasis Status Among Breast Cancer Patients. Front. Surg. 2022, 9, 797377. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Tadokoro, Y.; Nakagawa, M.; Morimoto, M.; Takechi, H.; Kondo, K.; Tangoku, A. Triple assessment of sentinel lymph node metastasis in early breast cancer using preoperative CTLG, intraoperative fluorescence navigation and OSNA. Breast Cancer 2014, 23, 202–210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).