Abstract
The Mediterranean diet, rich in plant-based foods, healthy fats, and herbs, has long been associated with a range of health benefits, including cardiovascular, neuroprotective, and anti-inflammatory effects. Recent studies suggest that certain components of this diet, particularly spices such as bay laurel, thyme, oregano, sage, and rosemary, may play a critical role in protecting the retina from oxidative damage, a key factor in blue-light-induced retinal degeneration. Blue light, emitted by digital screens and artificial lighting, has been implicated in the development of retinal conditions like age-related macular degeneration by inducing oxidative stress and inflammation. This review explores the potential of the herbs and spices commonly present in the Mediterranean diet to mitigate blue-light-induced retinal damage. These herbs are rich in polyphenols, flavonoids, essential oils, and terpenes, which offer antioxidant, anti-inflammatory, and antimicrobial properties, contributing to retinal health and reducing oxidative damage. By focusing on bioactive compounds such as eucalyptol (1,8-cineole), rosmarinic acid, carnosic acid, eugenol, and thymol, this article investigates how these herbs and spices might act as natural protectants against blue-light-induced stress and retinal degeneration. The findings highlight the promising role of these culinary staples in preventing retinal damage and offer insights into future dietary recommendations for eye health in an increasingly digital world.
1. Introduction
In recent nutrition research, the focus has shifted from examining the effects of individual nutrients or foods to exploring overall dietary patterns [1]. This change reflects the growing belief that a combination of foods and nutrients, through their synergistic interactions, plays a more significant role in influencing health outcomes than isolated nutrients alone.
The Mediterranean diet (MedDiet) is a term coined to identify a dietary pattern originating from the traditional cuisines of countries located around the Mediterranean Sea. It is most closely associated with the diet of Crete, much of Greece, and southern Italy from the early 1960s [2]. This diet, rich in whole plant foods, healthy fats, and fish, is recognized for its health benefits and is considered more than just a set of dietary guidelines, incorporating social, cultural, economic, and environmental aspects. In 2010, UNESCO declared the MedDiet an Intangible Cultural Heritage of Humanity [2,3]. In the initial document, four countries were mentioned—Greece, Italy, Morocco, and Spain—and the list was later expanded in 2013 by adding Croatia, Cyprus, and Portugal.
Key features of the traditional MedDiet include a high intake of plant foods; choosing fresh fruit as a typical dessert, with sweets containing sugars or honey being consumed rarely; a high intake of olive oil as the main source of fat (especially virgin and extra virgin); a moderate intake of dairy products (mainly in the form of cheese and yoghurt), with only up to four eggs consumed per week; a low to moderate intake of fish and poultry; a low intake of red meat; and moderate consumption of wine with meals [4].
Beyond the foundational foods of the MedDiet, it is important to note that Mediterranean cuisine is known for the use of predominantly locally sourced spices and aromatic herbs, as well as garlic and onion [5]. These are widely employed to enhance the palatability of plant-based dishes, which are central to the MedDiet. Rich in bioactive compounds (particularly flavonoids and polyphenols but also sulfur-containing substances, tannins, alkaloids, phenolic diterpenes, and vitamins), they may play a contributory role in the diet’s well-documented health benefits. Namely, plant- and spice-derived bioactive compounds have been shown to exert multiple beneficial effects, such as antioxidative and anti-inflammatory actions, the inhibition of tumor growth and carcinogenesis, and the modulation of metabolic parameters, including blood glucose and lipid profiles [4].
Digital lifestyles, characterized by the prolonged and frequent use of computers, smartphones, tablets, and other digital screens, have a significant impact on eye health. These effects can manifest as temporary discomfort and digital eye strain, also known as computer vision syndrome, a widespread multifactorial condition characterized by tired, dry, or burning eyes and blurred vision, but also as symptoms such as headaches and neck or shoulder pain [6,7]. Regarding their chronic effects, there is still no definite evidence on whether the excessive use of devices with digital screens causes permanent eye damage [8]. However, there are some indications that chronic exposure to digital devices may exacerbate the symptoms of underlying eye diseases [7,9,10]. Additionally, there are also concerns about whether screens’ blue light might be associated with eye damage [6,7,9].
The focus of this narrative review is exploring the protective potential of herbs and spices from the Mediterranean diet and their bioactive compounds against blue-light-induced retinal damage. By examining the interplay between modern lifestyle challenges, such as increased exposure to digital screens, and the traditional dietary practices of the Mediterranean region, we aim to highlight nutritional strategies that may contribute to preserving retinal health and preventing vision-related disorders.
2. The Mediterranean Diet: Key Components and Health Benefits
Designated by UNESCO as an Intangible Cultural Heritage of Humanity, the MedDiet is more than just a dietary pattern. It represents a holistic lifestyle deeply rooted in the culinary traditions, social practices, and cultural identities of the Mediterranean region, especially Greece and Southern Italy. As detailed in articles by Guasch-Ferré and Willett [4] and Sikalidis et al. [11], this way of eating emphasizes an abundance of plant-based foods. Vegetables, fruits, whole grains, legumes, nuts, and seeds form the cornerstone of the diet, providing significant amounts of fiber, vitamins, minerals, and antioxidants. Extra virgin olive oil, rich in healthy monounsaturated fats, is not merely an ingredient but a central element, used generously in cooking and as a flavorful dressing. Herbs and spices, such as basil, rosemary, oregano, thyme, cilantro, fennel, and mint, are preferred over salt, adding both distinctive flavors and a wealth of beneficial phytochemicals. The diet also includes moderate amounts of fish and poultry, with limited consumption of red meat and dairy products [4]. Red wine is often enjoyed in moderation with meals, rounding out the traditional Mediterranean dining experience [12].
The MedDiet is not completely uniform across the region. For example, while plant-based foods at the center and olive oil as the primary fat are constant elements, there are some variations in the uses of particular types of food. For example, in North Africa, couscous, vegetables, and legumes are important, unlike in Southern Europe, where pasta, polenta, rice, or potatoes with vegetables and legumes are heavily consumed. Also, in some cultures, alcoholic beverages are not included, mostly due to religious beliefs [4].
This dietary pattern, combined with regular physical activity and a socially engaging lifestyle, contributes to its well-documented health-promoting effects.
2.1. The Beneficial Effects of the Mediterranean Diet on Cardiovascular Health
Multiple large-scale epidemiological studies and randomized clinical trials, including the Seven Countries Studies, the Lyon Diet Heart Study, and the PREDIMED trial, have demonstrated the MedDiet’s efficacy in reducing the risk of cardiovascular disease or cardiovascular events by up to 30% in high-risk individuals [11]. Participants adhering to this diet showed a significant reduction in the incidence of myocardial infarction, stroke, and cardiovascular mortality.
It has been shown that the MedDiet improves plasma lipid profiles by increasing HDL cholesterol and lowering LDL cholesterol. Monounsaturated fats, especially from extra virgin oil and omega-3 fatty acids, reduce triglyceride levels, LDL oxidation, and lipid particle size, all factors that are crucial in preventing plaque formation in the arteries. Food rich in polyphenols (olive oil, red wine, vegetables, and fruits) inhibits the expression of pro-inflammatory genes [13], i.e., reduces the levels of inflammatory markers like C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), and activates nuclear factor-kappa B (NF-κB) expression, a key regulator of inflammation in the endothelial cells and macrophages. High intake of potassium, magnesium, fiber (from fruits, vegetables, and legumes), vitamins C and E, carotenoids, and polyphenols inhibits platelet-activating factor (PAF) and improves antioxidant defense, the bioavailability of nitric oxide, and endothelial function, all crucial for maintaining vascular responsiveness and preventing hypertension and atherosclerosis. While omega-3 fatty acids and polyphenols reduce platelet aggregation, low-glycemic-index food like whole grains, legumes, and vegetables reduces postprandial glycemia and insulin secretion, thus lowering the risk of metabolic syndrome and diabetes as one of the major cardiovascular risk factors [11]. The synergistic effects of all the above—lipid-lowering, anti-inflammatory, antioxidant, and antihypertensive actions—contribute to a significant reduction in the progression of atherosclerotic disease and cardiovascular risk.
The PREDIMED (Prevención con Dieta Mediterránea) trial, published in 2013 by Estruch et al. in the New England Journal of Medicine, confirmed the significant cardiovascular impacts of the MedDiet [14]. This controlled trial randomized 7447 subjects aged 55–80 years old at high cardiovascular risk but with no prior cardiovascular disease. They were randomly assigned into one of three groups: the MedDiet with added extra virgin olive oil, the MedDiet with mixed nuts added to it, or a control group that was told to follow a low-fat diet. Over a median follow-up of 4.8 years, the subjects on the MedDiet experienced about a 30% reduction in their risk of major cardiovascular events (heart attack, stroke, or cardiovascular death) versus that in the control group. This research also documented improvements in their blood pressure, lipid profiles, and inflammatory markers. These findings strongly attest to the fact that the MedDiet, particularly when supplemented with healthy fats like olive oil or nuts, is highly effective for the prevention of cardiovascular disease in high-risk populations [15].
2.2. The Neuroprotective Effects of the Mediterranean Diet
Emerging research and supporting clinical evidence [16,17] indicate the MedDiet’s neuroprotective role in maintaining cognitive health and reducing the risk of cerebrovascular events or neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease [18]. A high intake of polyphenols from fruits, vegetables, olive oil, and red wine, along with omega-3 fatty acids from fish, has been shown to reduce oxidative stress and chronic inflammation, both of which are implicated in neurodegenerative processes. These nutrients contribute to improved endothelial function and cerebral blood flow, thus reducing the risk of vascular-related cognitive impairments. It has also been shown that the MedDiet can reduce amyloid-beta accumulation and enhance neuronal signaling. Several studies have indicated that individuals who follow a Mediterranean-style diet perform better in cognitive tests and exhibit slower rates of brain atrophy and white matter degeneration [19]. This protective effect is thought to arise from the diet’s ability to modulate brain-derived neurotrophic factor (BDNF) and support synaptic plasticity, neurogenesis, and mitochondrial function, which are essential to the processes of learning and memory. Recently, it was described that the MedDiet, due to its high fiber content, fosters a healthy gut microbiome, which can influence brain health through the gut–brain axis.
A significant randomized controlled trial, the MedLey study, investigated the impact of the MedDiet on cognitive function among healthy older adults. The participants on the MedDiet showed significant improvements in several aspects of cognition, including memory, executive function, and processing speed, compared to those in the participants on a typical diet for six months. Such findings suggest that the MedDiet may enhance cognitive ability among the elderly [20].
A large-scale prospective cohort study utilizing data from the UK Biobank examined the relationship between MedDiet adherence and dementia risk. This study found that individuals with higher adherence to the MedDiet had a 23% lower risk of developing dementia, independent of genetic predisposition.
2.3. The Anti-Inflammatory Properties of the Nutrients Used in Mediterranean Cuisine
Chronic low-grade inflammation is implicated in the etiology of numerous chronic diseases, including cardiovascular disease, diabetes, cancer, and autoimmune conditions. The MedDiet’s rich content of anti-inflammatory compounds, including omega-3 fatty acids, polyphenols, flavonoids, and carotenoids, has been shown to suppress oxidative stress and key inflammatory pathways. Clinical evidence supporting the anti-inflammatory effects of the MedDiet [21] shows that regular intake of this dietary pattern is associated with decreased circulating levels of inflammatory biomarkers such as C-reactive protein (CRP) and pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. The MedDiet also promotes the production of anti-inflammatory mediators like IL-10. The high fiber intake from whole plant foods also supports healthy gut microbiota, which play a crucial role in modulating systemic inflammation and immune responses. Namely, the fermentation of fiber by gut bacteria produces short-chain fatty acids like butyrate, which have anti-inflammatory properties and help maintain intestinal barrier integrity [22]. One of the possible mechanisms of the MedDiet’s anti-inflammatory actions is linked to an increase in adiponectin levels and a reduction in the levels of leptin, a hormone that has recently emerged as a key mediator between metabolic responses and low-grade chronic inflammation [23]. Through a reduction in the expression of adhesion molecules like ICAM-1 and VCAM-1, the MedDiet contributes to decreasing leukocyte adhesion and vascular inflammation [24,25].
Another study by Vaziri from 2024 emphasized the MedDiet’s potential as a powerful defense against Alzheimer’s disease [18]. The diet’s rich composition of antioxidants, healthy fats, and anti-inflammatory compounds contributes to its protective effects on cognitive function. Potential mechanisms might involve vascular factors, glucose/lipid metabolism, and anti-inflammatory effects.
3. The Eye-Related Benefits of the Mediterranean Diet
Recent evidence suggests that the MedDiet offers significant benefits for ocular health. This is particularly relevant given the increasing prevalence of vision-threatening conditions such as age-related macular degeneration (AMD) and diabetic retinopathy (DR), both of which are strongly influenced by oxidative stress, inflammation, and microvascular dysfunction, as described above [26].
3.1. Age-Related Macular Degeneration
AMD is the leading cause of blindness among older adults and is characterized by the progressive degeneration of the macula, the central portion of the retina. It is the leading cause of blindness in older adults, particularly in industrialized countries, and is projected to affect 288 million people globally by 2040, with Asia bearing the highest burden [27]. AMD is characterized by damage to the macula, the central part of the retina responsible for detailed vision. There are two main forms of AMD: dry (atrophic, non-neovascular) and wet (neovascular). Dry AMD is characterized by the accumulation of drusen and gradual atrophy of the retinal pigment epithelium (RPE) cells, while wet AMD involves abnormal blood vessel growth under the retina and more rapid vision loss. Both types involve degeneration of the RPE cells and photoreceptors, processes that are closely linked to oxidative stress and chronic inflammation.
Recent research indicates that individuals with higher adherence to the MedDiet are at a lower risk of developing AMD. Nutrients such as lutein, zeaxanthin, vitamin C, vitamin E, and zinc, abundant in leafy greens, citrus fruits, nuts, and seeds, play a key role in retinal protection [28].
Moreover, the anti-inflammatory and antioxidant properties of olive oil and polyphenol-rich foods help reduce the oxidative damage that contributes to retinal cell death. The beneficial fats from fish and nuts also support the structural integrity of the retinal membranes. Clinical studies, including those conducted in Mediterranean populations, have demonstrated slower progression of AMD among individuals who follow this diet [29,30].
3.2. Diabetic Retinopathy
As a microvascular complication of diabetes, DR affects the retinal blood vessels, thus leading to vision impairment. Dietary interventions that support glycemic control, reduce inflammation, and improve vascular health are crucial in managing and preventing DR. The MedDiet addresses all of these factors through its low glycemic load, high fiber content, and abundance of bioactive compounds [31,32].
Omega-3 fatty acids from fish can reduce retinal inflammation and vascular permeability, while antioxidants from fruits, vegetables, and extra virgin olive oil help neutralize oxidative stress. Polyphenols such as resveratrol (found in grapes and red wine) and hydroxytyrosol (from olive oil) exhibit neuroprotective and anti-angiogenic effects that may prevent the abnormal blood vessel growth seen in advanced DR. Observational studies and clinical trials have linked adherence to the MedDiet with a lower risk and the reduced progression of diabetic retinopathy in patients with type 2 diabetes [31,33].
Recent clinical research has investigated the potential role of omega-3 fatty acids in preventing or slowing the progression of DR. The most comprehensive study to date is the ASCEND-Eye trial, a sub-study of the larger ASCEND (A Study of Cardiovascular Events in Diabetes) randomized controlled trial [34]. It is worth noting that while omega-3 fatty acids have demonstrated anti-inflammatory and vascular benefits in other contexts, their efficacy in the prevention or treatment of diabetic retinopathy remains unproven in large-scale human trials. Further research may explore different dosages, formulations, or patient populations to fully elucidate any potential benefits.
4. Blue Light and Retinal Damage
4.1. Blue Light Exposure from Digital Screens and Artificial Lighting
Blue light is a high-energy, short-wavelength (400–500 nm) part of the visible light spectrum. It is naturally present in sunlight, but modern lifestyles have significantly increased our exposure to artificial sources of blue light. LED screens, smartphones, tablets, computers, etc., emit substantial levels of blue light. While exposure to natural blue light during the day helps regulate circadian rhythms and mood, prolonged and intense artificial exposure, especially at night, raises potential concerns about ocular health [35].
High-energy short-wave blue light between 415 and 455 nm is the most harmful type, causing symptoms such as eye fatigue, dryness, blurred vision, and insomnia [10]. Unlike ultraviolet light, which is mostly absorbed by the cornea and the lens, blue light penetrates deeper and reaches the retina directly (Figure 1). This can cause cumulative photochemical stress on the retina and irreversible damage known as the “blue light hazard” [6]. Prolonged blue light exposure has been linked to eye diseases such as AMD, cataracts, and keratitis [10].
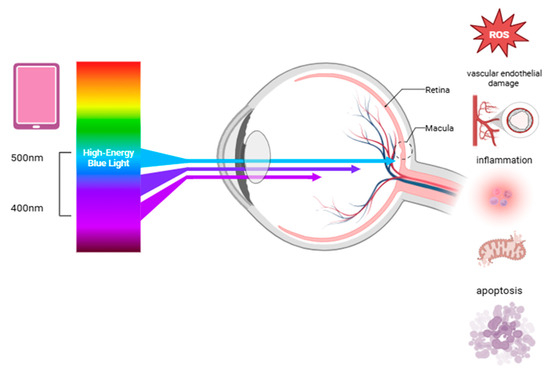
Figure 1.
The effects of blue light on retinal health, highlighting the processes that contribute to retinal damage and may increase the risk of degenerative eye diseases. Abbreviation: ROS, reactive oxygen species.
4.2. The Mechanisms of Oxidative Stress and Inflammation Caused by Blue Light
Blue light exposure can damage the eyes through several pathways, including oxidative stress, DNA damage, inflammation, mitochondrial dysfunction, apoptosis, impaired autophagy, and vascular endothelial damage (Figure 1) [36]. On the ocular surface, blue light can penetrate the eye and impact the corneal epithelial and endothelial cells, reducing their survival and increasing reactive oxygen species (ROS) and inflammatory markers like IL-1β [10]. The aging crystalline lens gradually absorbs more blue light, which can trigger ROS production in the lens epithelial cells, leading to apoptosis through the TGF-β/Smad3 pathway and contributing to cataract formation [6]. Damaged cells also release inflammatory signals, attracting immune cells and worsening tissue injury [9]. The retina is particularly vulnerable to oxidative stress due to its high metabolic activity and constant exposure to light and oxygen. Blue light can damage the retina primarily by inducing oxidative stress, especially in the retinal pigment epithelium (RPE) and photoreceptor cells. This damage is linked to the accumulation of lipofuscin, particularly its toxic component N-retinylidene-N-retinylethanolamin (A2E), which generates ROS under blue light [37,38]. These ROS are damaging because they react with cellular components like lipids, proteins, and the DNA. The retina contains large amounts of polyunsaturated fatty acids, particularly docosahexaenoic acid, which are especially prone to oxidation [33]. ROS cause endoplasmic reticulum stress, mitochondrial dysfunction, and lysosomal damage through the activation of pathways like MAPK and NF-κB [39,40], all contributing to RPE cell apoptosis. Blue light also disrupts calcium balance, further harming the mitochondria. Additionally, it elevates HIF-1α and VEGF expression, leading to retinal vascular endothelial dysfunction and potential neovascularization [41,42]. Prolonged exposure to low-intensity blue light can disrupt the retinal photoreceptors, while high-intensity blue light causes more severe damage. It inhibits cytochrome oxidase, disrupts mitochondrial function, reduces sodium–potassium ATPase activity, and leads to cell edema and photoreceptor degeneration [43]. Blue light also stimulates inflammatory signaling pathways, most notably NF-κB, a transcription factor that regulates genes involved in the immune response. The activation of NF-κB leads to the production of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β. These cytokines create a chronic inflammatory environment that contributes to structural damage in the retina over time [44]. Rod and cone cells are particularly vulnerable due to rhodopsin and S-opsin, which enhance photon capture and increase light-induced damage. Short-wavelength LEDs exacerbate this by aggregating visual proteins and damaging the cone cells [45]. Moreover, blue light can compromise the blood–retinal barrier by damaging RPE tight junctions, allowing the infiltration of inflammatory cells into the retinal tissues. This perpetuates oxidative injury and accelerates retinal degeneration [46].
4.3. The Connection Between Blue Light and Retinal Conditions
AMD is a complex, progressive, multifactorial disease that develops gradually over the years. Several known risk factors, including age, smoking, nutritional deficiencies, sunlight exposure, and genetic predisposition, are involved in the pathogenesis of the disease [47]. Recently, it was shown that prolonged exposure to blue light could also contribute to the development and worsening of the clinical picture of AMD. Namely, accumulation of the retinoid A2E, a key component of lipofuscin, further increases the risk of retinal cell apoptosis with age, as A2E is particularly sensitive to high-energy blue light [38]. Although blue light exposure is not the sole cause of AMD, it is considered a contributing risk factor, especially in individuals with genetic predispositions or existing retinal vulnerabilities. Experimental studies using animal models and in vitro retinal cultures have shown that blue light accelerates RPE cell death, impairs photoreceptor renewal, and increases drusen-like deposits—hallmarks of early AMD [46,48,49]. Additionally, with age, the natural protective mechanisms against blue light decline. The crystalline lens gradually yellows, filtering more blue light, but this process is less effective in older adults or those who have undergone cataract surgery. The macular pigments lutein and zeaxanthin, which absorb blue light and neutralize ROS, also decrease with age and poor diets, further reducing the eye’s defenses [50,51].
A clinical study investigating the effects of blue light exposure on retinal health was conducted in 2021 by Li et al. [52]. This study combined a clinical pilot investigation with an animal model to assess the impact of low-intensity blue light, similar to that emitted by smartphones, on retinal function and structure. The clinical component consisted of 25 healthcare providers who were assigned into two groups based on their duration of daily screen use. Multifocal electroretinography (mf-ERG) was used to measure retinal function. The findings indicated that increased screen exposure was linked to lower mf-ERG amplitudes in macular regions, reflecting damaged retinal function [52].
In conclusion, as electronic device use increases, the incidence of blue-light-related eye damage is expected to rise, particularly among the young and the elderly. By 2050, the prevalence of eye diseases like myopia, diabetic retinopathy, glaucoma, and in particular AMD, as a leading cause of blindness in older adults, is predicted to grow significantly. Future research will likely focus on understanding the role of blue light in these conditions, especially in younger and older populations [53].
5. Mediterranean Herbs and Spices: Bioactive Compounds and Their Properties
Mediterranean herbs such as bay laurel, thyme, oregano, sage, and rosemary are rich in bioactive compounds with impressive health-promoting attributes, most significantly for the protection of retinal health. These herbs contain powerful phenolic compounds like rosmarinic acid, carnosic acid, and thymol, which have been extensively researched for their antioxidant, anti-inflammatory, and antimicrobial properties [54].
Rosmarinic acid, which is present in abundance in oregano and rosemary, has been shown to inhibit oxidative stress and reduce inflammatory responses in the retinal cells and may retard the progress of age-related macular degeneration [55]. Carnosic acid, which is predominantly present in rosemary and sage, offers neuroprotection through its ability to scavenge free radicals and modulate inflammatory signaling pathways [56]. Thymol, a major thyme constituent, possesses strong antimicrobial and anti-inflammatory activity that may contribute to ocular surface integrity and the prevention of retinal infections [57]. These findings emphasize the potential of Mediterranean spices as dietary sources of retinal-integrity- and -function-enhancing compounds. Regarding their antioxidant activity, they neutralize ROS, inhibit lipid peroxidation, and increase endogenous antioxidants like glutathione [35].
Their anti-inflammatory action involves the suppression of the NF-κB and COX-2 pathways, reductions in pro-inflammatory cytokines, and matrix metalloproteinase (MMP) blockade to protect the retinal structure. In retinal health, MMPs participate in retinal damage, as their activity has the capacity to degrade structural proteins and plays a role in inflammatory processes. MMP inhibition decreases tissue injury and inflammation, and since these are the same effects brought about through the inhibition of MMP activity (e.g., by rosmarinic acid), the compounds that inhibit MMP activity have the potential to protect the retinal tissues against damage [58,59].
Bioactive compounds that originate from these herbs can disrupt microbial membranes to prevent infections that can worsen retinal inflammation, in the context of antimicrobial action [60]. Rosmarinic acid, one of the prominent bioactive molecules in rosemary, sage, thyme, and oregano, possesses significant antioxidant, anti-inflammatory, and antimicrobial activities that may safeguard retinal function.
In Table 1, a summary of the key bioactive components found in the most commonly used aromatic herbs in the MedDiet are presented, together with the mechanisms through which they might support retinal health.

Table 1.
Selected aromatic herbs, their key bioactive compounds, and their mechanisms related to retinal health.
Studies indicate that combining different herbal compounds can produce synergistic effects beneficial for retinal health. Mixed natural antioxidants often target multiple pathways simultaneously, enhancing their overall efficacy compared to that of individual compounds alone [65]. Similarly, traditional herbal formulas containing multiple herbs exhibit additive or synergistic interactions among their phytochemicals, improving outcomes such as the inhibition of retinal neovascularization by downregulating pro-angiogenic factors. These synergistic effects arise because the combined compounds can scavenge ROS, suppress pro-inflammatory cytokines, inhibit MMPs, and modulate multiple signaling pathways involved in retinal degeneration [66]. Therefore, multi-compound herbal mixtures hold promise as more effective interventions for preventing or slowing retinal diseases than single isolated compounds.
The European Food Safety Authority (EFSA) evaluates the health claims on bioactive compounds in the MedDiet according to solid scientific evaluations based on three criteria: the characterization of a substance, definition of its claimed effect, and cause–effect relationships [67]. The EFSA authorizes health claims about the MedDiet’s components using robust evidence (e.g., olive oil polyphenols) while restricting generic or unsubstantiated claims. Nutrient profiling exemptions ensure that traditional foods like herbs and vegetable oils remain eligible. Most MedDiet staples are not novel foods, though novel processing methods may trigger regulatory review [68]. Regarding novel food regulations, they must balance innovation with respect for traditional diets. Whole herbs/spaces are excluded from novel food status, but standardized extracts or purified bioactive compounds (e.g., eugenol from cloves) may require authorization [69].
6. The Protective Effects of Mediterranean Herbs and Spices Against Blue-Light-Induced Retinal Damage
Alongside the broader emphasis on antioxidant-rich foods, the MedDiet may offer indirect protection against blue-light-induced retinal damage by way of the bioactive compounds found in herbs and spices. These protective effects are most likely achieved through systemic antioxidant and anti-inflammatory mechanisms [54,61,63,70]. Specific studies on Mediterranean herbs/spices for this purpose are limited, but the key components align with established certain protective pathways (an increase in antioxidant defense, anti-inflammatory effects, and anti-angiogenesis). While Mediterranean herbs and spices are likely to be beneficial in enhancing the systemic antioxidant defenses, their specific role in retinal protection from blue light still remains hypothetical, as there are no specific targeted studies. For now, the existing evidence supports their inclusion as part of a broader nutrient-dense diet for ocular health (Table 2).

Table 2.
Ocular-health-protective effects of natural bioactive compounds found in Mediterranean herbs and spices in experimental models.
Kim et al. investigated the therapeutic effects of eucalyptol on diabetic retinal microvascular defects [71]. High glucose levels in diabetic conditions increase Aβ production, which damages the retinal cells. Eucalyptol significantly reduced the Aβ levels in both human retinal microvascular endothelial (RVE) cells and diabetic mice. Eucalyptol blocked apoptosis (cell death) in the retinal cells by restoring protective proteins (bcl-2) and reducing harmful ones (bax and caspase-12). Eucalyptol also reduced endoplasmic reticulum stress, which is a key contributor to retinal damage in diabetes. It blocked the PERK-eIF2α-ATF4-CHOP signaling pathway, which is activated by Aβ and high glucose. Eucalyptol significantly reduced the Aβ levels in both human RVE cells and diabetic mice (Table 2).
Another study on the effects of rosmarinic acid on retinal neovascularization showed its anti-angiogenic properties, as it suppressed retinal neovascularization by causing cell cycle arrest in the G2/M phase and increasing the expression of p21WAF1, a protein that regulates cell growth. It inhibited the proliferation and tube formation of the retinal endothelial cells in a dose-dependent manner. In a mouse model of retinopathy of prematurity, rosmarinic acid effectively reduced retinal neovascularization without causing retinal toxicity (Table 2) [72].
In animals exposed to intense light, carnosic acid administration preserved photoreceptor function and morphology. This protection is linked to the activation of the Nrf2 pathway, leading to the upregulation of endogenous antioxidant enzymes and a reduction in oxidative damage markers such as hyperoxidized peroxiredoxin 2 (Prx2).
Carnosic acid treatment has been observed to decrease the production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, thereby reducing inflammation-mediated retinal damage [76]. In the Pde6b (rd10) mouse model of retinitis pigmentosa, carnosic acid treatment resulted in the preservation of the photoreceptor cells and retinal structure. This neuroprotective effect is associated with the inhibition of the oxidative and endoplasmic reticulum stress pathways [77].
Inflammation has a significant role in the progression of retinal degenerative diseases. Eugenol has been shown to suppress the production of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, and may contribute to reducing retinal inflammation and subsequent neuronal damage [78].
Thymol’s antioxidant and anti-inflammatory effects observed in neuronal models suggest its potential therapeutic value in retinal neurodegenerative conditions [79]. Oxidative stress and inflammation are key contributors to retinal degenerative diseases such as AMD and retinitis pigmentosa; thus, thymol’s properties could be beneficial in this context. In a study involving a rotenone-induced model of Parkinson’s disease in rats, thymol treatment significantly reduced dopaminergic neuronal loss, oxidative stress markers, and pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. Additionally, thymol enhanced the activity of antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT) and increased glutathione (GSH) levels, indicating a bolstered endogenous antioxidant defense system [80].
7. Future Directions: Dietary Recommendations for Eye Health
Dietary recommendations for eye health should emphasize the integration of Mediterranean spices and herbs—such as bay laurel, thyme, oregano, sage, and rosemary—into daily meals given their rich content of bioactive compounds like quercetin, luteolin, and rosmarinic acid, which possess strong antioxidant and anti-inflammatory properties [70]. These phytochemicals may help combat oxidative stress and inflammation, both key contributors to retinal degeneration [81]. The development of targeted nutritional interventions, such as supplements or functional foods enriched with these specific compounds, holds promise for preventing or slowing the progression of retinal diseases [81]. Incorporating Mediterranean herbs such as bay laurel, thyme, oregano, sage, and rosemary into daily diets offers promising avenues for protecting eye health.
Rosemary contains potent antioxidants, including rosmarinic and carnosic acids, which have demonstrated protective effects against oxidative stress in the eyes. Studies suggest that rosemary extract may slow the progression and severity of AMD and delay the onset of cataracts, though most of this research has used concentrated extracts rather than culinary amounts. Incorporating rosemary into meals—such as with roasted vegetables or in soups or teas—may offer some benefit as part of an overall antioxidant-rich diet [82].
Both thyme and sage are rich in lutein, a carotenoid crucial for the health of the macula. Lutein acts as a natural shield, protecting the retina from oxidative damage and blue light exposure [83].
Oregano is another Mediterranean herb high in antioxidants and anti-inflammatory compounds. While specific clinical studies on oregano and eye health are limited, its general antioxidant properties support a reduction in oxidative stress, a key factor in the development of AMD and cataracts [84].
While direct evidence for bay laurel’s impact on eye health is limited, it is a staple in Mediterranean cuisine and contributes to the overall antioxidant profile of this diet.
In addition to the positive retinal health benefits of herbs and spices, the MedDiet is characterized by a high intake of fruits, vegetables, whole grains, legumes, nuts, olive oil, and herbs and is associated with a significantly reduced risk of age-related macular degeneration and other ocular diseases. Key nutrients for eye health found abundantly in this diet include lutein and zeaxanthin (leafy greens, herbs), vitamins C and E (citrus, nuts, and olive oil), omega-3 fatty acids (seafood and nuts), and zinc (legumes, seeds, and seafood) [29].
One suggestion is to incorporate Mediterranean herbs into daily meals to enhance antioxidant and anti-inflammatory intake and then combine herbs with other Mediterranean staples—leafy greens, colorful vegetables, nuts, olive oil, and fish—for synergistic effects. All of this alongside a reduction in salt, using herbs and spices to flavor foods in place of salt, can support both eye and cardiovascular health [85].
To advance clinical applications, further research is needed to isolate, characterize, and test the efficacy of these bioactive molecules in human trials, as well as to explore their synergistic effects with other MedDiet components like healthy fats and carotenoids [70]. Such studies could pave the way for precision nutrition strategies tailored to individuals at risk of blue-light-induced retinal degeneration and other ocular conditions. Suggestions for further research into bioactive compounds for clinical applications should prioritize human clinical trials to validate the preclinical findings, particularly for Mediterranean spices. Studies should investigate the synergistic interactions between carotenoids (e.g., lutein, zeaxanthin) and polyphenols (e.g., anthocyanins, quercetin) to optimize the antioxidant and anti-inflammatory effects while addressing potential absorption conflicts (e.g., the inhibition of lutein uptake by certain polyphenols) [86]. The research must also focus on bioavailability enhancements through novel delivery systems (e.g., nanoencapsulation) to improve the penetration of the ocular tissue by compounds like resveratrol [66,86]. Additionally, long-term trials are needed to assess the efficacy of bioactive combinations in preventing AMD and diabetic retinopathy, with an emphasis on the dose–response relationships and safety profiles [87]. Mechanistic studies should explore molecular pathways, including VEGF and TNF-α inhibition, to identify targets for precision nutrition strategies [88]. Finally, standardized extraction methods for spice-derived compounds and their integration into functional foods warrant exploration to bridge dietary recommendations and therapeutic applications [89].
8. Conclusions
The MedDiet, rich in plant-based foods, healthy fats, and herbs, has been associated with a variety of health benefits, including cardiovascular, neuroprotective, and anti-inflammatory effects. Recent research suggests that certain components of the MedDiet, particularly herbs such as bay leaf, thyme, oregano, sage, and rosemary, may play a key role in protecting the retina from oxidative damage, a key factor in blue-light-induced retinal degeneration. At the moment, there is a scarcity of evidence directly supporting the potential of these MedDiet staples; however, the findings presented in this review highlight the promising role of Mediterranean herbs and spices—as well as the bioactive compounds found in them—in preventing retinal damage, as well as other eye conditions related to modern digital lifestyles and the heavy use of blue-light-emitting devices.
Author Contributions
Conceptualization: K.P. and J.M.-P. Writing—original draft preparation: K.P., A.H.H., A.P., T.Č.-M. and J.M.-P. Writing—review and editing: K.P., A.H.H. and J.M.-P. Visualization: A.H.H. Supervision: K.P. and J.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant awarded by the University of Rijeka, Croatia, under project number uniri-iskusni-biomed-23-82 to J.M.-P.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMD | Age-related macular degeneration |
| ASCEND | A Study of Cardiovascular Events in Diabetes |
| A2E | N-retinylidene-N-retinylethanolamin |
| BDNF | Brain-derived neurotrophic factor |
| CA | Carnosic acid |
| CRP | C-reactive protein |
| DES | Digital eye strain |
| DNA | Deoxyribonucleic acid |
| DR | Diabetic retinopathy |
| EFSA | European Food Safety Authority |
| ER | Endoplasmic reticulum |
| HDL | High-density lipoprotein |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| LDL | Low-density lipoprotein |
| LED | Light-emitting diode |
| MAPK | Mitogen-activated protein kinase |
| MedDiet | Mediterranean diet |
| mf-ERG | Multifocal electroretinography |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor-kappa B |
| PAF | Platelet-activating factor |
| PREDIMED | Prevention with Mediterranean Diet |
| RA | Rosmarinic acid |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| RVE | Retinal microvascular endothelial |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor-alpha |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
References
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary Habits, Lipoprotein Metabolism and Cardiovascular Disease: From Individual Foods to Dietary Patterns. Crit. Rev. Food Sci. Nutr. 2021, 61, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Saulle, R.; Torre, G.L. The Mediterranean Diet, Recognized by UNESCO as a Cultural Heritage of Humanity. Ital. J. Public Health 2010, 7, 414–415. [Google Scholar] [CrossRef]
- Trichopoulou, A. Mediterranean Diet as Intangible Heritage of Humanity: 10 Years On. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1943–1948. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean Diet and Health: A Comprehensive Overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Dhaheri, A.S.A.; Alkhatib, D.H.; Jaleel, A.; Tariq, M.N.M.; Feehan, J.; Apostolopoulos, V.; Osaili, T.M.; Mohamad, M.N.; Ismail, L.C.; Saleh, S.T.; et al. Proximate Composition and Mineral Content of Spices Increasingly Employed in the Mediterranean Diet. J. Nutr. Sci. 2023, 12, e79. [Google Scholar] [CrossRef]
- Cougnard-Gregoire, A.; Merle, B.M.J.; Aslam, T.; Seddon, J.M.; Aknin, I.; Klaver, C.C.W.; Garhöfer, G.; Layana, A.G.; Minnella, A.M.; Silva, R.; et al. Blue Light Exposure: Ocular Hazards and Prevention—A Narrative Review. Ophthalmol. Ther. 2023, 12, 755–788. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, Y.; Zhao, Y.; Yang, Y.; An, G.; Liu, Z.; Qi, D. A Review on Eye Diseases Induced by Blue Light: Pathology, Model, Active Ingredients and Mechanisms. Front. Pharmacol. 2025, 16, 1513406. [Google Scholar] [CrossRef] [PubMed]
- Hipólito, V.; Coelho, J.M.P. Blue Light and Eye Damage: A Review on the Impact of Digital Device Emissions. Photonics 2023, 10, 560. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of Blue Light-Induced Eye Hazard and Protective Measures: A Review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Zhao, Z.-C.; Zhou, Y.; Tan, G.; Li, J. Research Progress about the Effect and Prevention of Blue Light on Eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Kelleher, A.H.; Kristo, A.S. Mediterranean Diet. Encyclopedia 2021, 1, 371–387. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.A.; Izuora, K.; Basu, A. Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 12762. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; PREDIMED INVESTIGATORS. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Nucci, D.; Sommariva, A.; Degoni, L.M.; Gallo, G.; Mancarella, M.; Natarelli, F.; Savoia, A.; Catalini, A.; Ferranti, R.; Pregliasco, F.E.; et al. Association between Mediterranean Diet and Dementia and Alzheimer Disease: A Systematic Review with Meta-Analysis. Aging Clin. Exp. Res. 2024, 36, 77. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Mediterranean Diet and Its Protective Mechanisms against Cardiovascular Disease: An Insight into Platelet Activating Factor (PAF) and Diet Interplay. Ann. Nutr. Disord. Ther. 2015, 2, 1016. [Google Scholar]
- Vaziri, Y. The Mediterranean Diet: A Powerful Defense against Alzheimer Disease—A Comprehensive Review. Clin. Nutr. ESPEN 2024, 64, 160–167. [Google Scholar] [CrossRef]
- Pelletier, A.; Barul, C.; Féart, C.; Helmer, C.; Bernard, C.; Periot, O.; Dilharreguy, B.; Dartigues, J.-F.; Allard, M.; Barberger-Gateau, P.; et al. Mediterranean Diet and Preserved Brain Structural Connectivity in Older Subjects. Alzheimer’s Dement. 2015, 11, 1023–1031. [Google Scholar] [CrossRef]
- Knight, A.; Bryan, J.; Wilson, C.; Hodgson, J.M.; Davis, C.R.; Murphy, K.J. The Mediterranean Diet and Cognitive Function among Healthy Older Adults in a 6-Month Randomised Controlled Trial: The MedLey Study. Nutrients 2016, 8, 579. [Google Scholar] [CrossRef]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, Q.; Li, Y.; Wei, L.; Zhang, Z.; Khan, A.; Khan, M.Z.; Wang, C. Butyrate Supplementation Improves Intestinal Health and Growth Performance in Livestock: A Review. Biomolecules 2025, 15, 85. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.K.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean Dietary Pattern, Inflammation and Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Reddy, A.; Gatta, P.D.; Mason, S.; Nicoll, A.J.; Ryan, M.; Itsiopoulos, C.; Abbott, G.; Johnson, N.A.; Sood, S.; Roberts, S.K.; et al. Adherence to a Mediterranean Diet May Improve Serum Adiponectin in Adults with Nonalcoholic Fatty Liver Disease: The MEDINA Randomized Controlled Trial. Nutr. Res. 2023, 119, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean Diet, Genetic Susceptibility, and Progression to Advanced Macular Degeneration: A Prospective Cohort Study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Jung, W.; Han, K.; Kim, B.; Hwang, S.; Yoon, J.M.; Park, J.; Lim, D.H.; Shin, D.W. Age-Related Macular Degeneration with Visual Disability Is Associated with Cardiovascular Disease Risk in the Korean Nationwide Cohort. J. Am. Heart Assoc. 2023, 12, e028027. [Google Scholar] [CrossRef] [PubMed]
- Murkey, S.P.; Agarwal, A.; Pandit, P.; Kumar, S.; Jaiswal, A. Unveiling the Spectrum of Ophthalmic Manifestations in Nutritional Deficiencies: A Comprehensive Review. Cureus 2023, 15, e50311. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, Y.; Yuan, Y.; Xiong, R.; Hu, Y.; Ning, K.; Ha, J.; Wang, W.; Han, X.; He, M. The Mediterranean Diet and Age-Related Eye Diseases: A Systematic Review. Nutrients 2023, 15, 2043. [Google Scholar] [CrossRef]
- Angelia, M.; Amelia, Y.S.; Pratama, K.G. Mediterranean Diet as a Modifiable Risk Factor for Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Tzu Chi Med. J. 2024, 36, 223–230. [Google Scholar] [CrossRef]
- Zooravar, D.; Soltani, P.; Khezri, S. Mediterranean Diet and Diabetic Microvascular Complications: A Systematic Review and Meta-Analysis. BMC Nutr. 2025, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. A Mediterranean Diet May Be Protective in the Development of Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 11145. [Google Scholar] [CrossRef]
- Zeppieri, M.; Gagliano, C.; D’Esposito, F.; Musa, M.; Gattazzo, I.; Zanella, M.S.; Rossi, F.B.; Galan, A.; Babighian, S. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA): A Targeted Antioxidant Strategy to Counter Oxidative Stress in Retinopathy. Antioxidants 2024, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Sammons, E.; Bowman, L.; Stevens, W.; Buck, G.; Wallendszus, K.; Hammami, I.; Parish, S.; Armitage, J.; ASCEND Collaborative Group. ASCEND-Eye: Rationale, Design and Baseline Characteristics for a Sub-Study of the ASCEND Randomised Trial, Exploring the Effects of Aspirin and Omega-3 Fatty Acids on Diabetic Retinopathy and Age-Related Macular Degeneration. Contemp. Clin. Trials Commun. 2023, 35, 101184. [Google Scholar] [CrossRef]
- Wang, P.; Chin, E.K.; Almeida, D. Antioxidants for the Treatment of Retinal Disease: Summary of Recent Evidence. Clin. Ophthalmol. 2021, 15, 1621–1628. [Google Scholar] [CrossRef]
- Sun, G.-F.; Qu, X.-H.; Jiang, L.-P.; Chen, Z.-P.; Wang, T.; Han, X.-J. The Mechanisms of Natural Products for Eye Disorders by Targeting Mitochondrial Dysfunction. Front. Pharmacol. 2024, 15, 1270073. [Google Scholar] [CrossRef] [PubMed]
- Boyer, N.P.; Higbee, D.; Currin, M.B.; Blakeley, L.R.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and N-Retinylidene-N-Retinylethanolamine (A2E) Accumulate in Retinal Pigment Epithelium in Absence of Light Exposure: Their Origin Is 11-Cis-Retinal. J. Biol. Chem. 2012, 287, 22276–22286. [Google Scholar] [CrossRef]
- Yang, B.; Yang, K.; Chen, Y.; Li, Q.; Chen, J.; Li, S.; Wu, Y. Exposure of A2E to Blue Light Promotes Ferroptosis in the Retinal Pigment Epithelium. Cell. Mol. Biol. Lett. 2025, 30, 22. [Google Scholar] [CrossRef]
- Basyal, D.; Lee, S.; Kim, H.J. Antioxidants and Mechanistic Insights for Managing Dry Age-Related Macular Degeneration. Antioxidants 2024, 13, 568. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive Oxygen Species in Biological Systems: Pathways, Associated Diseases, and Potential Inhibitors—A Review. Food Sci. Nutr. 2024, 12, 675–693. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Miwa, Y.; Kunimi, H.; Nakai, A.; Shoda, C.; Negishi, K.; Kurihara, T. Recent Insights into Roles of Hypoxia-Inducible Factors in Retinal Diseases. Int. J. Mol. Sci. 2024, 25, 10140. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.D.; Muniyandi, A.; Sishtla, K.; Kpenu, E.K.; Miller, W.P.; Kaplan, B.A.; Kim, L.A.; Liu, S.; Wan, J.; Qi, X.; et al. Ref-1 Redox Activity Regulates Retinal Neovascularization by Modulating Transcriptional Activation of HIF-1α. FASEB J. 2025, 39, e70348. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Núñez-Álvarez, C.; Del Olmo-Aguado, S.; Merrayo-Lloves, J. Visual Light Effects on Mitochondria: The Potential Implications in Relation to Glaucoma. Mitochondrion 2017, 36, 29–35. [Google Scholar] [CrossRef]
- Shafi, V.; Khan, N.A.; Kazmi, J.; Siddiqui, I. Cytokine-Driven NF-κB Activation in Retinal Cells and Its Impact on the Pathogenesis of Age-Related Macular Degeneration: A Systematic Review. medRxiv 2024. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shimazawa, M.; Sugitani, S.; Kudo, T.; Imai, S.; Inokuchi, Y.; Tsuruma, K.; Hara, H. Role of Endoplasmic Reticulum Stress in Light-Induced Photoreceptor Degeneration in Mice. J. Neurochem. 2013, 125, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, H.; Georgyev, V.; Wagen, C.; Hosseini, A.; Matsubara, J. Blue Light-Induced Phototoxicity in Retinal Cells: Implications in Age-Related Macular Degeneration. Front. Aging Neurosci. 2024, 16, 1509434. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.; Usategui-Martin, R.; Sanabria, M.R.; Fernandez-Perez, E.; Telleria, J.J.; Coco-Martin, R.M. Pathophysiology of Age-Related Macular Degeneration: Implications for Treatment. Ophthalmic Res. 2022, 65, 615–636. [Google Scholar] [CrossRef]
- Song, W.; Zhu, R.; Gao, W.; Xing, C.; Yang, L. Blue Light Induces RPE Cell Necroptosis, Which Can Be Inhibited by Minocycline. Front. Med. 2022, 9, 831463. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wu, M.-R.; Li, C.-H.; Cheng, H.-W.; Huang, S.-H.; Tsai, C.-H.; Lin, F.-L.; Ho, J.-D.; Kang, J.-J.; Hsiao, G.; et al. Editor’s Highlight: Periodic Exposure to Smartphone-Mimic Low-Luminance Blue Light Induces Retina Damage Through Bcl-2/BAX-Dependent Apoptosis. Toxicol. Sci. 2017, 157, 196–210. [Google Scholar] [CrossRef]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The Role of Lutein in Eye-Related Disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, M.; Wang, D.; Dong, G.; Chen, Z.; Li, S.; Sun, X.; Zeng, M.; Liao, H.; Chen, H.; et al. Blue Light from Cell Phones Can Cause Chronic Retinal Light Injury: The Evidence from a Clinical Observational Study and a SD Rat Model. BioMed Res. Int. 2021, 2021, 3236892. [Google Scholar] [CrossRef]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B.H. Green Tea Catechins as Brain-Permeable, Natural Iron Chelators-Antioxidants for the Treatment of Neurodegenerative Disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R. The Dietary Components Carnosic Acid and Carnosol as Neuroprotective Agents: A Mechanistic View. Mol. Neurobiol. 2016, 53, 6155–6168. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical Features and Therapeutic Potential of Rosmarinic Acid. Arch. Pharmacal Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Lim, R.R.; Hainsworth, D.P.; Mohan, R.R.; Chaurasia, S.S. Characterization of a Functionally Active Primary Microglial Cell Culture from the Pig Retina. Exp. Eye Res. 2019, 185, 107670. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorný, J. Natural Antioxidants from Herbs and Spices. Eur. J. Lipid Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Polyphenolic Characterization and Antioxidant Capacity of Laurus nobilis L. Leaf Extracts Obtained by Green and Conventional Extraction Techniques. Processes 2021, 9, 1840. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, P.; Mas-Sanchez, A.; Garriga, P. Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases. Molecules 2021, 26, 3407. [Google Scholar] [CrossRef]
- Amato, R.; Canovai, A.; Melecchi, A.; Pezzino, S.; Corsaro, R.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Dietary Supplementation of Antioxidant Compounds Prevents Light-Induced Retinal Damage in a Rat Model. Biomedicines 2021, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, T.; Kang, R.; Ren, F.; Liu, J.; Wang, J. Plant Bioactive Compounds Alleviate Photoinduced Retinal Damage and Asthenopia: Mechanisms, Synergies, and Bioavailability. Nutr. Res. 2023, 120, 115–134. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific Advice Related to Nutrient Profiling for the Development of Harmonised Mandatory Front-of-Pack Nutrition Labelling and the Setting of Nutrient Profiles for Restricting Nutrition and Health Claims on Foods. EFSA J. 2022, 20, e07259. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the Substantiation of Health Claims Related to Fruits and/or Vegetables (ID 1212, 1213, 1214, 1217, 1218, 1219, 1301, 1425, 1426, 1427, 1428, 1429, 1430) and to the “Mediterranean Diet” (ID 1423) Pursuant to Article 13(1) of Regulation (EC) No. 1924/2006. EFSA J. 2011, 9, 2245. [Google Scholar] [CrossRef]
- Zarbà, C.; Chinnici, G.; D’Amico, M. Novel Food: The Impact of Innovation on the Paths of the Traditional Food Chain. Sustainability 2020, 12, 555. [Google Scholar] [CrossRef]
- Trajkovska-Broach, A.; Trajkovska Petkoska, A. Mediterranean Herbs, Spices, and Medicinal Plants—Natural Remedies and Rich Sources of Bioactive Compounds. JSFA Rep. 2023, 3, 4–12. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, S.-H.; Yoon, Z.; Kim, J.; Kang, M.-K.; Kang, Y.-H. Eucalyptol Ameliorates Retinal Microvascular Defects through Modulating ER Stress and Angiopoietin–Tie Signaling in Diabetic Eyes. Int. J. Mol. Sci. 2024, 25, 7826. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, B.J.; Kim, J.H.; Yu, Y.S.; Kim, M.Y.; Kim, K.-W. Rosmarinic Acid Suppresses Retinal Neovascularization via Cell Cycle Arrest with Increase of p21WAF1 Expression. Eur. J. Pharmacol. 2009, 615, 150–154. [Google Scholar] [CrossRef]
- Rezaie, T.; McKercher, S.R.; Kosaka, K.; Seki, M.; Wheeler, L.; Viswanath, V.; Chun, T.; Joshi, R.; Valencia, M.; Sasaki, S.; et al. Protective Effect of Carnosic Acid, a Pro-Electrophilic Compound, in Models of Oxidative Stress and Light-Induced Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7847–7854. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, E.; Hazrati, R.; Saiah, G.V. Effects of Topical and Systemic Administration of Eugenia Caryophyllata Buds Essential Oil on Corneal Anesthesia and Analgesia. Res. Pharm. Sci. 2016, 11, 293–302. [Google Scholar] [CrossRef]
- Kanchan, D.M.; Kale, S.S.; Somani, G.S.; Kaikini, A.A.; Sathaye, S. Thymol, a Monoterpene, Inhibits Aldose Reductase and High-Glucose-Induced Cataract on Isolated Goat Lens. J. Pharm. Bioallied Sci. 2016, 8, 277–283. [Google Scholar] [CrossRef]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Kang, K.; Tarchick, M.J.; Yu, X.; Beight, C.; Bu, P.; Yu, M. Carnosic Acid Slows Photoreceptor Degeneration in the Pde6b(Rd10) Mouse Model of Retinitis pigmentosa. Sci. Rep. 2016, 6, 22632. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Y.; Li, J.; Wu, E.; Tang, T.; Singla, R.K.; Shen, B.; Zhang, M. Natural Products for the Treatment of Age-Related Macular Degeneration. Phytomedicine 2024, 130, 155522. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Meeran, M.F.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol, an All-Purpose Product. Catalysts 2022, 12, 48. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic Effects of Rosemary (Rosmarinus officinalis L.) and Its Active Constituents on Nervous System Disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Lawrenson, J.G. A Review of the Evidence for Dietary Interventions in Preventing or Slowing the Progression of Age-Related Macular Degeneration. Ophthalmic Physiol. Opt. 2014, 34, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Higashi, N.; Koga, K. Antioxidant and Antiinflammatory Activities of Oregano Extract. J. Health Sci. 2006, 52, 169–173. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Zhang, B.; Wang, S. Berries and Their Active Compounds in Prevention of Age-Related Macular Degeneration. Antioxidants 2024, 13, 1558. [Google Scholar] [CrossRef]
- Walia, A.; Gupta, A.K.; Sharma, V. Role of Bioactive Compounds in Human Health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Yuan, M.; Zhang, G.; Bai, W.; Han, X.; Li, C.; Bian, S. The Role of Bioactive Compounds in Natural Products Extracted from Plants in Cancer Treatment and Their Mechanisms Related to Anticancer Effects. Oxidative Med. Cell. Longev. 2022, 2022, 1429869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).