Interleukin Signatures as Prognostic Biomarkers in Ulcerative Colitis: From Immune Pathways to Clinical Prediction
Abstract
1. Introduction
2. Materials and Methods
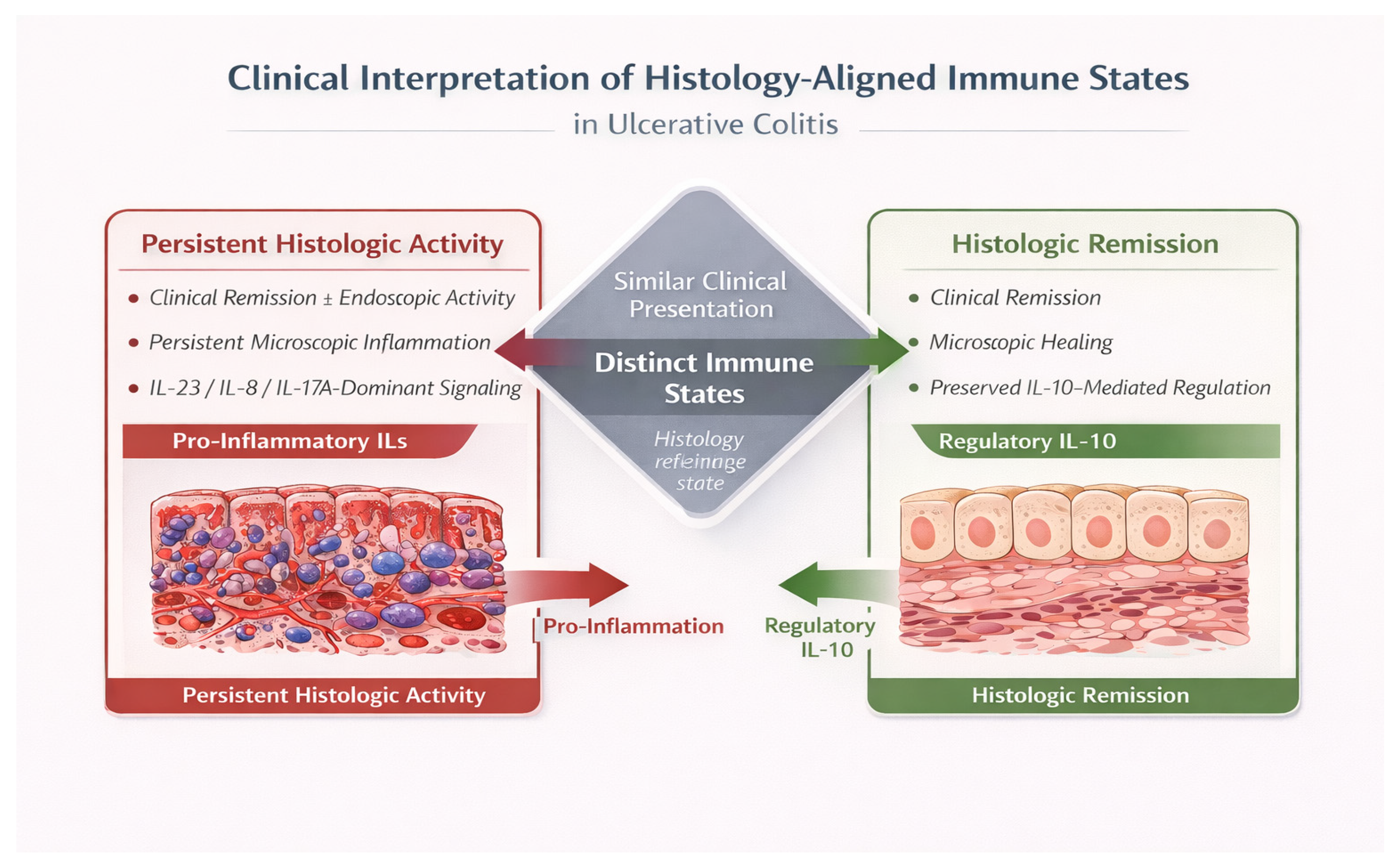
3. Rationale and Contemporary Relevance of Histology-Aligned Immune Frameworks
4. Immunopathogenesis of Ulcerative Colitis: IL Networks and Histologic Inflammation
4.1. IL-23 and Histologic Inflammation in Ulcerative Colitis
4.2. IL-10 and Failure of Immune Regulation in Ulcerative Colitis
4.3. IL-8 and IL-17A in Neutrophil Recruitment and Persistent Microscopic Inflammation
4.4. Integrated IL Patterns Associated with Histologic Activity in Ulcerative Colitis
4.5. Histologic Activity as a Stable Immune State Rather than a Transient Disease Phase
4.6. Histologic Remission as an Actively Maintained Regulatory Immune State
4.7. Clinical Implications of Histology-Aligned IL Patterns in Ulcerative Colitis
5. Limitations and Future Perspectives
5.1. Methodological Limitations
5.2. Conceptual Limitations
5.3. Translational Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kucharzik, T.; Stoll, R.; Lugering, N.; Domschke, W. Circulating anti-inflammatory cytokine IL-10 in patients with inflammatory bowel disease. Clin. Exp. Immunol. 1995, 100, 452–456. [Google Scholar] [CrossRef]
- Villanacci, V.; Antonelli, E.; Geboes, K.; Casella, G.; Bassotti, G. Histological healing in inflammatory bowel disease: A still unfulfilled promise. World J. Gastroenterol. 2013, 19, 968–978. [Google Scholar] [CrossRef]
- Schreiber, S.; Heinig, T.; Thiele, H.G.; Raedler, A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology 1995, 108, 1434–1444. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Marciniak, R.; Chudzicka-Strugała, I.; Wasilewska, A.; Drews, M.; Majewski, P.; Karpiński, T.; Zwoździak, B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch. Immunol. Ther. Exp. 2009, 57, 291–294. [Google Scholar] [CrossRef]
- Li, M.C.; He, S.H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Kvedaraite, E.; Lourda, M.; Ideström, M.; Chen, P.; Olsson-Åkefeldt, S.; Forkel, M.; Gavhed, D.; Lindforss, U.; Mjösberg, J.; Henter, J.-I.; et al. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut 2016, 65, 1632–1641. [Google Scholar] [CrossRef]
- Cayatte, C.; Joyce-Shaikh, B.; Vega, F.; Boniface, K.; Grein, J.; Murphy, E.; Blumenschein, W.M.; Chen, S.; Malinao, M.C.; Basham, B.; et al. Biomarkers of therapeutic response in the IL-23 pathway in inflammatory bowel disease. Clin. Transl. Gastroenterol. 2012, 3, e10. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, A.L.; Ilieș, M.; Vesa, Ș.C.; Seicean, R.; Din, S.; Iuga, C.A.; Seicean, A. Serum interleukin (IL)-23 and IL-17 profile in inflammatory bowel disease could differentiate between severe and non-severe disease. J. Pers. Med. 2021, 11, 1130. [Google Scholar] [CrossRef]
- Sewell, G.W.; Kaser, A. Interleukin-23 in the pathogenesis of inflammatory bowel disease and implications for therapeutic intervention. J. Crohns Colitis 2022, 16, ii3–ii19. [Google Scholar] [CrossRef] [PubMed]
- Mirsattari, D.; Seyyedmajidi, M.; Zojaji, H.; Haghazali, M.; Gooran Orimi, P.; Shoushtarizadeh, T.; Almasi, S. The relation between the level of interleukin-23 with duration and severity of ulcerative colitis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 49–53. [Google Scholar]
- Gheita, T.A.; El Gazzar, I.I.; El-Fishawy, H.S.; Aboul-Ezz, M.A.; Kenawy, S.A. Involvement of IL-23 in enteropathic arthritis with inflammatory bowel disease. Clin. Rheumatol. 2014, 33, 713–717. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Lappalainen, M.; Halme, L.; Turunen, U.; Saavalainen, P.; Einarsdottir, E.; Färkkilä, M.; Kontula, K.; Paavola-Sakki, P. Association of IL23R, TNFRSF1A, and HLA-DRB1*0103 allele variants with inflammatory bowel disease phenotypes in the Finnish population. Inflamm. Bowel Dis. 2008, 14, 1118–1124. [Google Scholar] [CrossRef]
- Einarsdottir, E.; Koskinen, L.L.E.; Dukes, E.; Kainu, K.; Suomela, S.; Lappalainen, M.; Ziberna, F.; Korponay-Szabo, I.R.; Kurppa, K.; Kaukinen, K.; et al. IL23R in the Swedish, Finnish, Hungarian and Italian populations: Association with IBD and psoriasis, and linkage to celiac disease. BMC Med. Genet. 2009, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.; Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 2007, 56, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23 as a drug target in autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef]
- Korta, A.; Kula, J.; Gomułka, K. The role of IL-23 in the pathogenesis and therapy of inflammatory bowel disease. Int. J. Mol. Sci. 2023, 24, 10172. [Google Scholar] [CrossRef]
- Noviello, D.; Mager, R.; Roda, G.; Borroni, R.G.; Fiorino, G.; Vetrano, S. IL23–IL17 axis in ulcerative colitis treatment: Successes and challenges. Front. Immunol. 2021, 12, 611256. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Balschun, T.; Karlsen, T.H.; Hedderich, J.; May, S.; Lu, T.; Schuldt, D.; Nikolaus, S.; Rosenstiel, P.; Krawczak, M.; et al. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat. Genet. 2008, 40, 713–715. [Google Scholar] [CrossRef]
- Andoh, A.; Yagi, Y.; Shioya, M.; Nishida, A.; Tsujikawa, T.; Fujiyama, Y. Mucosal cytokine network in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5154–5161. [Google Scholar] [CrossRef]
- Bamias, G.; Cominelli, F. Immunopathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 2007, 23, 365–369. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Bealgey, K.W.; Fang, Y.; Gong, Y.M.; Bao, S. Interleukin-23: Roles and implications. Int. J. Biochem. Cell Biol. 2009, 41, 733–735. [Google Scholar] [CrossRef]
- Ahern, P.P.; Izcue, A.; Maloy, K.J.; Powrie, F. The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 2008, 226, 147–159. [Google Scholar]
- Uhlig, H.H.; McKenzie, B.S.; Hue, S.; Thompson, C.; Joyce-Shaikh, B.; Stepankova, R.; Robinson, N.; Buonocore, S.; Tlaskalova-Hogenova, H.; Cua, D.J.; et al. Differential activity of IL-12 and IL-23 in mucosal inflammation. Immunity 2006, 25, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Okamoto, S.; Hisamatsu, T.; Kamada, N.; Chinen, H.; Saito, R.; Kitazume, M.T.; Nakazawa, A.; Sugita, A.; Koganei, K.; et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 2008, 57, 1682–1689. [Google Scholar] [CrossRef]
- Baskol, M.; Baskol, G.; Koçer, D.; Ozbakir, O.; Yucesoy, M. Advanced oxidation protein products: A novel marker of oxidative stress in ulcerative colitis. J. Clin. Gastroenterol. 2008, 42, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.D.; Wan, X.Q.; Liu, L.Y. Serum IL-23 and IL-17 in ulcerative colitis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011, 27, 203–206. [Google Scholar]
- De Nitto, D.; Sarra, M.; Cupi, M.L.; Pallone, F.; Monteleone, G. Targeting IL-23 and Th17-cytokines in inflammatory bowel diseases. Curr. Pharm. Des. 2010, 16, 3656–3660. [Google Scholar] [CrossRef]
- El Mezayen, R.; El Gazzar, M.; Myer, R.; High, K.P. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell 2009, 8, 553–565. [Google Scholar] [CrossRef]
- Kader, H.A.; Tchernev, V.T.; Satyaraj, E.; Lejnine, S.; Kotler, G.; Kingsmore, S.F.; Patel, D.D. Protein microarray analysis of disease activity in pediatric inflammatory bowel disease demonstrates elevated serum PLGF, IL-7, TGF-β1, and IL-12p40 levels in Crohn’s disease and ulcerative colitis patients in remission versus active disease. Am. J. Gastroenterol. 2005, 100, 414–423. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hayatbakhsh, M.M.; Zahedi, M.J.; Jalalpour, M.R.; Pakgohar, A. Serum interleukin-23 levels in patients with ulcerative colitis. Iran J. Immunol. 2011, 8, 183–188. [Google Scholar]
- Chakraborty, S. IL23 as a novel serum based biomarker in ulcerative colitis—An editorial. Gastroenterol. Hepatol. Bed Bench 2012, 5, 1–2. [Google Scholar]
- Kikly, K.; Liu, L.; Na, S.; Sedgwick, J.D. IL-23/Th17 axis as therapeutic target. Curr. Opin. Immunol. 2006, 18, 670–675. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Abraham, C.; Dulai, P.S.; Vermeire, S.; Sandborn, W.J. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 374–388.e4. [Google Scholar] [CrossRef]
- Soubières, A.A.; Poullis, A. Emerging biomarkers for the diagnosis and monitoring of inflammatory bowel diseases. Inflamm. Bowel Dis. 2016, 22, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Ishihara, S.; Yuki, T.; Fukuba, N.; Oshima, N.; Kazumori, H.; Sonoyama, H.; Yamashita, N.; Tada, Y.; Kusunoki, R.; et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016, 16, 47. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; McGovern, D.P.B. Using markers in IBD to predict disease and treatment outcomes: Rationale and a review of current status. Am. J. Gastroenterol. Suppl. 2016, 3, 17–26. [Google Scholar] [CrossRef]
- Kumar, S.; Ghoshal, U.C.; Aggarwal, R.; Saraswat, V.A.; Choudhuri, G. Severe ulcerative colitis: Prospective study of parameters determining outcome. J. Gastroenterol. Hepatol. 2004, 19, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Youssef, T.; Saleh, S.A.; Rund, A.; Montasser, I.; Mohsen, M.; Hazem, O. Evaluation of interleukin 23 (IL-23) as a non-invasive test of disease severity in patients with ulcerative colitis. Arab. J. Gastroenterol. 2018, 19, 116–120. [Google Scholar] [CrossRef]
- Fisher, S.A.; Tremelling, M.; Anderson, C.A.; Gwilliam, R.; Bumpstead, S.; Prescott, N.J.; Nimmo, E.R.; Massey, D.; Berzuini, C.; Johnson, C.; et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat. Genet. 2008, 40, 710–712. [Google Scholar] [CrossRef]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent IL-23 and IL-12 roles in autoimmunity. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Zidar, N.; Boštjančič, E.; Jerala, M.; Kojc, N.; Drobne, D.; Štabuc, B.; Glavač, D. Down-regulation of microRNAs of the miR-200 family and up-regulation of Snail and Slug in inflammatory bowel diseases—Hallmark of epithelial–mesenchymal transition. J. Cell. Mol. Med. 2016, 20, 1813–1824. [Google Scholar] [CrossRef]
- Uchiyama, K.; Takagi, T.; Murakami, E.; Naito, Y. Mucosal healing of ulcerative colitis based on endoscopic diagnosis, histopathology, and mucosal inflammatory mediators. Inflamm. Intest. Dis. 2025, 10, 233–245. [Google Scholar] [CrossRef]
- Rismo, R.; Olsen, T.; Cui, G.; Paulssen, E.J.; Christiansen, I.; Johnsen, K.; Florholmen, J.; Goll, R. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn’s disease. Scand. J. Gastroenterol. 2013, 48, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Pallone, F.; Monteleone, G. Interleukin-23 and Th17 cells in the control of gut inflammation. Mediat. Inflamm. 2009, 2009, 297645. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.; Hamedi, K.; Loftus, C.; Loftus, G.; Zhou, E.-R.; Arce, S. From cytokines to biomarkers: Mapping the immunopathology of inflammatory bowel disease. Cells 2025, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Systemic and local cytokine production in quiescent ulcerative colitis and its relationship to future relapse: A prospective pilot study. Inflamm. Bowel Dis. 2005, 11, 589–596. [Google Scholar] [CrossRef]
- Schreiber, S.; Fedorak, R.N.; Nielsen, O.H.; Wild, G.; Williams, C.N.; Nikolaus, S.; Jacyna, M.; Lashner, B.A.; Gangl, A.; Rutgeerts, P.; et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease: Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology 2000, 119, 1461–1472. [Google Scholar] [CrossRef]
- Nishida, Y.; Hosomi, S.; Yamagami, H.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; Kamata, N.; Tanigawa, T.; et al. Neutrophil-to-lymphocyte ratio for predicting loss of response to infliximab in ulcerative colitis. PLoS ONE 2017, 12, e0169845. [Google Scholar] [CrossRef]
- Yao, J.; Wei, C.; Wang, J.Y.; Zhang, R.; Li, Y.X.; Wang, L.S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World. J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Winer, S.; Travis, S.P.L.; Riddell, R.H. Systematic review: Histological remission in ulcerative colitis. Aliment. Pharmacol. Ther. 2014, 40, 807–821. [Google Scholar]
- de Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef]
- Biancheri, P.; Giuffrida, P.; Docena, G.H.; MacDonald, T.T.; Corazza, G.R.; Di Sabatino, A. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014, 25, 45–55. [Google Scholar] [CrossRef]
- Allocca, M.; Furfaro, F.; Fiorino, G.; Gilardi, D.; D’Alessio, S.; Danese, S. Can IL-23 be a good target for ulcerative colitis? Best Pract. Res. Clin. Gastroenterol. 2018, 32, 95–102. [Google Scholar] [CrossRef]
- Kurumi, H.; Yokoyama, Y.; Hirano, T.; Akita, K.; Hayashi, Y.; Kazama, T.; Isomoto, H.; Nakase, H. Cytokine profile in predicting the effectiveness of advanced therapy for ulcerative colitis: A narrative review. Biomedicines 2024, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.C.; Elsbury, S.K.; Pavli, P.; Doe, W.F. Interleukin 8: Cells of origin in inflammatory bowel disease. Gut 1996, 38, 90–98. [Google Scholar] [CrossRef]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef]
- Bertani, L.; Caviglia, G.P.; Antonioli, L.; Pellicano, R.; Fagoonee, S.; Astegiano, M.; Saracco, G.M.; Bugianesi, E.; Blandizzi, C.; Costa, F.; et al. Serum interleukin-6 and -8 as predictors of response to vedolizumab in inflammatory bowel diseases. J. Clin. Med. 2020, 9, 1323. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Tomiyasu, N.; Takaki, K.; Masuda, J.; Yamasaki, H.; Kuwaki, K.; Takeda, T.; Kitazaki, S.; Tsuruta, O.; Sata, M. Interleukin-10 in the pathophysiology of inflammatory bowel disease: Increased serum concentrations during the recovery phase. Mediat. Inflamm. 2006, 2006, 26875. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.R.; Gonçalves, P.; Magro, F.; Martel, F. Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease. Pharmacol. Res. 2020, 159, 104947. [Google Scholar] [CrossRef] [PubMed]
- Arnott, I.D.; Drummond, H.E.; Ghosh, S. Gut mucosal secretion of interleukin 1β and interleukin-8 predicts relapse in clinically inactive Crohn's disease. Dig. Dis. Sci. 2001, 46, 402–409. [Google Scholar] [CrossRef]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef]
- Uchiyama, K.; Takagi, T.; Mizushima, K.; Asaeda, K.; Kajiwara-Kubota, M.; Kashiwagi, S.; Toyokawa, Y.; Tanaka, M.; Hotta, Y.; Naito, Y.; et al. Mucosal interleukin-8 expression as a predictor of subsequent relapse in ulcerative colitis patients with Mayo endoscopic subscore 0. J. Gastroenterol. Hepatol. 2022, 37, 1034–1042. [Google Scholar] [CrossRef]
- Rosenberg, L.; Nanda, K.S.; Zenlea, T.; Gifford, A.; Lawlor, G.O.; Falchuk, K.R.; Wolf, J.L.; Cheifetz, A.S.; Goldsmith, J.D.; Moss, A.C. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission: Correlates of histological inflammation. Clin. Gastroenterol. Hepatol. 2013, 11, 991–996. [Google Scholar] [CrossRef]
- Okada, T.; Kanda, T.; Ueda, N.; Ikebuchi, Y.; Hashiguchi, K.; Nakao, K.; Isomoto, H. IL-8 and LYPD8 expression levels are associated with the inflammatory response in the colon of patients with ulcerative colitis. BMC Gastroenterol. 2020, 20, 85. [Google Scholar] [CrossRef]
- Louis, E.; Belaiche, J.; van Kemseke, C.; Franchimont, D.; de Groote, D.; Gueenen, V.; Mary, J.Y. A high serum concentration of interleukin-6 is predictive of relapse in quiescent Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 1997, 9, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Cătană, C.S.; Berindan Neagoe, I.; Cozma, V.; Magdaş, C.; Tăbăran, F.; Dumitrașcu, D.L. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 5823–5830. [Google Scholar] [CrossRef]
- Hue, S.; Ahern, P.; Buonocore, S.; Kullberg, M.C.; Cua, D.J.; McKenzie, B.S.; Powrie, F.; Maloy, K.J. IL-23 drives innate and T cell–mediated intestinal inflammation. J. Exp. Med. 2006, 203, 2473–2483. [Google Scholar] [CrossRef]
- Elson, C.O.; Cong, Y.; Weaver, C.T.; Schoeb, T.R.; McClanahan, T.K.; Fick, R.B.; Kastelein, R.A. Monoclonal anti–IL-23 reverses active colitis. J. Clin. Investig. 2007, 117, 995–1006. [Google Scholar]
- Geremia, A.; Arancibia-Cárcamo, C.V.; Fleming, M.P.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.L.; Powrie, F. IL-23–responsive innate lymphoid cells drive intestinal pathology. Nature 2011, 477, 340–344. [Google Scholar]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; Al Adham, Z.; Lavoie, S.; Ibourk, M.; et al. IL-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef]
- Begue, B.; Verdier, J.; Rieux-Laucat, F.; Goulet, O.; Morali, A.; Canioni, D.; Hugot, J.P.; Daussy, C.; Verkarre, V.; Pigneur, B.; et al. Defective IL-10 signaling defines a subset of inflammatory bowel disease. Gastroenterology 2011, 106, 1544–1555. [Google Scholar]
- Glocker, E.O.; Kotlarz, D.; Boztug, K.; Gertz, E.M.; Schäffer, A.A.; Noyan, F.; Perro, M.; Diestelhorst, J.; Allroth, A.; Murugan, D.; et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 2009, 361, 2033–2045. [Google Scholar] [CrossRef]
- Atreya, R.; Mudter, J.; Finotto, S.; Müllberg, J.; Jostock, T.; Wirtz, S.; Schütz, M.; Bartsch, B.; Holtmann, M.; Becker, C.; et al. Blockade of IL-6 trans-signaling suppresses colitis. Nat. Med. 2000, 6, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Mudter, J.; Neurath, M.F. IL-6 signaling in inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, M.; Simon, P.L.; Karmeli, F.; Rachmilewitz, D. Role of interleukin-1 in inflammatory bowel disease. Gastroenterology 1990, 98, 686–692. [Google Scholar]
- Daig, R.; Andus, T.; Aschenbrenner, E.; Falk, W.; Schölmerich, J.; Gross, V. Increased interleukin-8 expression in ulcerative colitis. Gut 1996, 38, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, L.; Hauser, C.; Zgraggen, K.; Wagner, H.; Hess, M.; Laissue, J.A.; Mueller, C. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am. J. Pathol. 1994, 144, 997–1007. [Google Scholar] [PubMed]
- Xue, M.; Shi, L.; Wang, W.; Chen, S.; Wang, L. An overview of molecular profiles in ulcerative colitis-related cancer. Inflamm. Bowel Dis. 2018, 24, 1883–1894. [Google Scholar] [CrossRef]
- Mosli, M.H.; Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Behling, C.; Kaplan, K.; Driman, D.K.; Shackelton, L.M.; Baker, K.A.; Macdonald, J.K.; et al. Histologic evaluation of ulcerative colitis: A systematic review of disease activity indices. Inflamm. Bowel Dis. 2014, 20, 564–575. [Google Scholar] [CrossRef]
- Marchal-Bressenot, A.; Salleron, J.; Boulagnon-Rombi, C.; Bastien, C.; Cahn, V.; Cadiot, G.; Diebold, M.-D.; Danese, S.; Reinisch, W.; Schreiber, S.; et al. Development and validation of the Nancy histological index. Gut 2017, 66, 43–49. [Google Scholar] [CrossRef]

| Study | Design | Biomarker Source | Histology Endpoint | Main Finding | Histology-Aligned Interpretation |
|---|---|---|---|---|---|
| [8] | Cross-sectional | Serum | Histologic severity | Serum IL-23 levels were higher in patients with increased histologic severity. | Elevated IL-23 reflects dominance of a pro-inflammatory immune state associated with sustained microscopic activity and neutrophil-driven tissue injury. |
| [11] | Case–control | Serum | Histologic activity | Serum IL-23 concentrations were increased in histologically active UC compared with inactive disease. | Increased IL-23 indicates persistent upstream inflammatory signaling aligned with an active histologic immune configuration rather than transient inflammation. |
| [10] | Cross-sectional | Serum | Histologic activity | IL-23 levels increased with greater histologic severity and longer disease duration. | Progressive elevation of IL-23 supports its role in stabilizing chronic inflammatory immune states underlying persistent histologic activity. |
| [41] | Prospective | Serum | Histologic severity | Elevated baseline serum IL-23 levels were associated with subsequent severe histologic disease. | Baseline IL-23 elevation identifies a pre-existing inflammatory immune state predisposing to persistent or progressive microscopic inflammation. |
| Study | Design | Biomarker Source | Histologic Endpoint | Main Finding | Histology-Aligned Interpretation |
|---|---|---|---|---|---|
| [1] | Cross-sectional | Serum | Histologic inactivity | Serum IL-10 levels were higher in histologically inactive disease compared with active inflammation. | Increased IL-10 reflects dominance of a regulatory immune state associated with suppression of microscopic inflammation and histologic quiescence. |
| [3] | Prospective | Serum | Histologic improvement | Higher baseline serum IL-10 levels were associated with subsequent histologic improvement. | Preserved IL-10 signaling indicates effective regulatory immune capacity favoring transition toward histologic remission. |
| [62] | Cohort | Serum | Histologic remission | IL-10 concentrations increased during histologic remission compared with active disease | Elevation of IL-10 characterizes restoration of regulatory immune balance accompanying microscopic healing. |
| [4] | Case–control | Serum | Microscopic inflammation | Reduced serum IL-10 levels were observed in patients with histologically active UC. | Reduced IL-10 reflects insufficient regulatory control permitting persistence of inflammatory immune states at the tissue level. |
| [55] | Prospective | Mucosal | Sustained histologic remission | Higher mucosal IL-10 expression was associated with maintenance of histologic remission. | Sustained mucosal IL-10 expression supports long-term stabilization of a regulatory immune state underlying durable microscopic healing. |
| Study | Design | Biomarker | Biomarker Source | Histologic Endpoint | Main Finding | Main Finding |
|---|---|---|---|---|---|---|
| [50] | Prospective | IL-8 | Mucosal | Histologic activity | Mucosal IL-8 expression was increased in histologically active UC compared with histologic remission. | Increased IL-8 reflects a neutrophil-dominant inflammatory immune state driving crypt abscess formation and persistent microscopic activity. |
| [59] | Cross-sectional | IL-8 | Mucosal | Histologic severity | IL-8 levels increased with greater histologic severity. | Progressive IL-8 elevation indicates amplification of neutrophil recruitment proportional to the intensity of histologic inflammation. |
| [71] | Cross-sectional | IL-8 | Serum | Nancy index | Higher serum IL-8 levels were observed in patients with histologic activity compared with inactivity. | Elevated circulating IL-8 mirrors ongoing tissue-level neutrophilic inflammation despite potential clinical quiescence. |
| [28] | Cross-sectional | IL-17A | Serum | Geboes score | Serum IL-17A levels were higher in histologically active disease. | Increased IL-17A reflects sustained Th17-driven effector activity stabilizing inflammatory immune states at the microscopic level. |
| [45] | Cross-sectional | IL-17A | Mucosal | Nancy index | Mucosal IL-17A expression correlated with severe histologic activity. | Enhanced mucosal IL-17A supports persistence of neutrophil-survival signals and limits spontaneous histologic resolution. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Martinos, N.; Lazaris, A.C.; Kroupis, C.; Kranidiotis, G.; Thomopoulou, G.-E. Interleukin Signatures as Prognostic Biomarkers in Ulcerative Colitis: From Immune Pathways to Clinical Prediction. Curr. Issues Mol. Biol. 2026, 48, 140. https://doi.org/10.3390/cimb48020140
Martinos N, Lazaris AC, Kroupis C, Kranidiotis G, Thomopoulou G-E. Interleukin Signatures as Prognostic Biomarkers in Ulcerative Colitis: From Immune Pathways to Clinical Prediction. Current Issues in Molecular Biology. 2026; 48(2):140. https://doi.org/10.3390/cimb48020140
Chicago/Turabian StyleMartinos, Nikolaos, Andreas C. Lazaris, Christos Kroupis, Georgios Kranidiotis, and Georgia-Eleni Thomopoulou. 2026. "Interleukin Signatures as Prognostic Biomarkers in Ulcerative Colitis: From Immune Pathways to Clinical Prediction" Current Issues in Molecular Biology 48, no. 2: 140. https://doi.org/10.3390/cimb48020140
APA StyleMartinos, N., Lazaris, A. C., Kroupis, C., Kranidiotis, G., & Thomopoulou, G.-E. (2026). Interleukin Signatures as Prognostic Biomarkers in Ulcerative Colitis: From Immune Pathways to Clinical Prediction. Current Issues in Molecular Biology, 48(2), 140. https://doi.org/10.3390/cimb48020140










