Abstract
Major Depressive Disorder (MDD) poses a significant global health burden, characterized by a complex and heterogeneous pathophysiology insufficiently targeted by conventional single-treatment approaches. This review presents an integrative framework incorporating network pharmacology, artificial intelligence (AI), and multi-omics technologies to advance a systems-level understanding and management of MDD. Its central contribution lies in moving beyond reductionist methods by embracing a holistic perspective that accounts for dynamic interactions within biological networks. The primary objective is to demonstrate how AI-powered integration of multi-omics data—spanning genomics, proteomics, and metabolomics—can enable the construction of predictive network models. These models are designed to uncover fundamental disease mechanisms, identify clinically relevant biotypes, and reveal novel therapeutic targets tailored to specific pathological contexts. Methodologically, the review examines the microbiota–gut–brain (MGB) axis as an illustrative case study, detailing its pathogenic roles through neuroimmune alterations, metabolic dysfunction, and disrupted neuro-plasticity. Furthermore, we propose a translational roadmap that includes AI-assisted biomarker discovery, computational drug repurposing, and patient-specific “digital twin” models to advance precision psychiatry. Our analysis confirms that this integrated framework offers a coherent route toward mechanism-based personalized therapies and helps bridge the gap between computational biology and clinical practice. Nevertheless, important challenges remain, particularly pertaining to data heterogeneity, model interpretability, and clinical implementation. In conclusion, we stress that future success will require integrating prospective longitudinal multi-omics cohorts, high-resolution digital phenotyping, and ethically aligned, explainable AI (XAI) systems. These concerted efforts are essential to realize the full potential of precision psychiatry for MDD.
1. Introduction
Depression, a debilitating mental disorder, poses a significant global health burden [1], affecting approximately 12% of adults globally and contributing substantially to disability and suicide [2]. Despite its high prevalence, the etiopathogenesis of depression remains elusive, largely due to its inherent complexity, multifactorial nature, and profound clinical heterogeneity [3]. Patients present with a wide spectrum of symptoms, severity, and treatment responses, underscoring that depression is not a single disease entity but rather a common clinical endpoint arising from a variety of underlying biological disturbances.
Despite the availability of various classes of antidepressants—including the less commonly prescribed monoamine oxidase inhibitors, and the widely used selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors—their clinical utility remains limited by a significant lag in efficacy [4,5], high rates of treatment resistance (approximately 30%) [3], and a multitude of adverse side effects that compromise patient adherence [6]. These limitations stem from a historical reductionist approach that targets single molecules or pathways, overlooking the polygenic and multisystemic nature of depression. This outdated “one-size-fits-all” paradigm underscores the critical unmet need for more precise strategies. Thus, the development of novel therapeutic agents remains a priority. This impasse necessitates a fundamental shift from a reductionist to a systems-level framework to deconvolute the disease’s complexity effectively.
The conceptualization of depression has evolved beyond neurotransmitter deficits to encompass dysregulations across multiple biological systems. Advances in genetics and systems biology have revealed that depression arises from the intricate interplay of polygenic risk scores [7], epigenetic modifications [8], dysregulated immune-inflammatory pathways [9], neuroendocrine (HPA axis) dysfunction [10], and alterations in gut–brain axis communication [11]. This complex network of interactions implies that effective intervention must move beyond isolated targets to modulate the entire disease-perturbed network. Thus, a systems-level understanding of depression requires a paradigm shift from a reductionist to a holistic perspective. Although multi-omics studies have catalogued molecular changes and network pharmacology has proposed new targets, these efforts have largely operated in isolation. The lack of integration between data generation and mechanistic interpretation remains a significant bottleneck, perpetuating the translational gap between discovery and effective therapeutics.
To address this challenge, an integrative framework combining network pharmacology, artificial Intelligence (AI), and multi-omics technologies emerges as a transformative, novel paradigm. Individually, each discipline offers unique and complementary capabilities: First, multi-omics (genomics, transcriptomics, proteomics, metabolomics) provides a comprehensive, multi-layer mapping of the molecular landscape underlying depression [12]. Building on this foundational data, Network Pharmacology constructs and analyzes the complex web of interactions between drugs, targets, and disease networks to predict therapeutic effects and identify multi-target interventions [13]. Finally, AI and Machine Learning (ML) are indispensable for integrating these massive, heterogeneous datasets, extracting meaningful patterns, and generating predictive, testable hypotheses [14]. Ultimately, this synergistic convergence—where AI dynamically interprets multi-omics data to inform and validate network pharmacology models—transforms these parallel tools into a unified, hypothesis-generating system.
This review aims to provide a comprehensive synthesis of this novel paradigm, outlining a pathway from data integration to therapeutic discovery. Throughout this review, the microbiota-gut–brain (MGB) axis is employed as a central, illustrative case study to demonstrate how each component of this paradigm—multi-omics, network pharmacology, and AI—can be integrated to decode a specific, complex pathophysiological system in depression. First, multi-omics profiling is detailed to unveil the molecular architecture of depression. This will be followed by a discussion of how network pharmacology models the mechanism of action of antidepressants and identifies potential multi-target interventions. Subsequently, the role of AI in integrating these data is highlighted for the discovery of diagnostic, prognostic, and treatment-response biomarkers, patient stratification (subtyping), and drug repurposing. The convergence of these approaches provides a more profound understanding of complex neuropsychiatric conditions and enhances the development of targeted treatment strategies, as illustrated in the integrative framework depicted in Figure 1. Finally, the review will conclude with a discussion of current challenges and future directions, proposing that this integrative approach is not merely an alternative but a necessity for decoding the heterogeneity of depression, thereby ushering in a new era of personalized molecular pharmacotherapeutics.
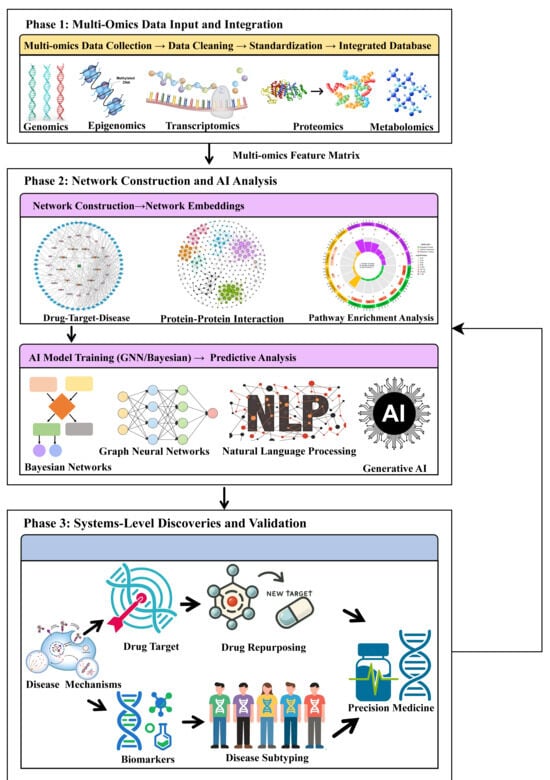
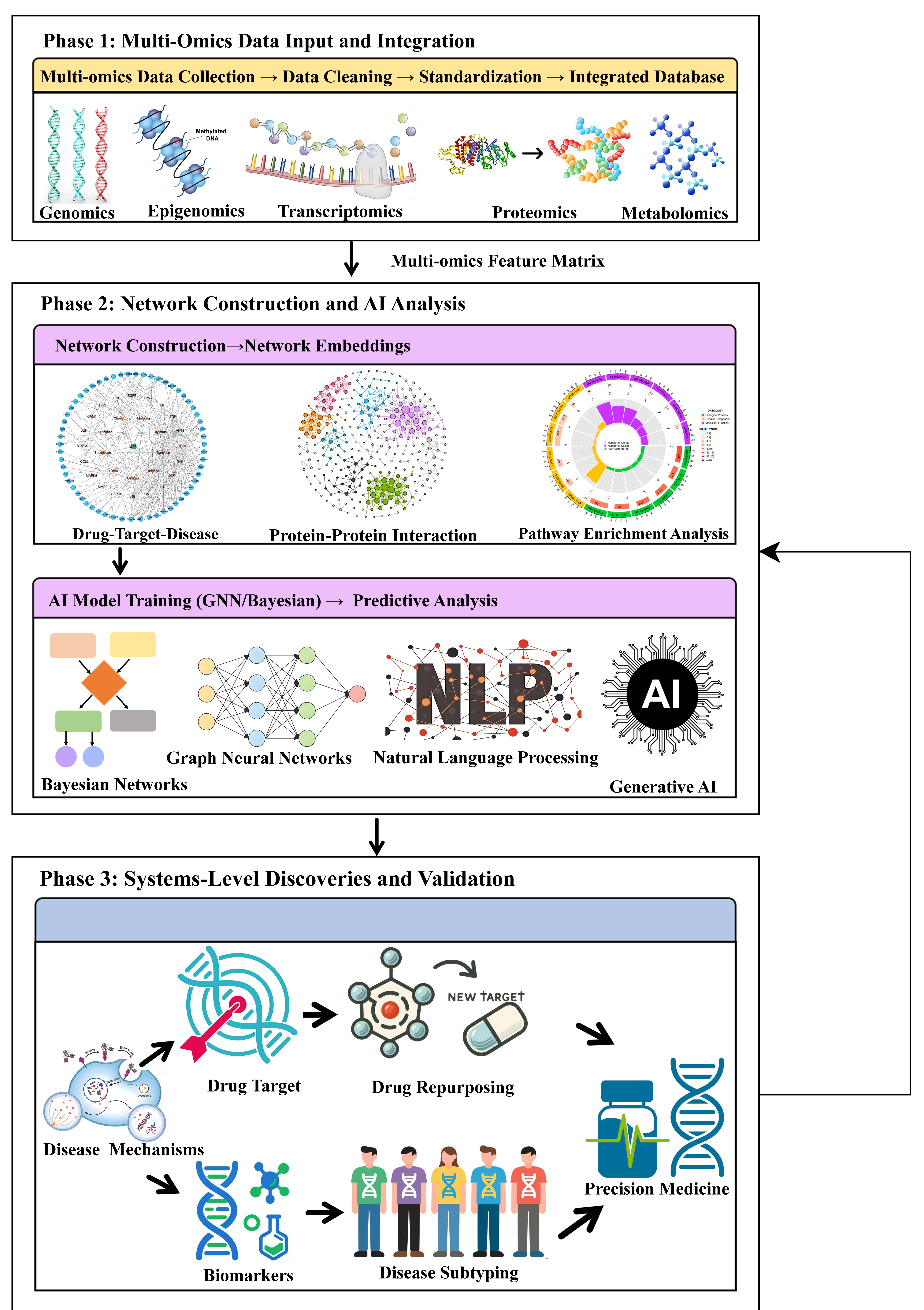
Figure 1.
Integrated workflow of network pharmacology and artificial intelligence (AI) for multi-omics analysis in major depressive disorder (MDD). The rightward arrow denotes the primary direction of the workflow and data transmission, while the vertical arrow indicates data transfer or sequential progression among phases. Phase 1 (Multi-omics data input and integration): Raw multi-omics data are collected, cleaned, standardized, and integrated into a unified database. Phase 2 (Network construction and AI analysis): Biological networks (e.g., drug–target–disease and protein–protein interaction) are constructed and converted into numerical embeddings. AI models—including graph neural networks, Bayesian networks, natural language processing, and generative AI—are then trained to generate predictive hypotheses. Phase 3 (systems-level discoveries and validation): Mechanistic insights (including disease mechanisms, novel targets, and biomarkers) inform translational applications such as drug repurposing and disease subtyping, ultimately enabling precision medicine strategies.
While the individual potential of multi-omics, network pharmacology, and AI in depression research has been recognized in existing literature, a critical synthesis gap remains. Previous reviews have often focused on one or two of these domains in isolation—for instance, discussing multi-omics biomarkers in depression [15], outlining the principles of network pharmacology [16], or exploring the role of the gut–brain axis [17]. However, there is a notable absence of comprehensive frameworks that explicitly integrate all three pillars—network pharmacology, AI, and multi-omics—into a cohesive paradigm to advance molecular therapeutics for depression.
To address these limitations, this review provides the first comprehensive synthesis. It proposes a unified, iterative framework in which AI serves as a central engine to dynamically interpret multi-omics data, thereby informing and validating context-aware network pharmacology models. The work makes three key contributions. First, it moves beyond isolated discussions to demonstrate the synergistic potential of integrating these three approaches. Second, it employs the MGB axis not merely as a topic of review but as a central, systems-level case study to concretely illustrate how the proposed framework can decode a specific, complex pathophysiological system—from multi-omics data integration and AI-driven pattern discovery to network-based target prioritization and therapeutic hypothesis generation. Finally, it outlines a clear translational pathway from large-scale data integration to novel diagnostic and therapeutic strategies, providing a strategic roadmap for researchers and clinicians in precision psychiatry. By bridging this methodological gap, this review aims to foster a new foundational perspective for future research in the field.
2. Methods
This systematic review aimed to identify all relevant studies on the integration of multi-omics, AI, and network pharmacology in depression research, with a specific focus on the MGB axis. To ensure the inclusion of the most current evidence, a comprehensive and reproducible search was conducted across three major electronic databases—PubMed/MEDLINE, Web of Science, and Google Scholar—with publication dates restricted to the past 5 years (from 2020 to 15 October 2025). The search utilized controlled terms with the following combinations: (“Depression” OR “Major Depressive Disorder”) AND (“Artificial Intelligence” OR “Machine Learning” OR “Deep Learning” OR “AI”); (“Depression” OR “Major Depressive Disorder”) AND (“Multi-Omics” OR “Metagenomics” OR “Proteomics” OR “Transcriptomics” OR “Metabolomics”); and (“Depression” OR “Major Depressive Disorder”) AND (“Microbiota-Gut-Brain Axis” OR “Gut-Brain Axis” OR “Gut Microbiota” OR “Microbiome”).
Following the search, all retrieved records were imported into EndNote for automatic and manual deduplication. Subsequently, study selection proceeded in two stages based on predefined eligibility criteria. The inclusion criteria encompassed original research or review articles focused on major depressive disorder in humans that explicitly integrated at least two of the three core domains (multi-omics, AI, network pharmacology) within the context of the MGB axis. Studies were excluded if they were not published in English, were conference abstracts, editorials, letters, or book chapters, or were based solely on animal or cell models without correlative human data. Titles and abstracts were screened first for relevance, followed by a full-text assessment of potentially eligible articles. To minimize selection bias, two reviewers independently conducted the entire screening process; any discrepancies were resolved through consensus or adjudication by a third reviewer.
Finally, data from the included studies were systematically extracted into a standardized form capturing key information, including authors, publication year, study design, types of omics data, specific AI/machine learning methods, network pharmacology approaches, key findings related to the MGB axis, and key conclusions for narrative synthesis.
3. AI-Powered Integration of Network Pharmacology and Multi-Omics
Traditional research paradigms have encountered significant limitations in deciphering the complexity of MDD. The failure of single-target strategies and the highly heterogeneous clinical presentations collectively indicate a fundamental need to shift from a static, reductionist perspective to a dynamic, systems-level one. This section builds upon these limitations to elaborate an integrative paradigm that converges network pharmacology, multi-omics, and AI. Here, AI acts not in isolation but as a central engine that unlocks the synergistic potential of the other two fields, collectively driving a methodological revolution in depression research. The core of this revolution lies in the deep integration of next-generation network pharmacology with multi-omics data, leveraging the computational power and advanced algorithms of AI to construct models that reflect the true complexity of the disease, ultimately enabling the seamless translation of big data into novel discoveries.
3.1. Multi-Omics Data Generation and Integration: A Foundation for Systems-Level Insight
Multi-omics technologies provide multifaceted and complementary biological insights into MDD. Their integration is fundamental for reconstructing causal disease networks—from genetic predisposition to physiological output—thereby advancing from associative findings to a mechanistic understanding of MDD.
3.1.1. Genomics: Defining Inherited Risk
Genome-wide association studies (GWAS) have identified numerous genetic loci associated with MDD risk. For example, the landmark study by Howard et al., which included over 807,553 cases, identified 102 independent variants, implicated 269 genes, and highlighted 15 gene sets associated with depression. These findings underscore pathways related to neuronal development and synaptic function [18]. Polygenic risk scores derived from these findings quantify individual genetic susceptibility, providing a foundational layer for understanding disease heritability [19].
3.1.2. Epigenomics: Bridging Environment and Genetics
Epigenetic mechanisms have emerged as crucial mediators connecting genetic predisposition and environmental exposures in the pathophysiology of depression. These mechanisms—including DNA methylation, hydroxymethylation, and histone modifications—produce stable alterations in gene expression without changing the underlying DNA sequence and have been consistently implicated in various psychiatric disorders [20]. Supporting this paradigm, a study by Efstathopoulos et al., which analyzed salivary DNA from a cohort of 1149 adolescents, demonstrated that methylation levels in exon 1 of the NR3C1 gene mediated the association between early life stress and the severity of depressive symptoms [21]. Such findings directly illustrate how life experiences “program” gene expression patterns to influence disease pathology.
3.1.3. Transcriptomics: Mapping Functional Gene Activity
RNA sequencing (RNA-seq) enables comprehensive profiling of global gene expression patterns in specific tissues or cell types under disease conditions, directly reflecting functional gene activity and serving as a primary resource for identifying key pathways and biomarkers [22]. Advancing beyond bulk tissue analysis, single-nucleus RNA sequencing (snRNA-seq) now enables the precise mapping of transcriptional alterations to specific cell populations, uncovering unprecedented cellular-resolution insights into disease mechanisms. Nagy et al. employed single-nucleus transcriptomics on the dorsolateral prefrontal cortex in MDD, revealing widespread, cell-type-specific gene expression changes. Specifically, deep-layer excitatory neurons and immature oligodendrocyte precursor cells were the most severely dysregulated, accounting for nearly half (47%) of all changes, directly implicating these populations in disease pathology and highlighting them for future investigative and therapeutic focus [23].
3.1.4. Proteomics: From Biomarker Discovery to Personalized Therapy
Proteomics plays an essential role in identifying biomarkers and informing personalized treatment strategies [24]. This is exemplified by the large-scale study by Bot et al., which analyzed 1589 participants and identified several depression-associated proteins implicated in critical processes such as immune response and cell communication [25], thereby fulfilling the core aim of biomarker discovery. Furthermore, the dynamic nature of the proteome is highlighted by studies showing that many inflammatory proteins exhibit significant concentration changes following antidepressant treatment [26], directly linking proteomic profiles to treatment response.
3.1.5. Metabolomics: Profiling Systemic Physiological Output
As the terminal downstream readout of physiological activity, metabolomics provides a unique window into the functional outputs of cellular processes and can reflect central nervous system activity across the blood–brain barrier [27]. Studies of young patients with MDD have revealed perturbations in specific metabolic pathways, including purine and fatty acid metabolism. Notably, inosine was identified as a potential independent diagnostic biomarker. Furthermore, the pathophysiology of MDD in this population appears to differ from that of adults, particularly in tryptophan metabolism [28]. The dysregulation of amino acid metabolism in MDD is characterized by elevated serum levels of glutamate, aspartate, and glycine, alongside decreased 3-hydroxykynurenine. Additionally, the serum levels of glutamate and phenylalanine show a correlation with depression severity, indicating their potential as clinical markers for severity-based stratification [29].
A summary of these multi-omics technologies and their contributions to MDD research is provided in Table 1.

Table 1.
Multi-omics technologies and their contributions to MDD research.
3.1.6. Integrative Multi-Omics: Towards a Unified Model
As highlighted in Table 1, integrating multiple omics layers is essential for reconstructing causal networks spanning from genetic variation to metabolic output. The individual omics layers, as detailed in the preceding sections, provide multifaceted but fragmented insights into MDD. Therefore, integrating these diverse data types through advanced computational methods is pivotal for constructing a unified systems-level model. A particularly promising frontier involves integrating host multi-omics data with gut metagenomic profiles, a fusion strategy that is critical for systematically elucidating the role of the MGB axis in MDD and, as detailed in later sections, paving the way for precision neuropsychiatry. This systemic approach is indispensable for dissecting disease heterogeneity, delineating molecular subtypes, and elucidating the mechanistic interplay between genetic predisposition, environmental triggers, and dysregulated biological pathways.
3.2. The Network Pharmacology Paradigm: From Single-Target to Systems-Level Intervention
3.2.1. Fundamentals of Network Pharmacology: From Single-Target to Network Medicine
The traditional organ-focused medical paradigm and the reductionist “one disease–one target–one drug” approach have substantially limited advances in understanding complex diseases and developing effective therapeutics. In contrast, network pharmacology (NP) has emerged as a discipline that transcends this limitation by adopting a systems-level perspective. First conceptualized by Hopkins [30], NP integrates systems biology, computational biology, and experimental validation through a data-driven framework to holistically decipher complex compound–body interaction networks [31].
Guided by the concept of “network targeting,” NP offers innovative strategies to elucidate how multi-compound or drug combinations act synergistically on key network proteins, thereby modulating shared disease-associated modules or signaling pathways [32]. This enables a fundamental shift from symptomatic treatment to targeting causal mechanisms in complex diseases [33].
Central to NP methodology is the construction and analysis of a “drug–target–disease” heterogeneous network, which integrates multi-source data, including protein–protein interactions to provide biological context; drug–target interactions (from databases such as STITCH, BindingDB, and ChEMBL); and disease-related genes/proteins from and disease-related genes/proteins (from GWAS and transcriptomic studies). Topological analyses—using metrics such as degree and betweenness centrality—allow for the identification of critical hub nodes and functional modules, which are often ideal targets for multi-target drug design.
While this static network approach represents a monumental leap from single-target thinking and remains a foundational tool, it ultimately provides only a static snapshot of a dynamic system. It inherently overlooks the temporal evolution of disease networks across different stages and their specific manifestations within distinct biological contexts, such as cell types or brain regions. Addressing these limitations is the primary goal of next-generation network pharmacology, which leverages artificial intelligence to model the full complexity of living systems [34,35].
3.2.2. Next-Generation Network Pharmacology: From Static Target Prediction to Dynamic, Context-Aware Network Construction
Building upon foundational static models, next-generation NP moves beyond the snapshot view by explicitly incorporating temporal dynamics and biological context into network construction. This shift is powered by the integration of artificial intelligence, which provides the computational tools necessary to infer and model these complex relationships. Advances are primarily unfolding in three interconnected areas:
Dynamic Network Modeling: Capturing Temporal Causality
Static networks reveal association, but dynamic models aim to infer causality and temporal progression. This is achieved by integrating temporal ML models with longitudinal multi-omics data (e.g., repeatedly sampled microbiomes, metabolomes, or transcriptomes over a treatment course) [36]. These models can simulate how a perturbation (e.g., a drug, a stressor) propagates through the molecular network over time, revealing cascading effects and critical intervention points. A compelling example is provided by Scutari et al., who employed dynamic Bayesian Networks (DBNs) to investigate the bidirectional causal relationships between skin diseases and mental disorders at an infodemiological level. Their model, which accounted for spatiotemporal dependencies and utilized bootstrap resampling for robust inference, successfully identified key skin-to-brain and brain-to-skin interaction pathways, demonstrating the unique capability of DBNs to capture reciprocal interactions across time points—a fundamental limitation of static network models [37]. Nevertheless, fundamental challenges in computational psychiatry—notably, the standardization and reproducibility of complex analytical workflows—persist.
Context-Specific Integration: Modeling Spatial and Cellular Heterogeneity
Biological networks are not universal; they vary widely across cell types, tissue microenvironments, and brain regions. Graph Neural Networks (GNNs) provide a powerful computational framework for this context-aware integration, enabling the merger of the core “drug–target–disease” network with multilayered contextual information. This includes leveraging single-cell RNA-seq data to infer cell-type-specific interaction networks, integrating spatial transcriptomics and proteomics data to decode the influence of cellular spatial organization on network topology, and incorporating compound properties to predict polypharmacy effects within a specific pathological context [38,39,40]. This approach aims to construct networks tailored to particular pathological states rather than employing a “one-size-fits-all” model, thereby paving the way for true precision psychiatry.
However, the effectiveness of GNNs in this domain is fundamentally constrained by a critical prerequisite: the quality and representativeness of the input network. A core and often underappreciated challenge is graph construction bias [41]. The performance and interpretability of GNNs are profoundly sensitive to how nodes and edges are defined in the initial biological network. This process is itself a hypothesis-driven modeling choice based on incomplete prior knowledge (e.g., protein–protein interaction databases), not an objective ground truth. Consequently, a biased or incomplete starting graph directly leads to biased model predictions and interpretations. This fundamental limitation, along with the broader methodological challenges it precipitates, is systematically elaborated in the section “Challenges and Future Directions for Next-Generation NP”.
Multi-Layered Network Fusion: Constructing Supernetworks of Systemic Dysregulation
The most holistic approach involves constructing multi-layered super-networks (or heterogeneous graphs) that vertically integrate disparate biological scales—genomic variation, epigenetic marks, gene expression, protein activities, and metabolic phenotypes—into a unified graph [42]. AI, particularly GNNs, is tasked with parsing the nonlinear relationships among these layers to identify cross-omic functional modules and key intermediary nodes that drive systemic dysregulation in MDD. This moves far beyond conventional PPI networks.
The construction of such multi-layered supernetworks represents a powerful approach to modeling MDD’s systemic dysregulation. However, the biological plausibility and utility of these AI-inferred networks face fundamental methodological challenges, particularly the sensitivity of their structure to initial assumptions and the lack of a definitive validation benchmark. To address this, we advocate for a multifaceted validation strategy (detailed in the section “Challenges and Future Directions for Next-Generation NP”) that combines in silico techniques, such as perturbation analysis, with ultimate experimental validation through wet-lab methods to establish causal biological plausibility.
Challenges and Future Directions for Next-Generation NP
Despite its promise, implementing next-generation network pharmacology faces several fundamental challenges that must be acknowledged.
First, data scarcity and heterogeneity pose a foundational bottleneck. Constructing dynamic and context-specific models demands vast amounts of high-quality, longitudinal, multi-modal data, which are often scarce, noisy, and costly to generate [43].
Second, graph construction bias remains a core methodological constraint. As discussed in the section “Context-Specific Integration: Modeling Spatial and Cellular Heterogeneity”, the structure and predictions of any network model are profoundly limited by the completeness and accuracy of the prior knowledge (e.g., from interaction databases) used to define the initial graph [44]. Models trained on biased or incomplete “prior graphs” risk producing misleading outputs.
Third, the validation of inferred networks lacks a “gold standard”. There is no absolute ground truth against which to benchmark computationally derived interactions [45]. Consequently, adopting a multifaceted validation strategy is imperative. This integrated approach comprises two critical components: Firstly, in silico validation employs techniques such as network perturbation analysis to assess the concordance between model-predicted key nodes and well-established essential genes or drug targets from independent databases [46]. Secondly, experimental validation is essential, wherein hypotheses concerning critical hubs and pathways require functional confirmation through wet-lab experiments, typically involving CRISPR-based knockdown or knockout in relevant cell models to establish causal roles [47].
Finally, model interpretability and generalizability pose significant translational barriers. The “black box” nature of complex models such as GNNs impedes clear biological interpretation [48]. More critically, models trained on specific cohorts often exhibit poor cross-cohort generalizability due to technical batch effects and population heterogeneity, limiting their broad clinical applicability.
3.3. AI as an Integrative Engine: From Multi-Omics Data to Mechanistic Insights in Depression
Understanding major depressive disorder as a complex systems-level disorder necessitates the integration of high-dimensional, multi-omics data. This integration poses significant analytical challenges related to dimensionality, heterogeneity, and dynamic complexity. AI serves as the essential engine for this integration, transforming these challenges into opportunities to generate novel, testable hypotheses about disease mechanisms. Rather than just a predictive toolkit, AI provides a framework to decipher the biological heterogeneity underlying clinical symptoms, model the dysregulated networks central to pathophysiology, and semantically bridge disparate data modalities—collectively advancing the field toward precision psychiatry.
3.3.1. Machine and Deep Learning: From Data Integration to Mechanistic Hypotheses
AI, particularly ML and DL, provides the essential computational framework for integrating high-dimensional, heterogeneous multi-omics data in depression research. Its value lies not merely in prediction, but in its capacity to generate data-driven, mechanistic hypotheses about disease heterogeneity.
Unsupervised learning addresses the core challenge of disease subtyping beyond symptomatic diagnosis. By identifying data-driven biotypes from integrated omics layers, these methods directly probe the biological heterogeneity underlying the clinical syndrome of MDD. For instance, hierarchical clustering of neurophysiological data by Drysdale et al. identified subtypes with distinct treatment response profiles, suggesting divergent underlying circuit dysfunctions [49]. Such approaches move the field towards a biology-based nosology.
Supervised learning addresses the challenge of integrating diverse biomarkers to improve prediction. By fusing features across genomic, epigenomic, and other domains, these models can improve the prediction of diagnosis or treatment outcome [50,51]. Critically, the feature importance metrics from models like Random Forests can highlight specific biological pathways (e.g., inflammation-related genes or specific methylation sites) worthy of further mechanistic investigation, thus bridging prediction and biological insight.
DL and GNNs offer a powerful paradigm for modeling complex, relational biological systems. In depression research, GNNs can analyze brain functional connectomes or molecular interaction networks to identify aberrant connectivity patterns or key pathogenic hubs, shifting the focus from individual biomarkers to dysfunctional systems-level properties [52]. Recent methodological innovations underscore this potential; for example, the Graph Frequency Attention Convolutional Neural Network incorporates electroencephalogram (EEG) signal frequency as an attention mechanism to prioritize clinically relevant neural rhythms. This model has shown superior performance in classifying treatment responses compared to established benchmarks, highlighting the utility of advanced GNNs in decoding complex brain dynamics and supporting clinical decisions [52].
3.3.2. Natural Language Processing: Bridging Subjective Experience and Biological Constructs
Natural Language Processing (NLP) has emerged as a pivotal tool for extracting quantifiable insights from unstructured textual data, offering a unique bridge between subjective patient experiences and objective biological measures in depression research [53]. By analyzing diverse digital footprints—from clinical notes and therapy transcripts to social media posts—NLP can identify subtle linguistic markers (e.g., emotional valence, semantic coherence, and first-person pronoun usage) that serve as digital phenotypes of depressive states [54,55].
While conventional machine learning models (e.g., Support Vector Machines, decision trees) provided foundational classification capabilities [54], the advent of large language models (LLMs) has markedly advanced the field. LLMs capture deeper contextual and semantic features specific to mental health language, thereby significantly improving the accuracy and generalizability of depression detection and progression-monitoring systems [55].
Critically, the value of NLP extends beyond standalone digital phenotyping. Its power is fully realized when linguistic features are integrated with other biological modalities. This multi-modal integration is exemplified by Dougherty et al., who combined NLP-derived features with electroencephalographic imaging to predict longitudinal responses to psilocybin therapy in treatment-resistant depression. Their model achieved high cross-validated accuracy, demonstrating that the combination of language-based and neurophysiological data outperforms either modality alone [56]. This approach underscores a central paradigm: NLP transforms qualitative narrative into structured data, enabling its correlation with genomic, metabolomic, or neuroimaging findings to construct a more comprehensive, multi-dimensional model of depression.
Thus, NLP operates not merely as an analytical tool but as a critical semantic translator, weaving a patient-generated narrative into the broader tapestry of multi-omics data. This integration is essential for advancing a precision psychiatry framework that is both biologically grounded and intimately informed by the lived experience of illness.
3.3.3. Generative AI: As a Novel Engine for Hypothesis Generation and Data Augmentation
Beyond its clinical applications, Generative AI (GAI) offers a transformative potential for mechanistic research in depression by addressing two fundamental bottlenecks in multi-omics integration: the scarcity of large-scale, well-annotated datasets and the difficulty in generating causal, testable hypotheses from high-dimensional data. Techniques such as generative adversarial networks and variational autoencoders can create high-fidelity synthetic multi-omics data [57], thereby augmenting limited clinical cohorts and enhancing the robustness of downstream analytical models [58]. This capability is critical for powering data-intensive deep learning approaches without exacerbating concerns of overfitting or compromising patient privacy.
More profoundly, GAI serves as a powerful engine for knowledge synthesis and the generation of mechanistic hypotheses. Researchers can leverage large language models (LLMs), pre-trained on vast biomedical corpora, to mine the literature and infer novel connections between disparate omics data, clinical symptoms, and known pathophysiology [59]. For instance, a GAI system could be prompted to propose novel gene regulatory networks underlying treatment-resistant depression by integrating transcriptomic data with known protein–protein interactions and pharmacogenetic databases, thus yielding specific, experimentally verifiable hypotheses.
This approach is exemplified by emerging studies employing GAI to design novel multi-epitope vaccines for major depression based on integrated genomic and proteomic data [60], or to predict the functional impact of non-coding genetic variants on neuronal gene regulation [61]. These applications demonstrate how GAI moves beyond pattern recognition to actively propose biomolecular mechanisms and therapeutic candidates that might remain obscured through conventional analysis.
Nevertheless, integrating GAI into the multi-omics research pipeline poses significant domain-specific challenges. These include the risk of generating biologically plausible but factually incorrect hallucinations [62], the embedded biases of training data that may perpetuate existing biomedical disparities [63], and the critical need for rigorous in vitro and in vivo validation of any AI-generated hypothesis. Future work must therefore prioritize the development of transparent, explainable, and biologically constrained GAI frameworks that can be seamlessly integrated into the iterative cycle of computational prediction and experimental validation, ultimately accelerating the path toward novel therapeutic targets.
A consolidated overview of these core AI approaches, their respective integrative functions, and exemplary applications is presented in Table 2.

Table 2.
Key artificial intelligence and computational approaches for integrating multi-omics data in depression research.
3.4. Data Integration and Analytical Framework: From Data to Systems-Level Insights
The central challenge in multi-omics research lies in integrating heterogeneous data types—spanning genomics, transcriptomics, epigenomics, and proteomics—with structured biomedical knowledge and artificial intelligence algorithms into a unified analytical framework. Among contemporary computational approaches, GNNs have emerged as one of the most promising avenues for this integration [64]. Their capability to natively represent biological systems as graphs—where nodes denote biological entities (e.g., genes, proteins, or metabolites) and edges represent functional or physical interactions—makes them particularly suitable for modeling complex, relational biomedical data.
3.4.1. Graph Neural Networks: A Technical Foundation
GNNs constitute a class of deep learning models designed to operate directly on graph-structured data. Architectures such as Graph Convolutional Networks, GraphSAGE, and Graph Attention Networks have achieved state-of-the-art performance across numerous bioinformatics tasks, including disease subtype prediction, drug repurposing, and biomarker discovery [65]. Initially developed for node-level classification, GNNs leverage message-passing mechanisms to propagate and aggregate feature information across adjacent nodes, enabling effective prediction and representation learning even with highly sparse and interconnected biological data [66].
3.4.2. Information Fusion and Inference via GNNs
Methodological advances have extended GNNs to multi-relational and heterogeneous graphs, facilitating the integration of diverse omics data types—such as transcriptomic, epigenomic, and spatial transcriptomic profiles—within a single model. Incorporation of external biological networks (e.g., protein–protein interactions, gene regulatory networks, and ligand–receptor databases) further enhances the contextual relevance and interpretability of GNN predictions. These integrative approaches allow models to infer functional biological relationships—such as regulatory mechanisms or intercellular signaling pathways—that are not readily apparent from omics data alone [65]. Nevertheless, several computational and practical challenges impede the broad application of GNNs in real-world biomedical contexts.
3.4.3. Current Challenges and Computational Strategies
A key limitation of many GNN architectures is their scalability to large biological graphs, constrained by high computational and memory demands. Furthermore, many models exhibit excessive reliance on local neighborhood information, constraining their ability to capture global graph topology and long-range dependencies—a critical shortcoming in applications such as genome-scale regulatory network inference. To mitigate this, one common strategy is to design deeper network architectures or incorporate hierarchical pooling mechanisms, thereby expanding the receptive field and promoting the integration of information from broader network contexts [65].
3.4.4. A Practical Roadmap for GNNs in Depression Research
Translating the theoretical strengths of GNNs into practical tools for depression research requires a domain-specific methodological framework that addresses salient challenges such as data heterogeneity, temporal sparsity, and interpretability.
The suitability of GNNs for multi-omics integration in neuropsychiatric research stems from their ability to explicitly incorporate biological priors—such as protein interaction networks or neuroimaging-derived connectomes—into an inductive learning framework [67]. This facilitates biologically grounded generalization, which is especially valuable in settings with limited sample sizes. Empirical validation of GNN-based approaches should include systematic benchmarking against alternative deep learning models, including transformers and multimodal autoencoders, using metrics such as predictive accuracy, robustness to noise, and interpretability [68,69]. Furthermore, the development and evaluation of hybrid or co-learning models that integrate the strengths of both paradigms represent a promising avenue for next-generation integrative analysis [70].
Constructing robust graph representations for MDD necessitates a comprehensive pre-processing and integration strategy to address data heterogeneity. This includes independent batch-effect correction for each omics layer prior to graph construction [71], incorporating adversarial domain adaptation within the GNN to minimize technical variability, and handling missing data via graph-aware imputation techniques [72] or multi-task architectures that jointly learn to impute missing values and predict clinical outcomes. To improve generalizability across independent cohorts or sequencing platforms, frameworks such as graph meta-learning or invariant representation learning can be employed to derive domain-agnostic features [73].
Model interpretability remains paramount when applying GNNs to high-dimensional, noisy biological data. Beyond using intrinsically interpretable architectures like GATs—which provide attention-based importance scores for nodes and edges—post hoc interpretation tools (e.g., GNNExplainer) can identify minimal predictive subgraphs that can be functionally annotated via pathway enrichment analysis [74]. Incorporating sparsity-inducing constraints during training can also promote simpler and more interpretable model structures.
Finally, modeling temporal dynamics in longitudinal multi-omics data—often sparse in MDD studies—requires specialized approaches. Techniques such as neural ordinary differential equations or Gaussian processes can reconstruct continuous individual trajectories from sparse observations, enabling the construction of dynamic graphs. Alternatively, temporal GNNs or dynamic embedding methods can directly model irregularly sampled time-series omics data. Incorporating ensemble learning strategies may further enhance the robustness and generalizability of dynamic forecasting models [75]. Together, these strategies form a comprehensive analytical workflow for translating multi-omics data into actionable insights within depression research.
4. A Systems-Level Case Study: The Gut–Brain Axis in Depression Pathophysiology
4.1. Identification of Key Molecular Modules and Pathways
Integrated multi-omics analyses have revealed core signaling pathway networks that extend beyond the classic monoaminergic hypothesis [76]. These include, but are not limited to, neuroinflammation [10], mitochondrial dysfunction [77], circadian rhythm disruptions [78], and neurotrophic factor signaling [79]. Collectively, these findings underscore the complex, interconnected biological architecture underlying depression and provide new mechanistic entry points for therapeutic intervention.
4.2. Parsing Disease Subtypes (Biotypes)
Unsupervised ML approaches applied to multi-omics data have enabled the identification of depression subtypes—referred to as biotypes—characterized by distinct molecular signatures [80]. These data-driven classifications move beyond symptom-based diagnoses and offer a biological foundation for precision subtyping, potentially guiding more targeted and effective treatment strategies.
4.3. The Gut–Brain Axis: A Central Interface
The gut microbiota is a highly complex, dynamically evolving consortium of diverse microorganisms—including bacteria, fungi, parasites, and viruses—that exhibits considerable geographic variation. These microbial communities engage in extensive intra- and interspecies interactions, as well as symbiotic relationships with the host, and play an essential role in maintaining normal physiological homeostasis [81]. The gut microbiota significantly modulates cognitive processes and behaviors via the MGB axis, an integrated network connecting the gastrointestinal tract and the central nervous system via neural, immune, and endocrine signaling mechanisms [82,83].
4.3.1. Neuroimmune and Neuroinflammatory Pathways
Building on the MGB axis, dysbiosis-induced systemic inflammation is a key mechanistic pathway linking the MGB axis to depression. An imbalanced gut microbiota can trigger the release of pro-inflammatory cytokines, which may cross the compromised blood–brain barrier and contribute directly to the development of depression symptoms [84]. A compelling mechanistic link involves matrix metalloproteinase-8 (MMP8), which is elevated in MDD patients and stress-vulnerable mice. MMP8 can traverse the blood–brain barrier, localize to reward-related regions such as the nucleus accumbens, and remodel the extracellular matrix, ultimately impairing social reward behavior [85,86]. This state of chronic inflammation is increasingly recognized as a central factor in depression pathophysiology, capable of damaging neuronal tissue and disrupting function [87].
4.3.2. Neurotransmitter and Metabolic Regulation
Beyond neuroimmune mechanisms, gut microbiota dysbiosis significantly influences neurotransmitter systems critically involved in MDD pathophysiology. A substantial proportion of neurotransmitters—including serotonin (5-HT), norepinephrine, dopamine, γ-aminobutyric acid (GABA), glutamate, and acetylcholine—or their precursors are produced by gut microbes or gut-enteroendocrine cells. These molecules enter the systemic circulation and subsequently affect brain function either by crossing the blood–brain barrier or by activating vagus nerve-mediated signaling [88,89].
Microbiota-derived metabolites further contribute to MDD through several parallel pathways. Short-chain fatty acids (SCFAs) modulate depressive-like behaviors via: (1) regulating the HPA axis [90]; (2) enhancing central serotonin synthesis via tryptophan metabolism [91]; and (3) exerting anti-inflammatory effects and reinforcing intestinal barrier integrity [92]. Additional microbial metabolic pathways implicated in MDD include bile acid metabolism and dysregulation of the tryptophan-kynurenine (Trp-Kyn) pathway, both of which may influence neuroactive signaling and immune activation [93,94].
4.3.3. Neuroplasticity and Structural Integration
In addition to systemic inflammation and metabolic regulation, the MGB axis influences depression pathophysiology through direct effects on neuroplasticity. Gut-derived signals, potentially mediated by microbial metabolites such as SCFAs, can regulate microglial development and function, thereby shaping neural circuitry and behavior [95]. Furthermore, impaired adult hippocampal neurogenesis—evidenced by reduced neural stem/progenitor cells, decreased granule neuron numbers, and lower dentate gyrus volume—represents a consistent morphological correlate of MDD [96,97,98]. The gut microbiota can modulate adult hippocampal neurogenesis and stress-related hormone secretion via the HPA axis, thereby participating in the regulation of mood and cognition [99]. These interrelated pathways are summarized in Figure 2.
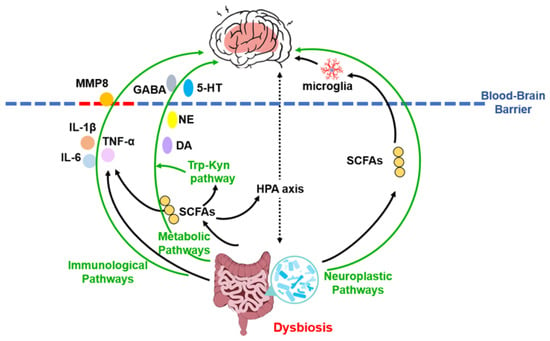
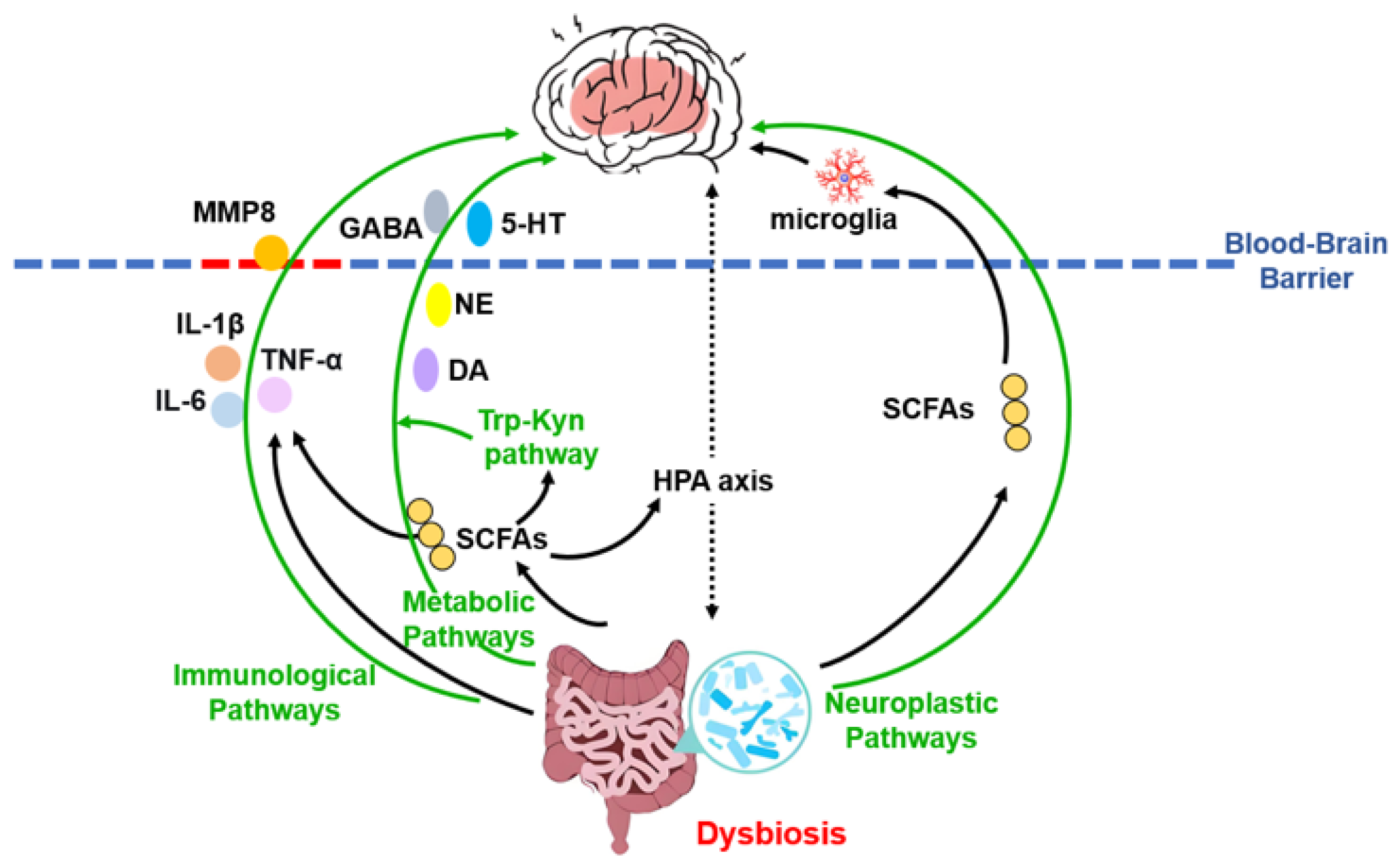
Figure 2.
Schematic overview of the microbiota–gut–brain axis mechanisms in depression. This integrative model illustrates how gut dysbiosis contributes to the pathophysiology of major depressive disorder through three core, interacting pathways: neuroimmune, metabolic, and neuroplastic. Solid arrows denote the direction of effects; green arrows highlight the three main mechanistic categories. The blood–brain barrier is represented by a dashed line, with red dashes indicating its compromised, permeable state. Abbreviations: MMP8, matrix metalloproteinase-8; 5-HT, serotonin; DA, dopamine; GABA, γ-aminobutyric acid; HPA, hypothalamic-pituitary-adrenal; IL, interleukin; NE, norepinephrine; SCFAs, short-chain fatty acids; Trp-Kyn, tryptophan-kynurenine.
Compelling evidence for the microbiota’s essential role comes from germ-free animal models; for instance, germ-free mice exhibit an immature enteric nervous system and defective sensory signaling, deficits which can be reversed by restoring a normal gut microbiota [100,101]. Clinically, a substantial proportion of MDD patients present with somatic manifestations indicative of MGB axis dysregulation, commonly encompassing gastrointestinal complaints, appetite loss, nausea, and metabolic irregularities [102]. Ultimately, integrating these mechanisms into molecular network models provides a more comprehensive understanding of depression’s systemic nature.
4.3.4. Evidence Heterogeneity and Translational Challenges
While preclinical evidence robustly supports the involvement of the MGB axis in depression pathophysiology, translating this evidence into effective clinical interventions remains complex and poses significant methodological challenges. Notably, several high-profile randomized controlled trials of probiotic supplementation (psychobiotics) have failed to demonstrate substantial antidepressant effects over the placebo in broad MDD populations [103,104]. This discrepancy underscores the complexity of translating mechanistic knowledge into effective therapies. Potential explanations for these mixed results include the vast heterogeneity in probiotic strains, formulations, and doses used across studies, which may not optimally target specific dysbiotic states or pathways relevant to depression. Furthermore, considerable inter-individual variability in baseline gut microbiota composition and host physiology suggests that effective interventions may need to be personalized rather than universal [105]. Adding to this complexity is the current lack of standardized treatment protocols. This is particularly relevant for patients with severe or chronic MDD, for whom modulating the gut microbiota alone may be insufficient, potentially necessitating combination with other therapeutic modalities [106].
These outcomes do not invalidate the MGB hypothesis but rather emphasize that modulating a complex, individualized ecosystem requires precision. Future research must therefore focus on biomarker-guided patient stratification (e.g., identifying inflammation- or tryptophan metabolism-dominant biotypes) and on testing targeted, next-generation psychobiotics or microbiome-based therapies in mechanistically aligned subpopulations. The integration of multi-omics profiling into clinical trial design is essential to identify responders and refine therapeutic strategies.
5. Translation and Application: From Big Data to Novel Diagnostic and Therapeutic Strategies
The rapidly evolving understanding of mechanisms of the MGB axis is fostering a transformative paradigm in translational psychiatry, enabling the conversion of multidimensional data into innovative diagnostic and therapeutic solutions. This paradigm shift is powered by the synergistic integration of multi-omics technologies with artificial intelligence, creating new pathways for biomarker discovery and precision medicine in depression. The computational foundation for this transformation is established through a suite of AI methodologies—from machine learning algorithms for pattern extraction to graph-based architectures for modeling biological networks—that collectively enable the systematic analysis of complex disease mechanisms and support the development of targeted interventions.
5.1. Biomarker Discovery and Validation
A single biomarker is insufficient to capture the high heterogeneity of MDD. Consequently, research strategies are shifting towards integrating multidimensional information from genomics, metagenomics, metabolomics, proteomics, and clinical data to discover and validate composite biomarker panels.
The integration of diverse high-dimensional datasets offers a powerful strategy for deriving multi-omics biosignatures, which can elucidate the biological underpinnings of clinical heterogeneity in depression. Specifically, Data Integration Analysis for Biomarker discovery using Latent components (DIABLO) employs a modified sparse partial least squares discriminant analysis to build predictive multi-omics signatures. These signatures are derived from the correlations within and between datasets to classify group membership, and the method applies to large, diverse datasets regardless of prior feature selection [17]. The identification of such data-driven biosignatures not only refines diagnostic precision but also directly informs the development of targeted therapeutics and personalized intervention strategies, as discussed in the following sections.
5.2. Drug Discovery and Repurposing
The discovery of the MGB axis has opened novel avenues for antidepressant drug development, with a core philosophy shifting from traditional “single-target” approaches to “network regulation” that better reflects the disease’s complexity.
5.2.1. Novel Multi-Target Drug Design
Building on the comprehensive pathophysiological framework of the MGB axis established in Section 3.3, NP analysis enables the identification of key, dysregulated nodes within this complex system. Novel therapeutic strategies aim to modulate multiple key nodes within this dysregulated network concurrently. The following approaches exemplify this rational design principle:
Targeting Neuroimmune Crosstalk: The established role of peripheral mediators, such as MMP8, presents a compelling therapeutic target. Rational drug design could aim to develop selective MMP8 inhibitors to prevent its detrimental remodeling of the nucleus accumbens extracellular matrix, thereby preserving social reward behavior.
Modulating Microbial Neurotransmitter Synthesis: The gut’s significant contribution to peripheral neurotransmitter precursors offers a novel avenue for intervention. One strategy involves developing small-molecule modulators that selectively influence microbial enzymes involved in the synthesis of tryptophan, tyrosine, or GABA.
Harnessing Metabolite Signaling Pathways: The beneficial effects of SCFAs via receptor-mediated mechanisms provide a blueprint for developing receptor agonists. Designing stable, bioavailable FFAR2/3 agonists could replicate the anti-inflammatory, barrier-strengthening, and HPA-axis-modulating effects of SCFAs. Similarly, correcting Trp-Kyn pathway dysregulation through Indoleamine 2,3-dioxygenase/Tryptophan 2,3-dioxygenase inhibitors represents a promising multi-target strategy.
Promoting Neuroplasticity: Future therapies may integrate the aforementioned strategies with agents that directly promote neurotrophic activity and neuronal resilience, thereby targeting the core morphological deficits of the disorder.
Collectively, these strategies establish a conceptual framework for multi-target intervention. Its practical realization critically depends on integrating network pharmacology with computational modeling to systematically decipher the interaction networks and pinpoint the optimal multi-target ligand combinations or repurposing opportunities for experimental testing.
5.2.2. AI-Driven Drug Repurposing
Drug repurposing is a cost- and time-efficient strategy that leverages existing knowledge of drug safety, pharmacokinetics, and manufacturing processes to identify new therapeutic applications, offering a viable alternative to traditional de novo drug development [107]. The integration of AI has transformed this strategy from a serendipitous process into a systematic, data-driven paradigm [108]. AI-powered computational frameworks can integrate multi-source biomedical data to efficiently predict novel drug-disease associations. This advancement is exemplified by high-throughput systems such as TensorFlow Drug Repurposer and DeepDrugRepurposing, which enable large-scale predictions by systematically analyzing multidimensional drug screening datasets [109].
The efficacy of these AI platforms stems from a suite of sophisticated computational and experimental methodologies. Computational methodologies include feature matching based on gene expression profiles (transcriptomics), metabolomics and proteomics, chemical structure similarity analysis, adverse drug reaction profiling, and molecular docking and GWAS. Complementary experimental approaches include retrospective clinical data analysis (e.g., electronic health records and post-marketing surveillance), the use of novel data sources (such as cancer cell line screening and patient-reported outcomes), target-binding assays, and phenotypic screening. Through these integrated strategies, researchers can systematically identify novel therapeutic indications for existing drugs in the treatment of depression across multiple dimensions [110].
A key advantage of the AI-driven approach is its ability to significantly accelerate the identification of repurposing candidates and streamline their progression into Phase II/III trials. By leveraging pre-existing pharmacological and toxicological data, AI models can accurately predict a drug’s efficacy and safety profile in new disease contexts, thereby substantially reducing both development timelines and associated costs [111].
In the context of depression, AI-driven repurposing has broadened the therapeutic landscape, with numerous agents across diverse therapeutic classes currently under investigation or approved based on computational predictions [110,112]. For instance, AI-based analyses of electronic health records and molecular interaction networks have suggested repurposing potential for various drug categories in bipolar disorder and depression. Successful examples include at least thirteen non-psychiatric drugs and twenty antidepressants that have been assigned new indications through computationally guided approaches [112].
AI models demonstrate particular efficacy in evaluating and prioritizing repurposing candidates from broad pharmacological categories, including anesthetics, GABA receptor modulators, second-generation antipsychotics, dopamine agonists, and N-Methyl-D-aspartic acid (NMDA) receptor antagonists. Furthermore, AI has identified potential antidepressant activity in seemingly unrelated drug classes, such as antibiotics, antidiabetics, Hydroxy Methyl Glutaryl-CoA reductase inhibitors (statins), angiotensin-converting enzyme inhibitors, and calcium channel blockers. These diverse agents, predicted to act via multi-target mechanisms, are hypothesized to exert antidepressant effects through pathways that align with their known pharmacological classes—a connection revealed through AI-driven pattern recognition and pathway analysis [113].
5.2.3. Modernization of Natural Products and Herbal Formulations
Natural products mediate their antidepressant effects through the synergistic actions of multiple bioactive constituents, which collectively target diverse molecular pathways to regulate the MGB axis in MDD [114].
Flavonoids, a class of polyphenolic compounds widely distributed in plants, exhibit a broad spectrum of biological activities, including antioxidant, anti-inflammatory, and neuroprotective properties [115]. Preclinical studies indicate that specific flavonoids exert antidepressant-like effects in animal models of depression [116]. For example, quercetin modulates key neurotransmitter systems such as NMDA and GABA receptors, downregulates pro-inflammatory cytokines (TNF-α, IL-1β), and activates the Nrf-2/ARE antioxidant pathway. It also influences brain-derived neurotrophic factor (BDNF) signaling and intracellular cascades, including PI3K/Akt and MAPK/ERK, underscoring its multi-target neuroprotective potential [117]. Puerarin, a major flavonoid derived from the root of Pueraria montana var. lobata (Ohwi) Maesen and S. M. Almeida, demonstrates neuroprotective efficacy by improving microcirculation and counteracting inflammatory and oxidative stress [118]. In Chronic Unpredictable Mild Stress (CUMS)-induced depressive rats, puerarin restored gut microbiota composition, reduced pathogenic bacteria such as Desulfovibrio and Verrucomicrobia, and enhanced hippocampal BDNF and IκB-α expression. It suppressed NF-κB activation, collectively contributing to its antidepressant effect [119].
Beyond flavonoids, alkaloids constitute another major class of plant-derived compounds that exhibit multi-target antidepressant effects via the MGB axis [120]. For instance, berberine, the primary bioactive alkaloid from Coptis chinensis Franch, modulates gut microbial composition by elevating Firmicutes and reducing Bacteroides, thereby altering SCFA profiles—specifically suppressing acetic and propionic acids while increasing isovaleric acid. These changes correlate with activation of the MGB axis, elevated hippocampal BDNF and monoamine levels, and alleviation of depressive-like behaviors [121].
Natural polysaccharides have attracted increasing interest due to their favorable safety profile and multi-target mechanisms, including antidepressant effects mediated through gut–brain communication [122]. Schisandra chinensis polysaccharide ameliorates depressive behaviors by inhibiting HPA axis overactivation and hepatic oxidative stress, restoring neurotrophic and synaptic plasticity, and modulating gut microbiota [123]. Similarly, Corydalis yanhusuo polysaccharides enhance monoamine neurotransmitters (Norepinephrine, Dopamine, 5-HT) and BDNF expression by regulating gut microbiota structure and SCFA metabolism while upregulating TPH-2 to mitigate neuronal damage in CUMS models [124].
Cryptotanshinone, a quinoid diterpene from Salvia miltiorrhiza Bunge, alleviates depression-like phenotypes by attenuating systemic inflammation and rebalancing gut microbiota (e.g., reducing Parabacteroides merdae), partly through modulation of the PI3K-AKT pathway [125]. Apple polyphenol extract rich in epicatechin and chlorogenic acid restores gut microbial homeostasis, inhibits NF-κB activation, enhances expression of tight junction proteins (occludin, ZO-1) in the gut and hippocampus, and normalizes HPA axis dysregulation, collectively contributing to its antidepressant efficacy [126]. Polygalae radix oligosaccharide esters increase beneficial gut bacteria (e.g., Actinobacteria and Firmicutes), reinforce intestinal barrier integrity, and modulate the tryptophan-kynurenine pathway to regulate brain neurotransmission [127]. Cuscutae Semen extract, whose primary active constituents include chlorogenic acid and hypericin, exhibits considerable antidepressant and anti-inflammatory activities. Studies indicate that these compounds ameliorate depressive-like behaviors induced by chronic unpredictable stress in mice, potentially via modulation of the gut microbiota, inhibition of NLRP3/NF-κB-mediated neuroinflammatory pathways, and preservation of synaptic integrity [128]. Saffron extract, derived from Crocus sativus L., increases the abundance of beneficial gut microbiota such as Akkermansia and Muribaculaceae flora, which are inversely associated with the neurotoxic metabolite dimethylamine. By reducing dimethylamine levels and modulating brain proteomic profiles, saffron extract exerts notable antidepressant effects [129]. The multi-target mechanisms of representative natural compounds targeting the MGB axis for antidepressant effects are systematically summarized in Table 3.

Table 3.
Natural products targeting the microbiota–gut–brain (MGB) axis.
In summary, these naturally derived compounds target the MGB axis through microbiota remodeling, anti-inflammatory and antioxidant mechanisms, and neuroendocrine regulation, offering promising scaffolds for the development of novel antidepressant agents. This approach provides a modern scientific interpretation for traditional medical knowledge and offers valuable molecular blueprints for new drug development.
5.3. Towards Precision Psychiatry
The long-term goal of precision medicine in depression is to transcend the current “one-size-fits-all” treatment paradigm. A promising, albeit aspirational, pathway lies in leveraging multi-omics data to create dynamic, in silico models of an individual’s pathophysiological state. These models, which aim to emulate the patient’s unique biological function, can be continuously updated with new data to simulate disease progression and virtually test interventions. This concept, sometimes described as building a “clinical digital twin” or a “digital self” [130], represents the ultimate endpoint of this endeavor.
Despite these challenges, a foundational framework is emerging. By integrating a patient’s gut metagenomic, plasma metabolomic, immunomic, and genomic data within probabilistic or mechanistic models, it becomes possible to generate hypotheses about underlying biological subsystems and their perturbation [131]. For instance, machine learning models trained on multi-omics datasets have already demonstrated the ability to stratify patients into biotypes with distinct inflammatory or metabolic profiles, which are predictive of treatment outcomes [49,132]. These models can be used in silico to simulate the potential effects, functioning not as definitive predictions but as data-driven decision-support tools.
The actionable output of such a system is a ranked set of personalized, evidence-based hypotheses [133]. For example, for a patient subgroup identified with significant gut dysbiosis and a plasma metabolome indicative of low-grade inflammation, the model might prioritize testing adjunctive anti-inflammatory nutraceuticals or specific probiotic regimens, as biomarker-defined inflammation has been shown to predict response to anti-inflammatory therapy in depression [105]. For those with profiles suggesting severe deficiencies in microbial metabolite production (e.g., SCFAs), a high-fiber dietary intervention might be a primary focus, given the established role of prebiotics in modulating gut–brain axis pathways relevant to depression [134]. This framework does not propose replacing clinician judgment but to augment it with insights derived from complex, high-dimensional data [135]. Its iterative refinement and validation through rigorous prospective clinical studies are the essential next steps for translation [135]. To delineate this translational pathway, Table 4 summarizes the principal translational applications of AI in depression management, encompassing core tasks, data types, and representative computational methods, thereby providing researchers with a systematic reference framework.

Table 4.
Translational applications of artificial intelligence in depression management.
6. Current Challenges and Future Perspectives
6.1. Challenges at the Data Level
The development and effectiveness of AI models are critically dependent on high-quality, well-structured, and comprehensive datasets. However, acquiring such data remains a formidable challenge. Existing datasets commonly suffer from limitations in accessibility, poor data quality, and internal inconsistencies, all of which compromise model reliability and lead to biased predictions [136]. The issues of data scarcity and heterogeneity are particularly pronounced in chemical medicine, where they significantly constrain the accuracy and generalizability of predictive models [137,138]. Techniques such as data augmentation and synthetic dataset generation offer viable pathways to address this bottleneck, effectively expanding dataset scale and diversity, thereby enhancing model performance. Nevertheless, these technical approaches must be applied cautiously to avoid introducing new biases that could further undermine model integrity [139].
6.2. Technical Challenges
A paramount technical challenge in AI involves the inherent opacity and limited interpretability of complex models, particularly DL architectures. These systems are frequently characterized as “black boxes” due to the considerable difficulty in elucidating their internal decision-making processes [140]. Despite their capacity to generate highly accurate predictions, the underlying rationale behind these outputs often remains obscure, raising significant concerns about the reliability and fairness of AI-driven predictions and decisions [111]. This lack of transparency poses a substantial barrier to adoption in critical sectors such as healthcare and finance, where understanding the basis for a decision is essential.
Furthermore, for network pharmacology, transitioning from relatively static “drug–target–disease” networks to dynamic, computable disease models constructed through the integration of AI and multi-omics data calls for the development of new, more sophisticated network analysis and validation tools.
To establish trust, ensure accountability, and facilitate regulatory compliance, AI models must exhibit a degree of interpretability accessible to stakeholders, including end-users and oversight bodies. The ability to audit and comprehend computational decision-making processes is a fundamental prerequisite for verifying fairness, identifying potential biases, and assuring reliability. While ongoing research continues to advance novel methodologies in XAI—such as the emerging Chemical-explainable Graph Neural Network, which demonstrates unique advantages in drug discovery [141]—achieving robust, universally applicable interpretability remains a significant, unresolved technical hurdle [111].
6.3. Biological Complexity
Multiple layers of biological complexity hinder the translation of biomedical discoveries into clinical applications. A central challenge lies in establishing causal relationships, which requires rigorously distinguishing correlation from causation [142]. This difficulty is compounded by the dynamic nature of biological processes, which are difficult to capture experimentally, and by the well-documented translational gap between animal models and human pathophysiology.
Although current AI systems excel at processing large-scale data, they remain limited in their ability to perform contextual reasoning and to adapt autonomously to novel or changing conditions—constraints that limit their effectiveness for modeling dynamic biological systems [111]. These limitations are starkly illustrated by the frequent failure of preclinical findings to translate to human outcomes. For example, Cataldi et al. report considerable discrepancies between animal and human studies of the gut–brain axis: mechanistic insights derived from highly controlled animal models often fail to generalize to heterogeneous human populations with diverse genetic backgrounds, diets, and lifestyles [143]. Thus, direct extrapolation from animal models to humans is inherently problematic. Future research should prioritize longitudinal human studies specifically designed to capture dynamic biological changes, rigorously control for confounders, and employ multi-omics technologies to bridge the translational gap and validate mechanistic pathways in humans [143].
6.4. Toward an Integrated Precision Psychiatry Pipeline
To translate the promise of AI and network pharmacology into clinical impact, future efforts must converge toward a coherent, patient-centered pipeline. This envisioned pipeline integrates causal discovery (through prospective multi-omics cohorts), dynamic monitoring (via digital phenotyping), and mechanism-targeted intervention (e.g., psychobiotics), all underpinned by interpretable AI and robust ethical frameworks.
6.4.1. Foundational Cohort Studies for Causal Discovery
Future research must prioritize large-scale, prospective cohort studies that deeply integrate multi-omics profiling (genomics, metabolomics, proteomics) with detailed clinical and lifestyle data. These cohorts are essential for moving beyond correlations to establishing causal mechanisms. Methodologies such as Mendelian randomization should be employed to rigorously test causal hypotheses regarding modifiable factors (e.g., specific microbial taxa and dietary components) and depression risk [142,144]. Such studies will yield high-quality, temporally resolved datasets needed to train more robust, generalizable, and causality-aware AI models, ultimately identifying mechanistically defined disease biotypes and novel therapeutic targets.
6.4.2. Dynamic Monitoring via Digital Phenotyping
Building on insights into emotional and cognitive variability preceding depressive episodes [145,146], the next step is to operationalize these findings for prevention. Future work should focus on developing multivariable dynamic models that synthesize data from wearables and smartphone sensors to track, in real-time, the central tendency, intra-individual variability, instability, and inertia of key states. The critical challenge is to translate these statistical patterns into clinically actionable, early warning systems. Success in this area will enable preemptive interventions for individuals at high risk, shifting the paradigm from reactive treatment to proactive management.
6.4.3. Novel Microbiota-Targeted Intervention
The gut microbiota has emerged as a critical pathophysiological factor in depression. Restoration of healthy microbiota can be achieved through several strategies, notably via psychobiotic interventions—live probiotics and prebiotics that confer neurological benefits. Primary approaches include (1) probiotics, (2) prebiotics, (3) dietary interventions, and (4) fecal microbiota transplantation (FMT) [147].
Psychobiotics, which include beneficial probiotics and prebiotics, alleviate depressive and anxiety-like behaviors through multiple pathways. These encompass modulation of cognitive-emotional processes, regulation of the hypothalamic–pituitary–adrenal (HPA) axis, reduction of pro-inflammatory cytokines (e.g., IL-1β, TNF-α), and influence on key neurotransmitters and neurotrophic factors such as BDNF, GABA, and glutamate [148,149,150]. For example, administration of Lactobacillus rhamnosus JB-1 significantly reduced depressive and anxious behaviors in murine models [150,151]. Preclinical studies further demonstrate that specific probiotic strains can normalize HPA axis hyperactivity and enhance BDNF expression, thereby improving mood and cognitive function [152,153].
Prebiotics, predominantly derived from high-fiber foods, ameliorate stress-induced anxiety and modulate gut microbial composition and metabolism, which, in turn, influence central nervous system development and function [154]. Specific prebiotic compounds, including galacto-oligosaccharides and resistant starch, exhibit efficacy in animal models of anxiety and depression via gut–brain axis communication [155,156]. Additionally, cocoa-derived flavanols demonstrate antidepressant effects, likely due to their anti-inflammatory and antioxidant properties [157].
Dietary patterns significantly shape the gut microbiota and impact depressive symptoms. The Mediterranean diet—rich in olive oil, fruits, vegetables, and fermented dairy—attenuates oxidative stress and inflammation through microbiota-mediated mechanisms [158]. Very-low-carbohydrate ketogenic diets show potential in managing neurological disorders, including treatment-resistant depression [159]. Calorie restriction may also confer antidepressant effects via weight loss and reduced inflammation, though further clinical evidence is needed [160].
FMT restores ecological balance in the gut microbiome and holds therapeutic promise for depression, anxiety, and autism spectrum disorder.
As discussed above, multiple gut-targeted strategies show promise. A comparative overview of these interventions is presented in Table 5.

Table 5.
Psychobiotic and dietary interventions for depression: mechanisms and evidence base.
A combinatorial treatment strategy integrating psychobiotics, prebiotics, tailored diets, and FMT may constitute a novel and effective paradigm for managing depression [106].
6.4.4. Ethical and Interpretable AI as Enabling Infrastructure
The entire pipeline depends on transparent and ethically governed AI tools. Advancing XAI methodologies is non-negotiable to ensure that model predictions (e.g., risk scores, treatment recommendations) are interpretable to clinicians and researchers, fostering trust and facilitating clinical adoption [111,141]. Concurrently, robust ethical frameworks must address critical issues of data privacy, algorithmic bias, equity in access, and accountability for AI-assisted decisions, ensuring that the march toward precision psychiatry does not compromise fairness or patient autonomy [111].
Beyond ethics and interpretability, the successful translation of this pipeline depends on addressing practical economic considerations and the implementation of best practices. The significant upfront investment required for multi-omics data acquisition, model development, and validation must be justified by demonstrating long-term cost-effectiveness, potentially through reducing ineffective treatment trials and enabling preventative care, as evidenced in health-economic analyses of digital interventions [161]. Furthermore, the adoption of best practices—such as rigorous external validation on diverse cohorts, proactive mitigation of dataset biases, and co-design of tools with end-users to ensure clinical utility—is essential to build reliable, generalizable, and actionable systems. These practices should be guided by evolving reporting standards and frameworks for clinical prediction models [162] to ensure robust integration into real-world healthcare workflows.
6.5. Limitations of This Review
This narrative review, while comprehensive, has several inherent limitations. First, its scope, centered on the integrative paradigm of AI, NP, and the gut–brain axis in depression, necessarily means that some adjacent areas (e.g., detailed pharmacogenomics of standard antidepressants, other neuroimmune pathways) are not covered in depth. Second, as a narrative synthesis, it is susceptible to selection bias because the cited literature reflects the authors’ curation of key developments rather than a systematic, exhaustive search of all available evidence. Finally, because the field is rapidly evolving, some of the most recent breakthroughs or clinical trial results may not be captured.
7. Conclusions
In conclusion, this review synthesizes and advocates for a fundamental shift in understanding and treating depression—from a reductionist, single-target approach to a dynamic, systems-level framework driven by the convergence of network pharmacology, multi-omics, and artificial intelligence. The gut–brain axis paradigm powerfully demonstrates depression as a network disorder, providing concrete mechanistic insights into biotypes and emphasizing the microbiome as a key therapeutic target. AI acts as an indispensable computational engine, transforming complex data into actionable hypotheses for drug discovery and personalized treatment. Although significant translational challenges remain—such as data integration, model interpretability, and biological validation—they represent critical frontiers for future research rather than fundamental limitations. Moving forward, success depends on fostering interdisciplinary collaboration to develop causality-aware, interpretable, and ethically governed AI tools rooted in diverse longitudinal data. Ultimately, this synergistic approach paves the way for redefining depression therapies by providing precise, mechanism-based, and highly personalized solutions, thereby bridging the gap between complex systems biology and effective clinical care.
Author Contributions
Conceptualization, L.Z.; data curation, K.C. and S.L. (Shun Li); writing original draft preparation, L.Z.; writing—review and editing, L.Z. and Z.W.; supervision, S.L. (Shengjie Liu); project administration, L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Projects of Natural Science Research of the Anhui Provincial Department of Education (Grant Nos. 2023AH052852 and 2024AH051448), the National Innovation and Entrepreneurship Training Program for College Students (Project ID: 202413619002), and the Fuyang Normal University Doctoral Research Start-up Fund (2025KYQD0077).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| MDD | Major Depressive Disorder |
| MGB | Microbiota–gut–brain |
| XAI | Explainable AI |
| HPA axis | Neuroendocrine |
| ML | Machine Learning |
| GWAS | Genome-wide association study |
| RNA-seq | RNA sequencing |
| snRNA-seq | single-nucleus RNA sequencing |
| NP | Network pharmacology |
| DBNs | Dynamic Bayesian Networks |
| GNNs | Graph Neural Networks |
| EEG | Electroencephalogram |
| NLP | Natural Language Processing |
| LLMs | large language models |
| GAI | Generative AI |
| MMP8 | matrix metalloproteinase-8 |
| 5-HT | serotonin |
| GABA | γ-aminobutyric acid |
| SCFAs | Short-chain fatty acids |
| Trp-Kyn | tryptophan-kynurenine |
| NMDA | N-Methyl-D-aspartic acid |
| BDNF | Brain-Derived Neurotrophic Factor |
| CUMS | Chronic Unpredictable Mild Stress |
| FMT | Fecal microbiota transplantation |
References
- Lu, B.; Lin, L.; Su, X. Global burden of depression or depressive symptoms in children and adolescents: A systematic review and meta-analysis. J. Affect. Disord. 2024, 354, 553–562. [Google Scholar] [CrossRef]
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef]
- Sampogna, G.; Toni, C.; Catapano, P.; Rocca, B.D.; Di Vincenzo, M.; Luciano, M.; Fiorillo, A. New trends in personalized treatment of depression. Curr. Opin. Psychiatry 2024, 37, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Sabella, D. Antidepressant Medications. Am. J. Nurs. 2018, 118, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, B.; Jose, J. Patient medication adherence: Measures in daily practice. Oman Med. J. 2011, 26, 155–159. [Google Scholar] [CrossRef]
- Als, T.D.; Kurki, M.I.; Grove, J.; Voloudakis, G.; Therrien, K.; Tasanko, E.; Nielsen, T.T.; Naamanka, J.; Veerapen, K.; Levey, D.F.; et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat. Med. 2023, 29, 1832–1844. [Google Scholar] [CrossRef]
- Benatti, B.M.; Adiletta, A.; Sgadò, P.; Malgaroli, A.; Ferro, M.; Lamanna, J. Epigenetic Modifications and Neuroplasticity in the Pathogenesis of Depression: A Focus on Early Life Stress. Behav. Sci. 2024, 14, 882. [Google Scholar] [CrossRef]
- Sempach, L.; Doll, J.P.K.; Limbach, V.; Marzetta, F.; Schaub, A.C.; Schneider, E.; Kettelhack, C.; Mählmann, L.; Schweinfurth-Keck, N.; Ibberson, M.; et al. Examining immune-inflammatory mechanisms of probiotic supplementation in depression: Secondary findings from a randomized clinical trial. Transl. Psychiatry 2024, 14, 305. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Anthony, D.C. Prebiotic and Probiotic Modulation of the Microbiota-Gut-Brain Axis in Depression. Nutrients 2023, 15, 1880. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, T.; Xu, M.; Lin, S.; Hu, B.; Chu, T.; Liu, B.; Xu, Y.; Ding, W.; Li, L.; et al. Spatial multi-omics: Deciphering technological landscape of integration of multi-omics and its applications. J. Hematol. Oncol. 2024, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D. Inhibition of inflammation by berberine: Molecular mechanism and network pharmacology analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lin, P.; Lin, F.; Chen, M.; Lin, C.; Lin, X.; Wu, L.; Zheng, M.; Chen, J. Identification of immune microenvironment subtypes and signature genes for Alzheimer’s disease diagnosis and risk prediction based on explainable machine learning. Front. Immunol. 2022, 13, 1046410. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Z.; Zhu, R.; Liu, Q.; Han, H.; Liu, N.; Shi, J.; Han, S.; Ma, N. Identifying potential pathogenic oxidative stress-related genes in depression through multi-omics summary-data-based Mendelian randomization analysis. J. Affect. Disord. 2025, 388, 119734. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yan, X.; Huang, H.; Guo, X.; Bai, M.; Wang, B.; Su, P.; Li, Y.; Xu, E. Integration of network pharmacology and experimental verification to reveal the active components and molecular mechanism of modified Danzhi Xiaoyao San in the treatment of depression. J. Ethnopharmacol. 2025, 336, 118739. [Google Scholar] [CrossRef]
- Foster, J.A.; Trivedi, M.H. The gut-brain axis in depression: Are multi-omics showing the way? Cell Rep. Med. 2024, 5, 101741. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Kendall, K.M.; Van Assche, E.; Andlauer, T.F.M.; Choi, K.W.; Luykx, J.J.; Schulte, E.C.; Lu, Y. The genetic basis of major depression. Psychol. Med. 2021, 51, 2217–2230. [Google Scholar] [CrossRef]
- Ochi, S.; Dwivedi, Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol. Psychiatry 2023, 28, 141–153. [Google Scholar] [CrossRef]
- Efstathopoulos, P.; Andersson, F.; Melas, P.A.; Yang, L.L.; Villaescusa, J.C.; Rȕegg, J.; Ekström, T.J.; Forsell, Y.; Galanti, M.R.; Lavebratt, C. NR3C1 hypermethylation in depressed and bullied adolescents. Transl. Psychiatry 2018, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Maitra, M.; Tanti, A.; Suderman, M.; Théroux, J.F.; Davoli, M.A.; Perlman, K.; Yerko, V.; Wang, Y.C.; Tripathy, S.J.; et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 2020, 23, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Kahwaji, H.; Nasr, S.; Baz, R.; Kim, Y.K.; Fakhoury, M. Precision Medicine in Depression: The Role of Proteomics and Metabolomics in Personalized Treatment Approaches. In Recent Advances and Challenges in the Treatment of Major Depressive Disorder; Advances in Experimental Medicine and Biology; Springer: Singapore, 2024; Volume 1456, pp. 359–378. [Google Scholar] [CrossRef]
- Bot, M.; Chan, M.K.; Jansen, R.; Lamers, F.; Vogelzangs, N.; Steiner, J.; Leweke, F.M.; Rothermundt, M.; Cooper, J.; Bahn, S.; et al. Serum proteomic profiling of major depressive disorder. Transl. Psychiatry 2015, 5, e599. [Google Scholar] [CrossRef]
- Liu, J.J.; Wei, Y.B.; Strawbridge, R.; Bao, Y.; Chang, S.; Shi, L.; Que, J.; Gadad, B.S.; Trivedi, M.H.; Kelsoe, J.R.; et al. Peripheral cytokine levels and response to antidepressant treatment in depression: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 339–350. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.; Ma, Y.; Yang, Y.; Pan, C.W.; Zhu, X.; Ke, C. Metabolomics on depression: A comparison of clinical and animal research. J. Affect. Disord. 2024, 349, 559–568. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Lan, X.; Cohen, D.; Zhang, Y.; Ravindran, A.V.; Yuan, S.; Zheng, P.; Coghill, D.; Yang, L.; et al. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents. Mol. Psychiatry 2019, 24, 1478–1488. [Google Scholar] [CrossRef]
- Ho, C.S.H.; Tay, G.W.N.; Wee, H.N.; Ching, J. The Utility of Amino Acid Metabolites in the Diagnosis of Major Depressive Disorder and Correlations with Depression Severity. Int. J. Mol. Sci. 2023, 24, 2231. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Liao, J.; Chen, Q.; Lu, X.; Fan, X. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin. J. Nat. Med. 2023, 21, 323–332. [Google Scholar] [CrossRef]
- Yagüe, E.; Sun, H.; Hu, Y. East Wind, West Wind: Toward the modernization of traditional Chinese medicine. Front. Neurosci. 2022, 16, 1057817. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Alagapan, S.; Choi, K.S.; Heisig, S.; Riva-Posse, P.; Crowell, A.; Tiruvadi, V.; Obatusin, M.; Veerakumar, A.; Waters, A.C.; Gross, R.E.; et al. Cingulate dynamics track depression recovery with deep brain stimulation. Nature 2023, 622, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Tyborowska, A.; Ikani, N.; Mocking, R.J.T.; Figueroa, C.A.; Schene, A.H.; Deco, G.; Kringelbach, M.L.; Cabral, J.; Ruhé, H.G. Depression recurrence is accompanied by longer periods in default mode and more frequent attentional and reward processing dynamic brain-states during resting-state activity. Hum. Brain Mapp. 2023, 44, 5770–5783. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Lugo-Martinez, J.; Bourguignon, N.; Mathee, K.; Lerner, B.; Bar-Joseph, Z.; Narasimhan, G. Dynamic Bayesian Networks for Integrating Multi-omics Time Series Microbiome Data. mSystems 2021, 6, e01105-20. [Google Scholar] [CrossRef]
- Scutari, M.; Kerob, D.; Salah, S. Inferring skin-brain-skin connections from infodemiology data using dynamic Bayesian networks. Sci. Rep. 2024, 14, 10266. [Google Scholar] [CrossRef]
- Veličković, P. Everything is connected: Graph neural networks. Curr. Opin. Struct. Biol. 2023, 79, 102538. [Google Scholar] [CrossRef]
- Ali, M.; Richter, S.; Ertürk, A.; Fischer, D.S.; Theis, F.J. Graph neural networks learn emergent tissue properties from spatial molecular profiles. Nat. Commun. 2025, 16, 8419. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, H.; Lin, H.; Long, H. Single-cell RNA-seq data analysis based on directed graph neural network. Methods 2023, 211, 48–60. [Google Scholar] [CrossRef]
- Zitnik, M.; Sosič, R.; Feldman, M.W.; Leskovec, J. Evolution of resilience in protein interactomes across the tree of life. Proc. Natl. Acad. Sci. USA 2019, 116, 4426–4433. [Google Scholar] [CrossRef]
- Corrivetti, G.; Monaco, F.; Vignapiano, A.; Marenna, A.; Palm, K.; Fernández-Arroyo, S.; Frigola-Capell, E.; Leen, V.; Ibarrola, O.; Amil, B.; et al. Optimizing and Predicting Antidepressant Efficacy in Patients with Major Depressive Disorder Using Multi-Omics Analysis and the Opade AI Prediction Tools. Brain Sci. 2024, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.R.; Weiss, S.T.; Glass, K.; Sharma, A. Network Medicine in the Age of Biomedical Big Data. Front. Genet. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Luck, K.; Kim, D.K.; Lambourne, L.; Spirohn, K.; Begg, B.E.; Bian, W.; Brignall, R.; Cafarelli, T.; Campos-Laborie, F.J.; Charloteaux, B.; et al. A reference map of the human binary protein interactome. Nature 2020, 580, 402–408. [Google Scholar] [CrossRef]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576.e516. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Hartenian, E.; Shi, X.; Scott, D.A.; Mikkelson, T.; Heckl, D.; Ebert, B.L.; Root, D.E.; Doench, J.G.; et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014, 343, 84–87. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Zhang, Y.; Mo, Y.K.; Wang, Y. XMR: An explainable multimodal neural network for drug response prediction. Front. Bioinform. 2023, 3, 1164482. [Google Scholar] [CrossRef]
- Drysdale, A.T.; Grosenick, L.; Downar, J.; Dunlop, K.; Mansouri, F.; Meng, Y.; Fetcho, R.N.; Zebley, B.; Oathes, D.J.; Etkin, A.; et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017, 23, 28–38, Erratum in Nat. Med. 2017, 23, 264. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, H.; Yu, C.; Jiang, J.; Duan, X. Application of machine learning in predicting the risk of postpartum depression: A systematic review. J. Affect. Disord. 2022, 318, 364–379. [Google Scholar] [CrossRef]
- Chen, B.; Jiao, Z.; Shen, T.; Fan, R.; Chen, Y.; Xu, Z. Early antidepressant treatment response prediction in major depression using clinical and TPH2 DNA methylation features based on machine learning approaches. BMC Psychiatry 2023, 23, 299. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Wang, F.; Wu, Z. Application of graph frequency attention convolutional neural networks in depression treatment response. Front. Psychiatry 2023, 14, 1244208. [Google Scholar] [CrossRef] [PubMed]
- Le Glaz, A.; Haralambous, Y.; Kim-Dufor, D.H.; Lenca, P.; Billot, R.; Ryan, T.C.; Marsh, J.; DeVylder, J.; Walter, M.; Berrouiguet, S.; et al. Machine Learning and Natural Language Processing in Mental Health: Systematic Review. J. Med. Internet Res. 2021, 23, e15708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Schoene, A.M.; Ji, S.; Ananiadou, S. Natural language processing applied to mental illness detection: A narrative review. NPJ Digit. Med. 2022, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Teferra, B.G.; Rueda, A.; Pang, H.; Valenzano, R.; Samavi, R.; Krishnan, S.; Bhat, V. Screening for Depression Using Natural Language Processing: Literature Review. Interact. J. Med. Res. 2024, 13, e55067. [Google Scholar] [CrossRef]
- Dougherty, R.F.; Clarke, P.; Atli, M.; Kuc, J.; Schlosser, D.; Dunlop, B.W.; Hellerstein, D.J.; Aaronson, S.T.; Zisook, S.; Young, A.H.; et al. Psilocybin therapy for treatment resistant depression: Prediction of clinical outcome by natural language processing. Psychopharmacology 2025, 242, 1553–1561. [Google Scholar] [CrossRef]
- Yelmen, B.; Decelle, A.; Ongaro, L.; Marnetto, D.; Tallec, C.; Montinaro, F.; Furtlehner, C.; Pagani, L.; Jay, F. Creating artificial human genomes using generative neural networks. PLoS Genet. 2021, 17, e1009303. [Google Scholar] [CrossRef]
- Xian, X.; Chang, A.; Xiang, Y.T.; Liu, M.T. Debate and Dilemmas Regarding Generative AI in Mental Health Care: Scoping Review. Interact. J. Med. Res. 2024, 13, e53672. [Google Scholar] [CrossRef]
- Sun, G.; Zhou, Y.H. AI in healthcare: Navigating opportunities and challenges in digital communication. Front. Digit. Health 2023, 5, 1291132. [Google Scholar] [CrossRef]
- Petrušić, I.; Ha, W.S.; Labastida-Ramirez, A.; Messina, R.; Onan, D.; Tana, C.; Wang, W. Influence of next-generation artificial intelligence on headache research, diagnosis and treatment: The junior editorial board members’ vision—Part 1. J. Headache Pain 2024, 25, 151. [Google Scholar] [CrossRef]
- Fraichot, A.; Favre, S.; Richard-Lepouriel, H. Case Report: Intranasal esketamine combined with a form of generative artificial intelligence in the management of treatment-resistant depression. Front. Psychiatry 2025, 16, 1536232. [Google Scholar] [CrossRef]
- Usuyama, N.; Wong, C.; Zhang, S.; Naumann, T.; Poon, H. Biomedical Natural Language Processing in the Era of Large Language Models. Annu. Rev. Biomed. Data Sci. 2025, 8, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, W.F.; Tejani, A.S. On the Opportunities and Risks of Foundation Models for Natural Language Processing in Radiology. Radiol. Artif. Intell. 2022, 4, e220119. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Wang, C.; Soh, W.K.; Rajapakse, J.C. Combining Neuroimaging and Omics Datasets for Disease Classification Using Graph Neural Networks. Front. Neurosci. 2022, 16, 866666. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hua, H.; Chen, S. Graph neural networks for single-cell omics data: A review of approaches and applications. Brief. Bioinform. 2025, 26, bbaf109. [Google Scholar] [CrossRef]
- Scarselli, F.; Gori, M.; Tsoi, A.C.; Hagenbuchner, M.; Monfardini, G. The graph neural network model. IEEE Trans. Neural Netw. 2009, 20, 61–80. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, S.; Chen, F.; Long, G.; Zhang, C.; Yu, P.S. A Comprehensive Survey on Graph Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4–24. [Google Scholar] [CrossRef]
- Ma, D.; Taher, M.R.H.; Pang, J.; Islam, N.U.; Haghighi, F.; Gotway, M.B.; Liang, J. Benchmarking and Boosting Transformers for Medical Image Classification. In Domain Adaptation and Representation Transfer; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2022; Volume 13542, pp. 12–22. [Google Scholar] [CrossRef]
- Torres, L.H.M.; Arrais, J.P.; Ribeiro, B. Combining graph neural networks and transformers for few-shot nuclear receptor binding activity prediction. J. Cheminform 2024, 16, 109. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, D.; Wang, Y.; Fu, Y.; Tian, Z. GTC: GNN-Transformer co-contrastive learning for self-supervised heterogeneous graph representation. Neural Netw. 2025, 181, 106645. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Gordon, D.; Petousis, P.; Zheng, H.; Zamanzadeh, D.; Bui, A.A.T. TSI-GNN: Extending Graph Neural Networks to Handle Missing Data in Temporal Settings. Front. Big Data 2021, 4, 693869. [Google Scholar] [CrossRef]
- Xiong, A.; Luo, Z.; Xia, Y.; Zou, Q.; Wei, L.; Zhang, Z.; Wang, T.; Wei, L.; Cui, F. An interpretable geometric graph neural network for enhancing the generalizability of drug-target interaction prediction. BMC Biol. 2025, 23, 350. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Fang, Z.; Gu, Y.; Liu, Z.; Long, Q.; Qiao, Z.; Qin, Y.; Shen, J.; Sun, F.; Xiao, Z.; et al. A Comprehensive Survey on Deep Graph Representation Learning. Neural Netw. 2024, 173, 106207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Peng, Y.; Huang, W.; Miao, X.; Cao, Y.; Wang, X.; Kong, X. A dynamic ensemble learning model for robust Graph Neural Networks. Neural Netw. 2025, 191, 107810. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cacho, A.; García-Gavilán, J.F.; Atzeni, A.; Konstanti, P.; Belzer, C.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Mela, V.; et al. Multi-omics approach identifies gut microbiota variations associated with depression. NPJ Biofilms Microbiomes 2025, 11, 68. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Liu, S.; Wang, Z. Dietary Acrylamide Induces Depression via SIRT3-Mediated Mitochondrial Oxidative Injury: Evidence from Multi-Omics and Mendelian Randomization. Curr. Issues Mol. Biol. 2025, 47, 836. [Google Scholar] [CrossRef]
- de Leeuw, M.; Verhoeve, S.I.; van der Wee, N.J.A.; van Hemert, A.M.; Vreugdenhil, E.; Coomans, C.P. The role of the circadian system in the etiology of depression. Neurosci. Biobehav. Rev. 2023, 153, 105383. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Kung, B.; Chiang, M.; Perera, G.; Pritchard, M.; Stewart, R. Unsupervised Machine Learning to Identify Depressive Subtypes. Healthc. Inform. Res. 2022, 28, 256–266. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota-gut-brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef]
- Reyes-Martínez, S.; Segura-Real, L.; Gómez-García, A.P.; Tesoro-Cruz, E.; Constantino-Jonapa, L.A.; Amedei, A.; Aguirre-García, M.M. Neuroinflammation, Microbiota-Gut-Brain Axis, and Depression: The Vicious Circle. J. Integr. Neurosci. 2023, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Lin, H.Y.; Chan, K.L.; Li, L.; Parise, L.F.; Alvarez, J.; Durand-de Cuttoli, R.; Aubry, A.V.; Muhareb, S.; Desland, F.; et al. Circulating myeloid-derived MMP8 in stress susceptibility and depression. Nature 2024, 626, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- AbdelKhalek, A.; Narayanan, S.K. Comparison between Symptomatic and Asymptomatic Mice after Clostridioides difficile Infection Reveals Novel Inflammatory Pathways and Contributing Microbiota. Microorganisms 2022, 10, 2380. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Siopi, E.; Galerne, M.; Rivagorda, M.; Saha, S.; Moigneu, C.; Moriceau, S.; Bigot, M.; Oury, F.; Lledo, P.M. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol. Psychiatry 2023, 28, 3002–3012. [Google Scholar] [CrossRef]
- Décarie-Spain, L.; Hayes, A.M.R.; Lauer, L.T.; Kanoski, S.E. The gut-brain axis and cognitive control: A role for the vagus nerve. Semin. Cell Dev. Biol. 2024, 156, 201–209. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Tian, T.; Mao, Q.; Xie, J.; Wang, Y.; Shao, W.H.; Zhong, Q.; Chen, J.J. Multi-omics data reveals the disturbance of glycerophospholipid metabolism caused by disordered gut microbiota in depressed mice. J. Adv. Res. 2022, 39, 135–145. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.; Wang, J.; Liu, Z.; Wang, X.; Kang, P.; Yang, C.; Liu, P.; Zhang, K. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin. Neurosci. 2022, 76, 321–328. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan—Kynurenine metabolic pathway in depression. Rev. Neurosci. 2023, 34, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Damiani, F.; Cornuti, S.; Tognini, P. The gut-brain connection: Exploring the influence of the gut microbiota on neuroplasticity and neurodevelopmental disorders. Neuropharmacology 2023, 231, 109491. [Google Scholar] [CrossRef]
- Boldrini, M.; Hen, R.; Underwood, M.D.; Rosoklija, G.B.; Dwork, A.J.; Mann, J.J.; Arango, V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol. Psychiatry 2012, 72, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Santiago, A.N.; Hen, R.; Dwork, A.J.; Rosoklija, G.B.; Tamir, H.; Arango, V.; John Mann, J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 2013, 38, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Galfalvy, H.; Dwork, A.J.; Rosoklija, G.B.; Trencevska-Ivanovska, I.; Pavlovski, G.; Hen, R.; Arango, V.; Mann, J.J. Resilience Is Associated With Larger Dentate Gyrus, While Suicide Decedents With Major Depressive Disorder Have Fewer Granule Neurons. Biol. Psychiatry 2019, 85, 850–862. [Google Scholar] [CrossRef]
- Ergang, P.; Vagnerová, K.; Hermanová, P.; Vodička, M.; Jágr, M.; Šrůtková, D.; Dvořáček, V.; Hudcovic, T.; Pácha, J. The Gut Microbiota Affects Corticosterone Production in the Murine Small Intestine. Int. J. Mol. Sci. 2021, 22, 4229. [Google Scholar] [CrossRef]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Mao, Y.K.; Bienenstock, J.; Foster, J.A.; Kunze, W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013, 25, 183–189, e88. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Kanoujia, J.; Mohana Lakshmi, S.; Patil, C.R.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. Drug Targets 2023, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Weth, F.R.; Hoggarth, G.B.; Weth, A.F.; Paterson, E.; White, M.P.J.; Tan, S.T.; Peng, L.; Gray, C. Unlocking hidden potential: Advancements, approaches, and obstacles in repurposing drugs for cancer therapy. Br. J. Cancer 2024, 130, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Jarallah, S.J.; Almughem, F.A.; Alhumaid, N.K.; Fayez, N.A.; Alradwan, I.; Alsulami, K.A.; Tawfik, E.A.; Alshehri, A.A. Artificial intelligence revolution in drug discovery: A paradigm shift in pharmaceutical innovation. Int. J. Pharm. 2025, 680, 125789. [Google Scholar] [CrossRef]
- Mohammadzadeh-Vardin, T.; Ghareyazi, A.; Gharizadeh, A.; Abbasi, K.; Rabiee, H.R. DeepDRA: Drug repurposing using multi-omics data integration with autoencoders. PLoS ONE 2024, 19, e0307649. [Google Scholar] [CrossRef]
- Mohammad Sadeghi, H.; Adeli, I.; Mousavi, T.; Daniali, M.; Nikfar, S.; Abdollahi, M. Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Life 2021, 11, 774. [Google Scholar] [CrossRef]
- Fu, C.; Chen, Q. The future of pharmaceuticals: Artificial intelligence in drug discovery and development. J. Pharm. Anal. 2025, 15, 101248. [Google Scholar] [CrossRef]
- Caban, A.; Pisarczyk, K.; Kopacz, K.; Kapuśniak, A.; Toumi, M.; Rémuzat, C.; Kornfeld, A. Filling the gap in CNS drug development: Evaluation of the role of drug repurposing. J. Mark. Access Health Policy 2017, 5, 1299833. [Google Scholar] [CrossRef]
- Verma, C.; Jain, K.; Saini, A.; Mani, I.; Singh, V. Exploring the potential of drug repurposing for treating depression. Prog. Mol. Biol. Transl. Sci. 2024, 207, 79–105. [Google Scholar] [CrossRef]
- Guo, C.; Huang, Q.; Wang, Y.; Yao, Y.; Li, J.; Chen, J.; Wu, M.; Zhang, Z.; E, M.; Qi, H.; et al. Therapeutic application of natural products: NAD(+) metabolism as potential target. Phytomedicine 2023, 114, 154768. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Chang, S.K.; Chen, Y.; Sheng, Z.; Jiang, Y.; Yang, B. The structure characteristics, biosynthesis and health benefits of naturally occurring rare flavonoids. Crit. Rev. Food Sci. Nutr. 2024, 64, 2490–2512. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Sharma, P.C.; Thakur, V.K.; Goyal, R.K. Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules 2021, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Chakraborty, P.; Dewanjee, S.; Arfin, S.; Das, S.S.; Dey, A.; Moustafa, M.; Mishra, P.C.; Jafari, S.M.; Jha, N.K.; et al. Neuropharmacological interventions of quercetin and its derivatives in neurological and psychological disorders. Neurosci. Biobehav. Rev. 2023, 144, 104955. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Ding, S.; Wang, Y.; Ye, L.; Chen, X.; Ma, H. Exploring the potential antidepressant mechanisms of puerarin: Anti-inflammatory response via the gut-brain axis. J. Affect. Disord. 2022, 310, 459–471. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Huang, M.; He, Y.; Tian, L.; Yu, L.; Cheng, Q.; Li, Z.; Gao, L.; Gao, S.; Yu, C. Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect. Disord. 2023, 325, 141–150. [Google Scholar] [CrossRef]
- Huo, J.; Wu, Z.; Sun, W.; Wang, Z.; Wu, J.; Huang, M.; Wang, B.; Sun, B. Protective Effects of Natural Polysaccharides on Intestinal Barrier Injury: A Review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef]
- Zhu, S.M.; Luo, F.Y.; Peng, J.; Luo, L.Y.; Xue, R.; Yang, Y.; Xu, R.; Zhai, Y.N.; Ma, H.; Li, C.W.; et al. The physicochemical characteristics and antidepressant-like effects of a polysaccharide-rich fraction from Schisandra chinensis (Turcz.) Baill in behavioral despair mice and olfactory bulbectomy-induced depression-like mice. J. Ethnopharmacol. 2024, 320, 117464. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Liao, X.; Deng, J.; Wang, Q.; Liang, J.; Yan, B. Corydalis yanhusuo Polysaccharides Ameliorate Chronic Stress-Induced Depression in Mice through Gut Microbiota-Derived Short-Chain Fatty Acid Activation of 5-Hydroxytryptamine Signaling. J. Med. Food 2023, 26, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.H.; Wang, S.Q.; Li, W.J.; Li, J.; Yin, Y.; Ye, F.F.; Guo, J.Y. Cryptotanshinone regulates gut microbiota and PI3K-AKT pathway in rats to alleviate CUMS induced depressive symptoms. Biomed. Pharmacother. 2023, 169, 115921. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, Z.; Qian, Q.; Yang, H.; Ma, J.; Luan, W.; Shang, S.; Li, X. Apple polyphenol extract ameliorates sugary-diet-induced depression-like behaviors in male C57BL/6 mice by inhibiting the inflammation of the gut-brain axis. Food Funct. 2024, 15, 2939–2959. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, T.; Wu, X.; Chen, X.; Wang, J.; Ba, Y. Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 13877. [Google Scholar] [CrossRef]
- Hou, L.; Yang, L.; Zhu, C.; Miao, J.; Zhou, W.; Tang, Y.; Meng, H.; Liu, S. Cuscutae semen alleviates CUS-induced depression-like behaviors in mice via the gut microbiota-neuroinflammation axis. Front. Pharmacol. 2023, 14, 1107781. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Connell, E.; Le Gall, G.; Pourtau, L.; Gaudout, D.; Angeloni, C.; Zallocco, L.; Ronci, M.; Giusti, L.; Müller, M.; et al. Saffron extract (Safr’Inside™) improves anxiety related behaviour in a mouse model of low-grade inflammation through the modulation of the microbiota and gut derived metabolites. Food Funct. 2022, 13, 12219–12233. [Google Scholar] [CrossRef]
- Maes, M. Precision Nomothetic Medicine in Depression Research: A New Depression Model, and New Endophenotype Classes and Pathway Phenotypes, and A Digital Self. J. Pers. Med. 2022, 12, 403. [Google Scholar] [CrossRef]
- Shao, X.; Wang, Y.; Geng, Z.; Liang, G.; Zhu, X.; Liu, L.; Meng, M.; Duan, L.; Zhu, G. Novel therapeutic targets for major depressive disorder related to oxidative stress identified by integrative multi-omics and multi-trait study. Transl. Psychiatry 2024, 14, 443. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Ye, J.; Sun, Z.; He, Y.; Zhao, Y.; Ren, S.; Zhang, G.; Liu, M.; Zheng, P.; et al. Multi-omics reveal microbial determinants impacting the treatment outcome of antidepressants in major depressive disorder. Microbiome 2023, 11, 195. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182, Correction in Curr. Nutr. Rep. 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Blanco-González, A.; Cabezón, A.; Seco-González, A.; Conde-Torres, D.; Antelo-Riveiro, P.; Piñeiro, Á.; Garcia-Fandino, R. The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies. Pharmaceuticals 2023, 16, 891. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Tsuji, S.; Hase, T.; Yachie-Kinoshita, A.; Nishino, T.; Ghosh, S.; Kikuchi, M.; Shimokawa, K.; Aburatani, H.; Kitano, H.; Tanaka, H. Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 92. [Google Scholar] [CrossRef]
- Chlap, P.; Min, H.; Vandenberg, N.; Dowling, J.; Holloway, L.; Haworth, A. A review of medical image data augmentation techniques for deep learning applications. J. Med. Imaging Radiat. Oncol. 2021, 65, 545–563. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Li, Y.; Deng, Y.; Zhao, H.; Hsieh, C.Y.; Hou, T. From Black Boxes to Actionable Insights: A Perspective on Explainable Artificial Intelligence for Scientific Discovery. J. Chem. Inf. Model. 2023, 63, 7617–7627. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, K.; Chandak, P.; Zitnik, M.; Gehlenborg, N. Extending the Nested Model for User-Centric XAI: A Design Study on GNN-based Drug Repurposing. IEEE Trans. Vis. Comput. Graph. 2023, 29, 1266–1276. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Poli, L.; Şahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The Effects of Physical Activity on the Gut Microbiota and the Gut-Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wei, J.; Chen, Q.; Hu, M.; Yu, L.; Mi, J.; Zhou, X.; Qin, D.; Wu, J.; Wu, A. Artificial intelligence-driven multi-omics approaches in Alzheimer’s disease: Progress, challenges, and future directions. Acta Pharm. Sin. B 2025, 15, 4327–4385. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.J.; Schat, E.; Smit, A.C.; Snippe, E.; Ceulemans, E. Monitoring emotional intensity and variability to forecast depression recurrence in real time in remitted adults. J. Consult. Clin. Psychol. 2024, 92, 505–516. [Google Scholar] [CrossRef]
- Snippe, E.; Smit, A.C.; Kuppens, P.; Burger, H.; Ceulemans, E. Recurrence of depression can be foreseen by monitoring mental states with statistical process control. J. Psychopathol. Clin. Sci. 2023, 132, 145–155. [Google Scholar] [CrossRef]
- Khoruts, A. Targeting the microbiome: From probiotics to fecal microbiota transplantation. Genome Med. 2018, 10, 80. [Google Scholar] [CrossRef]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef]
- McNutt, M.D.; Liu, S.; Manatunga, A.; Royster, E.B.; Raison, C.L.; Woolwine, B.J.; Demetrashvili, M.F.; Miller, A.H.; Musselman, D.L. Neurobehavioral effects of interferon-α in patients with hepatitis-C: Symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology 2012, 37, 1444–1454. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Ranuh, R.; Athiyyah, A.F.; Darma, A.; Risky, V.P.; Riawan, W.; Surono, I.S.; Sudarmo, S.M. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of intestinal microbiota on the gut-brain axis. Iran. J. Microbiol. 2019, 11, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J.; Michel, C. How to Manipulate the Microbiota: Prebiotics. In Microbiota of the Human Body; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 902, pp. 119–142. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain-Gut-Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Limbana, T.; Khan, F.; Eskander, N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus 2020, 12, e9966. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Massoudi, B.; Holvast, F.; Bockting, C.L.H.; Burger, H.; Blanker, M.H. The effectiveness and cost-effectiveness of e-health interventions for depression and anxiety in primary care: A systematic review and meta-analysis. J. Affect. Disord. 2019, 245, 728–743. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378, Correction in BMJ 2024, 385, q902. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).