New Alternative Techniques for Sentinel Lymph Node Biopsy
Abstract
:1. Introduction
2. Materials and Methods
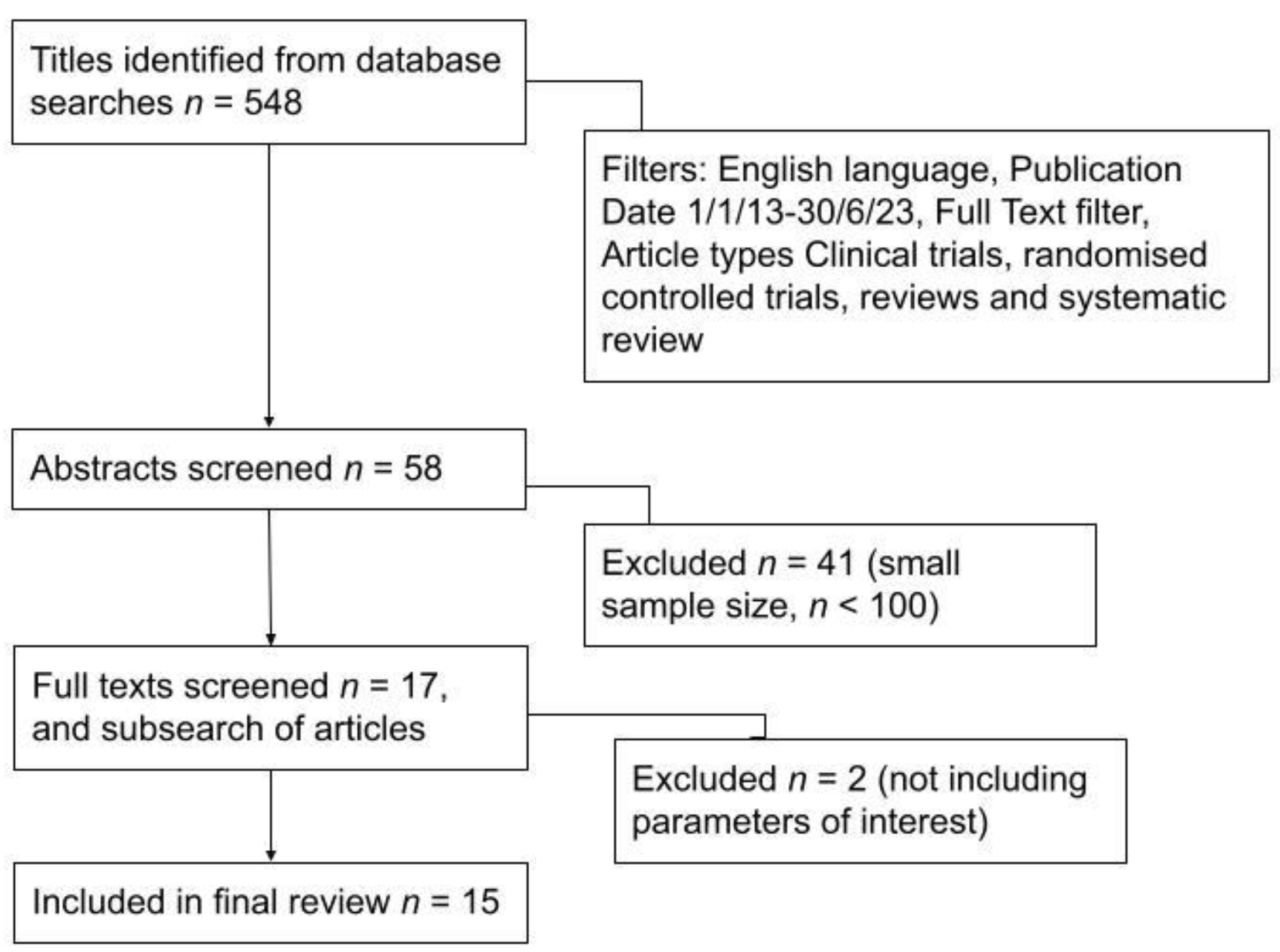
- Date of publication 1 January 2013 to 30 June 2023, narrowing the list to 242 results.
- Language of publication English only, further narrowing the list to 229 results.
- Article types filtered to only include clinical trials, randomised controlled trials, reviews and systematic reviews, narrowing the list to 59 results.
- Finally, a ‘full text’ filter was applied, which yielded a final 58 articles.
3. Results
3.1. Indocyanine Green (ICG) Fluorescence
3.2. Magnetic Tracers, Most Commonly SPIO (Superparamagnetic Iron Oxide)
3.3. Photosensitiser Talaporfin Sodium
3.4. Contrast-Enhanced Microbubbles
3.5. One-Step Nucleic Acid Amplification (OSNA)/Reverse Transcriptase Gene Therapy (Metasin)
3.6. Computed Tomography Lymphography (CTLG)
4. Discussion
4.1. Discussion of Techniques Evaluated in this Paper
4.2. Future Directions for Sentinel Node Biopsy in Breast Cancer
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breast Cancer Now: Breast Cancer Facts and Statistics. 2022. Available online: https://breastcancernow.org/about-us/why-we-do-it/breast-cancer-facts-and-statistics/ (accessed on 19 September 2023).
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef]
- Yamada, K.; Ogata, A.; Kaise, H.; Oda, M.; Kimura, F.; Komatsu, S.; Nakamura, Y.; Hosonaga, M.; Matsumura, M.; Kawate, T.; et al. Accuracy and validity of sentinel lymph node biopsy for breast cancer using a photosensitizer: 8-year follow-up. Lasers Surg. Med. 2013, 45, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Vaz, T.; Costa, S.; Peleteiro, B. Fluorescence-Guided: Sentinel Lymph Node Biopsy in Breast Cancer: Detection Rate and Diagnostic Accuracy. Acta Med. Port. 2018, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Maeda, N.; Yoshimura, K.; Oka, M. Intraoperative detection of sentinel lymph nodes in breast cancer patients using ultrasonography-guided direct indocyanine green dye-marking by real-time virtual sonography constructed with three-dimensional computed tomography-lymphography. Breast 2013, 22, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Bargon, C.A.; Huibers, A.; Young-Afat, D.A.; Jansen, B.A.; Borel-Rinkes, I.H.; Lavalaye, J.; van Slooten, H.-J.; Verkooijen, H.M.; van Swol, C.F.; Doeksen, A. Sentinel Lymph Node Mapping in Breast Cancer Patients Through Fluorescent Imaging Using Indocyanine Green. Ann. Surg. 2022, 276, 913–920. [Google Scholar] [CrossRef]
- Thongvitokomarn, S.; Polchai, N. Indocyanine Green Fluorescence Versus Blue Dye or Radioisotope Regarding Detection Rate of Sentinel Lymph Node Biopsy and Nodes Removed in Breast Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2020, 21, 1187–1195. [Google Scholar] [CrossRef]
- Goonawardena, J.; Yong, C.; Law, M. Use of indocyanine green fluorescence compared to radioisotope for sentinel lymph node biopsy in early-stage breast cancer: Systematic review and meta-analysis. Am. J. Surg. 2020, 220, 665–676. [Google Scholar] [CrossRef]
- Pouw, J.J.; Ahmed, M.; Anninga, B.; Schuurman, K.; Pinder, S.; Van Hemelrijck, M.; Pankhurst, Q.; Douek, M.; Haken, B.T. Comparison of three magnetic nanoparticle tracers for sentinel lymph node biopsy in an in vivo porcine model. Int. J. Nanomed. 2015, 10, 1235–1243. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Daskalakis, K.; Stålberg, P.; Olofsson, H.; Andersson, Y.; Eriksson, S.; Bergkvist, L.; Wärnberg, F. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br. J. Surg. 2017, 104, 1675–1685. [Google Scholar] [CrossRef]
- Man, V.; Wong, T.T.; Co, M.; Suen, D.; Kwong, A. Sentinel Lymph Node Biopsy in Early Breast Cancer: Magnetic Tracer as the Only Localizing Agent. Mol. Med. 2019, 43, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Argáez, C. Magnetic Localization System for Sentinel Lymph Node Biopsy: A Review of the Diagnostic Accuracy, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- Gkegkes, I.D.; Lavazzo, C. Contrast Enhanced Ultrasound (CEU) Using Microbubbles for Sentinel Lymph Node Biopsy in Breast Cancer: A Systematic Review. Acta Chir. Belg. 2015, 115, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Huxley, N.; Jones-Hughes, T.; Coelho, H.; Snowsill, T.; Cooper, C.; Meng, Y.; Hyde, C.; Mújica-Mota, R. A systematic review and economic evaluation of intraoperative tests [RD-100i one-step nucleic acid amplification (OSNA) system and Metasin test] for detecting sentinel lymph node metastases in breast cancer. Health Technol. Assess. 2015, 19, 1–215. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Farouk, O.; El-Badrawy, A.; Denewer, A.; Setit, A. Sentinel lymph node biopsy in breast cancer guided by CT lymphography; History, evolution and current applications. Breast Dis. 2021, 40, 219–225. [Google Scholar] [CrossRef]
- Krischer, B.; Forte, S.; Niemann, T.; Kubik-Huch, R.A.; Leo, C. Feasibility of breast MRI after sentinel procedure for breast cancer with superparamagnetic tracers. Eur. J. Surg. Oncol. 2018, 44, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Cykowska, A.; Marano, L.; D’Ignazio, A.; Marrelli, D.; Swierblewski, M.; Jaskiewicz, J.; Roviello, F.; Polom, K. New technologies in breast cancer sentinel lymph node biopsy; from the current gold standard to artificial intelligence. Surg. Oncol. 2020, 34, 324–335. [Google Scholar] [CrossRef]
- Mirzaei, N.; Wärnberg, F.; Zaar, P.; Leonhardt, H.; Bagge, R.O. Ultra-Low Dose of Superparamagnetic Iron Oxide Nanoparticles for Sentinel Lymph Node Detection in Patients with Breast Cancer. Ann. Surg. Oncol. 2023, 30, 5685–5689. [Google Scholar] [CrossRef]
- Vodicka, J.; Pesta, M.; Kulda, V.; Houfkova, K.; Vankova, B.; Sebek, J.; Skala, M.; Fichtl, J.; Prochazkova, K.; Topolcan, O. Prognostic Significance of Lymph Node Examination by the OSNA Method in Lung Cancer Patients—Comparison with the Standard Histopathological Procedure. Cells 2020, 9, 2611. [Google Scholar] [CrossRef]
- Ronsini, C.; Pasanisi, F.; Molitierno, R.; Iavarone, I.; Vastarella, M.G.; De Franciscis, P.; Conte, C. Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3831. [Google Scholar] [CrossRef]
- Holten-Rossing, H.; Talman, M.M.; Jylling, A.M.B.; Lænkholm, A.; Kristensson, M.; Vainer, B. Application of automated image analysis reduces the workload of manual screening of sentinel lymph node biopsies in breast cancer. Histopathology 2017, 71, 866–873. [Google Scholar] [CrossRef]
- Ha, R.; Chang, P.; Karcich, J.; Mutasa, S.; Fardanesh, R.; Wynn, R.T.; Liu, M.Z.; Jambawalikar, S. Axillary Lymph Node Evaluation Utilizing Convolutional Neural Networks Using MRI Dataset. J. Digit. Imaging 2018, 31, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Deng, L.; Jiang, Y.; Zhang, H. Application of the Machine-Learning Model to Improve Prediction of Non- Sentinel Lymph Node Metastasis Status Among Breast Cancer Patients. Front. Surg. 2022, 9, 797377. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Tadokoro, Y.; Nakagawa, M.; Morimoto, M.; Takechi, H.; Kondo, K.; Tangoku, A. Triple assessment of sentinel lymph node metastasis in early breast cancer using preoperative CTLG, intraoperative fluorescence navigation and OSNA. Breast Cancer 2014, 23, 202–210. [Google Scholar] [CrossRef] [PubMed]
| Paper Author, Year. | SLNB Detection Rate with ICG Dye (Compared to Alternative) |
|---|---|
| Yamamoto S, 2013 [6] | 99.6% (90.3% blue dye) |
| Vaz T, 2018 [5] | 91.4% (89.5% blue dye, 97.2% radioisotope) |
| Bargon CA, 2022 [7] | 96.1% (86.4% radioisotope) |
| Thongvitokomarn S, 2020 [8] | 69–100% (85–100% radioisotope) |
| Goonawardena J, 2020 [9] | 81.9–199% (85–199% radioisotope) |
| Paper Author, Year | SLNB Detection Rate with Magnetic Tracer (Compared to Alternative) |
|---|---|
| Pouw J, 2015 [10] | 100% retrieval rate Ferumoxytol/Sienna. 91.67% ferumoxide (alternative not reported) |
| Karakatsanis A, 2017 [11] | 95.6% SPIO (96.6% radioisotope) |
| Man V, 2019 [12] | 98.8% SPIO (alternative not reported) |
| Thompson W, 2020 [13] | 99.3% SPIO (98.6% radioisotope) |
| Paper Author, Year | SLNB Detection Rate with New Techniques (Compared to Alternative) |
|---|---|
| Yamada K, 2013 [4] | 81% talaporfin sodium (86% radioisotope) |
| Gkegkes ID, 2015 [14] | 9.3–55.2% contrast-enhanced ultrasound (CEU) sulphur hexafluoride gas microbubbles (alternative not reported) |
| Huxley N, 2015 [15] | 82.7–100% for one-step nucleic acid amplification (alternative not reported) |
| Hamdy O, 2021 [16] | 90.5% CTLG (alternative not reported) |
| Technique | Type of Tracer | Publication | Sample Size | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Indocyanine green fluorescence (ICG) [6] | Indocyanine green (ICG), blue dye as control | Japan, 2013 | 258 women | -ICG lasts 3 h post-injection, and thus can be injected outside the operating room -RVS detects metastatic nodes preoperatively so they can proceed directly to axillary dissection | -Injured lymph vessels cause ICG field contamination -Operating light needs to be turned off during fluorescence observation |
| Indocyanine green fluorescence (ICG) [5] | Indocyanine green (ICG) | Portugal, 2018 | 232 patients | -ICG is low-molecular-weight, non-toxic and non-radioactive -ICG’s low molecular weight and albumin binding aids SLN detection in neoadjuvant patients, as ICG can move through vessels obliterated by tumour cells/inflammation/previous surgery/RT-related fibrosis -Low cost of ICG | -ICG low tissue penetration (10–20 mm depth) -Need for a photodynamic chamber with ICG -Increased number of fluorescent lymph nodes removed and subsequent morbidity |
| Indocyanine green fluorescence (ICG) [7] | Indocyanine green (ICG) | Amsterdam, 2022 | 102 patients | -The ICG method did not increase detection/operative time -ICG is non-ionising and does not need special storage, handling procedures or legal permits -ICG fluorescence is five times less expensive than nanocolloid, and the fluorescent camera can be used for other indications | -ICG use for axillary SLNB has not yet been approved by the US FDA and the European Medicines Evaluation Agency (EMEA) -Difficult SLNB detection in high-BMI patients |
| Indocyanine green fluorescence (ICG) [8] | Indocyanine green (ICG) | Thailand, 2020 | 4216 SLNB procedures | -ICG has a short half-life and is absorbed into lymphatic vessels immediately -ICG is potentially available for use in pregnant women, with some human and animal studies | -ICG not visualised directly, and difficulty identifying SLNs from the screen -Insufficient data regarding long-term follow-up for ICG-SLNB |
| Indocyanine green fluorescence (ICG) [9] | Indocyanine green (ICG) | Australia, 2020 | 2301 patients | -Dual mapping with ICG + RI decreased false negative rate < 8.7% (compared to RI + BD) -ICG method costs USD 5–111 per patient (RI costs USD 331–420 per pt) | -Optimal ICG dose/concentration not yet standardised -ICG cannot be given to patients with iodine allergy |
| Magnetic tracer [10] | 1. Feraheme® (ferumoxytol) 2. Sienna+® (Fe/m) 3. Endorem® (ferumoxide) | London, 2015 | 18 mini-pigs | -59 mm tracer (Sienna®) is the best-performing tracer approved for human use in SLNB -Node localised within 30 min -Feraheme® and Sienna® tracer 100% retrieval rate (12/12 magnetic hotspots), ferumoxide retrieval rate 91.67% (11/12 magnetic hotspots) | -Skin discolouration at the injection site -Significantly higher number of nodes excised with 32 nm tracer as the small particle passes onto higher-level nodes |
| Magnetic tracer [11] | Superparamagnetic iron oxide (SPIO) nanoparticles | Sweden, 2017 | 338 patients | -SPIO costs EUR 225 and radioisotope costs EUR 252, with SPIO preoperative administration saving 20 min operating room time, further saving EUR 352.70 -SPIO resides in tissue for a prolonged period, so operation rescheduling does not require another injection, providing flexibility -SPIO simple administration only needs a portable probe and a room-temperature tracer | -Patients received injections of SPIO and radioisotope, and there was synergy bias -Skin staining in 39.9% of patients, with 41.2% still stained after 12 months -SPIO causes artefact on MRI, even in postop scans in the future |
| Magnetic tracer [12] | Superparamagnetic iron oxide (SPIO) | Hong Kong, 2019 | 328 females | -Outpatient clinic SPIO offers better preoperative logistics -No reported adverse reactions -SPIO alone in SLNB localisation saves an estimated USD 22,300 per year compared to conventional dual tracers | -Plastic operating instruments are required to avoid interference with the magnetometer -Persistent brown skin discolouration, and MRI scan artefact for up to 1.5 years |
| Magnetic tracer [13] | Superparamagnetic iron oxide (SPIO) | Canada, 2020 | 1834 patients | -Detection rate for SPIO was non-inferior to RI method -Magnetic localisation systems found to be generally safe (with minor skin staining) -Magnetic localisation was USD 27 cheaper than radioisotope method (USD 225 for magnetic localisation, USD 252 for RI) | -No guidelines available on use of magnetic localisation in sentinel node biopsy -No data on false positive rates (which would be helpful for avoiding unnecessary procedures and influencing policy decisions) |
| Photosensitiser talaporfin sodium [4] | Photosensitiser talaporfin sodium | Japan, 2013 | 21 women | -Talaporfin is metabolised in the liver, so is safe for renal failure patients -No adverse effects of talaporfin -Talaporfin identified SLNs and also the pathways to secondary SLNs | -No consistent correlation between fluorescence and pathological SLN metastasis -The amount of radioisotope and depth of injection may affect SLN identification |
| Contrast-enhanced ultrasound (CEU) using sulphur hexafluoride gas microbubbles [14] | Sulphur hexafluoride gas microbubbles | Greece, 2015 | 727 patients | -CEU method causes less trauma and architectural disruption to the lymph nodes’ precise sampling -The CEU method duration is roughly 25 min and does not cause discomfort to the patient—no published complications of this method so far | -Limited number of studies and small patient sample size—majority of existing evidence is from one centre |
| One-step nucleic acid amplification (OSNA)/ reverse transcriptase gene therapy (Metasin) [15] | UK, 2015 | 4604 patients 18 studies | -OSNA provides results within 30–45 min and can be used during breast surgery to determine if other lymph nodes should be removed at the same time -OSNA does not require mRNA extraction/purification from the tissue -Metasin test takes roughly 30 min in total for results | -OSNA QALY loss of 0.048 relative to histopathology, not cost-effective and less accurate -1% of breast tumours do not express CK19 MRA -Entire node analysis required as tumour cells may not be evenly distributed through the node | |
| CT lymphography [16] | Iopamidol | Egypt, 2021 | 835 patients | -CTLG 92.6% sensitivity, 88.6% specificity—potentially possible to omit SLN biopsy -CTLG is unaffected by lymph node fibrosis after neoadjuvant (90.5% detection rate post neoadjuvant) | -Further studies are needed to evaluate the use of CTLG in isolation compared to radioisotopes alone (rather than combination studies which introduce bias) |
| Review paper [17] | Switzerland, 2018 | 24 patients | -In 48% of the imaging cases, there was no restriction of MRI post magnetic tracer (average time since tracer injection was 42 months) | -40% impaired imaging, and 12% MRI impossible due to Sienna tracer residue -Sienna tracer causes skin discolouration in 19–40% of patients and persists in 8.6% after 15 months | |
| Review paper [18] | Italy, 2020 | Not reported | -Multimodal and hybrid techniques (preoperative CTLG + intraoperative SLNB with fluorescence navigation + OSNA) are an attractive option for institutions without nuclear medicine facilities and may help omit need for 2nd procedure in positive SLN patients | -Legislation problems with all new/hybrid techniques -Needs evaluation in larger, multi-centre studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramonian, S.; Chopra, S.; Vidya, R. New Alternative Techniques for Sentinel Lymph Node Biopsy. Medicina 2023, 59, 2077. https://doi.org/10.3390/medicina59122077
Subramonian S, Chopra S, Vidya R. New Alternative Techniques for Sentinel Lymph Node Biopsy. Medicina. 2023; 59(12):2077. https://doi.org/10.3390/medicina59122077
Chicago/Turabian StyleSubramonian, Subiksha, Sharat Chopra, and Raghavan Vidya. 2023. "New Alternative Techniques for Sentinel Lymph Node Biopsy" Medicina 59, no. 12: 2077. https://doi.org/10.3390/medicina59122077
APA StyleSubramonian, S., Chopra, S., & Vidya, R. (2023). New Alternative Techniques for Sentinel Lymph Node Biopsy. Medicina, 59(12), 2077. https://doi.org/10.3390/medicina59122077






