Abstract
Neuroinflammation represents a fundamental component in the development and progression of a wide range of neurological disorders, including neurodegenerative diseases, psychiatric conditions, and cerebral injuries. This review examines the complex mechanisms underlying neuroinflammatory responses, with a focus on the interactions between glial cells and neurons. The dualistic role of neuroinflammation is further investigated, highlighting its ability to promote neuroprotection in acute phases while also contributing to neuronal injury and degeneration during chronic activation. This review also considers innovative therapeutic approaches designed to target neuroinflammatory processes, like drug-based treatments and immune-modulating therapies. A thorough understanding of the regulatory balance within neuroinflammatory networks is essential for the development of effective treatments for several neurological pathologies. Finally, this review provides an integrative summary of current evidence and highlights emerging directions in neuroinflammation research.
1. Introduction
Neuroinflammation is a multifaceted biological response within the central nervous system (CNS) that has gained recognition as a pivotal contributor to the pathogenesis of numerous neurological diseases [1]. Once mainly seen as a transient protective response to infections, trauma, or neurotoxic injury, neuroinflammation is now understood to have a dual function (either maintaining CNS homeostasis or, on the other hand, driving neurodegeneration) [2,3]. This functional dichotomy is driven by a complex interaction between cellular components and molecular signaling pathways operating within the immune-privileged environment of the CNS [4,5].
At the cellular level, glial cells, particularly microglia and astrocytes, are essential in the regulation of the neuroinflammatory response [6]. Microglia, the resident innate immune cells of the CNS, typically sustain a surveillance phenotype under homeostatic conditions, constantly monitoring the neural milieu [7]. Upon exposure to pathological stimuli, like pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [8,9], microglia undergo a pronounced phenotypic shift, characterized by the release of pro-inflammatory mediators (e.g., TNF-α, IL-1β, and IL-6), reactive oxygen species (ROS), and nitric oxide (NO) [10,11,12]. These responses are regulated by the activation of some intracellular signaling pathways, including NF-κB, MAPKs (ERK, JNK, and p38), and the formation of the NLRP3 inflammasome [13,14,15]. Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), act as critical upstream sensors that trigger these cascades [16,17]. Astrocytes, once considered passive support cells, are now recognized as active contributors to the modulation of CNS inflammation [18]. Astrocytes, driven by microglial-derived factors such as IL-1α, TNF-α, and C1q, lose neuroprotective properties and secrete neurotoxic factors that compromise oligodendrocyte and neuronal viability [19,20]. Additionally, astrocytes regulate the permeability of the blood–brain barrier (BBB) through the production and secretion of VEGF and MMPs, thereby allowing the infiltration of peripheral immune cells, including T lymphocytes and monocytes [21]. On the other hand, neurons are not simply passive recipients of inflammatory signals but actively influence and regulate neuroimmune dynamics. This cell type expresses molecules such as CD200, CX3CL1, and neuregulins, which interact with glial receptors to maintain microglia in a homeostatic state [22,23,24]. However, persistent inflammatory stimuli suppress these regulatory signals, favoring a pro-inflammatory CNS environment [25].
At the molecular level, neuroinflammatory signaling involves a complex network of several transcription factors (such as NF-κB, STATs, and IRFs), kinases, and lipid mediators [26,27,28]. Bioactive lipids such as prostaglandins (notably PGE2) and leukotrienes (synthesized via COX and LOX pathways) amplify inflammatory responses [29,30]. Furthermore, alterations in kynurenine metabolism, a major pathway of tryptophan catabolism, lead to the accumulation of neurotoxic metabolites such as quinolinic acid, which exacerbate excitotoxicity through NMDA receptor overactivation [31].
Chronic neuroinflammation, in contrast with its acute counterpart, is now recognized as a central pathological mechanism in numerous neurodegenerative diseases [32]. In Alzheimer’s disease (AD), maintained microglial activation contributes to amyloid-beta (Aβ) plaques and τ-hyperphosphorylation [33]. In Parkinson’s disease (PD), inflammatory processes exacerbate dopaminergic neuronal loss in the substantia nigra, accompanied by elevated cytokine levels and oxidative stress [34]. In the same way, in traumatic brain injury (TBI) and ischemic stroke, the release of DAMPs initiates prolonged glial activation, secondary neurotoxicity, and lasting cognitive deficits [35,36]. Moreover, neuroinflammation is increasingly recognized as a critical contributor to the pathophysiology of several pediatric neurological disorders, including neuronal ceroid lipofuscinoses (NCLs) and Lafora disease (LD) [37,38]. In these conditions, persistent activation of immune pathways within the CNS underlies progressive neurodegeneration, resulting in the progressive worsening of cognitive and motor impairments. Emerging data indicate that the sustained presence of pro-inflammatory mediators not only reflects neuronal injury but may also play a causative role in driving disease progression during neurodevelopment [39]. Emerging evidence also implicates systemic factors (including gut–brain axis dysfunction and peripheral immune activation) as significant modulators of CNS inflammation [40,41].
Despite the complexity of neuroimmune signaling, several therapeutic strategies are under investigation. These include NLRP3 inhibitors (e.g., MCC950), tetracycline derivatives (e.g., minocycline), glucocorticoids, and biologics targeting key cytokines such as IL-1β (e.g., anakinra) and TNF-α (e.g., etanercept) [42,43,44,45]. Moreover, novel therapeutic strategies are focused on enhancing the resolution phase of inflammation through specialized pro-resolving mediators (SPMs), including resolvins, protectins, and maresins, which promote tissue repair while minimizing immunosuppression [46,47]. A failure in these resolution mechanisms can perpetuate chronic inflammation, cellular stress, and ongoing neurodegeneration [48].
In this context, the present review aims to provide an in-depth analysis of the cellular and molecular mechanisms governing neuroinflammation, emphasizing its dual role in maintaining CNS homeostasis and promoting neurodegeneration. Moreover, this review will explore its involvement in some neurological disorders, as well as current and emerging therapeutic strategies targeting inflammatory pathways. Gaining an understanding of these mechanisms is crucial for the development of more effective treatments for neurological diseases linked to neuroinflammation. Such insights may also contribute to early diagnosis and prevention strategies, ultimately improving patient outcomes.
2. Mechanisms of Neuroinflammation
Neuroinflammation represents a highly regulated and complex response of the CNS to diverse stimuli like infections, injuries, and neurodegenerative events [1]. While acute, well-regulated inflammation can support tissue repair and homeostasis, dysregulated inflammatory activity is a hallmark of various CNS pathologies, including AD and PD [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The orchestration of this biological response relies on the dynamic crosstalk among glial cells within the CNS, peripheral immune components, soluble pro-inflammatory factors, and complex intracellular signaling cascades. These pathways initiate and sustain the inflammatory response, ultimately influencing neuronal viability and function.
2.1. Cellular Mediators
Neurons, the fundamental building blocks of the nervous system, are highly specialized cells responsible for transmitting electrical and chemical signals throughout the body [49]. These specialized cells establish highly complex neural networks that mediate signal transmission between distinct regions of the brain and spinal cord, thereby orchestrating the full spectrum of cognitive, sensory, and motor functions [50]. Neurons are characterized by their unique structure, which includes a cell body, dendrites that receive signals, and an axon that transmits signals to other neurons or muscles [51]. However, these cells do not operate in isolation but interact with a variety of other cells, including glial cells (microglia, astroglia, and oligodendroglia), which provide structural support, protection, and nourishment (Figure 1) [52].
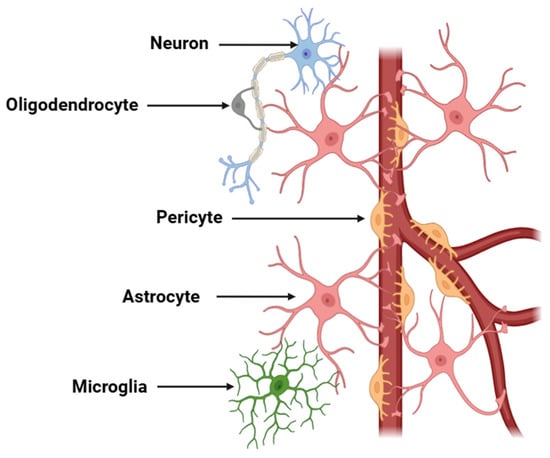
Figure 1.
Schematic representation of a neurovascular unit. The diagram illustrates the components of a neurovascular unit, which include neurons, astrocytes, endothelial cells, pericytes, microglia, and the basement membrane. This unit is critical for maintaining cerebral homeostasis, regulating BBB permeability, and facilitating neurovascular coupling. Neuronal activity is coupled with vascular responses via astrocytic end-feet and endothelial signaling mechanisms.
Microglial cells are the resident immune cells of the CNS, playing a key role in maintaining homeostasis, modulating neuronal function, and responding to pathological conditions [53]. These glial cells are derived from yolk sac progenitors early in development and persist throughout life, acting as the first line of defense against injury or infection in the brain and spinal cord [54]. Under physiological conditions, microglial cells maintain a surveillant state, extending and retracting their processes to continuously survey the surrounding microenvironment for signs of homeostatic imbalance [55]. In response to various signals, including injury, infection, or neurodegeneration, microglia can become activated, transforming into a more amoeboid morphology [56]. This activation is characterized by the release of many pro-inflammatory mediators, which can lead to a cascade of neuroinflammatory responses [57]. While microglial activation is fundamental for initiating the inflammatory response to protect the CNS from harmful stimuli, chronic activation of microglia is detrimental [58]. Sustained neuroinflammation, driven by overactive microglia, contributes to neuronal damage, synaptic loss, and even cell death, perpetuating a cycle of chronic inflammation that exacerbates neurodegenerative processes. Under neuroinflammatory conditions, microglia can adopt different polarization states (pro-inflammatory -M1- or anti-inflammatory -M2-) depending on the pathological context and the specific signals present in the microenvironment [59]. The balance between these phenotypes is crucial in determining the trajectory of the inflammatory response: an M1-dominant state may exacerbate neurodegeneration, whereas an M2-dominant profile is associated with tissue repair and neuroprotective effects [60,61]. Moreover, the activation of microglia in neuroinflammation has been associated with changes in BBB permeability, increased oxidative stress, and the release of neurotoxic factors, all of which contribute to a disrupted neuronal environment [62].
Astrocytes, a major class of glial cells, play essential roles in maintaining CNS homeostasis, providing metabolic and structural support to neurons, and modulating synaptic transmission and plasticity [63]. These stellate cells are widely distributed throughout the brain and spinal cord, where they execute numerous vital functions, such as regulating the integrity of the BBB, maintaining ionic equilibrium, clearing excess neurotransmitters, and supporting cellular energy metabolism [64]. Astrocytes are essential for maintaining the extracellular environment, offering structural support to neurons, and serving as intermediaries in the communication between neurons and blood vessels [65]. During neuroinflammation, astrocytes may undergo “reactive astrogliosis”, a process in which they alter their morphology, gene expression, and function in response to various pathological stimuli [66]. While reactive astrocytes attempt to protect the CNS by secreting pro-inflammatory molecules and promoting tissue repair, this response can also have significant deleterious effects when it becomes chronic [67]. In neuroinflammatory states, astrocytes release many pro-inflammatory molecules, which can exacerbate neuronal damage, synaptic dysfunction, and BBB disruption [68,69]. Moreover, reactive astrocytes can engage in bidirectional interactions with microglia, thereby amplifying the inflammatory response and contributing to a self-perpetuating cycle of neural damage and repair that ultimately compromises neuroprotective mechanisms [70]. The balance between neuroinflammation and neuroprotection in astrocytes is delicate, as excessive or prolonged activation of these cells can lead to neuronal death, glial scar formation, and impaired recovery [71].
Finally, oligodendrocytes are a specialized class of glial cells within the CNS, chiefly responsible for the formation of myelin sheaths that insulate axons and ensure the propagation of the electrical signals [72]. Oligodendrocytes originate from oligodendrocyte precursor cells (OPCs), which are distributed throughout the CNS and have the capacity to differentiate into mature oligodendrocytes in response to some cues [73]. The myelination process is essential for the proper functioning of the nervous system, and any disruption in the production or maintenance of myelin can lead to severe neurological conditions, including multiple sclerosis (MS), leukodystrophies, and other demyelinating diseases [74,75]. In situations of neuroinflammation, oligodendrocytes become critically affected. During the neuroinflammation process, pro-inflammatory cytokines exert cytotoxic effects on oligodendrocytes, leading to impaired myelin regeneration and promoting neurodegenerative processes [76]. These molecules can also alter the delicate balance between oligodendrocyte progenitor cell differentiation and oligodendrocyte maturation, inhibiting the remyelination process that is crucial for recovery following demyelination [77]. In diseases such as MS, the immune system erroneously attacks the myelin-producing oligodendrocytes, leading to widespread inflammation that disrupts the normal function of oligodendrocytes [78]. In this context, oligodendrocytes not only become the target of immune-mediated destruction but also play an active role in modulating inflammation. They can secrete various signaling molecules that either exacerbate or resolve the inflammatory response [79]. For instance, oligodendrocytes may release factors that promote the activation of microglia, which can further worsen inflammation [80]. However, oligodendrocytes can also secrete several anti-inflammatory molecules that regulate microglial homeostasis [81].
2.2. Pro-Inflammatory Mediators and Their Signaling Pathways
Neuroinflammation (Figure 2), as a tightly regulated immunological response within the CNS, comprises a cascade of highly orchestrated molecular events primarily mediated by glial cells as well as other cell types, including endothelial cells, pericytes, infiltrating immune cells, and neurons themselves [1,2,3,4,5]. In the context of chronic neuroinflammation, beyond canonical PRR-mediated activation, cytokine receptor signaling pathways (most notably the JAK/STAT axis) play a key role in orchestrating the transcriptional programs of both pro-inflammatory and anti-inflammatory phenotypes in glial cells [82]. Neuroinflammation begins when PRRs (TLRs, NLRs, and RLRs) detect PAMPs and/or DAMPs in microglial cells (Figure 3) [83,84].
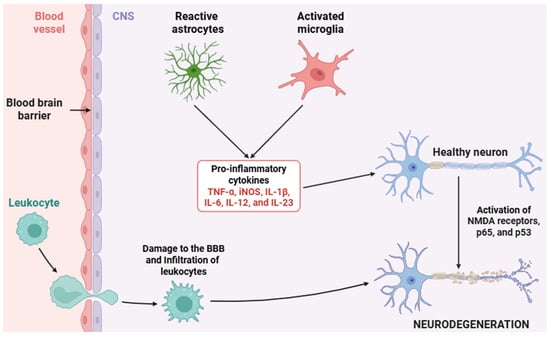
Figure 2.
Schematic representation of the neuroinflammatory process. This diagram depicts the key cellular and molecular events involved in neuroinflammation, including the activation of microglia and astrocytes, the release of several pro-inflammatory cytokines, and the recruitment of peripheral immune cells. These processes contribute to BBB disruption, neuronal dysfunction, and tissue damage. Abbreviations: CNS (central nervous system), BBB (blood–brain barrier), TNF-α (tumor necrosis factor alpha), iNOS (inducible nitric oxide synthase), IL-1β (interleukin 1 beta), IL-6 (interleukin 6), IL-12 (interleukin 12), IL-23 (interleukin 23), NMDA (N-methyl-D-aspartate), p65 (NF-κB p65 -RelA- subunit), and p53 (tumor protein 53).
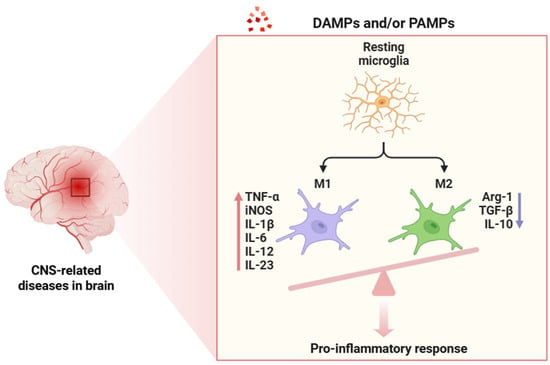
Figure 3.
Activation process of resident microglia following exposure to DAMPs or PAMPs. This interaction triggers a strong polarization toward the pro-inflammatory M1 phenotype, whereas the anti-inflammatory M2 phenotype is minimally represented. The red arrow indicates gene upregulation, whereas the blue arrow shows downregulation. Abbreviations: DAMPs (damage-associated molecular patterns), PAMPs (pathogen-associated molecular patterns), CNS (central nervous system), TNF-α (tumor necrosis factor alpha), iNOS (inducible nitric oxide synthase), IL-1β (interleukin-1 beta), IL-6 (interleukin 6), IL-12 (interleukin 12), IL-23 (interleukin 23), Arg-1 (arginase 1), TGF-β (transforming growth factor beta), and IL-10 (interleukin 10).
TLR4 binds LPS (from Gram-negative bacteria) or endogenous ligands like HMGB1, S100B proteins, and Hsp proteins [85,86]. Upon ligand binding, TLR4 dimerizes and recruits adaptor proteins (mainly MyD88 and TRIF), thereby initiating two intracellular signaling pathways [87,88]. The MyD88-dependent pathway activates IL-1R-associated kinases (IRAK1 and IRAK4), which in turn phosphorylate TRAF6, culminating in the activation of the IκB kinase (IKK) complex [89]. This phosphorylates the protein IκBα, targeting it for ubiquitination and proteasomal degradation, allowing the dissociation and nuclear translocation of NF-κB dimers (p50/p65) to translocate into the nucleus and initiate transcription of several pro-inflammatory genes such as TNF-α, IL-1β, IL-6, iNOS, and COX-2 [90,91]. In parallel, MAPKs activation triggers the activation of numerous transcription factors, such as AP-1 and CREB, further amplifying the transcriptional response [92,93]. The TRIF-dependent arm of TLR4 signaling activates TBK1 and IKKε kinases, leading to phosphorylation and activation of IRF3, which induces transcription of IFN-γ, essential in antiviral responses and microglial priming [94].
On the other hand, the NLRP3 inflammasome, perhaps the most studied in neuroinflammation, is a tripartite complex formed by the NLRP3 sensor protein, the adaptor ASC, and procaspase-1 [95]. Upon activation by signals such as mitochondrial ROS, K+ efflux, and lysosomal destabilization, NLRP3 undergoes conformational change, oligomerizes, and recruits ASC through PYD interactions [96]. Through CARD-CARD interactions, ASC permits the recruitment and activation of procaspase-1, triggering its autocatalytic cleavage into active caspase-1. Thereafter, caspase-1 catalyzes the proteolytic processing of pro-IL-1β and pro-IL-18 into their functional, secreted cytokine forms and contributes to a potent pro-inflammatory environment [97]. Another axis involves purinergic receptors, particularly P2X7 and P2Y12, which respond to extracellular ATP (released during injuries) [98]. P2X7, a ligand-gated cation channel, permits Ca2+ and Na+ influx and K+ efflux upon ATP binding, which serves as a second signal for NLRP3 inflammasome activation [99]. Meanwhile, P2Y12 mediates directed microglial process extension toward injury foci through PI3K/AKT and Rac1 pathways, a mechanism crucial for early neuroimmune surveillance and repair [100].
The arachidonic acid pathway has a profound impact on the neuroinflammatory process. Inflammatory stimuli evoke the expression of cytosolic phospholipase A2 (cPLA2), which hydrolyzes membrane lipids to release arachidonic acid [101]. This substrate is then converted by COX-2 into prostaglandin H2 (PGH2), which is further metabolized by specific prostaglandin synthases into bioactive prostanoids, mainly prostaglandin E2 (PGE2) [102]. PGE2 binds to EP receptors (EP1-EP4), each coupled to distinct G-proteins, initiating various downstream effects ranging from increased cAMP and Ca2+ mobilization to altered gene expression via PKA and CREB [103]. In neurons, PGE2 signaling can exacerbate excitotoxicity by modulating glutamate receptor function, whereas in glial cells, PGE2 promotes cytokine production and impairs the resolution of inflammation [104].
ROS and RNS represent non-cytokine inflammatory mediators with strong effects. Microglial NOX2 becomes activated upon integrin engagement or TLR signaling and catalyzes the one-electron reduction of molecular oxygen to superoxide, which can dismutate into hydrogen peroxide or form peroxynitrite in the presence of NO [105,106]. NO is produced by iNOS, whose transcription is upregulated by NF-κB and STAT1 in response to IFN-γ and TNF-α [107]. Peroxynitrite, a highly reactive oxidant, nitrates tyrosine residues in proteins, damages DNA, and impairs mitochondrial function, thereby promoting neuronal injury and neurodegeneration [108,109].
The complement system constitutes a key component of the innate immune response within the CNS, with classical complement pathway components (especially C1q, C3, and C5a) playing prominent roles in neuroimmune modulation [110]. Under pathological conditions, this pathway becomes aberrantly activated. C1q, the starting molecule of the classical cascade, selectively binds to altered, damaged, or apoptotic synapses and neuronal elements, serving as a molecular tag for downstream complement activation [111]. This engagement triggers the proteolytic cleavage of complement component C3 into its active fragments, C3a and C3b [112]. C3b serves as a potent opsonin, decorating synaptic membranes and apoptotic cells, thus targeting them for phagocytic clearance by microglia via CR3 (also known as CD11b/CD18) [113]. Simultaneously, the cleavage of C5 yields C5a, a pro-inflammatory anaphylatoxin [114]. C3a and C5a exert their biological effects through certain GPCRs (C3aR and C5aR1, respectively) located on glial cells [115,116]. Activation of these receptors enhances microglial chemotaxis, promotes morphological activation, and induces the release of pro-inflammatory cytokines, amplifying the neuroinflammatory milieu [115,116]. While this pathway contributes to host defense and homeostatic clearance under acute conditions, dysregulated activation can lead to highly pathological consequences, including disruption of synaptic integrity and promotion of neuroinflammation [117,118]. Chronic complement activity leads to synaptic pruning, even in the absence of infection and/or injury, thereby compromising synaptic integrity and plasticity [119].
On the other hand, astrocytes, while traditionally viewed as support cells, are active participants in neuroinflammation [18]. Upon stimulation by IL-1β, TNF-α, or TLR agonists, astrocytes upregulate their own expression of pro-inflammatory cytokines, chemokines (such as CCL2 and CXCL10), adhesion molecules (e.g., ICAM-1 and VCAM-1), and acute-phase proteins [68]. These molecules enable leukocyte recruitment across the BBB, whose permeability is compromised during neuroinflammation through the action of MMPs, particularly MMP-2 and MMP-9 [120]. MMPs degrade extracellular matrix components and tight junction proteins like occludin and claudin-5, destabilizing the BBB and enabling infiltration of monocytes, T cells, and neutrophils [121]. Infiltrated immune cells release extra pro-inflammatory mediators, forming a positive feedback loop [122].
Finally, the resolution phase of neuroinflammation is an actively regulated process mediated by SPMs, which are biosynthesized from ω-3 polyunsaturated fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) via the enzymatic activity of lipoxygenases, especially 15-lipoxygenase (15-LOX) [123,124]. These lipid mediators include resolvins (e.g., RvD1 and RvE1), protectins (e.g., PD1), maresins (e.g., MaR1), and lipoxins (LXA4), which exert their pro-resolving effects through binding to certain GPCRs such as GPR32 (RvD1), ALX/FPR2 (LXA4), and ChemR23 (RvE1) [125,126]. The activation of these receptors on glial cells (e.g., microglia) inhibits NF-κB signaling, activates AMPK, and induces PPARγ, thereby promoting a phenotypic shift toward reparative functions [127,128]. These include enhanced phagocytosis of apoptotic cells and myelin debris, suppression of pro-inflammatory cytokines, and increased secretion of neurotrophic factors such as BDNF and IGF-1, facilitating the restoration of homeostasis [129,130,131,132,133]. The failure in SPM biosynthesis or receptor signaling contributes to persistent neuroinflammation, which is a defining pathological feature of some neurodegenerative diseases [134].
3. The Dual Role of Neuroinflammation
Neuroinflammation is elicited by a broad spectrum of pathogenic or injurious stimuli, including microbial infections [135], mechanical trauma [136], cerebral ischemia [137], and a range of neurodegenerative processes [138]. This biological phenomenon is characterized by the activation of resident glial cells, which undergo profound phenotypic and functional transformations upon stimulation [7,18]. Activated glial cells release many bioactive molecules that can exert either cytoprotective or cytotoxic effects, depending on the context, intensity, and duration of the inflammatory insult [139,140].
In its acute and transient manifestation, neuroinflammation is widely regarded as a highly coordinated and adaptive physiological response that serves to maintain the structural and functional integrity of the CNS in response to injury or various cellular stressors [141]. This regulated process involves a cascade of molecular and cellular events primarily orchestrated by resident immune cells of the CNS, including microglia and astrocytes, and to a lesser extent by infiltrating peripheral immune cells when the BBB is compromised [142]. One of the principal roles of acute neuroinflammation is the facilitation of phagocytic clearance of apoptotic cells, infectious agents, and cellular debris, thereby preventing the excessive deposition of neurotoxic substances and reducing subsequent damage to the surrounding neural parenchyma [131,143,144,145]. This immune-driven clearance process is further supported by the activation of intracellular signaling cascades that facilitate structural tissue remodeling, thereby facilitating the re-establishment of a permissive environment for neuronal regeneration [146]. Notably, acute neuroinflammation has been shown to stimulate neurogenesis through the upregulation of growth factors such as BDNF and VEGF, which also play a role in promoting angiogenesis, with neurogenesis and vascular remodeling being critical determinants of functional recovery in damaged areas of the CNS [147,148].
Furthermore, this form of neuroinflammatory response plays a key role in the restoration and preservation of CNS homeostasis by acting as a component of the innate immune surveillance system [25]. The CNS has traditionally been considered immunoprivileged, largely due to the protective role of the BBB combined with a limited population of APCs; however, recent studies reveal that an important degree of immunosurveillance is sustained within the CNS, allowing for rapid initiation of responses to both endogenous and exogenous insults [149]. In this context, acute neuroinflammation functions not only as a protective mechanism but also as a dynamic mediator of crosstalk between the neural and immune systems, ensuring rapid detection and mitigation of disturbances to CNS integrity to prevent chronic dysfunction [150]. The resolution phase of this inflammatory response is crucial, marked by the suppression of pro-inflammatory signaling and the induction of anti-inflammatory and pro-resolving pathways, which collectively serve to terminate inflammation and mitigate its potential role in the progression of neurodegenerative pathology [151].
However, when the neuroinflammatory milieu becomes chronic, the same glial-derived mediators that confer protection can instead instigate a cascade of deleterious events [152]. Prolonged activation of microglia and astrocytes promotes a sustained pro-inflammatory milieu, characterized by the persistent upregulation of molecules such as TNF-α, IL-1β, and iNOS [10,11,12]. This pathological state contributes to increased oxidative stress, glutamate-mediated excitotoxicity, and dysregulated synaptic activity [153,154,155]. Chronic neuroinflammation has been implicated in the propagation of some CNS pathologies, including but not limited to AD [156], PD [157], MS [158], and ALS [159]. In these contexts, neuroinflammation is not merely a secondary consequence of neurodegeneration but an active driver of disease progression.
The dichotomous nature of neuroinflammation (simultaneously neuroprotective and neurodestructive) highlights the critical importance of precise spatiotemporal regulation of inflammatory signaling pathways [160]. The molecular crosstalk between neurons, glial cells, endothelial cells, and peripheral immune components ultimately determines the trajectory of the inflammatory response [161]. A pivotal aspect of this process is the tightly regulated interplay between pro-inflammatory (e.g., NF-κB and STAT1) and anti-inflammatory (e.g., IL-10 and TGF-β) signaling cascades, which ultimately dictates whether inflammation is resolved or evolves into a detrimental, chronic state [162].
Emerging evidence also challenges traditional binary classifications of glial phenotypes, such as the M1/M2 dichotomy for microglia, or the A1/A2 paradigm for astrocytes. Instead, reactive glial cells are increasingly recognized as occupying a continuum of activation states, influenced by a confluence of intrinsic and extrinsic factors (including aging, sex, genetic predisposition, systemic immune status, microbiome composition, and environmental exposures) [163,164,165,166,167,168]. The nature of glial phenotypes presents a major obstacle for therapeutic intervention, as broad modulation of inflammatory pathways may unintentionally compromise neuroprotective functions or miss critical functional nuances.
Effective strategies must therefore account for the temporal and spatial heterogeneity of glial responses, as well as the molecular cues driving phenotype transitions. A precise understanding of glial signaling networks is essential to selectively target detrimental processes while preserving or enhancing reparative functions.
4. Therapeutic Strategies Targeting Neuroinflammation
Therapeutic strategies targeting neuroinflammation represent a crucial frontier in the management of a wide range of neurological diseases, including AD, PD, and MS, among others [169,170,171]. Due to the multifactorial aspects of neuroinflammation, therapeutic approaches must be tailored to modulate specific molecular and cellular pathways involved in the inflammatory response within the CNS. Current pharmacological interventions can be categorized into established therapies, including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and monoclonal antibodies, and into novel experimental therapies, such as microglial modulators and inflammasome inhibitors, each with distinct molecular targets and mechanisms of action.
4.1. Established Therapies
4.1.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs constitute a widely utilized class of pharmacological agents, known for their analgesic, antipyretic, and anti-inflammatory effects [172]. Their main mechanism of action involves the inhibition of COX enzymes, specifically COX-1 and COX-2 [173]. These enzymes are integral to the biosynthetic pathway that converts arachidonic acid (a polyunsaturated fatty acid liberated from membrane phospholipids during cellular stress or injury) into a range of prostanoids, such as prostaglandins, prostacyclins, and thromboxanes [174]. These lipid mediators orchestrate crucial aspects of the inflammatory response, including vasodilation, increased vascular permeability, and the recruitment of immune cells to sites of tissue injury or infection [175]. In contrast with COX-1, which is constitutively expressed and crucial for maintaining physiological functions such as gastrointestinal mucosal protection and renal blood flow, COX-2 is an inducible enzyme whose expression is markedly upregulated by several inflammatory stimuli (e.g., pro-inflammatory cytokines, bacterial pathogens, and injuries) [176]. Within the CNS, COX-2 upregulation occurs in glial cells as well as in neurons under pathological conditions [177,178].
Beyond COX inhibition, many NSAIDs also exert COX-independent anti-inflammatory effects. By interfering with NF-κB activation and nuclear translocation, NSAIDs extend their anti-inflammatory and neuroprotective effects beyond prostaglandin inhibition [179]. Additionally, certain NSAIDs have been shown to modulate peroxisome proliferator-activated receptors (PPARs), particularly PPARγ, providing an additional mechanism through which NSAIDs may attenuate neuroinflammatory processes [180].
Despite their therapeutic potential, the long-term use of NSAIDs is limited by their well-documented adverse effects profile. Long-term NSAID therapy is linked to gastrointestinal complications (including gastritis, peptic ulcer disease, and gastrointestinal bleeding) primarily due to COX-1 inhibition [181]. Renal adverse effects, such as decreased renal perfusion, salt and water retention, and potential acute kidney injury, arise from impaired prostaglandin-mediated renal vasodilation [182]. Moreover, cardiovascular risks, such as hypertension, myocardial infarction, and stroke, are particularly pronounced with selective COX-2 inhibitors (termed as coxibs) [183].
Addressing these safety concerns has prompted the exploration of strategies such as selective COX-2 inhibition [184], dual COX/LOX (lipoxygenase) inhibition [185], development of NO-donating NSAIDs [186], and co-administration of gastroprotective agents like proton pump inhibitors (e.g., omeprazole and lansoprazole) [187]. Nevertheless, achieving an optimal balance between therapeutic efficacy and safety remains a significant challenge in the clinical application of NSAIDs for chronic neuroinflammatory conditions.
Table 1 provides an overview of treatments involving NSAIDs aimed at addressing neuroinflammation in different pathological conditions. This table outlines those NSAIDs used in clinical settings, highlighting their therapeutic effectiveness.

Table 1.
NSAID-based therapeutic approaches for pathologies associated with neuroinflammatory processes. Abbreviations: MS (multiple sclerosis), AD (Alzheimer’s disease), PD (Parkinson’s disease), RR (relative risk), CI (confidence interval), OR (odd ratio), NSAID (non-steroidal anti-inflammatory drug), TLR4 (Toll-Like receptor 4), TNF-α (tumor necrosis factor alpha), BDNF (brain-derived neurotrophic factor), Nrf-2 (nuclear factor erythroid 2-related factor 2), UPDRS (Unified Parkinson’s Disease Rating Scale), TBI (traumatic brain injury), COX (cyclooxygenase), ALS (amyotrophic lateral sclerosis), TDP-43 (TAR DNA-binding protein 43), and LC3 (microtubule-associated protein 1A/1B-light chain 3).
4.1.2. Corticosteroids
Corticosteroids, including dexamethasone and methylprednisolone, exert their anti-inflammatory effects through the activation of the glucocorticoid receptor (GR), a ligand-activated transcription factor that belongs to the nuclear receptor superfamily [197]. Upon entering target cells, corticosteroids bind to the GR in the cytoplasm, inducing its conformational change and dissociation from Hsp proteins (e.g., HSP70 and HSP90), facilitating its translocation into the nucleus [198]. Once in the nucleus, the GR–ligand complex binds to specific DNA sequences known as glucocorticoid response elements (GREs) located in the promoter regions of some target genes, recruiting coactivators such as steroid receptor coactivator-1 (SRC-1) and p300 [199,200,201]. This interaction promotes the transcription of anti-inflammatory genes (e.g., IL-10) while inhibiting the expression of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 [202,203]. Furthermore, the GR exerts transcriptional interference with several transcription factors, especially NF-κB (by binding to the p65 subunit of NF-κB, it prevents its translocation to the nucleus and DNA binding) [204] and AP-1 (by sequestering AP-1 activators-such as c-Jun-, inhibiting their ability to promote the transcription of pro-inflammatory genes) [205].
Additionally, corticosteroids inhibit the expression of some adhesion molecules, such as ICAM-1 and VCAM-1, thereby reducing immune cell recruitment to inflamed tissues [206,207]. Corticosteroids also promote the apoptosis of activated immune cells, particularly T and B lymphocytes, through the induction of pro-apoptotic proteins including BIM and FASL, eliciting some extrinsic and intrinsic apoptotic pathways [208,209]. In contrast, corticosteroids decrease the permeability of the BBB, preventing the infiltration of peripheral immune cells into the CNS, thereby limiting neuroinflammation [210].
Despite their anti-inflammatory actions, corticosteroids are associated with numerous adverse effects, including immunosuppression [211], metabolic alterations [212], osteoporosis [213], and neuropsychiatric disturbances [214], especially with prolonged use, underscoring the importance of vigilant monitoring and dose modulation in clinical settings.
Table 2 presents an overview of corticosteroid treatments targeting neuroinflammation in different pathological conditions. It highlights the corticosteroids utilized in clinical settings, emphasizing their therapeutic effectiveness.

Table 2.
Corticosteroid-based therapeutic strategies for disorders linked to neuroinflammatory processes. Abbreviations: MS (multiple sclerosis), RRMS (relapsing–remitting multiple sclerosis), MRI (magnetic resonance imaging), and IFNβ-1a (interferon beta-1a).
4.2. Experimental Approaches
4.2.1. Monoclonal Antibodies
Monoclonal antibody (mAb) therapies have emerged as a groundbreaking strategy in the treatment of neuroinflammatory disorders, offering unparalleled specificity in molecular targeting, thereby enabling precise modulation of pathological pathways within the CNS [222]. A leading example of this therapeutic progress is natalizumab, an anti-α4 integrin mAb. α4 integrin is a cell adhesion molecule expressed on the surface of leukocytes, including T-cells, monocytes, and granulocytes [223]. By binding to α4 integrin, natalizumab blocks its interaction with VCAM-1, thereby preventing the adhesion and subsequent transmigration of leukocytes across the BBB [224]. This mechanism is particularly significant in the context of MS, where autoreactive immune cells infiltrate the CNS, triggering inflammation that leads to demyelination and neurodegeneration [225].
Similarly, monoclonal antibodies targeting TNF-α have demonstrated therapeutic efficacy in neuroinflammatory diseases. Anti-TNF-α antibodies, including infliximab and adalimumab, neutralize both soluble and membrane-bound forms of TNF-α, thereby inhibiting its downstream signaling pathways [226,227]. These antibodies prevent the activation of TNF receptors (TNFR1 and TNFR2), which are involved in the induction of inflammatory cascades, oxidative stress, and neuronal cell death [228,229]. Its inhibition can impair the immune response, leaving patients susceptible to opportunistic infections, such as tuberculosis, fungal infections, and viral reactivation [230,231,232].
In recent years, mAbs targeting other cytokines implicated in chronic CNS inflammation have gained attention. Canakinumab, a mAb that selectively targets and neutralizes IL-1β, has shown promise in modulating the IL-1β signaling pathway. Through the inhibition of IL-1β binding to its receptor (IL-1R), canakinumab reduces the downstream production of other inflammatory mediators, including IL-6, IL-8, and MMPs, which contribute to BBB disruption and neuronal damage [233,234,235]. Similarly, tocilizumab, a mAb that antagonizes the IL-6 receptor (IL-6R), has been developed as a treatment for autoimmune diseases, and its potential in modulating neuroinflammation is under investigation [236]. Blocking IL-6R signaling reduces the expression of pro-inflammatory cytokines in the brain, thus providing a targeted approach to attenuate neuroinflammation [237].
Another area of significant interest is the development of monoclonal antibodies targeting Aβ plaques, which are critical in the pathogenesis of AD. Aβ plaques are neurotoxic protein aggregates that accumulate in the brains of individuals with AD, driving neuroinflammation, synaptic dysfunction, and neurodegeneration [238]. The accumulation of Aβ plaques triggers the activation of glial cells, leading to the release of many pro-inflammatory cytokines and the exacerbation of neuronal injury [239]. mAbs, such as aducanumab, have been developed to specifically target and promote the clearance of Aβ plaques [240]. These antibodies recognize both soluble and insoluble forms of Aβ, facilitating their clearance via microglial phagocytosis or by enhancing amyloid elimination [241]. By reducing Aβ accumulation and mitigating the associated neuroinflammatory response, aducanumab has shown promise in slowing disease progression and improving cognitive function in AD patients [242].
Moreover, additional mAbs have been introduced to target other key inflammatory mediators in neuroinflammatory disorders. Eculizumab, a mAb targeting the complement protein C5, has been shown to attenuate the activation of the complement system, which plays a role in the pathogenesis of diseases such as neuromyelitis optica spectrum disorder (NMOSD) [243]. Through the inhibition of C5, eculizumab prevents the assembly of the membrane attack complex (MAC), thereby diminishing the inflammation and tissue damage associated with complement activation [244]. Further advances have been made in targeting the IL-17 pathway, a key driver of autoimmune neuroinflammation. Monoclonal antibodies such as secukinumab and ixekizumab, which target IL-17A, have been successfully used in the treatment of psoriasis and ankylosing spondylitis [245,246], and their potential in CNS disorders is under exploration [247,248,249]. Finally, mAbs targeting IL-23, a cytokine upstream of IL-17 production, have shown promise in reducing inflammation in preclinical models of neuroinflammatory conditions [250].
The development of mAb therapies for neuroinflammatory disorders is continuously advancing, with ongoing research aimed at discovering new targets and optimizing therapeutic efficacy. Strategies include the refinement of antibody selectivity, improving delivery across the BBB, and minimizing adverse effects, such as increased susceptibility to infections [251]. Furthermore, combination therapies targeting numerous pro-inflammatory pathways are under investigation as potential strategies to improve clinical outcomes in patients with complex neuroinflammatory disorders [252]. As our understanding of the molecular mechanisms underlying neuroinflammation continues to deepen, mAb-based therapies hold great promise in the treatment of a broad spectrum of CNS disorders.
Table 3 details the treatments previously referenced, assessed in certain clinical trials, alongside additional interventions not previously cited, to highlight the broad spectrum of therapeutic strategies available for neuroinflammation-associated disorders.

Table 3.
Experimental immunotherapeutic treatments targeting various CNS disorders characterized by neuroinflammation. Abbreviations: mAb (monoclonal antibody), MS (multiple sclerosis), CD20 (cluster of differentiation 20), CD52 (cluster of differentiation 52), RRMS (relapsing–remitting multiple sclerosis), PPMS (primary progressive multiple sclerosis), NMOSD (neuromyelitis optica spectrum disorder), C5 (complement component 5), IL-6R (interleukin 6 receptor), CD19 (cluster of differentiation 19), AD (Alzheimer’s disease), PD (Parkinson’s disease), CNS (central nervous system), NPSLE (neuropsychiatric systemic lupus erythematosus), AAV (anti-neutrophil cytoplasmic antibody-associated vasculitis), and BAFF (B-cell activating factor).
4.2.2. Other Innovative Therapeutic Strategies
Recent advances in the development of novel microglial modulators offer promising therapeutic strategies designed to restore microglial function and completely mitigate the harmful consequences of dysregulated activation. One such class of modulators includes colony-stimulating factor 1 receptor (CSF1R) inhibitors, such as PLX5622. CSF1R is a receptor critical for microglial survival, proliferation, and maintenance, as it transduces signals required for the development of microglia in the CNS [268]. Inhibition of CSF1R signaling by these compounds results in the depletion or phenotypic reprogramming of microglia, favoring their transition to a quiescent or neuroprotective state [269]. This reprogramming reduces the production of pro-inflammatory cytokines and ROS, thus mitigating the neurotoxic effects of overactive microglia [270,271]. CSF1R inhibition has been shown to impair the survival signals necessary for microglial proliferation, effectively reducing microglial numbers and dampening chronic neuroinflammation [272].
Another promising approach to modulate microglial activity involves targeting the fractalkine receptor (CX3CR1), which mediates communication between neurons and microglia. CX3CR1 antagonists are designed to restore homeostatic interactions between microglia and neurons, thereby mitigating the adverse consequences of excessive microglial activation, including aberrant synaptic pruning and neurotoxicity [273,274]. This strategy seeks to enhance the neuroprotective functions of microglia while preventing them from overreacting to minor stimuli, a characteristic factor in neuroinflammatory diseases [275]. Moreover, pharmacological agents that enhance PPARγ signaling, like pioglitazone, have shown promise in modulating microglial polarization [276]. On the other hand, selective NLRP3 inhibitors, like MCC950, block the activation of NLRP3 by preventing its ATPase-dependent oligomerization. This inhibition attenuates caspase-1 activation and the subsequent release of pro-inflammatory cytokines without interfering with other aspects of the innate immune response [277]. In preclinical models, NLRP3 inhibition has been shown to reduce microglial activation, BBB disruption, and neuronal death, highlighting its potential as a therapeutic approach in neuroinflammatory disorders [278].
Beyond direct inflammasome inhibition, other experimental strategies focus on targeting downstream effectors of inflammasome activation, such as gasdermin D. Gasdermin D is the executor of pyroptosis, a form of programmed cell death that is often associated with inflammatory responses [279]. Pyroptosis exacerbates tissue injury and contributes to disease progression in neuroinflammatory conditions. By inhibiting gasdermin D-mediated pyroptosis, these strategies aim to minimize neuronal damage and preserve tissue integrity [280].
Collectively, these innovative strategies constitute a multifactorial approach to modulating neuroinflammation, preserving neural tissue integrity, and enhancing therapeutic outcomes in neurodegenerative disorders. By selectively reprogramming microglia and inhibiting harmful inflammatory processes, these therapies hold great potential for mitigating the devastating effects of chronic neuroinflammation and preserving CNS function.
5. Future Directions and Research Gaps
Although considerable progress has been made in elucidating the molecular pathways and cellular mechanisms involved in neuroinflammation, several critical challenges persist that limit the advancement of effective diagnostic tools and therapeutic interventions. Among the foremost research imperatives is the identification and rigorous validation of highly specific, sensitive, and reliable biomarkers capable of distinguishing homeostatic and beneficial neuroinflammatory activity from chronic and huge neuroinflammatory responses [281]. Current clinical and experimental methodologies lack the resolution to differentiate adaptive immune activation (crucial for tissue repair and neuroprotection) from maladaptive inflammatory cascades that exacerbate neuronal injury and promote neurodegeneration [282]. The development of such biomarkers would enable earlier and precise detection of neuroinflammatory components in neurological pathologies, support the stratification of patients in clinical trials, and permit real-time monitoring of therapeutic efficacy [283].
Another major limitation in the field is the lack of physiologically relevant and translationally predictive experimental models. Conventional in vitro models largely fail to replicate the multicellular interactions, spatial organization, and dynamic microenvironment characteristic of the human CNS [284]. Likewise, widely used animal models often lack construct or face validity, especially in the context of chronic neurodegenerative diseases, aging, and comorbid systemic conditions [285]. A growing body of evidence underscores the urgent need for more sophisticated models (such as human-derived organoids and microfluidic brain-on-a-chip platforms), and genetically modified animal models that more faithfully emulate the human neuroimmune microenvironment (to effectively bridge the translational divide between preclinical and clinical research) [286,287]. These models should incorporate innate and adaptive immune responses, temporal dynamics, and the influence of systemic immune modulation to provide a more comprehensive understanding of neuroinflammatory processes across different disease stages.
Moreover, the long-term consequences of modulating neuroinflammation (especially via broad-spectrum or prolonged anti-inflammatory interventions) remain insufficiently characterized. While suppression of inflammation may yield short-term symptomatic relief or neuroprotection, accumulating evidence suggests that persistent inhibition of immune signaling within the CNS might interfere with critical physiological functions, such as synaptic plasticity, neurogenesis, and clearance of cellular debris [288,289,290]. Glial cells exhibit a high degree of functional plasticity, and their roles in disease are highly context-dependent. Therefore, indiscriminate targeting of these glial populations or their associated cytokine networks may lead to unintended consequences, including impaired neural repair, increased susceptibility to infections, or disruption of neuroimmune homeostasis [288,291]. Additionally, the long-term impact of modulating neuroinflammation, through the application of broad-spectrum or sustained anti-inflammatory treatments, remains inadequately defined [292].
Furthermore, clinical studies are crucial to evaluate the safety, efficacy, and mechanistic impact of anti-inflammatory and immunomodulatory therapies over extended time periods [293]. This research should incorporate a multimodal approach, combining neuroimaging, fluid biomarkers, electrophysiology, and comprehensive neurocognitive assessments, to accurately capture the multifaceted effects of treatment. Integrating systems biology, single-cell transcriptomics, and machine learning may also offer novel insights into patient-specific inflammatory signatures and therapeutic responsiveness, paving the way toward personalized medicine in the treatment of neuroinflammatory disorders [294,295,296].
In summary, addressing these research gaps requires a multidisciplinary effort that spans basic neuroscience, immunology, systems biology, and clinical research. The future of neuroinflammation research depends on the development of innovative tools to elucidate its dualistic nature, the creation of precise and representative models, and a comprehensive understanding of how therapeutic modulation influences the delicate balance between neuroprotection and neurotoxicity throughout the lifespan and within the context of complex neurological disorders.
6. Conclusions
Neuroinflammation is a complex and multifaceted process that plays both protective and deleterious roles in the CNS. This dual nature underscores its critical involvement in the pathogenesis and progression of some neurological disorders, including neurodegenerative diseases, TBI, and autoimmune conditions. Mechanistically, neuroinflammation is orchestrated by a dynamic interplay of glial cell activation, cytokine release, and BBB dysfunction, which contribute to neuronal damage or repair depending on the context.
While acute neuroinflammatory responses might serve protective and regenerative functions, chronic or dysregulated inflammation is strongly associated with neuronal degeneration. Therefore, therapeutic strategies targeting neuroinflammation must be calibrated to preserve its beneficial aspects while mitigating its harmful consequences.
Emerging interventions, like immunomodulatory agents, cytokine inhibitors, and glial-targeted therapies, exhibit encouraging results in preclinical and clinical studies. A deeper understanding of the molecular pathways governing neuroinflammation will be pivotal for developing precision-based treatments tailored to individual disease states and stages.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 15-LOX | 15-lipoxygenase |
| A1 | A1 phenotype astroglia |
| A2 | A2 phenotype astroglia |
| AAV | Anti-neutrophil cytoplasmic antibody-associated vasculitis |
| AD | Alzheimer’s disease |
| ALX/FPR2 | ALX/formyl peptide receptor 2 |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator protein 1 |
| Arg-1 | Arginase 1 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| APC | Antigen-presenting cell |
| ATP | Adenosine triphosphate |
| Aβ | Amyloid beta |
| BAFF | B-cell activating factor |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BIM | Bcl-2 interacting mediator of cell death |
| C1q | Complement component 1q |
| C3 | Complement component 3 |
| C3a | Complement component 3a |
| C3aR | Complement component 3a receptor |
| C3b | Complement component 3b |
| C5 | Complement component 5 |
| C5a | Complement component 5a |
| C5aR1 | Complement component 5a receptor 1 |
| cAMP | Cyclic adenosine monophosphate |
| CARD | Caspase activation and recruitment domain |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CD11b | Cluster of differentiation 11b |
| CD18 | Cluster of differentiation 18 |
| CD19 | Cluster of differentiation 19 |
| CD20 | Cluster of differentiation 20 |
| CD52 | Cluster of differentiation 52 |
| CD200 | Cluster of differentiation 200 |
| ChemR23 | Chemokine-like receptor 1 |
| CI | Confidence interval |
| c-Jun | c-Jun proto-oncogene |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| COX-1 | Cyclooxygenase 1 |
| COX-2 | Cyclooxygenase 2 |
| cPLA2 | Cytosolic phospholipase A2 |
| CR3 | Complement receptor 3 |
| CREB | cAMP response element-binding protein |
| CSF1R | Colony-stimulating factor 1 receptor |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| CX3CR1 | C-X3-C motif chemokine receptor 1 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DAMP | Damage-associated molecular pattern |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid |
| EP | E-prostanoid receptor (PGE2 receptor) |
| EP1 | E-type prostanoid receptor 1 |
| EP2 | E-type prostanoid receptor 2 |
| EP3 | E-type prostanoid receptor 3 |
| EP4 | E-type prostanoid receptor 4 |
| EPA | Eicosapentaenoic acid |
| ERK | Extracellular signal-regulated kinase |
| FASL | Fas ligand |
| GPCR | G protein-coupled receptor |
| GPR32 | G protein-coupled receptor 32 |
| GR | Glucocorticoid receptor |
| GRE | Glucocorticoid response element |
| HMGB1 | High mobility group box 1 |
| Hsp | Heat shock protein |
| HSP70 | Heat shock protein 70 |
| HSP90 | Heat shock protein 90 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IFN-β | Interferon beta |
| IFNβ-1a | Interferon beta-1a |
| IGF-1 | Insulin-like growth factor 1 |
| IKK | IκB kinase |
| IKKε | IκB kinase epsilon |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-17 | Interleukin 17 |
| IL-17A | Interleukin 17A |
| IL-1R | Interleukin 1 receptor |
| IL-1α | Interleukin 1 alpha |
| IL-1β | Interleukin 1 beta |
| IL-23 | Interleukin 23 |
| IL-6 | Interleukin 6 |
| IL-6R | Interleukin 6 receptor |
| iNOS | Inducible nitric oxide synthase |
| IRAK1 | Interleukin 1 receptor-associated kinase 1 |
| IRAK4 | Interleukin 1 receptor-associated kinase 4 |
| IRF | Interferon regulatory factor |
| IRF3 | Interferon regulatory factor 3 |
| IκBα | Inhibitor of κB alpha |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LD | Lafora disease |
| LOX | Lipoxygenase |
| LXA4 | Lipoxin A4 |
| M1 | M1 phenotype microglia |
| M2 | M2 phenotype microglia |
| mAb | Monoclonal antibody |
| MaR1 | Maresin 1 |
| MAC | Membrane attack complex |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| MMP-2 | Matrix metalloproteinase 2 |
| MMP-9 | Matrix metalloproteinase 9 |
| MRI | Magnetic resonance imaging |
| MS | Multiple sclerosis |
| MyD88 | Myeloid differentiation primary response 88 |
| NCLs | Neuronal ceroid lipofuscinoses |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B-cells |
| NLR | NOD-like receptor |
| NLRP3 | NLR family pyrin domain containing 3 |
| NMDA | N-methyl-D-aspartate |
| NMOSD | Neuromyelitis optica spectrum disorder |
| NO | Nitric oxide |
| NOX2 | NADPH oxidase 2 |
| NPSLE | Neuropsychiatric systemic lupus erythematosus |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| NSAID | Non-steroidal anti-inflammatory drug |
| OPC | Oligodendrocyte precursor cell |
| OR | Odds ratio |
| P2X7 | Purinergic receptor P2X, ligand-gated ion channel 7 |
| P2Y12 | Purinergic receptor P2Y, G-protein coupled, 12 |
| p300 | E1A binding protein p300 |
| p38 | p38 mitogen-activated protein kinase |
| p50 | NF-κB p50 subunit |
| p53 | Tumor protein 53 |
| p65 | NF-κB p65 (RelA) subunit |
| PAMP | Pathogen-associated molecular pattern |
| PD | Parkinson’s disease |
| PD1 | Protectin D1 |
| PGE2 | Prostaglandin E2 |
| PGH2 | Prostaglandin H2 |
| PI3K/AKT | Phosphoinositide 3-kinase/Protein kinase B pathway |
| PPAR | Peroxisome proliferator-activated receptor |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| pro-IL-18 | Pro-interleukin 18 |
| pro-IL-1β | Pro-interleukin 1 beta |
| PRR | Pattern recognition receptor |
| PYD | Pyrin domain |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RLR | RIG-I-like receptor |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RR | Relative risk |
| RRMS | Relapsing–remitting multiple sclerosis |
| RvD1 | Resolvin D1 |
| RvE1 | Resolvin E1 |
| S100B | S100 calcium-binding protein B |
| SPM | Specialized pro-resolving mediators |
| SRC-1 | Steroid receptor coactivator-1 |
| STAT | Signal transducer and activator of transcription |
| STAT1 | Signal transducer and activator of transcription 1 |
| TBI | Traumatic brain injury |
| TBK1 | TANK-binding kinase 1 |
| TDP-43 | TAR DNA-binding protein 43 |
| TGF-β | Transforming growth factor beta |
| TLR | Toll-like receptor |
| TLR4 | Toll-like receptor 4 |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNFR2 | Tumor necrosis factor receptor 2 |
| TNF-α | Tumor necrosis factor alpha |
| TRAF6 | TNF receptor-associated factor 6 |
| TRIF | TIR-domain-containing adapter-inducing interferon beta |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
References
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, A.G.; Zgavc, T.; Kooijman, R.; Hachimi-Idrissi, S.; Sarre, S.; Michotte, Y. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J. Neuroinflamm. 2010, 7, 74. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Mechanisms and Emerging Regulators of Neuroinflammation: Exploring New Therapeutic Strategies for Neurological Disorders. Curr. Issues Mol. Biol. 2024, 47, 8. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Di Vito, A.; Donato, G.; Tomassoni, D. Molecular and Cellular Mechanisms of Neuroinflammation. Biomed. Res. Int. 2017, 2017, 8417183. [Google Scholar] [CrossRef]
- Afridi, R.; Bhusal, A.; Tsuda, M.; Ryu, H.; Suk, K. Function of Glial Cells in Neuroinflammatory and Neuroimmunological Responses II. Cells 2023, 12, 1750. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Figuera-Losada, M.; Rojas, C.; Slusher, B.S. Inhibition of microglia activation as a phenotypic assay in early drug discovery. J. Biomol. Screen 2014, 19, 17–31. [Google Scholar] [CrossRef]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018, 11. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, C.; Ma, X.; Luo, W.; Zheng, S.G.; Qiu, W. Nuclear Factor κB (NF-κB)-Mediated Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef]
- Gradisnik, L.; Velnar, T. Astrocytes in the central nervous system and their functions in health and disease: A review. World J. Clin. Cases 2023, 11, 3385–3394. [Google Scholar] [CrossRef]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef]
- Bouvier, D.S.; Fixemer, S.; Heurtaux, T.; Jeannelle, F.; Frauenknecht, K.B.M.; Mittelbronn, M. The Multifaceted Neurotoxicity of Astrocytes in Ageing and Age-Related Neurodegenerative Diseases: A Translational Perspective. Front. Physiol. 2022, 13, 814889. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood-Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol Sci. 2023, 24, 17146. [Google Scholar] [CrossRef] [PubMed]
- Manich, G.; Recasens, M.; Valente, T.; Almolda, B.; González, B.; Castellano, B. Role of the CD200-CD200R Axis During Homeostasis and Neuroinflammation. Neuroscience 2019, 405, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Hippensteel, R.; Shimizu, S.; Nicolai, J.; Fatatis, A.; Meucci, O. Interactions between chemokines: Regulation of fractalkine/CX3CL1 homeostasis by SDF/CXCL12 in cortical neurons. J. Biol. Chem. 2010, 285, 10563–10571. [Google Scholar] [CrossRef]
- Ledonne, A.; Mercuri, N.B. On the Modulatory Roles of Neuregulins/ErbB Signaling on Synaptic Plasticity. Int. J. Mol. Sci. 2019, 21, 275. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S.; Müller, V. From Homeostasis to Neuroinflammation: Insights into Cellular and Molecular Interactions and Network Dynamics. Cells 2025, 14, 54. [Google Scholar] [CrossRef]
- Mehta, S.L.; Arruri, V.; Vemuganti, R. Role of transcription factors, noncoding RNAs, epitranscriptomics, and epigenetics in post-ischemic neuroinflammation. J. Neurochem. 2024, 168, 3430–3448. [Google Scholar] [CrossRef]
- Lee, S.H.; Suk, K. Emerging roles of protein kinases in microglia-mediated neuroinflammation. Biochem. Pharmacol. 2017, 146, 1–9. [Google Scholar] [CrossRef]
- David, S.; López-Vales, R. Bioactive Lipid Mediators in the Initiation and Resolution of Inflammation after Spinal Cord Injury. Neuroscience 2021, 466, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.V.; Bastos, L.F.; Limborço-Filho, M.; Fiebich, B.L.; de Oliveira, A.C. Role of prostaglandins in neuroinflammatory and neurodegenerative diseases. Mediat. Inflamm. 2012, 2012, 946813. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhang, S.; Li, C.; Zhang, L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: Implications for cerebral ischemia and neurodegenerative diseases. Neurobiol. Aging 2020, 87, 1–10. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Pajares, M.; I Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Chan, A.; Ouyang, J.; Nguyen, K.; Jones, A.; Basso, S.; Karasik, R. Traumatic brain injuries: A neuropsychological review. Front. Behav. Neurosci. 2024, 18, 1326115. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Takahashi, K.; Nelvagal, H.R.; Lange, J.; Cooper, J.D. Glial Dysfunction and Its Contribution to the Pathogenesis of the Neuronal Ceroid Lipofuscinoses. Front. Neurol. 2022, 13, 886567. [Google Scholar] [CrossRef]
- Della Vecchia, S.; Marchese, M.; Santorelli, F.M. Glial Contributions to Lafora Disease: A Systematic Review. Biomedicines 2022, 10, 3103. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Saint-Faust, M. Neuroinflammation in the Developing Brain: Risk Factors, Involvement of Microglial Cells, and Implication for Early Anesthesia. Anesth. Analg. 2019, 128, 718–725. [Google Scholar] [CrossRef]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in neuroinflammation and synaptic dysfunction: A focus on Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation from Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Prakash, R.; Kumari, N.; Ali Khan, M.; Quaiyoom Khan, A.; Uddin, S.; Verma, S.; Ab Robertson, A.; Boltze, J.; Shadab Raza, S. MCC950 reduces autophagy and improves cognitive function by inhibiting NLRP3-dependent neuroinflammation in a rat model of Alzheimer’s disease. Brain Behav. Immun. 2024, 116, 70–84. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, J.; Wu, L.; Xu, G.; Dai, J.; Liu, X. Tetracycline inhibits local inflammation induced by cerebral ischemia via modulating autophagy. PLoS ONE 2012, 7, e48672. [Google Scholar] [CrossRef]
- Komoltsev, I.G.; Gulyaeva, N.V. Brain Trauma, Glucocorticoids and Neuroinflammation: Dangerous Liaisons for the Hippocampus. Biomedicines 2022, 10, 1139. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Chowdhury, P.; Bhowmik, P.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Targeting Cytokine-Mediated Inflammation in Brain Disorders: Developing New Treatment Strategies. Pharmaceuticals 2025, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.; Ulu, A.; Hanson, C.; Cameron-Smith, E.; Bertoni, J.; Wuebker, J.; Fisher, A.; Siu, K.C.; Marmelat, V.; Adamec, J.; et al. Role of Specialized Pro-resolving Mediators in Reducing Neuroinflammation in Neurodegenerative Disorders. Front. Aging Neurosci. 2022, 14, 780811. [Google Scholar] [CrossRef]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized Pro-Resolving Mediators in Neuroinflammation: Overview of Studies and Perspectives of Clinical Applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Sammons, M.; Popescu, M.C.; Chi, J.; Liberles, S.D.; Gogolla, N.; Rolls, A. Brain-body physiology: Local, reflex, and central communication. Cell 2024, 187, 5877–5890. [Google Scholar] [CrossRef]
- Florio, T.M. Emergent Aspects of the Integration of Sensory and Motor Functions. Brain Sci. 2025, 15, 162. [Google Scholar] [CrossRef]
- Powers, R.M.; Hevner, R.F.; Halpain, S. The Neuron Navigators: Structure, function, and evolutionary history. Front. Mol. Neurosci. 2023, 15, 1099554. [Google Scholar] [CrossRef] [PubMed]
- Demmings, M.D.; da Silva Chagas, L.; Traetta, M.E.; Rodrigues, R.S.; Acutain, M.F.; Barykin, E.; Datusalia, A.K.; German-Castelan, L.; Mattera, V.S.; Mazengenya, P.; et al. (Re)building the nervous system: A review of neuron-glia interactions from development to disease. J. Neurochem. 2025, 169, e16258. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells as Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Ding, Y.; Wang, L.; Zhu, Y.J. Current Knowledge of Microglia in Traumatic Spinal Cord Injury. Front. Neurol. 2022, 12, 796704. [Google Scholar] [CrossRef]
- Waisman, A.; Ginhoux, F.; Greter, M.; Bruttger, J. Homeostasis of Microglia in the Adult Brain: Review of Novel Microglia Depletion Systems. Trends Immunol. 2015, 36, 625–636. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Liu, D.; Hsueh, S.C.; Tweedie, D.; Price, N.; Glotfelty, E.; Lecca, D.; Telljohann, R.; deCabo, R.; Hoffer, B.J.; Greig, N.H. Chronic inflammation with microglia senescence at basal forebrain: Impact on cholinergic deficit in Alzheimer’s brain haemodynamics. Brain Commun. 2024, 6, fcae204. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Lyu, J.; Xie, D.; Bhatia, T.N.; Leak, R.K.; Hu, X.; Jiang, X. Microglial/Macrophage polarization and function in brain injury and repair after stroke. CNS Neurosci. Ther. 2021, 27, 515–527. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.L.; Singh, S.K.; Campos-Campos, J.; Colmena, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Caballero, O.; Jorda, A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int. J. Mol. Sci. 2023, 24, 8434. [Google Scholar] [CrossRef] [PubMed]
- Rupareliya, V.P.; Singh, A.A.; Butt, A.M.; Hariharan, A.; Kumar, H. The “molecular soldiers” of the CNS: Astrocytes, a comprehensive review on their roles and molecular signatures. Eur. J. Pharmacol. 2023, 959, 176048. [Google Scholar] [CrossRef]
- Hösli, L.; Zuend, M.; Bredell, G.; Zanker, H.S.; Porto de Oliveira, C.E.; Saab, A.S.; Weber, B. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 2022, 39, 110599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; Cao, Y.; Yang, J. Astrocyte-Mediated Neuroinflammation in Neurological Conditions. Biomolecules 2024, 14, 1204. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Ye, Q.; Jo, J.; Wang, C.Y.; Oh, H.; Zhan, J.; Choy, T.J.; Kim, K.I.; D’Alessandro, A.; Reshetnyak, Y.K.; Jung, S.Y.; et al. Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a CCL2-CCR2 pathway and NO dysregulation. Cell Rep. 2024, 43, 114193. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Moulson, A.J.; Squair, J.W.; Franklin, R.J.M.; Tetzlaff, W.; Assinck, P. Diversity of Reactive Astrogliosis in CNS Pathology: Heterogeneity or Plasticity? Front. Cell. Neurosci. 2021, 15, 703810. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef]
- Ettle, B.; Schlachetzki, J.C.M.; Winkler, J. Oligodendroglia and Myelin in Neurodegenerative Diseases: More Than Just Bystanders? Mol. Neurobiol. 2016, 53, 3046–3062. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Wang, F.; Huang, N.X.; Xiao, L.; Mei, F. Oligodendrocytes and myelin: Active players in neurodegenerative brains? Dev. Neurobiol. 2022, 82, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Pukos, N.; Goodus, M.T.; Sahinkaya, F.R.; McTigue, D.M. Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia 2019, 67, 2178–2202. [Google Scholar] [CrossRef]
- Zveik, O.; Rechtman, A.; Brill, L.; Vaknin-Dembinsky, A. Anti- and pro-inflammatory milieu differentially regulate differentiation and immune functions of oligodendrocyte progenitor cells. Immunology 2024, 171, 618–633. [Google Scholar] [CrossRef] [PubMed]
- López-Muguruza, E.; Matute, C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. [Google Scholar] [CrossRef]
- Boccazzi, M.; Raffaele, S.; Fumagalli, M. Not only myelination: The immune-inflammatory functions of oligodendrocytes. Neural Regen. Res. 2022, 17, 2661–2663. [Google Scholar]
- Chen, J.Q.A.; Wever, D.D.; McNamara, N.B.; Bourik, M.; Smolders, J.; Hamann, J.; Huitinga, I. Inflammatory microglia correlate with impaired oligodendrocyte maturation in multiple sclerosis. Front. Immunol. 2025, 15, 1522381. [Google Scholar] [CrossRef]
- Gualerzi, A.; Lombardi, M.; Verderio, C. Microglia-oligodendrocyte intercellular communication: Role of extracellular vesicle lipids in functional signalling. Neural Regen. Res. 2021, 16, 1194–1195. [Google Scholar]
- Zelic, M.; Blazier, A.; Pontarelli, F.; LaMorte, M.; Huang, J.; Tasdemir-Yilmaz, O.E.; Ren, Y.; Ryan, S.K.; Shapiro, C.; Morel, C.; et al. Single-cell transcriptomic and functional studies identify glial state changes and a role for inflammatory RIPK1 signaling in ALS pathogenesis. Immunity 2025, 58, 961–979.e8. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Maliar, N.L.; Talbot, E.J.; Edwards, A.R.; Khoronenkova, S.V. Microglial inflammation in genome instability: A neurodegenerative perspective. DNA Repair 2024, 135, 103634. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like receptor 4: A potential therapeutic target for multiple human diseases. Biomed. Pharmacother. 2023, 166, 115338. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Laird, M.H.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leukoc. Biol. 2009, 85, 966–977. [Google Scholar] [CrossRef]
- Shalaby, K.H.; Al Heialy, S.; Tsuchiya, K.; Farahnak, S.; McGovern, T.K.; Risse, P.A.; Suh, W.K.; Qureshi, S.T.; Martin, J.G. The TLR4-TRIF pathway can protect against the development of experimental allergic asthma. Immunology 2017, 152, 138–149. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Chu, F.; Zhu, J.; Jin, T. Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Front. Mol. Neurosci. 2020, 12, 314. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Xu, J.; Xiao, K.; Xu, Y.; Guo, T.; Zhang, L.; Wang, J.; Zheng, H. β-TrCP Restricts Lipopolysaccharide (LPS)-Induced Activation of TRAF6-IKK Pathway Upstream of IκBα Signaling. Front. Immunol. 2018, 9, 2930. [Google Scholar] [CrossRef]
- Dhillon, B.; Aleithan, F.; Abdul-Sater, Z.; Abdul-Sater, A.A. The Evolving Role of TRAFs in Mediating Inflammatory Responses. Front. Immunol. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Leiva, A.; Peña, M.; Müller, M.; Debandi, A.; Hidalgo, C.; Carrasco, M.A.; Jaimovich, E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J. Cell. Physiol. 2006, 209, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Mota, M.; Pizzi, M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim. Biophys. Acta 2016, 1862, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Almouhanna, F.; Chausse, B. Interferon γ: A master cytokine in microglia-mediated neural network dysfunction and neurodegeneration. Trends Neurosci. 2022, 45, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Torres, V.; Kielian, T. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J. Neurochem. 2011, 119, 736–748. [Google Scholar] [CrossRef]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef]
- Tewari, M.; Michalski, S.; Egan, T.M. Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human. Cells 2024, 13, 161. [Google Scholar] [CrossRef]
- Irino, Y.; Nakamura, Y.; Inoue, K.; Kohsaka, S.; Ohsawa, K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J. Neurosci. Res. 2008, 86, 1511–1519. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chen, Y.T.; Hu, X.L.; Cai, W.T.; Wang, X.Y.; Ni, W.F.; Zhou, K.L. Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma. Neural Regen. Res. 2023, 18, 258–266. [Google Scholar] [PubMed]
- Akundi, R.S.; Candelario-Jalil, E.; Hess, S.; Hüll, M.; Lieb, K.; Gebicke-Haerter, P.J.; Fiebich, B.L. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 2005, 51, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q. Prostaglandin EP2 receptor: Novel therapeutic target for human cancers (Review). Int. J. Mol. Med. 2018, 42, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, Y.; Hou, R.; Hao, J.; Jiang, J. Inhibiting the PGE2 Receptor EP2 Mitigates Excitotoxicity and Ischemic Injury. ACS Pharmacol. Transl. Sci. 2020, 3, 635–643. [Google Scholar] [CrossRef]
- Surace, M.J.; Block, M.L. Targeting microglia-mediated neurotoxicity: The potential of NOX2 inhibitors. Cell. Mol. Life Sci. 2012, 69, 2409–2427. [Google Scholar] [CrossRef]
- Hu, C.F.; Wu, S.P.; Lin, G.J.; Shieh, C.C.; Hsu, C.S.; Chen, J.W.; Chen, S.H.; Hong, J.S.; Chen, S.J. Microglial Nox2 Plays a Key Role in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2021, 12, 638381. [Google Scholar] [CrossRef]
- Liu, P.W.; Chen, M.F.; Tsai, A.P.; Lee, T.J. STAT1 mediates oroxylin a inhibition of iNOS and pro-inflammatory cytokines expression in microglial BV-2 cells. PLoS ONE 2012, 7, e50363. [Google Scholar] [CrossRef]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin; Ali, A. Pathophysiological Role of Peroxynitrite Induced DNA Damage in Human Diseases: A Special Focus on Poly(ADP-ribose) Polymerase (PARP). Indian J. Clin. Biochem. 2015, 30, 368–385. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, J.M.T.; Chang, R.C.C.; Wong, G.T.C. The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration. Biomolecules 2022, 12, 337. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Pei, H. C1q and central nervous system disorders. Front. Immunol. 2023, 14, 1145649. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fan, L.; Xia, S.; Yang, Q.; Zhang, Z.; Chen, H.; Zeng, H.; Fu, X.; Peng, Y.; Xu, C.; et al. Role of complement C1q/C3-CR3 signaling in brain injury after experimental intracerebral hemorrhage and the effect of minocycline treatment. Front. Immunol. 2022, 13, 919444. [Google Scholar] [CrossRef] [PubMed]
- Ramaglia, V.; Hughes, T.R.; Donev, R.M.; Ruseva, M.M.; Wu, X.; Huitinga, I.; Baas, F.; Neal, J.W.; Morgan, B.P. C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc. Natl. Acad. Sci. USA 2012, 109, 965–970. [Google Scholar] [CrossRef]
- Guo, R.F.; Ward, P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, T.; Bosco, D.B.; Xie, M.; Zheng, J.; Dheer, A.; Ying, Y.; Wu, Q.; Lennon, V.A.; Wu, L.J. The complement C3-C3aR pathway mediates microglia-astrocyte interaction following status epilepticus. Glia 2021, 69, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.; Schartz, N.D.; Balderrama-Gutierrez, G.; Liang, H.Y.; Chu, S.H.; Selvan, P.; Gomez-Arboledas, A.; Petrisko, T.J.; Fonseca, M.I.; Mortazavi, A.; et al. Modulation of C5a–C5aR1 signaling alters the dynamics of AD progression. J. Neuroinflamm. 2022, 19, 178. [Google Scholar] [CrossRef]
- Orsini, F.; De Blasio, D.; Zangari, R.; Zanier, E.R.; De Simoni, M.G. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front. Cell. Neurosci. 2014, 8, 380. [Google Scholar] [CrossRef]
- Warwick, C.A.; Keyes, A.L.; Woodruff, T.M.; Usachev, Y.M. The complement cascade in the regulation of neuroinflammation, nociceptive sensitization, and pain. J. Biol. Chem. 2021, 297, 101085. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. Immunotargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef]
- Song, J.; Wu, C.; Korpos, E.; Zhang, X.; Agrawal, S.M.; Wang, Y.; Faber, C.; Schäfers, M.; Körner, H.; Opdenakker, G.; et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015, 10, 1040–1054. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Salsinha, A.S.; Socodato, R.; Rodrigues, A.; Vale-Silva, R.; Relvas, J.B.; Pintado, M.; Rodríguez-Alcalá, L.M. Potential of omega-3 and conjugated fatty acids to control microglia inflammatory imbalance elicited by obesogenic nutrients. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2023, 1868, 159331. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, M.; Chiurchiù, V. Specialized Pro-resolving Lipid Mediators and Glial Cells: Emerging Candidates for Brain Homeostasis and Repair. Front. Cell. Neurosci. 2021, 15, 673549. [Google Scholar] [CrossRef]
- Dokalis, N.; Prinz, M. Resolution of neuroinflammation: Mechanisms and potential therapeutic option. Semin. Immunopathol. 2019, 41, 699–709. [Google Scholar] [CrossRef]
- Ji, R.R. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 273–293. [Google Scholar] [CrossRef]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef]
- Neurath, M.F. Resolution of inflammation: From basic concepts to clinical application. Semin. Immunopathol. 2019, 41, 627–631. [Google Scholar] [CrossRef]
- Chen, Y.; Kou, Y.; Ni, Y.; Yang, H.; Xu, C.; Fan, H.; Liu, H. Microglia efferocytosis: An emerging mechanism for the resolution of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2025, 22, 96. [Google Scholar] [CrossRef]
- Wu, S.Y.; Pan, B.S.; Tsai, S.F.; Chiang, Y.T.; Huang, B.M.; Mo, F.E.; Kuo, Y.M. BDNF reverses aging-related microglial activation. J. Neuroinflamm. 2020, 17, 210. [Google Scholar] [CrossRef]
- Wan, Y.; Gao, W.; Zhou, K.; Liu, X.; Jiang, W.; Xue, R.; Wu, W. Role of IGF-1 in neuroinflammation and cognition deficits induced by sleep deprivation. Neurosci. Lett. 2022, 776, 136575. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.A.; Planel, E.; Terrando, N. Impaired Resolution of Inflammation in Alzheimer’s Disease: A Review. Front. Immunol. 2017, 8, 1464. [Google Scholar] [CrossRef] [PubMed]
- Lotz, S.K.; Blackhurst, B.M.; Reagin, K.L.; Funk, K.E. Microbial Infections Are a Risk Factor for Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 691136. [Google Scholar] [CrossRef] [PubMed]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain-Immune Interactions and Neuroinflammation After Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Bernaus, A.; Blanco, S.; Sevilla, A. Glia Crosstalk in Neuroinflammatory Diseases. Front. Cell. Neurosci. 2020, 14, 209. [Google Scholar] [CrossRef]
- Oyarce, K.; Cepeda, M.Y.; Lagos, R.; Garrido, C.; Vega-Letter, A.M.; Garcia-Robles, M.; Luz-Crawford, P.; Elizondo-Vega, R. Neuroprotective and Neurotoxic Effects of Glial-Derived Exosomes. Front. Cell. Neurosci. 2022, 16, 920686. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Mol. Neurobiol. 2023, 60, 923–959. [Google Scholar] [CrossRef]
- Zhu, Y.; Webster, M.J.; Mendez Victoriano, G.; Middleton, F.A.; Massa, P.T.; Weickert, C.S. Molecular Evidence for Altered Angiogenesis in Neuroinflammation-Associated Schizophrenia and Bipolar Disorder Implicate an Abnormal Midbrain Blood-Brain Barrier. Schizophr. Bull. 2024, sbae184. [Google Scholar] [CrossRef]
- Wang, Y.; Leak, R.K.; Cao, G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front. Cell. Neurosci. 2022, 16, 980722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal. Transduct. Target Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef]
- Mancuso, M.R.; Kuhnert, F.; Kuo, C.J. Developmental angiogenesis of the central nervous system. Lymphat. Res. Biol. 2008, 6, 173–180. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015, 36, 637–650. [Google Scholar] [CrossRef]
- Johnson, H.J.; Koshy, A.A. Understanding neuroinflammation through central nervous system infections. Curr. Opin. Neurobiol. 2022, 76, 102619. [Google Scholar] [CrossRef]
- Schwartz, M.; Baruch, K. The resolution of neuroinflammation in neurodegeneration: Leukocyte recruitment via the choroid plexus. EMBO J. 2014, 33, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Viviani, B.; Boraso, M.; Marchetti, N.; Marinovich, M. Perspectives on neuroinflammation and excitotoxicity: A neurotoxic conspiracy? Neurotoxicology 2014, 43, 10–20. [Google Scholar] [CrossRef]
- Mottahedin, A.; Ardalan, M.; Chumak, T.; Riebe, I.; Ek, J.; Mallard, C. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front. Cell. Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, M.; Foss, J.F.; Giordano, T. Neuroinflammation, its Role in Alzheimer’s Disease and Therapeutic Strategie. J. Prev. Alzheimers Dis. 2023, 10, 686–698. [Google Scholar] [CrossRef]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef]
- Peng, W.; Xie, Y.; Liao, C.; Bai, Y.; Wang, H.; Li, C. Spatiotemporal patterns of gliosis and neuroinflammation in presenilin 1/2 conditional double knockout mice. Front. Aging Neurosci. 2022, 14, 966153. [Google Scholar] [CrossRef]
- Tian, L.; Ma, L.; Kaarela, T.; Li, Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J. Neuroinflamm. 2012, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Taipa, R.; das Neves, S.P.; Sousa, A.L.; Fernandes, J.; Pinto, C.; Correia, A.P.; Santos, E.; Pinto, P.S.; Carneiro, P.; Costa, P.; et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 2019, 76, 125–132. [Google Scholar] [CrossRef]
- Bowirrat, A. Immunosenescence and Aging: Neuroinflammation Is a Prominent Feature of Alzheimer’s Disease and Is a Likely Contributor to Neurodegenerative Disease Pathogenesis. J. Pers. Med. 2022, 12, 1817. [Google Scholar] [CrossRef]
- McCarthy, M.M. Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology 2019, 44, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Jagodic, M.; Piehl, F.; Wallström, E. Genetics of autoimmune neuroinflammation. Curr. Opin. Immunol. 2006, 18, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Murtaj, V.; Belloli, S.; Di Grigoli, G.; Pannese, M.; Ballarini, E.; Rodriguez-Menendez, V.; Marmiroli, P.; Cappelli, A.; Masiello, V.; Monterisi, C.; et al. Age and Sex Influence the Neuro-inflammatory Response to a Peripheral Acute LPS Challenge. Front. Aging Neurosci. 2019, 11, 299. [Google Scholar] [CrossRef]
- Caldarelli, M.; Rio, P.; Marrone, A.; Ocarino, F.; Chiantore, M.; Candelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut-Brain Axis: Focus on Sex Differences in Neuroinflammation. Int. J. Mol. Sci. 2024, 25, 5377. [Google Scholar] [CrossRef]
- O’Callaghan, J.P.; Miller, D.B. Neuroinflammation disorders exacerbated by environmental stressors. Metabolism 2019, 100S, 153951. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Stocchi, F.; Bravi, D.; Emmi, A.; Antonini, A. Parkinson disease therapy: Current strategies and future research priorities. Nat. Rev. Neurol. 2024, 20, 695–707. [Google Scholar] [CrossRef]
- Talanki Manjunatha, R.; Habib, S.; Sangaraju, S.L.; Yepez, D.; Grandes, X.A. Multiple Sclerosis: Therapeutic Strategies on the Horizon. Cureus 2022, 14, e24895. [Google Scholar] [CrossRef] [PubMed]
- Arfeen, M.; Srivastava, A.; Srivastava, N.; Khan, R.A.; Almahmoud, S.A.; Mohammed, H.A. Design, classification, and adverse effects of NSAIDs: A review on recent advancements. Bioorg. Med. Chem. 2024, 112, 117899. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, C.J. COX-1 and COX-2 inhibitors. Best. Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50, S423–S428. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Temel, S.G.; Kahveci, Z. Cyclooxygenase-2 expression in astrocytes and microglia in human oligodendroglioma and astrocytoma. J. Mol. Histol. 2009, 40, 369–377. [Google Scholar] [CrossRef]
- Hiskens, M.I.; Schneiders, A.G.; Fenning, A.S. Selective COX-2 Inhibitors as Neuroprotective Agents in Traumatic Brain Injury. Biomedicines 2024, 12, 1930. [Google Scholar] [CrossRef]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Puhl, A.C.; Milton, F.A.; Cvoro, A.; Sieglaff, D.H.; Campos, J.C.; Bernardes, A.; Filgueira, C.S.; Lindemann, J.L.; Deng, T.; Neves, F.A.; et al. Mechanisms of peroxisome proliferator activated receptor γ regulation by non-steroidal anti-inflammatory drugs. Nucl. Recept. Signal. 2015, 13, e004. [Google Scholar] [CrossRef]
- Radi, Z.A.; Khan, N.K. Effects of cyclooxygenase inhibition on the gastrointestinal tract. Exp. Toxicol. Pathol. 2006, 58, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Drożdżal, S.; Lechowicz, K.; Szostak, B.; Rosik, J.; Kotfis, K.; Machoy-Mokrzyńska, A.; Białecka, M.; Ciechanowski, K.; Gawrońska-Szklarz, B. Kidney damage from nonsteroidal anti-inflammatory drugs-Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 2021, 9, e00817. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Sabzwari, S.R.A.; Vargova, V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus 2017, 9, e1144. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Miyake, S.; Mizuno, M.; Oka, N.; Kusunoki, S.; Yamamura, T. Selective COX-2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX-2-independent pathway. Brain 2006, 129, 1984–1992. [Google Scholar] [CrossRef]
- Irrera, N.; Bitto, A. Evidence for using a dual COX 1/2 and 5-LOX inhibitor in neurodegenerative diseases. Neural Regen. Res. 2017, 12, 1077–1078. [Google Scholar]
- Ajmone-Cat, M.A.; Bernardo, A.; Greco, A.; Minghetti, L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals 2010, 3, 1949–1965. [Google Scholar] [CrossRef]
- Gwee, K.A.; Goh, V.; Lima, G.; Setia, S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: Risks versus benefits. J. Pain Res. 2018, 11, 361–374. [Google Scholar] [CrossRef]
- Tsau, S.; Emerson, M.R.; Lynch, S.G.; LeVine, S.M. Aspirin and multiple sclerosis. BMC Med. 2015, 13, 153. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Ding, P.; Gorenflo, M.P.; Zhu, X.; Xu, R. Aspirin Use and Risk of Alzheimer’s Disease: A 2-Sample Mendelian Randomization Study. J. Alzheimers Dis. 2023, 92, 989–1000. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Schwarzschild, M.A.; Ascherio, A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011, 76, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Badawoud, A.M.; Ali, L.S.; Abdallah, M.S.; El Sabaa, R.M.; Bahaa, M.M.; Elmasry, T.A.; Wahsh, E.; Yasser, M.; Eltantawy, N.; Eldesoqui, M.; et al. The relation between Parkinson’s disease and non-steroidal anti-inflammatories; a systematic review and meta-analysis. Front. Pharmacol. 2024, 15, 1434512. [Google Scholar] [CrossRef]
- Khrieba, M.O.; Hegazy, S.K.; Mustafa, W.; El-Haggar, S.M. Repurposing celecoxib as adjuvant therapy in patients with Parkinsonian disease: A new therapeutic dawn: Randomized controlled pilot study. Inflammopharmacology 2024, 32, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, D.; Hallan, D.R.; Staub, J.; Rizk, E.; Zacko, J.C. Early Celecoxib use in Patients with Traumatic Brain Injury. Neurocrit. Care 2024, 40, 886–897. [Google Scholar] [CrossRef]
- Tsai, C.P.; Lin, F.C.; Lee, J.K.; Lee, C.T. Aspirin use associated with amyotrophic lateral sclerosis: A total population-based case-control study. J. Epidemiol. 2015, 25, 172–177. [Google Scholar] [CrossRef]
- Salomon-Zimri, S.; Pushett, A.; Russek-Blum, N.; Van Eijk, R.P.A.; Birman, N.; Abramovich, B.; Eitan, E.; Elgrart, K.; Beaulieu, D.; Ennist, D.L.; et al. Combination of ciprofloxacin/celecoxib as a novel therapeutic strategy for ALS. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Staels, B.; Dacquet, C.; Spedding, M.; Laudet, V. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 2006, 58, 685–704. [Google Scholar] [CrossRef]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef]
- Stockner, T.; Sterk, H.; Kaptein, R.; Bonvin, A.M. Molecular dynamics studies of a molecular switch in the glucocorticoid receptor. J. Mol. Biol. 2003, 328, 325–334. [Google Scholar] [CrossRef]
- Meijer, O.C.; Kalkhoven, E.; van der Laan, S.; Steenbergen, P.J.; Houtman, S.H.; Dijkmans, T.F.; Pearce, D.; de Kloet, E.R. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology 2005, 146, 1438–1448. [Google Scholar] [CrossRef]
- Van Moortel, L.; Verhee, A.; Thommis, J.; Houtman, R.; Melchers, D.; Delhaye, L.; Van Leene, C.; Hellemans, M.; Gevaert, K.; Eyckerman, S.; et al. Selective Modulation of the Human Glucocorticoid Receptor Compromises GR Chromatin Occupancy and Recruitment of p300/CBP and the Mediator Complex. Mol. Cell. Proteom. 2024, 23, 100741. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.P.; Greulich, F.; Ansari, S.A.; Uhlenhaut, N.H. Anti-inflammatory glucocorticoid action: Genomic insights and emerging concepts. Curr. Opin. Pharmacol. 2020, 53, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- González, M.V.; Jiménez, B.; Berciano, M.T.; González-Sancho, J.M.; Caelles, C.; Lafarga, M.; Muñoz, A. Glucocorticoids antagonize AP-1 by inhibiting the Activation/phosphorylation of JNK without affecting its subcellular distribution. J. Cell. Biol. 2000, 150, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Kimmel, S.C.; Levin, R.I.; Martiniuk, F.; Weissmann, G. A mechanism for the antiinflammatory effects of corticosteroids: The glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1992, 89, 9991–9995. [Google Scholar] [CrossRef]
- Atsuta, J.; Plitt, J.; Bochner, B.S.; Schleimer, R.P. Inhibition of VCAM-1 expression in human bronchial epithelial cells by glucocorticoids. Am. J. Respir. Cell. Mol. Biol. 1999, 20, 643–650. [Google Scholar] [CrossRef]
- Fukuzuka, K.; Edwards, C.K., 3rd; Clare-Salzer, M.; Copeland, E.M., 3rd; Moldawer, L.L.; Mozingo, D.W. Glucocorticoid and Fas ligand induced mucosal lymphocyte apoptosis after burn injury. J. Trauma. 2000, 49, 710–716. [Google Scholar] [CrossRef]
- López-Royuela, N.; Balsas, P.; Galán-Malo, P.; Anel, A.; Marzo, I.; Naval, J. Bim is the key mediator of glucocorticoid-induced apoptosis and of its potentiation by rapamycin in human myeloma cells. Biochim. Biophys. Acta 2010, 1803, 311–322. [Google Scholar] [CrossRef]
- Salvador, E.; Shityakov, S.; Förster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef]
- Mustafa, S.S. Steroid-induced secondary immune deficiency. Ann. Allergy Asthma Immunol. 2023, 130, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Durham, H.; Glover, L.; Ather, O.; Phillips, V.; Nemes, S.; Cousens, L.; Blomgran, P.; Ambery, P. Metabolic adverse events associated with systemic corticosteroid therapy-a systematic review and meta-analysis. BMJ Open 2022, 12, e061476. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef]
- Nasereddin, L.; Alnajjar, O.; Bashar, H.; Abuarab, S.F.; Al-Adwan, R.; Chellappan, D.K.; Barakat, M. Corticosteroid-Induced Psychiatric Disorders: Mechanisms, Outcomes, and Clinical Implications. Diseases 2024, 12, 300. [Google Scholar] [CrossRef]
- Durelli, L.; Cocito, D.; Riccio, A.; Barile, C.; Bergamasco, B.; Baggio, G.F.; Perla, F.; Delsedime, M.; Gusmaroli, G.; Bergamini, L. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: Clinical-immunologic correlations. Neurology 1986, 36, 238–243. [Google Scholar] [CrossRef]
- Barkhof, F.; Tas, M.W.; Frequin, S.T.; Scheltens, P.; Hommes, O.R.; Nauta, J.J.; Valk, J. Limited duration of the effect of methylprednisolone on changes on MRI in multiple sclerosis. Neuroradiology 1994, 36, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Havrdova, E.; Zivadinov, R.; Krasensky, J.; Dwyer, M.G.; Novakova, I.; Dolezal, O.; Ticha, V.; Dusek, L.; Houzvickova, E.; Cox, J.L.; et al. Randomized study of interferon beta-1a, low-dose azathioprine, and low-dose corticosteroids in multiple sclerosis. Mult. Scler. 2009, 15, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H.; Probasco, J.; Irani, S.R.; Ances, B.; Benavides, D.R.; Bradshaw, M.; Christo, P.P.; Dale, R.C.; Fernandez-Fournier, M.; Flanagan, E.P.; et al. Autoimmune Encephalitis Alliance Clinicians Network. Autoimmune encephalitis: Proposed recommendations for symptomatic and long-term management. J. Neurol. Neurosurg. Psychiatry 2021, 92, 897–907. [Google Scholar] [CrossRef]
- Abbatemarco, J.R.; Yan, C.; Kunchok, A.; Rae-Grant, A. Antibody-mediated autoimmune encephalitis: A practical approach. Cleve. Clin. J. Med. 2021, 88, 459–471. [Google Scholar] [CrossRef]
- Stingl, C.; Cardinale, K.; Van Mater, H. An Update on the Treatment of Pediatric Autoimmune Encephalitis. Curr. Treatm. Opt. Rheumatol. 2018, 4, 14–28. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, X.; He, Z.; Tang, D.; Li, Y.; Li, P. Efficacy of dexamethasone combined with intravenous immunoglobulin for the treatment of pediatric autoimmune encephalitis. Front. Neurol. 2025, 16, 1512908. [Google Scholar] [CrossRef] [PubMed]
- Kothari, M.; Wanjari, A.; Acharya, S.; Karwa, V.; Chavhan, R.; Kumar, S.; Kadu, A.; Patil, R. A Comprehensive Review of Monoclonal Antibodies in Modern Medicine: Tracing the Evolution of a Revolutionary Therapeutic Approach. Cureus 2024, 16, e61983. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Coisne, C.; Mao, W.; Engelhardt, B. Cutting edge: Natalizumab blocks adhesion but not initial contact of human T cells to the blood-brain barrier in vivo in an animal model of multiple sclerosis. J. Immunol. 2009, 182, 5909–5913. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, K. Natalizumab for multiple sclerosis: The dilemma of NOVA. Lancet Neurol. 2022, 21, 579–581. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Liu, L.; Gong, X.; Liu, J.; Sun, L.; Liu, X.; Wu, L.; Chen, L.; Wang, L.; et al. Fine Comparison of the Efficacy and Safety Between GB242 and Infliximab in Patients with Rheumatoid Arthritis: A Phase III Study. Rheumatol. Ther. 2022, 9, 175–189. [Google Scholar] [CrossRef]
- Traczewski, P.; Rudnicka, L. Adalimumab in dermatology. Br. J. Clin. Pharmacol. 2008, 66, 618–625. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Strangfeld, A.; Listing, J. Infection and musculoskeletal conditions: Bacterial and opportunistic infections during anti-TNF therapy. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1181–1195. [Google Scholar] [CrossRef]
- Kim, S.Y.; Solomon, D.H. Tumor necrosis factor blockade and the risk of viral infection. Nat. Rev. Rheumatol. 2010, 6, 165–174. [Google Scholar] [CrossRef]
- Li, Y.; Ye, R.; Dai, H.; Lin, J.; Cheng, Y.; Zhou, Y.; Lu, Y. Exploring TNFR1: From discovery to targeted therapy development. J. Transl Med. 2025, 23, 71. [Google Scholar] [CrossRef]
- Torene, R.; Nirmala, N.; Obici, L.; Cattalini, M.; Tormey, V.; Caorsi, R.; Starck-Schwertz, S.; Letzkus, M.; Hartmann, N.; Abrams, K.; et al. Canakinumab reverses overexpression of inflammatory response genes in tumour necrosis factor receptor-associated periodic syndrome. Ann. Rheum Dis. 2017, 76, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.L.; Nidorf, S.M. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: Lessons from the CANTOS trial. J. Thorac. Dis. 2018, 10, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Weickert, T.W.; Jacomb, I.; Lenroot, R.; Lappin, J.; Weinberg, D.; Brooks, W.S.; Brown, D.; Pellen, D.; Kindler, J.; Mohan, A.; et al. Adjunctive canakinumab reduces peripheral inflammation markers and improves positive symptoms in people with schizophrenia and inflammation: A randomized control trial. Brain Behav. Immun. 2024, 115, 191–200. [Google Scholar] [CrossRef]
- Parisi, S.; Ditto, M.C.; Ghellere, F.; Panaro, S.; Piccione, F.; Borrelli, R.; Fusaro, E. Update on tocilizumab in rheumatoid arthritis: A narrative review. Front. Immunol. 2025, 16, 1470488. [Google Scholar] [CrossRef]
- Yu, Y.M.; Jin, G.H.; Zhong, C.; Qian, H.; Wang, L.; Zhan, F. Exploring the role of interleukin-6 receptor blockade in epilepsy and associated neuropsychiatric conditions through a mendelian randomization study. World J. Psychiatry 2024, 14, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Cadiz, M.P.; Gibson, K.A.; Todd, K.T.; Nascari, D.G.; Massa, N.; Lilley, M.T.; Olney, K.C.; Al-Amin, M.M.; Jiang, H.; Holtzman, D.M.; et al. Aducanumab anti-amyloid immunotherapy induces sustained microglial and immune alterations. J. Exp. Med. 2024, 221, e20231363. [Google Scholar] [CrossRef]
- Nisticò, R.; Borg, J.J. Aducanumab for Alzheimer’s disease: A regulatory perspective. Pharmacol. Res. 2021, 171, 105754. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Masaki, K.; Tanaka, E.; Matsushita, T.; Isobe, N. The Efficacy of Eculizumab in the Acute Phase of Neuromyelitis Optica Spectrum Disorder: A Case Series Study. Cureus 2024, 16, e73205. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, S.; Fyfe, B.; Weiss, L.; Xiao, X.; Zhang, Y.; Smith, R.J. Eculizumab and recurrent C3 glomerulonephritis. Pediatr. Nephrol. 2013, 28, 1975–1981. [Google Scholar] [CrossRef]
- Malagoli, P.; Dapavo, P.; Amerio, P.; Atzori, L.; Balato, A.; Bardazzi, F.; Bianchi, L.; Cattaneo, A.; Chiricozzi, A.; Congedo, M.; et al. Secukinumab in the Treatment of Psoriasis: A Narrative Review on Early Treatment and Real-World Evidence. Dermatol. Ther. 2024, 14, 2739–2757. [Google Scholar] [CrossRef] [PubMed]
- Shelton, S.K.; Bai, S.R.; Jordan, J.K.; Sheehan, A.H. Ixekizumab: A Review of Its Use for the Management of Moderate to Severe Plaque Psoriasis. Ann. Pharmacother. 2019, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Huang, L.; Flores, J.; Ding, Y.; Li, P.; Peng, J.; Zuo, G.; Zhang, J.H.; Lu, J.; Tang, J.P. Secukinumab attenuates reactive astrogliosis via IL-17RA/(C/EBPβ)/SIRT1 pathway in a rat model of germinal matrix hemorrhage. CNS Neurosci. Ther. 2019, 25, 1151–1161. [Google Scholar] [CrossRef]
- Liu, S.; Deng, S.; Ding, Y.; Flores, J.J.; Zhang, X.; Jia, X.; Hu, X.; Peng, J.; Zuo, G.; Zhang, J.H.; et al. Secukinumab attenuates neuroinflammation and neurobehavior defect via PKCβ/ERK/NF-κB pathway in a rat model of GMH. Exp. Neurol. 2023, 360, 114276. [Google Scholar] [CrossRef]
- Li, Q.; Han, X.; Dong, M.; Bai, L.; Zhang, W.; Liu, W.; Wang, F.; Zhu, X. FDA-Approved Secukinumab Alleviates Glial Activation and Immune Cell Infiltration in MPTP-Induced Mouse Model of Parkinson’s Disease. Inflammation 2025, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Langrish, C.L.; McKenzie, B.; Joyce-Shaikh, B.; Stumhofer, J.S.; McClanahan, T.; Blumenschein, W.; Churakovsa, T.; Low, J.; Presta, L.; et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Investig. 2006, 116, 1317–1326. [Google Scholar] [CrossRef]
- Gklinos, P.; Papadopoulou, M.; Stanulovic, V.; Mitsikostas, D.D.; Papadopoulos, D. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Ruck, T.; Nimmerjahn, F.; Wiendl, H.; Lünemann, J.D. Next-generation antibody-based therapies in neurology. Brain 2022, 145, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Cree, B.A.C.; Hauser, S.L. Ocrelizumab and Other CD20+ B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 835–841. [Google Scholar] [CrossRef]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef]
- Pittock, S.J.; Berthele, A.; Fujihara, K.; Kim, H.J.; Levy, M.; Palace, J.; Nakashima, I.; Terzi, M.; Totolyan, N.; Viswanathan, S.; et al. Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019, 381, 614–625. [Google Scholar] [CrossRef]
- Yamamura, T.; Kleiter, I.; Fujihara, K.; Palace, J.; Greenberg, B.; Zakrzewska-Pniewska, B.; Patti, F.; Tsai, C.P.; Saiz, A.; Yamazaki, H.; et al. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019, 381, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Fujihara, K.; Paul, F.; Cutter, G.R.; Marignier, R.; et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): A double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019, 394, 1352–1363. [Google Scholar] [CrossRef]
- Thaler, F.S.; Zimmermann, L.; Kammermeier, S.; Strippel, C.; Ringelstein, M.; Kraft, A.; Sühs, K.W.; Wickel, J.; Geis, C.; Markewitz, R.; et al. Rituximab Treatment and Long-term Outcome of Patients with Autoimmune Encephalitis: Real-world Evidence from the GENERATE Registry. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1088. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, S.T.; Moon, J.; Sunwoo, J.S.; Byun, J.I.; Lim, J.A.; Kim, T.J.; Shin, Y.W.; Lee, K.J.; Jun, J.S.; et al. Tocilizumab in Autoimmune Encephalitis Refractory to Rituximab: An Institutional Cohort Study. Neurotherapeutics 2016, 13, 824–832. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Boess, F.G.; Taylor, K.I.; Ricci, B.; Mollenhauer, B.; Poewe, W.; Boulay, A.; Anzures-Cabrera, J.; Vogt, A.; Marchesi, M.; et al. A Phase II Study to Evaluate the Safety and Efficacy of Prasinezumab in Early Parkinson’s Disease (PASADENA): Rationale, Design, and Baseline Data. Front. Neurol. 2021, 12, 705407. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Fraser, K.; Yang, M.; Fox, T.; Hirschhorn, E.; Njingti, E.; Scott, D.; Bedell, B.J.; Kistner, K.M.; Cedarbaum, J.M.; et al. Cinpanemab in Early Parkinson Disease: Evaluation of Biomarker Results From the Phase 2 SPARK Clinical Trial. Neurology 2024, 102, e209137. [Google Scholar] [CrossRef]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef]
- Navarra, S.V.; Guzmán, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.; Thomas, M.; Kim, H.Y.; León, M.G.; et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 721–731. [Google Scholar] [CrossRef]
- Hu, B.; Duan, S.; Wang, Z.; Li, X.; Zhou, Y.; Zhang, X.; Zhang, Y.W.; Xu, H.; Zheng, H. Insights Into the Role of CSF1R in the Central Nervous System and Neurological Disorders. Front. Aging Neurosci. 2021, 13, 789834. [Google Scholar] [CrossRef]
- Hupp, S.; Iliev, A.I. CSF-1 receptor inhibition as a highly effective tool for depletion of microglia in mixed glial cultures. J. Neurosci. Methods 2020, 332, 108537. [Google Scholar] [CrossRef]
- Jovanovic, J.; Liu, X.; Kokona, D.; Zinkernagel, M.S.; Ebneter, A. Inhibition of inflammatory cells delays retinal degeneration in experimental retinal vein occlusion in mice. Glia 2020, 68, 574–588. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Weigel, T.K.; Poffenberger, C.N.; Herkenham, M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J. Neurosci. 2019, 39, 5594–5605. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, E.; Severson, P.L.; Hohsfield, L.A.; Crapser, J.; Zhang, J.; Burton, E.A.; Zhang, Y.; Spevak, W.; Lin, J.; Phan, N.Y.; et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 3758. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. Ther. 2022, 231, 107989. [Google Scholar] [CrossRef]
- Ridderstad Wollberg, A.; Ericsson-Dahlstrand, A.; Juréus, A.; Ekerot, P.; Simon, S.; Nilsson, M.; Wiklund, S.J.; Berg, A.L.; Ferm, M.; Sunnemark, D.; et al. Pharmacological inhibition of the chemokine receptor CX3CR1 attenuates disease in a chronic-relapsing rat model for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2014, 111, 5409–5414. [Google Scholar] [CrossRef]
- Suresh, P.; Phasuk, S.; Liu, I.Y. Modulation of microglia activation and Alzheimer’s disease: CX3 chemokine ligand 1/CX3CR and P2X7R signaling. Tzu. Chi. Med. J. 2021, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Qi, M.; Zhang, X.; Wu, D. Pioglitazone Modulates Microglia M1/M2 Polarization Through PPAR-γ Pathway and Exerts Neuroprotective Effects in Experimental Subarachnoid Hemorrhage. Mol. Neurobiol. 2025, 62, 5930–5946. [Google Scholar] [CrossRef]
- Barczuk, J.; Siwecka, N.; Lusa, W.; Rozpędek-Kamińska, W.; Kucharska, E.; Majsterek, I. Targeting NLRP3-Mediated Neuroinflammation in Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2022, 23, 8979. [Google Scholar] [CrossRef]
- Schwaid, A.G.; Spencer, K.B. Strategies for Targeting the NLRP3 Inflammasome in the Clinical and Preclinical Space. J. Med. Chem. 2021, 64, 101–122. [Google Scholar] [CrossRef]
- Imre, G. Pyroptosis in health and disease. Am. J. Physiol.-Cell Physiol. 2024, 326, C784–C794. [Google Scholar] [CrossRef]
- Xia, L.; Liu, L.; Cai, Y.; Zhang, Y.; Tong, F.; Wang, Q.; Ding, J.; Wang, X. Inhibition of Gasdermin D-Mediated Pyroptosis Attenuates the Severity of Seizures and Astroglial Damage in Kainic Acid-Induced Epileptic Mice. Front. Pharmacol. 2022, 12, 751644. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.R.; Guillot-Sestier, M.V.; Town, T. The role of the immune system in neurodegenerative disorders: Adaptive or maladaptive? Brain Res. 2015, 1617, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Angiulli, F.; Conti, E.; Zoia, C.P.; Da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Blood-Based Biomarkers of Neuroinflammation in Alzheimer’s Disease: A Central Role for Periphery? Diagnostics 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef]
- Ma, C.; Seong, H.; Li, X.; Yu, X.; Xu, S.; Li, Y. Human Brain Organoid: A Versatile Tool for Modeling Neurodegeneration Diseases and for Drug Screening. Stem Cells Int. 2022, 2022, 2150680. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Moosavi Basri, S.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019, 56, 8489–8512. [Google Scholar] [CrossRef]
- Ousman, S.S.; Kubes, P. Immune surveillance in the central nervous system. Nat. Neurosci. 2012, 15, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Faravelli, I.; Velardo, D.; Podestà, M.A.; Ponticelli, C. Immunosuppression-related neurological disorders in kidney transplantation. J. Nephrol. 2021, 34, 539–555. [Google Scholar] [CrossRef]
- Clarke, G.J.B.; Follestad, T.; Skandsen, T.; Zetterberg, H.; Vik, A.; Blennow, K.; Olsen, A.; Håberg, A.K. Chronic immunosuppression across 12 months and high ability of acute and subacute CNS-injury biomarker concentrations to identify individuals with complicated mTBI on acute CT and MRI. J. Neuroinflamm. 2024, 21, 109. [Google Scholar] [CrossRef]
- Castro, I.; Ruiz, J.; Tasias, M.; Montero, M.; Salavert, M. Central nervous system infections in immunocompromised patients. Rev. Esp. Quimioter. 2018, 31 (Suppl. S1), 56–61. [Google Scholar] [PubMed]
- Lee, D.H.; Lee, J.Y.; Hong, D.Y.; Lee, E.C.; Park, S.W.; Lee, Y.K.; Oh, J.S. Pharmacological Treatment for Neuroinflammation in Stress-Related Disorder. Biomedicines 2022, 10, 2518. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.; Giaccone, G.; Calvert, H.; Lobbezoo, M.; Eisenhauer, E.A. Targeted agents: How to select the winners in preclinical and early clinical studies? Eur. J. Cancer 2012, 48, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, F.; Beltrán, E.; Gerdes, L.A.; Becher, B. Single-cell multiomics in neuroinflammation. Curr. Opin. Immunol. 2022, 76, 102180. [Google Scholar] [CrossRef]
- Li, S.; Lu, C.; Zhao, Z.; Lu, D.; Zheng, G. Uncovering neuroinflammation-related modules and potential repurposing drugs for Alzheimer’s disease through multi-omics data integrative analysis. Front. Aging Neurosci. 2023, 15, 1161405. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, R.Y.; Dong, M.Y.; Qian, Y.L.; Wang, X.H.; Xia, W.W.; Shen, Y.; Zhang, K.Z. Integrated Analysis of Single-Cell and Transcriptome Data Reveals the Role and Regulatory Mechanisms of Neuroinflammation in Parkinson’s Disease. Inflammation 2025, 1–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).