The Property of a Key Amino Acid Determines the Function of Farnesyl Pyrophosphate Synthase in Sporobolomyces pararoseus NGR
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strains and Growth Conditions
2.3. Cloning of Genes Encoding the FPPS Enzymes from S. pararoseus NGR
2.4. Bioinformatic Analysis of Putative FPPS from S. pararoseus
2.5. Construction of Expression Vectors
2.6. Protein Expression and Purification
2.7. Determination of Enzymatic Activity
2.8. Preparation and Activity Assays of FPPS Mutants
3. Results
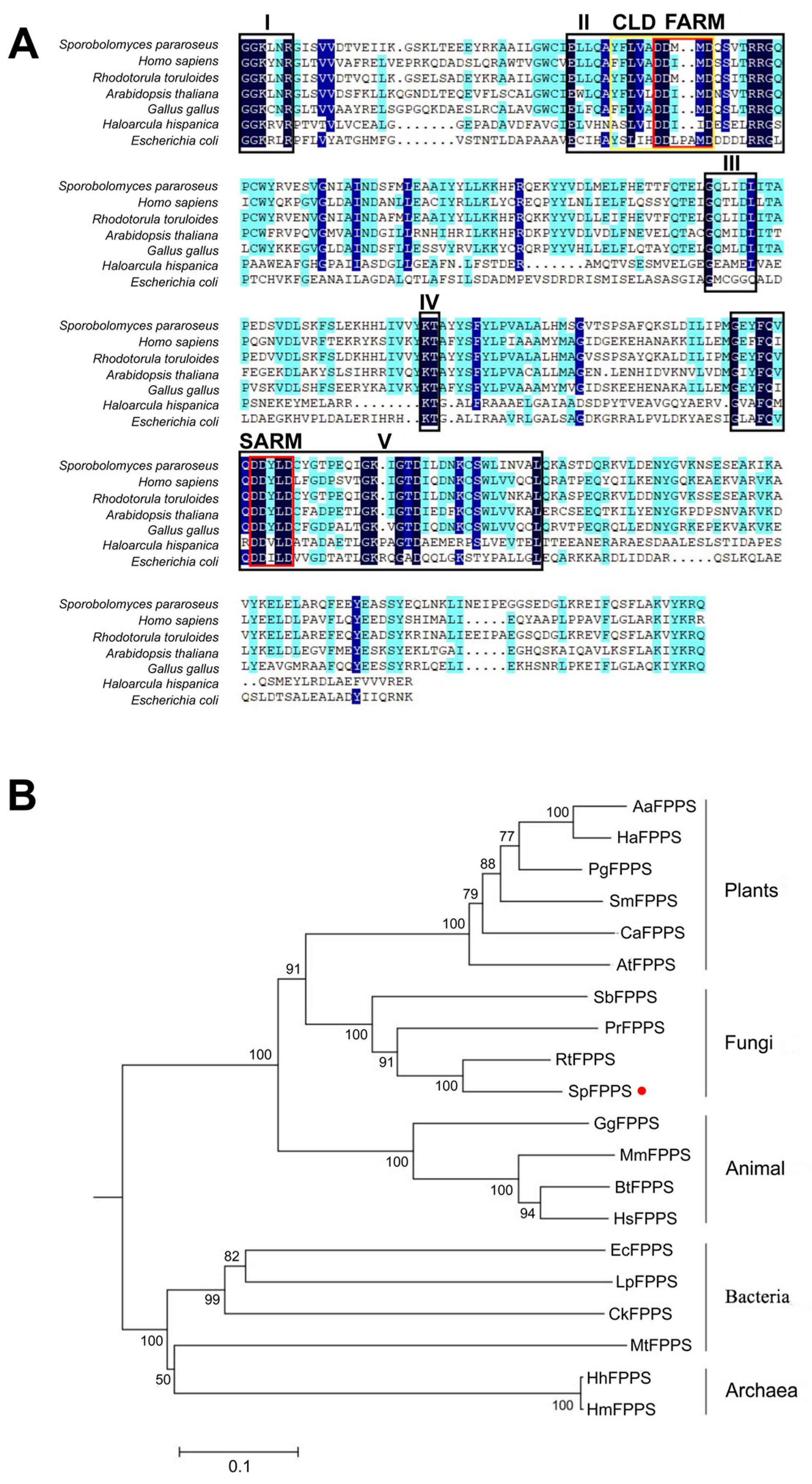
3.1. Putative FPPS Gene Cloning and Sequence Analysis
3.2. Sequence Analysis of SpFPPS Protein
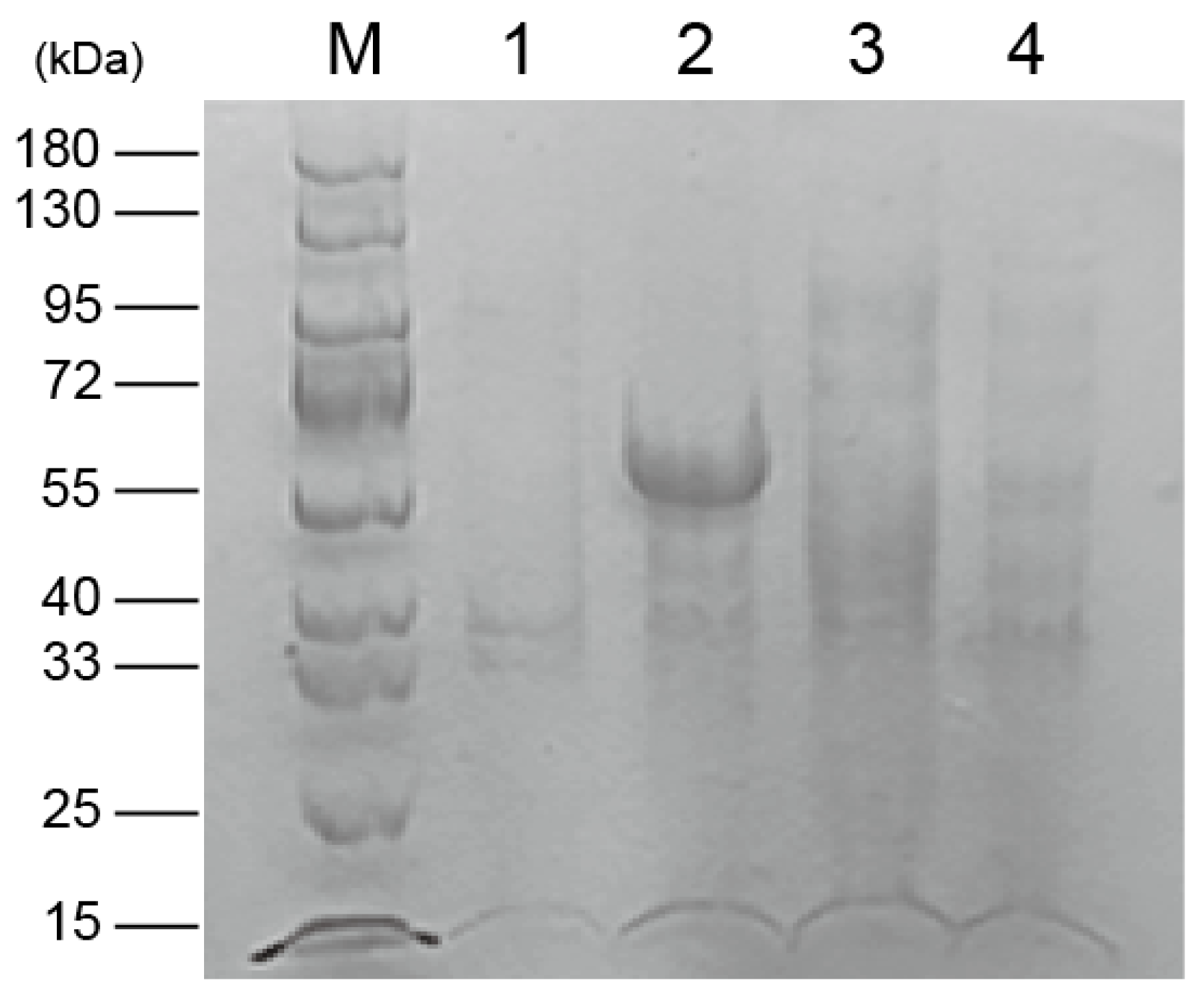
3.3. Expression and Purification of Recombinant Protein pET32a-SpFPPS
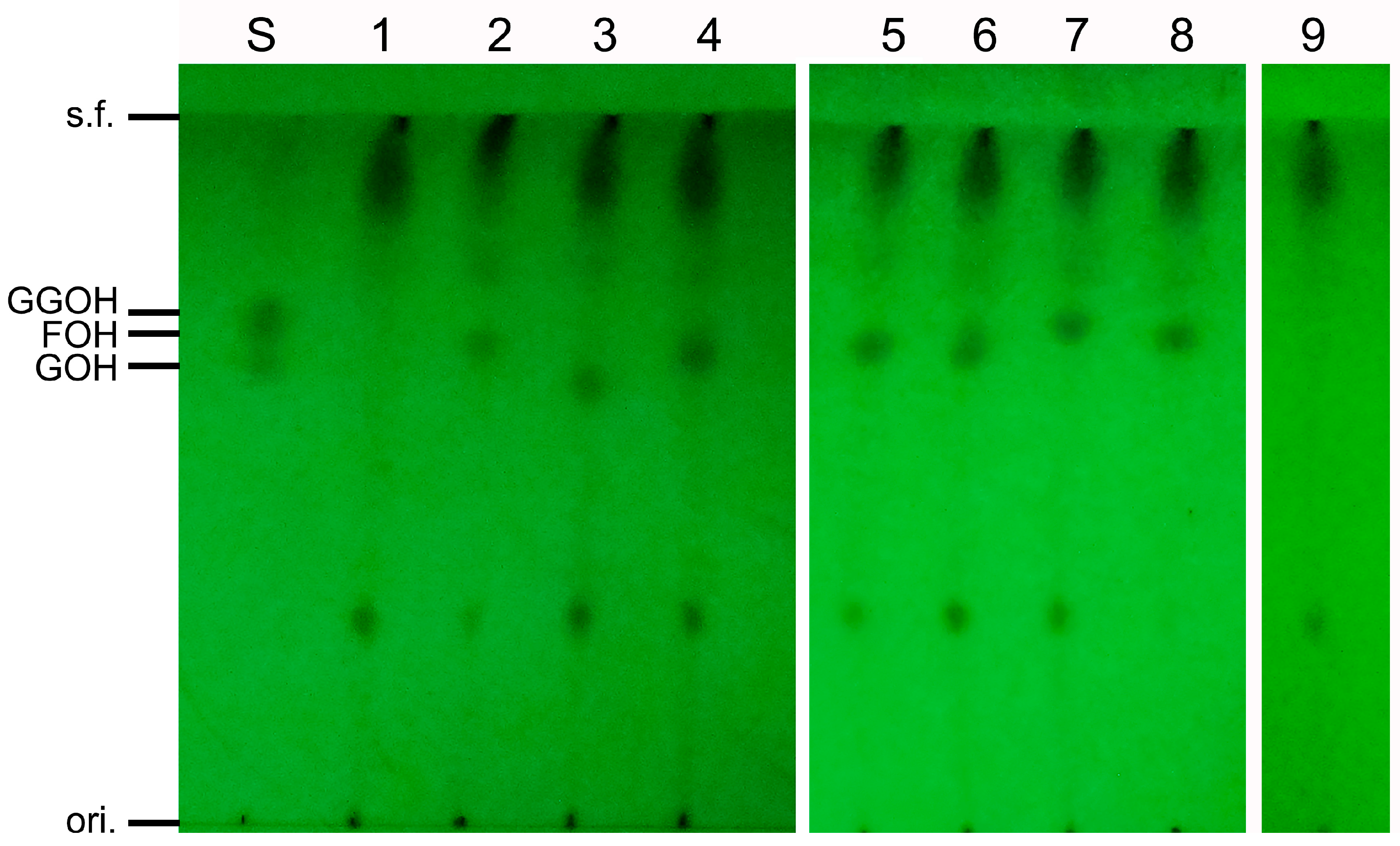
3.4. Analysis of In Vitro Activity of SpFPPS
3.5. Design of Mutant SpFPPS
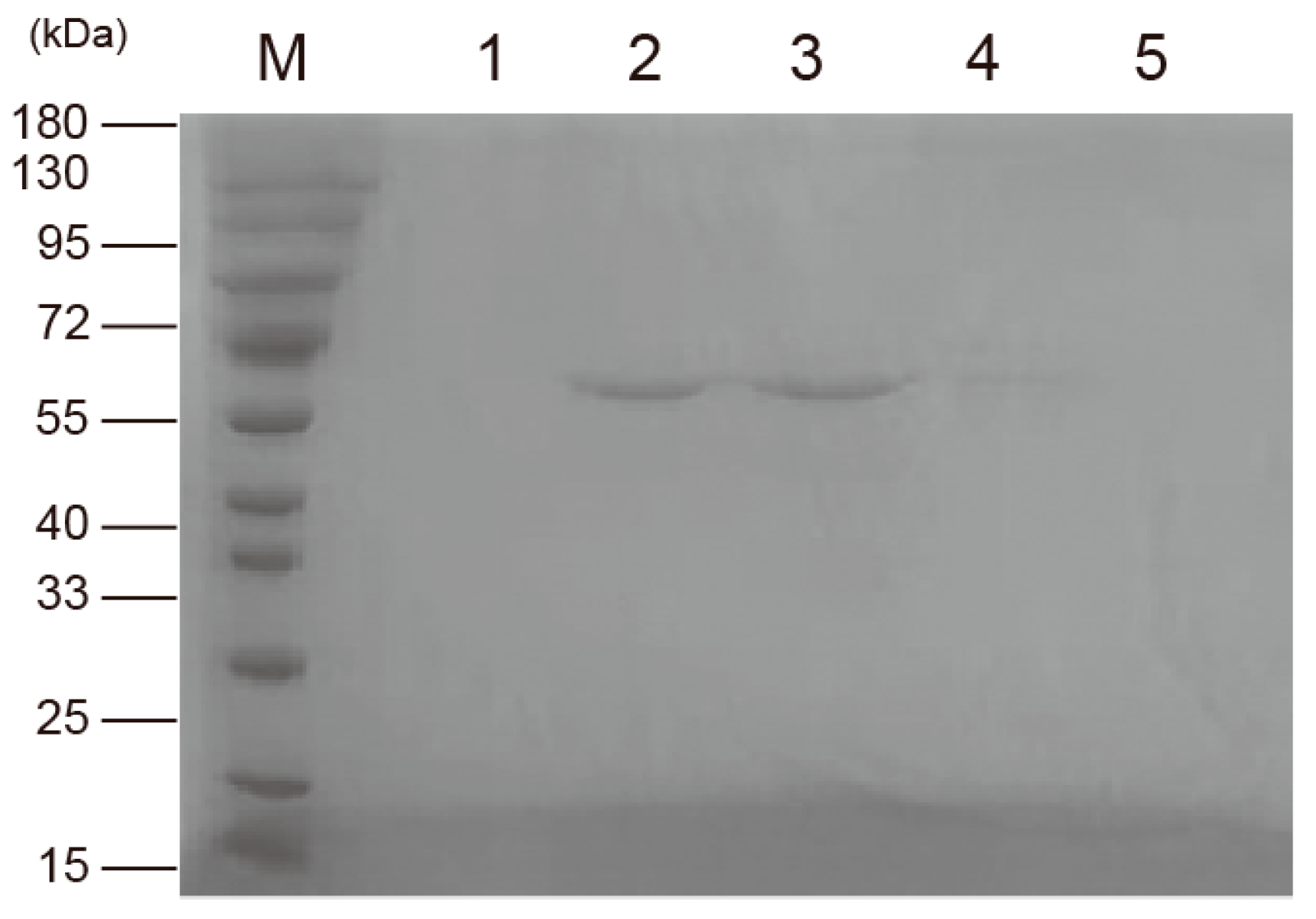
3.6. Construction, Expression and Purification of SpFPPS Mutants
3.7. Mutant SpFPPS Enzymatic Activity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; Katsimpouras, C.; Stephanopoulos, G. Novel Strategies and Platforms for Industrial Isoprenoid Engineering. Trends Biotechnol. 2020, 38, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Karst, F.; Plochocka, D.; Meyer, S.; Szkopinska, A. Farnesyl diphosphate synthase activity affects ergosterol level and proliferation of yeast Saccharomyces cerevisae. Cell Biol. Int. 2004, 28, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L. The isoprene rule and the biogenesis of terpenic compounds. Experientia 1953, 9, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Swiezewska, E.; Danikiewicz, W. Polyisoprenoids: Structure, biosynthesis and function. Prog. Lipid Res. 2005, 44, 235–258. [Google Scholar] [CrossRef]
- Ogura, K.; Koyama, T. Enzymatic Aspects of Isoprenoid Chain Elongation. Chem. Rev. 1998, 98, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Vandermoten, S.; Haubruge, É.; Cusson, M. New insights into short-chain prenyltransferases: Structural features, evolutionary history and potential for selective inhibition. Cell. Mol. Life Sci. 2009, 66, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Liang, P. Reaction Kinetics, Catalytic Mechanisms, Conformational Changes, and Inhibitor Design for Prenyltransferases. Biochemistry 2009, 48, 6562–6570. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.U.O.K. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 521–547. [Google Scholar] [CrossRef]
- Eberhardt, N.L.; Rilling, H.C. Prenyltransferase from Saccharomyces cerevisiae. Purification to homogeneity and molecular properties. J. Biol. Chem. 1975, 250, 863–866. [Google Scholar] [CrossRef]
- Tarshis, L.C.; Yan, M.; Poulter, C.D.; Sacchettini, J.C. Crystal Structure of Recombinant Farnesyl Diphosphate Synthase at 2.6-.ANG. Resolution. Biochemistry 1994, 33, 10871–10877. [Google Scholar] [CrossRef] [PubMed]
- Winkelblech, J.; Fan, A.; Li, S. Prenyltransferases as key enzymes in primary and secondary metabolism. Appl. Microbiol. Biotechnol. 2015, 99, 7379–7397. [Google Scholar] [CrossRef]
- Nürenberg, G.; Volmer, D.A. The analytical determination of isoprenoid intermediates from the mevalonate pathway. Anal. Bioanal. Chem. 2012, 402, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, A.S.A.D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim. Pol. 2005, 52, 45–55. [Google Scholar]
- Ohnuma, S.; Narita, K.; Nakazawa, T.; Ishida, C.; Takeuchi, Y.; Ohto, C.; Nishino, T. A Role of the Amino Acid Residue Located on the Fifth Position before the First Aspartate-rich Motif of Farnesyl Diphosphate Synthase on Determination of the Final Product. J. Biol. Chem. 1996, 271, 30748–30754. [Google Scholar] [CrossRef]
- Li, C.; Li, B.; Zhang, N.; Wei, N.; Wang, Q.; Wang, W.; Xie, Y.; Zou, H. Salt stress increases carotenoid production of Sporidiobolus pararoseus NGR via torulene biosynthetic pathway. J. Gen. Appl. Microbiol. 2019, 65, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Meléndez Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for Biosynthesis of Natural Product Drugs Using Engineered Microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Song, J.; Wei, N.; Li, B.; Zou, H.; Han, X. A single desaturase gene from red yeast Sporidiobolus pararoseus is responsible for both four- and five-step dehydrogenation of phytoene. Gene 2016, 590, 169–176. [Google Scholar] [CrossRef]
- Csernetics, Á.; Nagy, G.; Iturriaga, E.A.; Szekeres, A.; Eslava, A.P.; Vágvölgyi, C.; Papp, T. Expression of three isoprenoid biosynthesis genes and their effects on the carotenoid production of the zygomycete Mucor circinelloides. Fungal Genet. Biol. 2011, 48, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Daudonnet, S.; Karst, F.; Tourte, Y. Expression of the farnesyldiphosphate synthase gene of Saccharomyces cerevisiae in tobacco. Mol. Breed. 1997, 3, 137–145. [Google Scholar] [CrossRef]
- Han, J.; Liu, B.; Ye, H.; Wang, H.; Li, Z.; Li, G. Effects of Overexpression of the Endogenous Farnesyl Diphosphate Synthase on the Artemisinin Content in Artemisia annua L. J. Integr. Plant Biol. 2006, 48, 482–487. [Google Scholar] [CrossRef]
- Koyama, T.; Obata, S.; Osabe, M.; Takeshita, A.; Yokoyama, K.; Uchida, M.; Ishino, T.; Ogura, T. Thermostable Farnesyl Diphosphate Synthase of Bacillus stearothermophilus: Molecular Cloning, Sequence Determination, Overproduction, and Purification. J. Biochem. 1993, 113, 263–355. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bouvier, F.; Rahier, A.; Camara, B. Biogenesis, molecular regulation and function of plant isoprenoids. Prog. Lipid Res. 2005, 44, 357–429. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Koyama, T.; Ogura, K. Efficient enzymatic hydrolysis of polyprenyl pyrophosphates. Biochim. Biophys. Acta 1982, 712, 716–718. [Google Scholar]
- Lao, Y.M.; Jin, H.; Zhou, J.; Zhang, H.J.; Zhu, X.S.; Cai, Z.H. A Novel Hydrolytic Activity of Tri-Functional Geranylgeranyl Pyrophosphate Synthase in Haematococcus pluvialis. Plant Cell Physiol. 2018, 59, 2536–2548. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Li, B.; Xu, Q.; Song, J.; Wei, N.; Wang, W.; Zou, H. Increased torulene accumulation in red yeast Sporidiobolus pararoseus NGR as stress response to high salt conditions. Food Chem. 2017, 237, 1041–1047. [Google Scholar] [CrossRef]
- Taban, A.H.; Tittiger, C.; Blomquist, G.J.; Welch, W.H. Isolation and characterization of farnesyl diphosphate synthase from the cotton boll weevil, Anthonomus grandis. Arch. Insect Biochem. 2009, 71, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Grabińska, K.; Palamarczyk, G. Dolichol biosynthesis in the yeast Saccharomyces cerevisiae: An insight into the regulatory role of farnesyl diphosphate synthase. Fems Yeast Res. 2002, 2, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Alcaino, J.; Romero, I.; Niklitschek, M.; Sepúlveda, D.; Rojas, M.C.; Baeza, M.; Cifuentes, V. Functional characterization of the Xanthophyllomyces dendrorhous farnesyl pyrophosphate synthase and geranylgeranyl pyrophosphate synthase encoding genes that are involved in the synthesis of isoprenoid precursors. PLoS ONE 2014, 9, e96626. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K.; Schulbach, M.C.; Mahapatra, S.; Baulard, A.R.; Vissa, V.; Brennan, P.J.; Crick, D.C. Identification of a novel class of ω,E,E-farnesyl diphosphate synthase from Mycobacterium tuberculosis. J. Lipid Res. 2004, 45, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Cervantes, M.; Gallagher, C.E.; Zhu, C.; Wurtzel, E.T. Maize cDNAs Expressed in Endosperm Encode Functional Farnesyl Diphosphate Synthase with Geranylgeranyl Diphosphate Synthase Activity. Plant Physiol. 2006, 141, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Vandermoten, S.; Charloteaux, B.; Santini, S.; Sen, S.E.; Béliveau, C.; Vandenbol, M.; Francis, F.; Brasseur, R.; Cusson, M.; Haubruge, E. Characterization of a novel aphid prenyltransferase displaying dual geranyl/farnesyl diphosphate synthase activity. FEBS Lett. 2008, 582, 1928–1934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsiao, Y.; Jeng, M.; Tsai, W.; Chuang, Y.; Li, C.; Wu, T.; Kuoh, C.; Chen, W.; Chen, H. A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X)2–4D motif. Plant J. 2008, 55, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Gershenzon, J. Cloning and characterization of two different types of geranyl diphosphate synthases from Norway spruce (Picea abies). Phytochemistry 2008, 69, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Wa Chtler, B.; Temp, U.; Krekling, T.; SeݩGuin, A.; Gershenzon, J. A Bifunctional Geranyl and Geranylgeranyl Diphosphate Synthase Is Involved in Terpene Oleoresin Formation in Picea abies. Plant Physiol. 2010, 152, 639–655. [Google Scholar] [CrossRef]
- Hayato Ueoka, K.S.T.M.; Hirobumi Yamamoto, N.S.H.S. A Cytosol-Localized Geranyl Diphosphate Synthase from Lithospermum erythrorhizon and Its Molecular Evolution. Plant Physiol. 2020, 182, 1933–1945. [Google Scholar] [CrossRef]
- Jordão, F.M.; Gabriel, H.B.; Alves, J.M.; Angeli, C.B.; Bifano, T.D.; Breda, A.; de Azevedo, M.F.; Basso, L.A.; Wunderlich, G.; Kimura, E.A.; et al. Cloning and characterization of bifunctional enzyme farnesyl diphosphate/geranylgeranyl diphosphate synthase from Plasmodium falciparum. Malar. J. 2013, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Li, Z.; Miranda, K.; Oldfield, E.; Moreno, S.N.J. The Farnesyl-diphosphate/Geranylgeranyl-diphosphate Synthase of Toxoplasma gondii Is a Bifunctional Enzyme and a Molecular Target of Bisphosphonates. J. Biol. Chem. 2007, 282, 30804–30816. [Google Scholar] [CrossRef] [PubMed]
- Navale, G.R.; Dharne, M.S.; Shinde, S.S. Metabolic engineering and synthetic biology for isoprenoid production in Escherichia coli and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.; Moreira, D. Origins and Early Evolution of the Mevalonate Pathway of Isoprenoid Biosynthesis in the Three Domains of Life. Mol. Biol. Evol. 2010, 28, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Song, H.; Liu, H.; Liu, P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 2013, 17, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, B.A.; Poulter, C.D. Chain elongation in the isoprenoid biosynthetic pathway. Curr. Opin. Chem. Biol. 1997, 1, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, C.; Zhang, N.; Li, C.; Wang, Y.; Li, B. Functional verification and characterization of a type-III geranylgeranyl diphosphate synthase gene from Sporobolomyces pararoseus NGR. Front. Microbiol. 2022, 13, 1032234. [Google Scholar] [CrossRef]
- Li, Z.; Cintrón, R.; Koon, N.A.; Moreno, S.N.J. The N-Terminus and the Chain-Length Determination Domain Play a Role in the Length of the Isoprenoid Product of the Bifunctional Toxoplasma gondii Farnesyl Diphosphate Synthase. Biochemistry 2012, 51, 7533–7540. [Google Scholar] [CrossRef]


| Enzymes | Organism | Products | Reference |
|---|---|---|---|
| FPPS | Zea mays L. | FPP, GGPP | [35] |
| FPPS | Myzus persicae | GPP, FPP | [36] |
| GPPS | Phalaenopsis bellina | GPP, FPP | [37] |
| GPPS | Picea abies (Norway spruce) | GPP, FPP, GGPP | [38] |
| GPPS | Picea abies | GPP, GGPP | [39] |
| GPPS | Lithospermum erythrorhizon | GPP, FPP | [40] |
| FPPS | Plasmodium falciparum | FPP, GGPP | [41] |
| FPPS | Toxoplasma gondii | FPP, GGPP | [42] |
| GGPPS | Haematococcus pluvialis | GPP, FPP, GGPP | [29] |
| SpFPPS-Y90A | S. pararoseus | FPP, GGPP | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, N.; Yan, J.; Li, C.; Zeng, N.; Wang, D.; Li, Z.; Li, B.; An, Y. The Property of a Key Amino Acid Determines the Function of Farnesyl Pyrophosphate Synthase in Sporobolomyces pararoseus NGR. Curr. Issues Mol. Biol. 2024, 46, 3108-3121. https://doi.org/10.3390/cimb46040195
Wang Y, Zhang N, Yan J, Li C, Zeng N, Wang D, Li Z, Li B, An Y. The Property of a Key Amino Acid Determines the Function of Farnesyl Pyrophosphate Synthase in Sporobolomyces pararoseus NGR. Current Issues in Molecular Biology. 2024; 46(4):3108-3121. https://doi.org/10.3390/cimb46040195
Chicago/Turabian StyleWang, Yunjiao, Ning Zhang, Jianyu Yan, Chunwang Li, Nan Zeng, Dandan Wang, Zijing Li, Bingxue Li, and Yingfeng An. 2024. "The Property of a Key Amino Acid Determines the Function of Farnesyl Pyrophosphate Synthase in Sporobolomyces pararoseus NGR" Current Issues in Molecular Biology 46, no. 4: 3108-3121. https://doi.org/10.3390/cimb46040195
APA StyleWang, Y., Zhang, N., Yan, J., Li, C., Zeng, N., Wang, D., Li, Z., Li, B., & An, Y. (2024). The Property of a Key Amino Acid Determines the Function of Farnesyl Pyrophosphate Synthase in Sporobolomyces pararoseus NGR. Current Issues in Molecular Biology, 46(4), 3108-3121. https://doi.org/10.3390/cimb46040195






