Abstract
Per and polyfluoroalkyl substances (PFAS) are a class of synthetic, persistent environmental pollutants detected in biological systems and increasingly recognized for their harmful effects on human health. The liver, being a central organ in the metabolism of xenobiotics, is profoundly affected by these compounds and is a main target of PFAS-induced toxicity. The purpose of the present Scoping Review is to investigate the multiple and complex mechanisms behind PFAS hepatotoxicity, taking into consideration evidence from preclinical in vivo models. Using electronic databases (PubMed and Google Scholar), a total of 38 studies were found eligible to be extensively explored to gather information regarding PFAS toxicity toward hepatic lipid metabolism, oxidative stress, injury and inflammation. Moreover, the parental exposure of these chemicals on the offspring will be discussed as well. As illustrated in the proposed graphical abstract, PFAS exposure has been linked to the triggering of oxidative stress phenomena, mitochondrial dysfunction and hepatic inflammatory infiltrate with sex specific effects in rodents. The predominant effects manifest as the overproduction of reactive oxygen species (ROS), the disruption of hepatic lipid metabolism, and the activation of several nuclear transcription factors involved in lipid regulation, with PPAR-α being the most prominent. Considering their strong bioaccumulative properties and persistence in both the environment and the human body, legacy and emerging PFAS should be regarded as potent toxicants with a distinctive role in the onset of metabolic diseases and as a pressing issue to be addressed within regulatory policies.
1. Introduction
PFAS (per- and polyfluoroalkyl substances), also known as “forever chemicals”, are a heterogeneous group of human-made compounds used in industries and consumer products since the 1940s [1].
According to the Organisation for Economic Co-operation and Development (OECD), a representative of 38 industrialized countries in America, Asia and Europe, PFAS are defined as “fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it)” (Data accessed: 22 November 2024). In their document “Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance” published in 2021, it is stated that the PFAS family counts a total of more than 4730 chemicals, comprehensive of the “legacy PFAS” perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) and the “emerging PFAS”, slightly modified molecules such as the 6:2 chlorinated perfluoroalkyl ether sulfonic acid (6:2 Cl-PFESA) [2].
The high-energy C-F (carbon-fluorine) bond provides significant thermal and chemical stability to PFAS, which are likely to persist over long periods in the environment once introduced into it. Industrial wastewater treatment plants contribute directly to PFAS dissemination, contaminating soil, water, and food sources. Additionally, several industrial sectors, including food packaging, firefighting foams, water-repellent clothing, medical devices, inks and paints are considered some of the main sources of PFAS emission into the air [1,3].
Due to their high resistance, bioaccumulative and toxic properties, PFAS and their subcategories are considered a serious concern for human health [4].
Several studies have linked PFAS exposure to the onset of a wide variety of diseases and adverse health effects in humans. Relevant to the toxicity of these chemicals were found to be the magnitude, duration and individual features (i.e., sex, age, occupation) with infants and children as the most vulnerable subjects. Pathological mechanisms directly linked to PFAS exposure include the impairment of immune and endocrinological functions, the dysregulation of serum lipid profile and glucose metabolism and a higher risk of developing cardiometabolic disorders and different forms of cancer (e.g., kidney, prostate, testicles) [5].
PFAS’s pleiotropic toxicity is also relevant to the liver. This organ plays a crucial role in several vital physiological processes, including macronutrient and xenobiotic metabolism, immune system functions, and endocrinological regulation [6].
The hepatotoxicity exerted by PFAS and their subcategories is being evaluated due to the partially unknown effects of these compounds. Numerous vivo studies on rodents highlighted positive correlations between PFAS and the rise in markers of liver damage as well as an increased risk of developing steatosis and Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) [7,8,9].
The mechanisms proposed behind the hepatotoxicity are numerous and involve the ability to bind and activate multiple nuclear receptor pathways, to promote liver enlargement, the deposition of lipid droplets in hepatocytes, the induction of oxidative stress, the dysregulation of the liver’s antioxidant enzymes and many more [9].
Taking into account PFAS broad spreading into the ecosystem and their partially undiscovered mode of action, the current review aims to identify the mechanisms behind per- and polyfluoroalkyl substances-induced hepatotoxicity in experimental animal models. The main focus has been directed on the toxicants’ ability to interfere with hepatic lipid metabolism and gene expression and elicit oxidative stress and inflammation. Furthermore, the effects of maternal PFAS exposure and the consequent metabolic alterations in the offspring will also be discussed.
2. Materials and Methods
The current Scoping Review was conducted following the Sigma extension for Scoping Review protocol “Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist” provided by the Journal “Current Issues in Molecular Biology” in the section “Instructions for Authors” [10].
Scoping reviews serve as a useful tool in scientific literature to map and provide an overview of the evidence and studies available on a specific topic. The checklist outlined in the protocol consists of 20 required items and two optional ones. Considering the pleiotropic toxicity of PFAS and the numerous contexts in which they are studied, this framework is valuable to apply.
The rationale behind exploring the hepatotoxicity of PFAS in experimental animal models through a scoping review lies in the selection of the studies. To clarify, the features of the protocol allow for a more specific investigation of a targeted organ while minimizing the interference of other exogenous factors.
2.1. Eligibility Criteria
The inclusion and exclusion criteria considered for conducting the present scoping review are provided in Figure 1. The studies’ screening process was performed by one author (G.T.) examining the title, abstract and Materials and Methods Section. Two other authors (A.V., L.P.) then revised the list of potentially eligible studies. With the intent of the review being to evaluate the hepatotoxicity of PFAS, specifically in experimental animal models, all studies whose main focus was on humans or articles that performed experiments on human cells were considered unsuitable for inclusion.
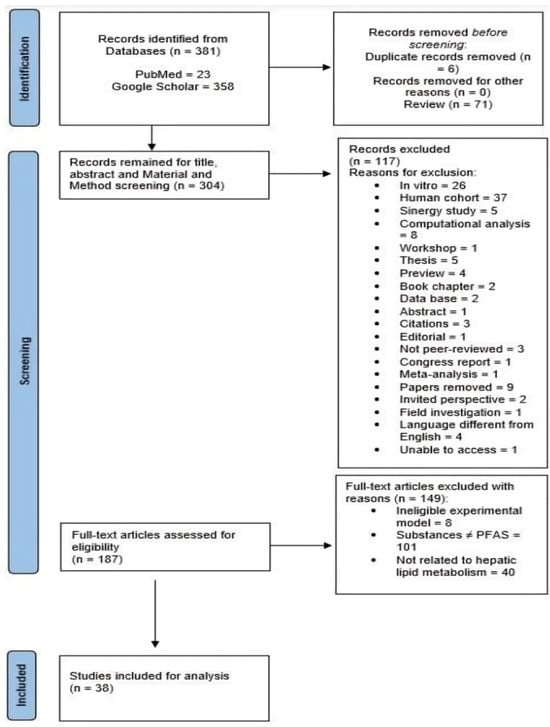
Figure 1.
Preferred reporting items for systematic review and meta-analysis (PRISMA) flow chart of included studies. Source: Page MJ et al. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. The papers (n = 381) identified from databases PubMed and Google Scholar underwent a first rationale selection that led to the exclusion of 77 papers (n = 6 duplicates; n = 71 reviews). This led to the screening of the remaining papers (n = 304) for Title, Abstract and Materials and Methods Section. Coherently with the purpose of the present Scoping Review, a total of 117 studies were excluded (see the reasons for exclusion in Figure 1 above). The papers left (n = 187) were fully screened and assessed for eligibility based on the criteria reported in Figure 1 above. The remaining studies (n = 38) were confirmed as suitable to be included for the analysis and discussion.
2.2. Search and Selection of Sources of Evidence
The screening process was conducted from the 1st of November 2024 till the 6th of January 2025 using electronic databases such as Google Scholar and PubMed. The search strategy on Google Scholar’s platform was conducted using the specific string as follows:
“(pfas OR pfos OR pfna OR pfhxs OR pfoa) AND (mouse OR rat OR in vivo OR zebrafish OR frog OR drosophila OR hamster OR rabbit OR pig) AND “hepatic lipid metabolism””. Regarding the research on the electronic database PubMed, the string used was “PFAS and hepatic lipid metabolism”, selecting 2021–2024 as the time range.
In extent, all the articles published between the years 2021 and 2024, containing simultaneously one word from the first and second bracket and the exact phrase “hepatic lipid metabolism” were considered for inclusion. Out of the 381 articles initially identified in the years of reference, only 38 were eligible to be included and analyzed in the present scoping review. The selection process was performed by examining the title, abstract and the Materials and Methods Section to assess the conformity of the studies with the purpose of our work. Mandatory criteria for the article to be included were the usage of the English language and the employment of experimental animal models to evaluate the impact of PFAS specifically on the hepatic lipid metabolism through biochemical, molecular, histological and omics techniques. All the articles included had to be related to the mechanisms of PFAS concerning the liver, its functions, steatosis, the alteration of biomarkers of oxidative stress and inflammation, and the qualitative and quantitative alterations of lipids, metabolites, proteins, and gene expression of well-established biomarkers of liver function.
2.3. Data Extraction and Charting
Individually, two reviewers (G.T. and, M.S.) were responsible for the data screening and extraction. Discrepancies were resolved by consensus with the other three authors (A.V., L.P. and L.C.) to ensure accuracy. Results obtained were charted according to the main pathological effects exerted by PFAS, coherently with the aims of the present work as follows:
- (i)
- PFAS and hepatic lipid metabolism
- (ii)
- PFAS and oxidative stress
- (iii)
- PFAS and liver injury and inflammation
- (iv)
- Effects of maternal PFAS exposure on the liver’s offspring
All charts concerning the studies’ examination are structured reporting:
- -
- Year and reference of the study
- -
- Animal model employed
- -
- Typology of PFAS administered
- -
- Intervention’s modality and duration
- -
- Method of analysis
- -
- Results
3. Results
3.1. Selection of Sources of Evidence
A total of 381 studies were identified from the two databases employed, PubMed (n = 23) and Google Scholar (n = 358), using the chosen search strings and considering the publication period from 2021 to 2024. The graphical representation of the evidence source selection process is illustrated in the PRISMA 2020 flow diagram for new systematic reviews, which includes searches of databases and registers only (Figure 1). Initially, 76 records were excluded from the analysis: 6 due to duplication and 71 because they were reviews. The remaining 304 records were screened based on their title, abstract and Materials and Methods Section. Coherently with the purpose of the present Scoping Review, 117 papers were removed (the reasons for exclusion are shown in Figure 1). In the final step of the screening process, the remaining 187 papers were screened as full-text articles: 8 of them were excluded due to the ineligibility of the experimental models, 101 studies employed substances different from PFAS and 40 were not related to the effects of PFAS on the hepatic lipid metabolism. As a result, 38 studies were considered suitable for the present Scoping Review. A summary of the included evidence and the relative data synthesis is presented in Table 1.

Table 1.
Summary of included studies reporting the year of publication, reference and country of the research group, paper title, animal model and PFAS administered.
3.2. Characteristics of Sources of Evidence
Consistent with the rationale of the present study, all the included studies were conducted between 2021 and 2024 using preclinical animal models, specifically rodents (mice, n = 24; rats, n = 7), zebrafish (n = 7). Out of the 38 studies, 19 took place in China, 15 in the USA, 1 in Norway, 1 in the Netherlands, 1 in Luxembourg, and 1 in Finland. The chemicals administered in the different research projects belonged to the legacy and new generation’s PFAS and were given to experimental animal models singularly or in combination.
4. Discussion
4.1. PFAS and Oxidative Stress
A growing body of in vivo studies converges on a central, recurring theme: oxidative stress is a key mechanism underlying PFAS-induced toxicity, affecting diverse species and biological systems.
From zebrafish to rodents, PFAS exposure disrupts redox homeostasis, drives lipid peroxidation, and undermines antioxidant defenses, ultimately impairing liver function, metabolism, and immune regulation (Table 2). Yang et al. (2023) and Khan et al. (2023) [12,13] found that PFOS and PFOA exposure in mice activated PPARα and downstream oxidative genes like ACOX1, promoting ROS overproduction. These molecular shifts not only triggered oxidative injury but also altered lipid metabolism and liver function, highlighting the convergence between redox imbalance and metabolic dysregulation in PFAS toxicity. The findings by Gadi et al. (2023) [27] in zebrafish further support this mechanism. Low concentrations of PFOA and HFBA altered expression of stress-related genes including DDIT3, BAXA, and IL-6, implicating oxidative stress pathways such as the unfolded protein response (UPR), senescence, and inflammation. These effects were particularly pronounced in Spns1-mutant zebrafish with impaired autophagy, underlining how PFAS toxicity is exacerbated in systems with already-compromised stress responses. Chen et al. (2022) [25] extended this understanding to PFBS and PFOS, reporting severe oxidative imbalance, decreased antioxidant enzyme activity, and disrupted hepatic lipid metabolism in mice. PFOS had a more potent effect, emphasizing that both legacy and newer PFAS variants are capable of redox disruption, though to varying degrees.

Table 2.
Schematic summary of selected studies on PFAS and oxidative stress in preclinical animal models.
Moreover, the study by Wang et al. (2022) [28] comparing PFOS and H-PFMO2OSA reaffirmed this pattern. Both compounds elevated ROS levels and impaired antioxidant enzymes like superoxide dismutase and catalase, reinforcing the notion that oxidative stress is a unifying toxicological feature across PFAS classes. Though often studied in isolation, PFAS compounds rarely exist alone in the environment. Roth et al. (2024) [22] demonstrated that exposure to a PFAS mixture significantly elevated circulating cholesterol and bile acids in mice while reducing fecal bile acid excretion. Although oxidative stress markers were not directly assessed, these metabolic disturbances, especially in conjunction with increased ASBT expression in the ileum, suggest that oxidative and inflammatory pathways may underlie impaired cholesterol catabolism and rising cardiovascular risk. Collectively, these studies paint a coherent and concerning picture: PFAS exposure consistently induces oxidative stress, a process tightly linked to lipid metabolism disruption, inflammation, and long-term organ damage. Across multiple models and exposure types, redox imbalance emerges not merely as a consequence of PFAS toxicity but as a driver of broader physiological dysfunction. Importantly, this oxidative stress does not operate in isolation; it interacts with pathways regulating bile acid cycling, immune signaling, and autophagy, potentially compounding PFAS toxicity. Given the persistence of PFAS in the environment and their bioaccumulative nature, future research should focus on the chronic and transgenerational consequences of oxidative stress induced by PFAS. There is a pressing need to identify molecular biomarkers of redox disruption and develop intervention strategies—nutritional, pharmacological, or regulatory—to mitigate the health risks posed by ongoing environmental PFAS exposure.
4.2. PFAS and Liver Injury and Inflammation
A robust and growing body of evidence demonstrates that exposure to both legacy and emerging PFAS induces liver injury and inflammation through diverse, yet interconnected, molecular and physiological pathways. From histopathological changes to transcriptomic and metabolomic alterations, these compounds consistently disrupt hepatic homeostasis, particularly by altering lipid metabolism, immune signaling, and gut–liver axis communication (Table 3).

Table 3.
Schematic summary of selected studies on PFAS liver injury and inflammation in preclinical animal models.
He et al. (2024) [42] provided single-cell resolution of PFOS-induced hepatic changes, showing profound alterations in cell populations, especially pro-inflammatory macrophages and fibroblasts, in female mice. This immunological remodeling, paired with hepatocyte dysfunction, revealed a complex inflammatory and fibrotic response that underscores sex-specific vulnerabilities to PFAS exposure. Pan et al. (2021) and Zhao et al. (2023) [17,38] further highlighted the inflammatory potential of PFOS alternatives like 6:2 Cl-PFESA. These studies linked liver damage not only to disrupted lipid metabolism but also to gut microbiota alterations, demonstrating that PFAS can activate hepatic inflammation through microbiome-mediated endocrine and metabolic crosstalk. Similarly, Wang et al. (2022) [11] and Junaid et al. (2024) [49] confirmed that gut dysbiosis and co-contaminant exposure (e.g., nanoplastics) amplify liver injury in zebrafish models, supporting the relevance of the gut-liver axis in PFAS-induced hepatotoxicity. Attema et al. (2022) [14] and Yang et al. (2023) [12] delved into nuclear receptor signaling, showing that PFOA and GenX act through PPARα, PXR, and CAR to disrupt hepatic lipid metabolism and promote steatosis. These changes were particularly evident in high-fat diet models, suggesting that underlying metabolic stress exacerbates PFAS toxicity. This was echoed by Liu et al. (2023) [23] and He et al. (2022) [37], who demonstrated that dietary restriction or high-fat diets potentiate PFAS-induced liver injury, reinforcing the importance of nutritional status in modulating toxicity outcomes. Gadi et al. (2023) found that low, environmentally relevant levels of PFOA and HFBA upregulated inflammatory and stress-response genes such as TNF-α, IL-6, and DDIT3 in zebrafish embryos, particularly in autophagy-deficient models, suggesting that PFAS exposure may overwhelm cellular defense mechanisms and lead to early signs of MAFLD [27]. Metabolomic insights from Meng et al. (2023) [20] revealed that perinatal PFBS exposure in rats disrupts genes and metabolites related to lipid synthesis and oxidation, with potential long-term effects on liver health in offspring. Chen et al. (2022) [25] and Li et al. (2024) [35] corroborated these findings by linking PFOS and F-53B,trade neme of 6:2 Cl-PFESA and substitute for PFOS, exposure to hepatic inflammation and oxidative stress, suggesting conserved toxicity pathways across PFAS variants. Importantly, Lee et al. (2023) [15] revealed a systemic dimension to PFAS-induced hepatotoxicity: PFOS exposure not only disrupted liver metabolism but also impaired testicular function, with altered hepatokines like FGF-21 and RBP-4 linking hepatic and reproductive dysfunction. Sands et al. (2024) [40] added a novel layer by comparing the epigenetic and transcriptomic impacts of PFOA and LiTFSI, a new PFAS analog, in mice, finding that both compounds induced inflammation and lipid dysregulation, albeit through distinct molecular signatures. Finally, Roth et al. (2024) [22] demonstrated that PFAS exposure impairs bile acid cycling by downregulating intestinal ASBT, elevating circulating cholesterol and disrupting lipid clearance, which may initiate or exacerbate hepatic inflammation and metabolic liver disease.
In summary, these studies collectively paint a compelling portrait of PFAS-induced hepatotoxicity, marked by inflammation, lipid accumulation, and immune dysregulation. The convergence of evidence across species, exposure routes, and PFAS classes, legacy and novel, highlights a critical need for more comprehensive risk assessments, especially in populations facing dietary or metabolic vulnerabilities. Future research should prioritize the long-term and multigenerational consequences of PFAS exposure on liver health and identify actionable intervention points to mitigate their growing public health impact.
4.3. Effects of Prenatal PFAS Exposure on Offspring Health
An increasing number of studies reveal that prenatal and perinatal exposure to PFAS, whether through maternal or paternal routes, can profoundly and persistently alter the metabolic health of offspring. The findings reported in Table 4 underscore a concerning reality: early developmental windows represent critical periods of heightened vulnerability to environmental toxicants, with PFAS acting as potent disruptors of metabolic programming, immune balance, and epigenetic integrity across generations. Meng et al. (2023) [20] provided clear evidence that maternal exposure to PFBS during gestation and lactation disrupts lipid metabolism in rat offspring. Their integrative transcriptomic and metabolomic analysis revealed dysregulation in hepatic fatty acid synthesis and degradation pathways, indicating long-lasting perturbations in lipid homeostasis. These metabolic imbalances may predispose offspring to chronic metabolic conditions, highlighting the enduring impact of in utero PFAS exposure. Extending this concern beyond maternal influence, Maxwell et al. (2024) [33] demonstrated that paternal exposure to PFAS mixtures can reprogram offspring metabolism via epigenetic modifications. Specifically, altered sperm DNA methylation patterns led to transcriptional changes in liver and adipose tissue of the next generation, particularly affecting lipid metabolism and inflammatory pathways. This study not only underscores the importance of paternal environmental history but also positions sperm epigenetics as a crucial vector for transgenerational PFAS toxicity.

Table 4.
Schematic summary of selected studies on prenatal PFAS exposure on offspring health.
Fetal susceptibility is further emphasized by Blake et al. (2022) [34], who showed that gestational exposure to either PFOA or its replacement GenX impairs hepatic function in both pregnant mice and their fetuses. Fetal livers displayed distinct transcriptomic alterations, particularly in genes linked to oxidative stress, lipid metabolism, and inflammation. These data point to a direct hepatotoxic effect of PFAS during intrauterine development, with potential long-term consequences for hepatic health and systemic metabolism.
The role of the gut-liver axis in mediating developmental PFAS toxicity was explored by Liu et al. (2024) [36], who found that perinatal PFOS exposure compromised intestinal integrity and altered gut microbiota composition in offspring. These changes were linked to elevated hepatic inflammatory markers, suggesting that early-life PFOS exposure disrupts immune homeostasis via gut-liver crosstalk, a pathway increasingly recognized in chronic liver disease development.
Similarly, Yi et al. (2024) [43] identified that maternal PFOS exposure leads to hepatic lipid accumulation and inflammation in adult female offspring, driven by shifts in microbiota and impaired autophagy. These disruptions contributed to enhanced hepatic lipid deposition and chronic inflammation, reinforcing that the microbiome-gut-liver axis and autophagic processes are key mechanistic targets of developmental PFAS toxicity. Metabolic dysfunction was also evident in the findings of Shao et al. (2021) [44], where early-life PFOA exposure induced obesity and lipid dysregulation in male offspring. Remarkably, dietary supplementation with chlorogenic acid attenuated these effects by modulating metabolic and oxidative stress pathways. This not only strengthens the link between PFAS exposure and early-onset obesity but also suggests the potential for dietary interventions to mitigate PFAS-induced damage during critical developmental windows.
In summary, prenatal and early-life exposure to PFAS, whether maternal or paternal, emerges as a significant determinant of offspring health, capable of reshaping metabolic, immunological, and epigenetic landscapes. The persistent alterations observed across studies point to a heightened risk for metabolic disorders, liver inflammation, and obesity in exposed progeny. Moreover, the involvement of the gut-liver axis, autophagy, and sperm epigenetics unveils complex, multi-systemic routes by which PFAS imprint long-term biological effects. These findings call for heightened regulatory attention to reproductive PFAS exposure and further research into protective interventions that could buffer developing organisms from these enduring toxic insults.
4.4. Effects of PFAS on Hepatic Lipid Metabolism
The compiled findings from recent in vivo studies consistently demonstrate that both legacy and emerging PFAS compounds exert significant hepatotoxic effects across diverse species, including rodents and zebrafish, as well as various genetic and dietary models. Central to these effects is the disruption of hepatic lipid homeostasis (Table 5), mediated through a combination of direct nuclear receptor activation, notably of PPARα, and secondary consequences of oxidative stress, inflammation, and mitochondrial dysfunction. These studies also reinforce the critical roles of sex, dose, exposure duration, and host genetics in modulating susceptibility to PFAS-induced liver injury. One of the most consistent findings across studies is the elevation of serum liver enzymes—ALT and AST—as indicators of hepatocellular injury. Mouse studies by He et al. (2024) [42], Yang et al. (2023) [12], and Zhao et al. (2023) [38] all reported increased levels of these enzymes following PFOS and PFOA exposure, signaling membrane damage and cell death. Interestingly, these effects were often sex-dependent, with He et al. (2024) [42] noting greater elevations in females, whereas Khan et al. (2023) [13] found that males exhibited a greater increase in hepatosomatic index, suggesting that sex hormones or differential expression of PFAS transporters and receptors could influence toxicodynamics.

Table 5.
Schematic summary of selected studies on PFAS and hepatic lipid metabolism in preclinical animal models.
Liver enlargement, or hepatomegaly, is another common feature of PFAS exposure. Studies by Lee et al. (2023) [15], Attema et al. (2022) [14], and Kirkwood-Donelson et al. (2024) [19] documented increased liver weights across exposure to various PFAS including GenX, PFOS, and PFBA. While hepatomegaly may initially represent an adaptive response, chronic enlargement often precedes overt liver dysfunction. Disruption of bile acid metabolism—reported in Roth et al. (2024) [22] and Wang et al. (2022) [11]—adds another dimension, potentially linking hepatomegaly to impaired cholesterol turnover and enterohepatic signaling. Lipid metabolism is a particularly sensitive target of PFAS-induced hepatic toxicity. Multiple studies have demonstrated alterations in both hepatic and systemic lipid profiles, although the direction of these changes varies. Stoffels et al. (2023) [26] and Roth et al. (2024) [22] observed increased liver fat and serum cholesterol, whereas Liu et al. (2023) and Attema et al. (2024) [23,31] reported reduced plasma lipids alongside hepatic triglyceride accumulation. These discordant results likely reflect complex changes in lipid synthesis, oxidation, and transport. Indeed, transcriptomic and lipidomic analyses consistently implicate dysregulation of pathways such as fatty acid β-oxidation, lipogenesis, and cholesterol metabolism, often mediated through nuclear receptor pathways including PPARs, AMPK, and FXR. Enzymes like ACLY, ACC, and FASN frequently emerge as key nodes altered by PFAS, indicating systemic shifts in energy metabolism. Steatosis—excessive lipid deposition within liver cells—is a common histopathological outcome. Both zebrafish and rodent studies have shown PFAS-induced steatosis, suggesting that these effects are conserved across vertebrates. For instance, zebrafish exposed to PFHxS and F-53B in the studies by Liao et al. (2024) [18] and Gu et al. (2024) [30] exhibited lipid accumulation within the liver.
In rodents, He et al. (2022) [37] and Salter et al. (2021) [41] confirmed these findings with histological evidence of steatosis following PFHxS and PFOS exposure. This phenotype may reflect increased lipid synthesis, impaired fatty acid breakdown, or compromised VLDL secretion. Importantly, steatosis serves not only as a biomarker of PFAS hepatotoxicity but may also mark the early stages of non-alcoholic fatty liver disease, raising significant public health concerns.
Nuclear receptors—particularly, but not exclusively, PPARα—play a central mechanistic role in PFAS toxicity. The study by Pan et al. (2021) [17] proposes PPAR-γ and -α isoform activation as the driving factors for the promotion of hepatic metabolic lipid disorder and the consequent liver accumulation. Both transcriptional nuclear activators were found to be upregulated following 6:2 Cl-PFESA ten-week exposure period, together with genes known to play a pivotal role in triglyceride synthesis and hepatic lipid deposition. In this context, the research group highlights a marked decrease in the levels of acyl-carnitine, a central amino acid in fatty acid transport at the mitochondrial level.
Activation of PPARα promotes fatty acid oxidation and suppresses lipid synthesis and inflammation. Multiple studies have implicated PPARα activation in mediating PFAS effects. In the study by Liao et al. (2024) [18], PFHxS-induced lipid dysregulation in zebrafish was reversed by PPARα antagonism, confirming this receptor as a key molecular target. Conversely, Attema et al. (2024) [31] showed that even in PPARα-deficient mice, high-dose PFOA exposure still altered lipid profiles, suggesting that additional or parallel mechanisms may come into play depending on dose or compound. FXR and FGF15 signaling pathways, especially in bile acid regulation and hepatic glucose handling, were also implicated in studies by Wang et al. (2024) [32], indicating broader endocrine disruption.
Emerging PFAS analogs such as F-53B, GenX, and PFBA further complicate the toxicological landscape. Initially marketed as safer alternatives, many of these compounds demonstrate comparable or even enhanced hepatotoxicity. For example, F-53B exposure in studies by Wang et al. (2022) [28], Gu et al. (2024) [30], and Li et al. (2024) [35] induced steatosis, inflammation, and metabolic dysregulation, raising serious concerns about its use as a PFOS replacement. Conversely, newer compounds like LiTFSI, studied by Zhao et al. (2023) and Sands et al. (2024), showed minimal liver toxicity, indicating that not all substitutes are equally harmful [38,40]. Nonetheless, these findings highlight the need for rigorous safety evaluation of any PFAS alternative before widespread use.
Diet significantly modulates PFAS toxicity. The study by He et al. (2022) [37] demonstrates that high-fat diets can exacerbate PFAS-induced liver injury, while Deng et al. (2022) [24] showed that dietary fiber could mitigate PFOS toxicity and reduce systemic burden. Interestingly, the work by Liu et al. (2023) [23] underscores the role of chronic dietary restriction in aggravating the negative impact on liver function and parameters. These findings point to diet as a modifiable risk factor in PFAS exposure outcomes, and they emphasize the interplay between nutrition, gut microbiota, and xenobiotic metabolism. Genetic background also influences PFAS susceptibility. Use of genetically engineered models—like PPARα knockout mice or zebrafish with altered autophagy pathways—offers valuable insights into the mechanisms driving individual variation in response. Attema et al. (2022) [14] showed that PFOA exposure altered lipid metabolism even in the absence of PPARα, while Gadi et al. (2023) [27] highlighted the importance of autophagic processes in zebrafish models of PFAS exposure. These models help pinpoint key molecular pathways and may inform future risk stratification efforts. The consistency of findings across species supports a conserved mode of hepatotoxic action for PFAS. From zebrafish embryos to adult mice, PFAS exposure results in liver enlargement, enzyme elevation, lipid dysregulation, and histological damage. However, the severity and precise manifestations depend on the compound’s chain length, chemical structure, and functional group. Long-chain PFAS like PFOS and PFOA tend to bioaccumulate more readily and induce stronger effects, but short-chain and novel compounds are not necessarily benign. Their physicochemical properties influence tissue distribution, cellular uptake, and receptor binding, contributing to differential toxicity profiles.
Collectively, these findings have major regulatory and public health implications. The assumption that newer, short-chain PFAS replacements are inherently safer is increasingly being questioned. The toxicological data for GenX and F-53B, for example, suggest significant hepatic risks, warranting a more cautious approach to replacement chemistry. Given the persistence and widespread detection of PFAS in environmental and human matrices, regulatory bodies must prioritize comprehensive toxicity testing and enforce stricter exposure limits. Public health strategies should also address the role of diet and genetic predisposition in modulating risk. The study by He et al. (2024) [42] provides compelling evidence for the molecular underpinnings of PFHxS toxicity. In early life-stage zebrafish, environmentally relevant concentrations of PFHxS disrupted lipid profiles and activated the PPARα signaling pathway. Lipidomic analyses revealed dysregulation of glycerophospholipids, sphingolipids, and sterol lipids. Molecular docking studies suggested PFHxS binds PPARα with greater affinity than oleic acid, and co-exposure to a PPARα antagonist reversed lipid disruptions, strongly implicating PPARα as the molecular initiating event. Similarly, Khan et al. (2023) [13] examined chronic exposure to a PFAS mixture in A/J mice, reflecting real-world exposure conditions. Their results revealed liver accumulation of PFAS and associated increases in male body weight and hepatosomatic index. Lipidomic analysis identified 207 differentially abundant lipids, with alterations in key fatty acids. Gene and protein expression studies showed sex-specific modulation of lipid regulators, including PPARs, LXRs, RXR, FASN, and SREBP, along with altered glutathione reductase activity—pointing to both oxidative stress and disrupted nuclear receptor signaling.
In the work by Heintz et al. (2022) [29], mice were exposed to HFPO-DA (GenX) at doses up to 5 mg/kg/day. Transcriptomic analysis confirmed strong PPARα activation, with enrichment of peroxisomal and mitochondrial fatty acid oxidation genes at lower doses. Higher doses led to activation of complement, apoptosis, and cell cycle pathways, all correlating with histological liver injury. These findings confirm that GenX hepatotoxicity operates through PPARα-mediated pathways, further challenging the presumed safety of PFAS replacements.
Lastly, Hari et al. (2024) [16] explored the shared mechanisms of PFAS and PAH-induced steatosis using transcriptomic data and molecular initiating event predictions. PFAS exposure led to marked upregulation of fatty acid oxidation genes and downregulation of lipid transporters. The findings also revealed sex-specific gene responses: male rats exhibited suppression of gluconeogenesis and bile acid synthesis genes, while lipid synthesis gene Scd was upregulated. These results support a multifactorial, PPARα-centered model of PFAS-induced hepatic steatosis with clear sex-based divergence.
4.5. Comparison of Legacy and Emerging PFAS Hepatotoxicity Mechanisms
According to the document “Multi-Industry Per- and Polyfluoroalkyl Substances (PFAS) Study” (United States Environmental Protection Agency, 2021), emerging PFAS were introduced as alternative compounds following concerns regarding legacy PFAS’ bioaccumulative properties, environmental persistence and adverse health effects in humans [50]. Despite the efforts, these new compounds share several hepatotoxic mechanisms with the legacy ones and may pose health and environmental risks comparable to, or exceeding, those of their predecessors [51].
Our comparative analysis highlights that exposure to both long-chain PFAS, such as PFOS and PFOA, and their short-chain or alternative counterparts, including GenX, F-53B, PFBS, and PFBA, leads to the activation of PPARα [14,18,29]. As previously discussed, this nuclear receptor plays a pivotal role in regulating lipid homeostasis, and its sustained activation by PFAS results in enhanced peroxisomal fatty acid oxidation, lipid accumulation, and hepatic hypertrophy [19]. These changes are accompanied by increased oxidative stress, mitochondrial dysfunction, and depletion of antioxidant defenses, such as superoxide dismutase and catalase, culminating in hepatocellular injury and steatosis [11,35,38]. Our findings support that the fundamental hepatotoxic mode of action is conserved across PFAS classes.
However, notable distinctions emerge when comparing the capillarity of their effects. Legacy PFAS often trigger cholestatic effects and bile acid dysregulation [32]. Certain emerging PFAS, such as GenX and PFBS, appear to preferentially activate oxidative and endoplasmic reticulum stress pathways [20,27]. Moreover, newer compounds such as GenX are proposed to primarily exert their effects through PPARα activation, while the legacy compound PFOA induces hepatotoxicity via the activation of multiple nuclear receptors, including PXR and CAR [14]. Furthermore, emerging PFAS such as HFPO-DA exhibit reduced bioaccumulation compared to legacy PFAS due to their greater hydrophilicity and consequently more rapid clearance, as reported in the study by M. Thomson et al. (2019) [52].
The same result is confirmed in the study by Wang et al. (2022) [28] included in the present scoping review. After the 28-day intervention, adult male mice reported higher concentrations of PFOS compared to H-PFMO2OSA at 0.2, 1 and 5 mg/kg/day in both serum and liver. Nonetheless, emerging PFAS can elicit comparable or even stronger acute responses at equivalent molar concentrations. For instance, in the same study, the H-PFMO2OSA-treated groups suffered greater toxicological impact at the same doses than the PFOS counterparts regarding body weight, ALT and AST activity and liver histology.
Fortunately, this does not always apply to all the representatives of the emerging PFAS class. Indeed, as reported by Li et al. (2017) [53], the serum decline rate and corresponding half-life of PFHxS was remarkably longer compared to the PFOS and PFOA ones (5.3 years vs. 3.4 years and 2.7 years).
Additionally, Sands et al. (2024) [40] indicate lower levels of LiTFSI compared to PFOA in the serum of male mice after 14 and 30 days of exposure, but the same trend does not replicate for the liver compartment, where LiTFSI concentration aligns with the long-chain PFAS. Despite the shared tropism, the newer compound is associated with less profound liver toxicity compared to the legacy representative.
5. Conclusions
In summary, PFAS-induced hepatotoxicity is a complex, multifactorial process involving nuclear receptor signaling, oxidative stress, the activation of inflammatory pathways and metabolic reprogramming. Findings from in vivo models consistently demonstrate that both legacy and replacement PFAS can significantly disrupt liver function, often in a sex-, dose-, and diet-dependent manner. A particularly relevant aspect that emerged from delving into the PFAS-related toxicity is the transgenerational effect, supporting their view as epigenetic, immune and metabolic disruptor also in the offspring following maternal and perinatal exposure. Moreover, our findings support the notion that structural modifications do not necessarily translate into reduced hepatotoxic potency and that intra-group chemical structure variations play a distinct role in hepatotoxicity and tropism for storage in the organism. Therefore, while emerging PFAS may pose a lower risk of long-term bioaccumulation, replacing long-chain PFAS with shorter-chain or structurally modified analogs primarily reduces their persistence rather than their intrinsic toxicity, with notable exceptions. From a public health perspective, severe and proactive regulatory policies that reflect the latest mechanistic science should be applied promptly. Main goals might be represented by reducing the introduction of these compounds into the environment and promoting large-scale awareness campaigns to increase public understanding of these substances. The authors also share the opinion that while we might not be able to completely avoid the exposure of the currently present compounds in the water system and soils, the identification of novel compounds with material-wise comparable properties, while precisely characterizing the chemical groups responsible for toxicity and bioaccumulative potential, should be a key focus in the development of safer PFAS alternatives.
Author Contributions
Conceptualization, G.T., L.C., L.P. and A.V.; methodology, G.T. and A.V.; validation, G.T., L.P. and A.V.; formal analysis, G.T.; investigation, G.T., L.P. and A.V.; data curation, G.T., L.C. and M.S.; writing—original draft preparation, G.T., L.P. and A.V.; writing—review and editing, G.T., L.P. and A.V.; visualization, G.T. and A.V.; supervision, L.P. and A.V.; project administration, G.T., L.P. and A.V.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Authors L.C. and M.S. were employed by the company Flashtox Srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABCB11 | ATP-binding cassette, sub-family B member 11 |
| ABCC3 | ATP Binding Cassette Subfamily C Member 3 |
| ABCC4 | ATP Binding Cassette Subfamily C Member 4 |
| ACADM | Acyl-Coenzyme A dehydrogenase |
| ACC | Acetyl-CoA carboxylase |
| ACLY | ATP Citrate Lyase |
| ACOX1 | Acyl-CoA Oxidase 1 |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AMPK | AMP activated protein kinase |
| APOA1 | Apolipoprotein A1 |
| APOA5 | Apolipoprotein A5 |
| ASBT | Apical sodium-dependent bile acid transporter |
| AST | Aspartate aminotransferase |
| BAXA | BCL2 associated X, apoptosis regulator a |
| BSA | Bovine serum albumin |
| CAR | Chimeric antigen receptor |
| Cd14 | Cluster of differentiation 14 |
| Cd36 | Cluster of differentiation 36 |
| 6:2 Cl-PFESA | 6:2 Chlorinated perfluoroalkyl ether sulfonic acid |
| Col1α | Collagen type I alpha |
| CPT1A | Carnitine palmitoyltransferase 1A |
| CPT2 | Carnitine palmitoyltransferase 2 |
| Csf1ra | Colony stimulating factor 1 receptor, a |
| Cyp4a14 | Cytochrome P450 omega-hydroxylase 4A14 |
| Cyp4a1-3 | Cytochrome P450 omega-hydroxylase 4A1-3 |
| Cyp7a1 | Cytochrome P450 omega-hydroxylase 7A1 |
| DDIT3 | DNA-damage-inducible transcript 3 |
| DEGs | Differentially Expressed Genes |
| EDEM1 | ER degradation enhancing alpha-mannosidase like protein 1 |
| Ehhadh | Enoyl-CoA, Hydratase/3-Hydroxyacyl CoA Dehydrogenase |
| F-53B | 6:2 Cl-PFESA |
| FABP1 | Fatty Acid Binding Protein 1 |
| FAT | Fatty Acid Transporter |
| FATP2 | Fatty Acid Transport Protein 2 |
| FASN | Fatty acid synthase |
| FGF-21 | Fibroblast growth factor 21 |
| FXR | Farnesoid X Receptor |
| GPAM | Glycerol-3-phosphate acyltransferase, mitochondrial |
| GGT | Gamma-glutamyl transferase |
| GOT | Glutamic-oxaloacetic transaminase |
| GPT | Glutamic-pyruvic transaminase |
| GSTM1 | Glutathione S-transferase mu 1 |
| HA | Humic acid |
| HDL | High density lipoprotein |
| HFBA | Heptafluorobutyric Acid |
| H-PFMO2OSA | Nafion byproduct 2 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| LC3 | Protein LC3 |
| LDL | Low density lipoprotein |
| LiTFSI | Lithium bis(trifluoromethyl sulfonyl)imide |
| Lpl | Lipoprotein lipase |
| LXR | Liver X receptor |
| m-TOR | Mechanistic target of rapamycin |
| MAFLD | Metabolic dysfunction-Associated Fatty Liver Disease |
| MDA | Malondialdehyde |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MTTP | Microsomal triglyceride transfer protein |
| MUFA | Mono unsatureated fatty acids |
| NEFA | Non-esterified fatty acids |
| Nfkbiaa | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha a |
| NLRP3 | NLR family pyrin domain containing 3 |
| NPs | Nanoplastics |
| NTCP | Sodium taurocholate co-transporting polypeptide |
| OBS | Sodium p-perfluorous nonenoxybenzenesulfonate |
| OECD | Organisation for Economic Co-operation and Development |
| Ostb | Organic solute transporter beta |
| P62 | Protein 62 |
| PFAS | Per- and polyfluoroalkyl substances |
| PFBS | Perfluorobutanesulfonic acid |
| PFDA | Perfluorodecanoic acid |
| PFNA | Perfluorononanoic acid |
| PFHxS | Perfluorohexanesulfonic acid |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctanesulfonic acid |
| PFTrDA | Perfluorotridecanoic acid |
| PFuDA | Perfluoroundecanoic acid |
| PFTA | Perfluorotetradecanoic acid |
| PFTeDA | Perfluorotetradecanoic acid |
| PND | Post-natal day |
| PPAR | Peroxisome Proliferator-Activated Receptors |
| Prkcda | Protein kinase C delta type |
| PXR | Pregnane X receptor |
| RBP-4 | Retinol-Binding Protein 4 |
| RXR | Retinoic X receptor |
| ROS | Reactive Oxygen Species |
| SCD | Stearoyl-CoA desaturase |
| SLC10A1 | Solute carrier family 10 member 1 |
| SM | Sphingoyelin |
| Spns1 | Spinster homolog 1 |
| SREBP | Sterol Regulatory Element-Binding Protein |
| T-AOC | Total Antioxidant Capacity |
| TBA | Total bile acid |
| TC | Total cholesterol |
| TG | Triglycerides |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-aplha |
| UPR | Unfolded protein response |
| VLDL | Very low density lipoprotein |
References
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Djordjevic, A.B.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- OECD. Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Corsini, E.; Iulini, M.; Galbiati, V.; Maddalon, A.; Pappalardo, F.; Russo, G.; Hoogenboom, R.L.A.P.; Beekmann, K.; Janssen, A.W.F.; Louisse, J.; et al. EFSA Project on the Use of NAMs to Explore the Immunotoxicity of PFAS. EFSA Support. Publ. 2024, 21, 8926E. [Google Scholar] [CrossRef]
- Nian, M.; Zhou, W.; Feng, Y.; Wang, Y.; Chen, Q.; Zhang, J. Emerging and Legacy PFAS and Cytokine Homeostasis in Women of Childbearing Age. Sci. Rep. 2022, 12, 6517. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.; Rock, S.; Stratakis, N.; Eckel, S.P.; Walker, D.I.; Valvi, D.; Cserbik, D.; Jenkins, T.; Xanthakos, S.A.; Kohli, R.; et al. Exposure to Per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2022, 130, 046001. [Google Scholar] [CrossRef]
- Das, K.P.; Wood, C.R.; Lin, M.J.; Starkov, A.A.; Lau, C.; Wallace, K.B.; Corton, J.C.; Abbott, B.D. Perfluoroalkyl Acids-Induced Liver Steatosis: Effects on Genes Controlling Lipid Homeostasis. Toxicology 2017, 378, 37–52. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Puckett, H.; Oliver, J.; Nielsen, G.; Heiger-Bernays, W.; Webster, T.F. Perfluorooctanoic Acid Activates Multiple Nuclear Receptor Pathways and Skews Expression of Genes Regulating Cholesterol Homeostasis in Liver of Humanized PPARα Mice Fed an American Diet. Toxicol. Appl. Pharmacol. 2020, 405, 115204. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Liu, S.; Wang, C.; Jin, Y.; Lai, H.; Tu, W. Aberrant Hepatic Lipid Metabolism Associated with Gut Microbiota Dysbiosis Triggers Hepatotoxicity of Novel PFOS Alternatives in Adult Zebrafish. Environ. Int. 2022, 166, 107351. [Google Scholar] [CrossRef]
- Yang, W.; Ling, X.; He, S.; Cui, H.; Yang, Z.; An, H.; Wang, L.; Zou, P.; Chen, Q.; Liu, J.; et al. PPARα/ACOX1 as a Novel Target for Hepatic Lipid Metabolism Disorders Induced by per- and Polyfluoroalkyl Substances: An Integrated Approach. Environ. Int. 2023, 178, 108138. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.A.; Grønnestad, R.; Krøkje, Å.; Bartosov, Z.; Johanson, S.M.; Müller, M.H.B.; Arukwe, A. Alteration of Hepato-Lipidomic Homeostasis in A/J Mice Fed an Environmentally Relevant PFAS Mixture. Environ. Int. 2023, 173, 107838. [Google Scholar] [CrossRef] [PubMed]
- Attema, B.; Janssen, A.W.F.; Rijkers, D.; van Schothorst, E.M.; Hooiveld, G.J.E.J.; Kersten, S. Exposure to Low-Dose Perfluorooctanoic Acid Promotes Hepatic Steatosis and Disrupts the Hepatic Transcriptome in Mice. Mol. Metab. 2022, 66, 101602. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Lam, T.K.Y.; Tang, H.C.; Ho, T.C.; Wan, H.T.; Wong, C.K.C. PFOS-Elicited Metabolic Perturbation in Liver and Fatty Acid Metabolites in Testis of Adult Mice. Front. Endocrinol. 2023, 14, 1302965. [Google Scholar] [CrossRef]
- Hari, A.; AbdulHameed, M.D.M.; Balik-Meisner, M.R.; Mav, D.; Phadke, D.P.; Scholl, E.H.; Shah, R.R.; Casey, W.; Auerbach, S.S.; Wallqvist, A.; et al. Exposure to PFAS Chemicals Induces Sex-Dependent Alterations in Key Rate-Limiting Steps of Lipid Metabolism in Liver Steatosis. Front. Toxicol. 2024, 6, 1390196. [Google Scholar] [CrossRef]
- Pan, Z.; Miao, W.; Wang, C.; Tu, W.; Jin, C.; Jin, Y. 6:2 Cl-PFESA Has the Potential to Cause Liver Damage and Induce Lipid Metabolism Disorders in Female Mice through the Action of PPAR-γ. Environ. Pollut. 2021, 287, 117329. [Google Scholar] [CrossRef]
- Liao, H.; He, Y.J.; Zhang, S.; Kang, X.; Yang, X.; Xu, B.; Magnuson, J.T.; Wang, S.; Zheng, C.; Qiu, W. Perfluorohexanesulfonic Acid (PFHxS) Induces Hepatotoxicity through the PPAR Signaling Pathway in Larval Zebrafish (Danio Rerio). Environ. Sci. Technol. 2024, 58, 22894–22906. [Google Scholar] [CrossRef]
- Kirkwood-Donelson, K.I.; Chappel, J.; Tobin, E.; Dodds, J.N.; Reif, D.M.; DeWitt, J.C.; Baker, E.S. Investigating Mouse Hepatic Lipidome Dysregulation Following Exposure to Emerging Per- and Polyfluoroalkyl Substances (PFAS). Chemosphere 2024, 354, 141654. [Google Scholar] [CrossRef]
- Meng, X.; Yu, G.; Luo, T.; Zhang, R.; Zhang, J.; Liu, Y. Transcriptomics Integrated with Metabolomics Reveals Perfluorobutane Sulfonate (PFBS) Exposure Effect during Pregnancy and Lactation on Lipid Metabolism in Rat Offspring. Chemosphere 2023, 341, 140120. [Google Scholar] [CrossRef]
- He, Y.J.; Liao, H.; Yang, G.; Qiu, W.; Xuan, R.; Zheng, G.; Xu, B.; Yang, X.; Magnuson, J.T.; Schlenk, D.; et al. Perfluorohexanesulfonic Acid (PFHxS) Impairs Lipid Homeostasis in Zebrafish Larvae through Activation of PPARα. Environ. Sci. Technol. 2024, 58, 16258–16268. [Google Scholar] [CrossRef]
- Roth, K.; Yang, Z.; Agarwal, M.; Birbeck, J.; Westrick, J.; Lydic, T.; Gurdziel, K.; Petriello, M.C. Exposure of Ldlr−/−Mice to a PFAS Mixture and Outcomes Related to Circulating Lipids, Bile Acid Excretion, and the Intestinal Transporter ASBT. Environ. Health Perspect. 2024, 132, 087007. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Zhang, D.; Li, H.; Shao, X.; Xie, P.; Li, J. Novel Insights into Perfluorinated Compound-Induced Hepatotoxicity: Chronic Dietary Restriction Exacerbates the Effects of PFBS on Hepatic Lipid Metabolism in Mice. Environ. Int. 2023, 181, 108274. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Durham, J.; Liu, J.; Zhang, X.; Wang, C.; Li, D.; Gwag, T.; Ma, M.; Hennig, B. Metabolomic, Lipidomic, Transcriptomic, and Metagenomic Analyses in Mice Exposed to PFOS and Fed Soluble and Insoluble Dietary Fibers. Environ. Health Perspect. 2022, 130, 117003. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Mu, H.; Li, H.; Liu, S.; Zhu, M.; Bu, Y.; Wu, B. Effects of Perfluorobutane Sulfonate and Perfluorooctane Sulfonate on Lipid Homeostasis in Mouse Liver. Environ. Pollut. 2022, 315, 120403. [Google Scholar] [CrossRef]
- Stoffels, C.B.A.; Angerer, T.B.; Robert, H.; Poupin, N.; Lakhal, L.; Frache, G.; Mercier-Bonin, M.; Audinot, J.N. Lipidomic Profiling of PFOA-Exposed Mouse Liver by Multi-Modal Mass Spectrometry Analysis. Anal. Chem. 2023, 95, 6568–6576. [Google Scholar] [CrossRef]
- Gadi, S.; Niture, S.; Hoang, H.; Qi, Q.; Hatcher, C.; Huang, X.; Haider, J.; Norford, D.C.; Leung, T.C.; Levine, K.E.; et al. Deficiency of Spns1 Exacerbates Per- and Polyfluoroalkyl Substances Mediated Hepatic Toxicity and Steatosis in Zebrafish (Danio Rerio). Toxicology 2023, 499, 153641. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, J.; Guo, H.; Sheng, N.; Guo, Y.; Dai, J. Comparative Hepatotoxicity of a Novel Perfluoroalkyl Ether Sulfonic Acid, Nafion Byproduct 2 (H-PFMO2OSA), and Legacy Perfluorooctane Sulfonate (PFOS) in Adult Male Mice. Environ. Sci. Technol. 2022, 56, 10183–10192. [Google Scholar] [CrossRef]
- Heintz, M.M.; Chappell, G.A.; Thompson, C.M.; Haws, L.C. Evaluation of Transcriptomic Responses in Livers of Mice Exposed to the Short-Chain PFAS Compound HFPO-DA. Front. Toxicol. 2022, 4, 937168. [Google Scholar] [CrossRef]
- Gu, X.; Yang, H.; Wu, L.; Fu, Z.; Zhou, S.; Zhang, Z.; Liu, Y.; Zhang, M.; Liu, S.; Lu, W.; et al. Contribution of Gut Microbiota to Hepatic Steatosis Following F-53B Exposure from the Perspective of Glucose and Fatty Acid Metabolism. J. Hazard. Mater. 2024, 480, 136104. [Google Scholar] [CrossRef]
- Attema, B.; Kummu, O.; Pitkänen, S.; Weisell, J.; Vuorio, T.; Pennanen, E.; Vorimo, M.; Rysä, J.; Kersten, S.; Levonen, A.L.; et al. Metabolic Effects of Nuclear Receptor Activation in Vivo after 28-Day Oral Exposure to Three Endocrine-Disrupting Chemicals. Arch. Toxicol. 2024, 98, 911–928. [Google Scholar] [CrossRef]
- Wang, X.; Lv, Y.; Qiang, X.; Liang, S.; Li, R.; Zhan, J.; Liu, J. Perfluorooctanoic Acid (PFOA) and Its Alternative Perfluorobutanoic Acid (PFBA) Alter Hepatic Bile Acid Profiles via Different Pathways. Sci. Total Environ. 2024, 950, 175312. [Google Scholar] [CrossRef]
- Maxwell, D.A.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) Alter Sperm Methylation and Long-Term Reprogramming of Offspring Liver and Fat Transcriptome. Environ. Int. 2024, 186, 108577. [Google Scholar] [CrossRef]
- Blake, B.E.; Miller, C.N.; Nguyen, H.; Chappell, V.A.; Phan, T.P.; Phadke, D.P.; Balik-Meisner, M.R.; Mav, D.; Shah, R.R.; Fenton, S.E. Transcriptional Pathways Linked to Fetal and Maternal Hepatic Dysfunction Caused by Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide-Dimer Acid (HFPO-DA or GenX) in CD-1 Mice. Ecotoxicol. Environ. Saf. 2022, 248, 114314. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Wang, A.; Shan, S.; Wang, X.; Wang, Y.; Wan, J.; Ning, P.; Hong, C.; Tian, H.; et al. Hepatotoxicity Induced in Rats by Chronic Exposure to F–53B, an Emerging Replacement of Perfluorooctane Sulfonate (PFOS). Environ. Pollut. 2024, 346, 123544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, G.; Medsker, H.; Luo, T.; Meng, X.; Wang, C.; Feng, L.; Zhang, J. Perinatal Exposure to Perfluorooctane Sulfonate and the Risk of Hepatic Inflammation in Rat Offspring: Perturbation of Gut-Liver Crosstalk. Environ. Res. 2024, 259, 119442. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, J.; Zhang, X.X. Environmental Exposure to Low-Dose Perfluorohexanesulfonate Promotes Obesity and Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet. Environ. Sci. Pollut. Res. 2022, 29, 49279–49290. [Google Scholar] [CrossRef]
- Zhao, N.; Kong, Y.; Yuan, Q.; Wei, Z.; Gu, J.; Ji, C.; Jin, H.; Zhao, M. The Toxic Mechanism of 6:2 Cl-PFESA in Adolescent Male Rats: Endocrine Disorders and Liver Inflammation Regulated by the Gut Microbiota-Gut-Testis/Liver Axis. J. Hazard. Mater. 2023, 459, 132155. [Google Scholar] [CrossRef]
- Renyer, A.; Ravindra, K.; Wetmore, B.A.; Ford, J.L.; DeVito, M.; Hughes, M.F.; Wehmas, L.C.; MacMillan, D.K. Dose Response, Dosimetric, and Metabolic Evaluations of Replacement PFAS Perfluoro-(2,5,8-Trimethyl-3,6,9-Trioxadodecanoic) Acid (HFPO-TeA). Toxics 2023, 11, 951. [Google Scholar] [CrossRef]
- Sands, M.; Zhang, X.; Laws, M.; Spinella, M.; Erdogan, Z.M.; Irudayaraj, J. Comparative Hepatotoxicity of Novel Lithium Bis(Trifluoromethanesulfonyl)Imide (LiTFSI, Ie. HQ-115) and Legacy Perfluorooctanoic Acid (PFOA) in Male Mice: Insights into Epigenetic Mechanisms and Pathway-Specific Responses. Environ. Int. 2024, 185, 108556. [Google Scholar] [CrossRef]
- Salter, D.M.; Wei, W.; Nahar, P.P.; Marques, E.; Slitt, A.L. Perfluorooctanesulfonic Acid (PFOS) Thwarts the Beneficial Effects of Calorie Restriction and Metformin. Toxicol. Sci. 2021, 182, 82–95. [Google Scholar] [CrossRef]
- He, X.; Sun, Z.; Sun, J.; Chen, Y.; Luo, Y.; Wang, Z.; Linghu, D.; Song, M.; Cao, C. Single-Cell Transcriptomics Reveal the Microenvironment Landscape of Perfluorooctane Sulfonate-Induced Liver Injury in Female Mice. Sci. Total Environ. 2024, 940, 173562. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Shi, J.; Wang, L.; Wang, D.; Wang, Y.; Song, J.; Xin, L.; Jiang, F. Maternal PFOS Exposure in Mice Induces Hepatic Lipid Accumulation and Inflammation in Adult Female Offspring: Involvement of Microbiome-Gut-Liver Axis and Autophagy. J. Hazard. Mater. 2024, 470, 134177. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Xu, J.; Xu, C.; Weng, Z.; Liu, Q.; Zhang, X.; Liang, J.; Li, W.; Zhang, Y.; Jiang, Z.; et al. Early-Life Perfluorooctanoic Acid Exposure Induces Obesity in Male Offspring and the Intervention Role of Chlorogenic Acid. Environ. Pollut. 2021, 272, 115974. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a Mixture of Legacy, Alternative, and Replacement per- and Polyfluoroalkyl Substances (PFAS) Results in Sex-Dependent Modulation of Cholesterol Metabolism and Liver Injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef]
- Pfohl, M.; Marques, E.; Auclair, A.; Barlock, B.; Jamwal, R.; Goedken, M.; Akhlaghi, F.; Slitt, A.L. An ‘Omics Approach to Unraveling the Paradoxical Effect of Diet on Perfluorooctanesulfonic Acid (PFOS) and Perfluorononanoic Acid (PFNA)-Induced Hepatic Steatosis. Toxicol. Sci. 2021, 180, 277–294. [Google Scholar] [CrossRef]
- Jackson, T.W.; Lambright, C.S.; Evans, N.; Wehmas, L.C.; MacMillan, D.K.; Bangma, J.; Gray, L.E.; Conley, J.M. Exploring Maternal and Developmental Toxicity of Perfluoroalkyl Ether Acids PFO4DA and PFO5DoA Using Hepatic Transcriptomics and Serum Metabolomics. Sci. Total Environ. 2024, 953, 175978. [Google Scholar] [CrossRef]
- Kaye, E.; Marques, E.; Agudelo Areiza, J.; Modaresi, S.M.S.; Slitt, A. Exposure to a PFOA, PFOS and PFHxS Mixture during Gestation and Lactation Alters the Liver Proteome in Offspring of CD-1 Mice. Toxics 2024, 12, 348. [Google Scholar] [CrossRef]
- Junaid, M.; Liu, S.; Yue, Q.; Wang, J. Exacerbated Interfacial Impacts of Nanoplastics and 6:2 Chlorinated Polyfluorinated Ether Sulfonate by Natural Organic Matter in Adult Zebrafish: Evidence through Histopathology, Gut Microbiota, and Transcriptomic Analysis. J. Hazard. Mater. 2024, 476, 135038. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. United States Environmental Protection Agency Multi-Industry Per-and Polyfluoroalkyl Substances (PFAS) Study-2021 Preliminary Report; United States Environmental Protection Agency: Washington, DC, USA, 2021.
- Zhang, Y.; Zhou, Y.; Dong, R.; Song, N.; Hong, M.; Li, J.; Yu, J.; Kong, D. Emerging and Legacy Per- and Polyfluoroalkyl Substances (PFAS) in Fluorochemical Wastewater along Full-Scale Treatment Processes: Source, Fate, and Ecological Risk. J. Hazard. Mater. 2024, 465, 133270. [Google Scholar] [CrossRef]
- Thompson, C.M.; Fitch, S.E.; Ring, C.; Rish, W.; Cullen, J.M.; Haws, L.C. Development of an Oral Reference Dose for the Perfluorinated Compound GenX. J. Appl. Toxicol. 2019, 39, 1267. [Google Scholar] [CrossRef]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-Lives of PFOS, PFHxS and PFOA after End of Exposure to Contaminated Drinking Water. Occup. Environ. Med. 2017, 75, 46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).