Abstract
Microplastics and nanoplastics (MNPs) are becoming an increasingly severe global problem due to their widespread distribution and complex impact on living organisms. Apart from their environmental impact, the effects of MNPs on living organisms have also continued to attract attention. The harmful impact of MNPs has been extensively documented in marine invertebrates and larger marine vertebrates like fish. However, the research on the toxicity of these particles on mammals is still limited, and their possible effects on humans are poorly understood. Considering that MNPs are commonly found in food or food packaging, humans are primarily exposed to them through ingestion. It would be valuable to investigate the potential harmful effects of these particles on gut health. This review focuses on recent research exploring the toxicological impacts of micro- and nanoplastics on the gut, as observed in human cell lines and mammalian models. Available data from various studies indicate that the accumulation of MNPs in mammalian models and human cells may result in adverse consequences, in terms of epithelial toxicity, immune toxicity, and the disruption of the gut microbiota. The paper also discusses the current research limitations and prospects in this field, aiming to provide a scientific basis and reference for further studies on the toxic mechanisms of micro- and nanoplastics.
1. Introduction
Microplastics (MPs) are tiny particles derived from plastics, or synthetic or semisynthetic polymers produced from hydrocarbon or biomass materials. Most plastics are petroleum-derived polymers that consist of “molecules of high relative molecular mass, whose structure essentially comprises multiple repetitions of derived units, from molecules of low relative molecular mass” [1]. These polymers, like polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC), polyethylene terephthalate (PET), and polystyrene (PS), are non-biodegradable [2].
Plastic is a widely used material in industrial applications and its production has been consistently increasing over the years. In 2016, annual plastic production reached 300 million tons. However, if this trend continues, it is estimated that approximately 25 million tons of plastic waste will be produced by 2050 [3,4,5,6,7]. Plastic is a key component in a diverse range of industrial and consumer products, including cosmetics, detergents, paints, synthetic fertilizers, and pesticides, among others. Unfortunately, MPs have been detected in various food products, such as processed foods, beer, seafood, and sugar-sweetened beverages [8,9].
This widespread plastic contamination, which has been attributed to the limited recycling efforts and the absence of regulatory frameworks, has had a substantial impact on aquatic, terrestrial, and atmospheric environments. The issue of plastic pollution has become a pressing concern as it is now present in almost all water bodies, including oceans, seas, rivers, and lakes, thereby posing a significant threat to biodiversity and public health [10].
Plastic waste, once discarded in nature, is exposed to different factors, including physical (e.g., ultraviolet radiation and temperature), chemical (e.g., salinity, pH, and corrosive agents), and biological (e.g., bacteria, microalgae, and plankton). These factors decompose plastic waste into particles of different sizes and ecological impact. Three main classification groups are commonly used to describe plastic waste based on its particle size: macro- (>25 mm), meso- (between 5 and 25 mm), and microplastics (MPs < 5 mm). Additionally, the intentional production and further degradation of microplastics can generate smaller waste particles, known as nanoplastics (NPs < 1 µm) [11].
Microplastics are categorized by their origin. “Primary” microplastics are intentionally created at the microscale, while “secondary” microplastics come from the fragmentation of larger plastics [12].
Microplastic pollution is widespread in soil environments, including agricultural soils, greenhouses, and coastal, industrial, and floodplain soils. This type of pollution is a result of the inappropriate management and unsustainable use of plastic waste and agricultural processes [13,14]. Microplastic pollution is also a significant issue in aquatic environments, such as the marine environment, where plastic debris can be found on the sea floor, surface, and shoreline [15]. It has been estimated that 80% of the plastic pollution in oceans and seas comes from land [16]. Microplastics have also been detected in freshwater, including lakes, rivers, and groundwater. These particles mainly come from urban pollution, shipping, fishing, tourism, oil and gas platforms, wastewater treatment plants, discharged personal care products, textiles, and packaging [17]. Furthermore, microplastics have been found in the atmospheric fallout in both megacities and sparsely populated areas [6,18,19,20,21], and suspended atmospheric microplastics have also been repeatedly detected in indoor air [22,23].
The wide distribution of microplastics and nanoplastics (MNPs) promotes contamination by different animal species, especially by integumentary exposure, inhalation, and ingestion [24,25,26]. Specifically, for mammals and humans, the inhalation of nanomaterials and ingestion of contaminated water, sea salt, and seafood are the main routes of exposure to these plastic particles [27].
Although previously considered safe and inert materials, the negative biological impact of the contamination of microplastics and nanoplastics has been demonstrated recently [26,28,29]. As a result, the smallest particles (such as 10 μm and 2.5 μm) can penetrate organs like lungs and intestines, as well as cells like enterocytes and macrophages. These particles are recognized as foreign elements that stimulate immune response and oxidative stress [29,30]. Due to their difficulty in being cleared biologically, particles can accumulate and result in chronic inflammation, potentially leading to the development of tumors [29,31,32]. In addition, microplastics and nanoplastics pose a high toxicological risk, as they contain hazardous additives like plasticizers, flame retardants, stabilizers, dyes, antistatic agents, lubricants, sliding agents, curing agents, foaming agents, and biocides [33]. It is worth noting that microplastics have the potential to adopt a fibrous form, which is commonly referred to as “microplastic fibers” [34]. The contamination of environments with microplastic fibers is probably as much as, or even more than, that caused by microplastic particles [35,36]. Because of their elongated shape, microplastic fibers have a higher potential for bioaccumulation and can cause direct harm to organisms or lead to adverse effects [37].
It is currently not feasible to conduct clinical studies that analyze the health risks of MNPs in humans due to ethical concerns. As a result, we do not have a clear understanding of the health impact of MNPs on humans. We are unaware of the extent to which humans can absorb and accumulate MNPs, and the pharmacokinetic and pharmacodynamic mechanisms associated with them [38].
However, it is still a major concern that MNPs could have toxicological effects on the entire intestinal system, especially through ingestion, which remains one of the main exposure mechanisms of these particles.
This paper aims to present an objective overview of the potential impacts that these polymers could have on the intestinal system by highlighting the toxicological effects related to MNPs for in vivo mammalian and in vitro human cell studies found in the literature. This review focuses specifically on the toxicological effects of MNPs on the gut and the complex immunological system related to it, examining the various interrelationships that these particles have with the gut microbiota. These studies cannot provide clinical data. However, they can lay an important foundation for future research by providing an overview of these issues.
2. Main Pathogenetic Mechanisms of MNP-Induced Cell Toxicity
Experimental models have revealed that the mechanisms of membrane damage, oxidative stress, immune response, and genotoxicity contribute to the toxicity of MNPs.
Among them, the cytotoxicity of MNPs was mainly attributed to membrane damage and oxidative stress [39]. Particles can damage the plasma membrane, which is often observed with cationic particles [40,41]. Polyethylene nanoparticles have been found to penetrate the hydrophobic milieu of the bilayer of the plasma membrane and cause structural changes [42]. Endocytosed particles can permeabilize the endosomal and lysosomal membrane and interact with intracellular organelles [43,44].
Reactive oxygen species (ROS) can be generated during plastic polymerization and particle processing and, upon interaction with the bioenvironment, cause cellular stress [45]. On the other hand, the direct or indirect impairment of DNA through the translocation of particles or ROS into the nucleus and damage to the DNA replication or repair mechanism may contribute to the genotoxicity of particles [46,47].
In mammalian cells, MNPs can cause nuclear membrane disruption, oxidative stress, the release of damage-associated molecular patterns, and the downstream activation of inflammatory and apoptotic and necrotic pathways [44,48].
The absorption of micro- or nanoplastics can lead to the loss of integrity of plasma, endosomal, and nuclear membranes, causing pore formation in membranes and the subsequent generation of ROS from mitochondria. Elevated levels of intracellular ROS can cause mitochondrial damage due to increased mitochondrial Ca2+, concomitant mitochondrial membrane depolarization, the release of pro-apoptotic factors from mitochondria, the reduction of ATP, the release of damage-associated molecular patterns (DAMPs) from mitochondria or other organelles, resulting in the production of pro-inflammatory cytokines, and, finally, the activation of cell death pathways, leading to apoptosis or necrosis [38].
3. MNPs and the Intestinal System
The main source of exposure to microplastic and nanoplastic particles is through the ingestion of food or water that is contaminated with these particles. Plastics inevitably find their way into the food chain and carry contaminants that can affect intestinal homeostasis. Studies have found the presence of microplastics and nanoplastics in many types of foods, including fruits, vegetables, marine products, livestock (such as chickens), and drinking water [49,50,51,52,53]. Other foods such as sugar, honey, beer, cow’s milk, and sea salt have also been found to contain microplastics [9,52,54,55,56]. These particles have even been found in the gastrointestinal contents of more than 220 different marine species, such as mussels, oysters, clams, and common shrimp, as well as in various seafood products [57,58,59].
The most commonly detected polymers in food and drinking water are polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), and polyethylene terephthalate (PET). Polyamide (PA), acrylic, or acrylic-related compounds, polyesters, and polymethyl methacrylate (PMMA) are also detected, but less frequently [60].
It is uncertain whether the ingestion of MNPs poses a significant risk to the intestinal system, given the conflicting data on human exposure and the biodistribution of these particles. It has been observed that human adults can potentially ingest up to 458,000 microplastic particles per annum through tap water and 3,569,000 microplastic particles per annum through bottled water [61]. However, there exists a considerable variation in the estimates of human exposure to microplastics due to differences in the type of plastic and experimental methodologies employed in various studies [9]. In a recent study, Schawabl et al. endeavored to estimate human contamination by measuring the amount of microplastics in the feces of eight healthy volunteers. The study established an average of 20 microplastic particles per 10 g of feces, ranging in size from 50 to 500 μm, and belonging to nine types of plastics, with PP and PET being the most prevalent [62,63].
The distribution of micro- and nanoplastics after ingestion is not well-understood. Due to the stability of plastic materials, enzymatic or chemical degradation is challenging, especially since mammalian intestines lack specialized enzymes for plastic degradation. This means that plastic particles are not significantly degraded during digestion. Larger microplastics (>150 μm) remain attached to the intestinal mucus layer, directly contacting the apical part of intestinal epithelial cells. Smaller particles (<150 μm), however, can cross the intestinal mucus layer. The uptake of micro- and nanoplastics depends on their size and occurs through various mechanisms, including transcytosis through microfold cells, endocytosis through enterocytes, persorption (which is the passage through crevices at the end of the villus, following the loss of enterocytes), and paracellular uptake [64,65,66,67].
When micro- and nanoplastics are ingested, most of them are excreted through feces [68,69], while a small portion stays in the intestine for several days [68]. In the gut, MNPs can cause damage and inflammation by entering the bloodstream, spreading to other tissues, and persisting for prolonged periods [70]. The bioavailability of MNPs after oral intake depends on intestinal translocation. In a study of three intestinal cell models [71], it was observed that the size and surface chemistry of the particles influenced translocation, with 50 nm nanoparticles having a higher translocation rate than 100 nm NPs. The translocation of MNPs is influenced by various factors, including the characteristics of plastic particles and animal behavior and development [72].
Despite the low rate of intestinal absorption, exposure to significant amounts of micro- and nanoparticles could lead to systemic toxicity, as their small size allows them to penetrate deep into organs. Specifically, a study by Walczak et al. investigating the impact of in vitro gastrointestinal digestion on the protein crown of PS-NPs revealed that, after digestion, translocation was 4 times higher for positively charged NPs and 80 and 1.7 times higher for two different types of negatively charged NPs. In vitro digestion also reduced the presence of higher-molecular-weight proteins, shifting the protein content of the corona toward lower-molecular-weight proteins [73].
Comprehensive studies of the 55 most widely used polymer types developed a model for ranking the hazard of each polymer, according to the monomer chemicals that formed the polymer. The most hazardous polymers were those produced from carcinogenic, mutagenic, or both monomers. Hazard classification data were mainly taken from Annex VI of the EU Classification, Labelling and Packaging (CLP) regulation which is based on the UN Globally Harmonized System (GHS). However, while this approach determined a high ranking for polyurethanes, epoxy resins, and polyvinylchloride and styrene polymers, no hazard classification was available for many of the listed substances, such as suspected endocrine disruptors, due to the lack of safety data [67,74].
3.1. Toxicity of Micro-/Nanoplastics in the Intestine
The effects of MNPs on the intestinal system and gut microbiota in mammals and humans, and the associated mechanisms, are still not fully understood. Figure 1 summarizes the main postulated toxicological effects of MNPs on the intestinal system.
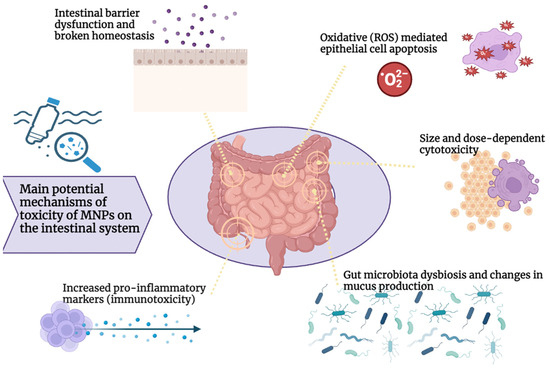
Figure 1.
Main potential mechanisms of MNP toxicity on the intestinal system.
Studies have shown that microparticles have a harmful impact on the intestines of invertebrates and vertebrates like fish. For instance, research conducted on Caenorhabditis elegans, Artemia parthenogenetica zooplankton larvae, and Eisenia fetida earthworm has revealed that intestinal oxidative damage is a significant mechanism in microplastic toxicity. Moreover, exposure to microparticles was found to be associated with the progression of cellular deformations and enterocyte decomposition [75,76,77]. Further evidence comes from studies that involved oral exposure to microplastics in aquatic vertebrates such as rainbow trout, juvenile intertidal fish Girella laevifrons, juvenile large yellow croaker Larimichthys crocea, or Oryzias melastigma. Overall, these studies have found that PS microbeads and nanoparticles cause a decrease in digestive enzyme activity (lipase, trypsin, and lysozyme) [78,79], and induce goblet cell enlargement and increased mucus secretion [78,80], the secretion of proinflammatory cytokines like Tumor Necrosis Factor α (TNF α), Interferon γ (IFN γ), and Interleukin-6 (IL-6) [78], leukocyte infiltration, hyperemia, and the loss of villi and crypt cells [81]. Interestingly, intestinal levels of oxidative stress enzymes were found to be modified in opposite ways between nano- and microparticles [80].
The toxic effects of microplastics on the gut have been assessed in several aquatic species, pointing out inflammation, genotoxicity, and oxidative stress responses [82]. Several studies have been conducted to investigate the effects of PS on the intestines of zebrafish. Exposure to PS beads resulted in an increase in the secretion of proinflammatory cytokines such as Interleukin-1α (IL1α), Interleukin-1β (IL1β), and IFN γ. This exposure also enhanced the activity of enzymes that respond to excessive oxidative stress. It was observed that exposure to PS was associated with reduced levels of antioxidant enzyme diamine oxidase and of D-lactate, which could indicate increased intestinal permeability [83]. Furthermore, a single-cell analysis revealed a dysfunction of intestinal cell populations, a decrease in the detoxification/antioxidant capacity of enterocytes, and a decrease in the cell chemotaxis of secretory cells.
It appears that the impact of microplastics on the intestinal epithelium depends not only on the size of the particles but also on their shape. In fact, when exposed to microplastic fibers, the volume of mucus in the intestine of zebrafish declined sharply. Additionally, both microplastic fibers and fragments led to a decrease in intestinal D-lactate, caused inflammation in the intestine, and increased the activity of superoxide dismutase [84]. Exposure to PVC induced a histological alteration in the intestine of European sea bass Dicentrarchus labrax L. [85], increasing the globet cell number, villus thickness, and expression of intestinal nuclear factor E2-related factor 2 (Nrf2). On the other side, exposure to irregularly shaped high-density PE and PS particles determined an epithelial detachment, increase in the neutrophil count, and decrease in the globet cell count in the intestine of zebrafish [86].
Notwithstanding the available evidence, the data from in vitro and in vivo studies in mammalian models are comparatively restricted and conflicting (Table 1).

Table 1.
Major studies investigating the potential effects of MNPs on the intestinal system in human cell lines and mammalian models.
In 2018, Abdelkhaliq et al. showed no cytotoxicity of polystyrene (PS) particles (50 nm and 200 nm) on Caco-2 cells at a concentration of 250 mg/mL for 10 to 120 min of exposure [87]. Accordingly, with 1 to 30 mg/mL, laser-ablated, approximately 100 nm PET particles, no impact on Caco-2 cell viability and no inflammation was measured up to 24 h of incubation [88]. Similarly, Hesler et al., in 2019, showed the absence of toxicity at a concentration below 100 mg/mL PS particles (between 40–52 nm and 457–477 nm) after 24 h of incubation [89]. A significant decrease of Caco-2 cell viability was only measured at very high concentrations of 4–10 µm PS particles (1 × 108 particles per mL) after 48 h of incubation. Furthermore, in investigating the effect of PS particles on the macrophage cell line THP-1, no effect on cell polarization was detected after particle exposure [91]. According to a recent study, when HRT-18 and CMT-93 epithelial cell lines were exposed to PS microparticles (with a diameter of 4.8–5.8 µm, a concentration of 1 mg/mL, and a time between 6 and 48 h), it resulted in a significant increase in cytotoxicity in both cell lines. However, only CMT-93 cells showed an increase in oxidative stress activity [92]. Moreover, after being tested at various concentrations for 48 h, polyethylene (PE) microplastics between 30 and 140 μm caused a significant reduction in Caco-2 cell viability at high concentrations (1000 mg/L) [90].
Notably, a recent comparative systematic analysis monitored the influence of small microplastics, of size 50–100 nm, on human colon cells and human colon organoids, and in vivo in a mouse model. According to the authors, the viability of colon organoids decreased by over 20% when exposed to concentrations of 5 mg/mL of MPs. This exposure also led to an increase in the expression of genes linked to inflammation, apoptosis, and immunity. Additionally, in vivo data from a murine model indicated that 50 nm MPs accumulated in several mouse organs, including the colon, after 7 days of exposure [98].
Several studies in mice exposed to PS microspheres have shown a transcriptional decrease in major genes related to mucin expression, such as mucin 1 (Muc1) and Klf4 [93,94], and to ion transport, such as cystic fibrosis transmembrane conductance regulator (Cftr), Na-K-2Cl cotransporter 1 (Nkcc1), Na+/H+ exchanger 3 (Nhe3), anoctamin 1 (Ano1), and solute carrier family 26 member 6 (Slc26a6) [93]. In a research study conducted on mouse models, it was found that exposure to a mixture of microplastics ranging from 1 µm to 10 µm in size, at a volume of 10 mL/kg, and for a total of one dose for three weeks, did not lead to any evidence of intestinal inflammation [91].
Accordingly, in a recent study, mice were fed with 5 μm pristine and fluorescent polystyrene MP for 6 weeks [93]. The results revealed that PS-MPs were observed in the intestine of mice, and reduced the intestinal mucus secretion, thus causing damage to the intestinal barrier function. Similarly, male mice exposed to polystyrene MP from 0.5 and 50 μm at 1000 μg/L for 5 weeks exhibited decreased intestinal mucus secretion following oral exposure [94]. On the other hand, when mice were exposed to different amounts of polyethylene microplastics, it led to histological inflammation in their colon and duodenum. Specifically, exposure to PE-MP (10–150 μm) at various concentrations (2, 20, and 200 μg/g for 5 weeks) resulted in the increased secretion of proinflammatory cytokines and higher levels of toll-like receptor 4 (TLR4), c-Jun, and interferon regulatory factor 5 [95].
Virgin PE spheres with a size between 45 and 53 μm and a concentration of 0.2 g/L (1.5 × 105 particles/L) after 30 days of exposure have been found to cause impaired intestinal permeability in mouse models [96]. Another study on mammals confirms that exposure to MNPs may cause adverse effects on the intestinal system. When exposed to PS-NPs and PS-MPs (50 nm, 500 nm, and 5000 nm at a concentration of 20 mL/kg body weight for 28 days), there was a combined exposure that caused intestinal barrier dysfunction by the apoptosis of epithelial cells through ROS production in the mouse model [97]
In terms of toxicity, a mention must be made of the ability of MPs to transport pollutants and plasticizers. In this review, considering the focus of this paper; we will only refer to pollutants and plasticizers having the greatest potential to harm the gut system.
Chemical compounds called plasticizers can expose humans through occupational exposure, product use, or transfer from plastic packaging [33,107]. Exposure to these compounds can occur through ingestion, inhalation, and skin contact [108]. Among the various plasticizers, phthalates (PAEs) are known to be harmful to human health according to several studies. This group of chemicals is a major concern as they have been identified as endocrine-metabolic disruptors, which can affect the reproductive system based on available evidence from human epidemiological studies [109]. Numerous reports have found high levels of phthalate contamination in drinking water and various foods, including meat, oil, fats, dairy products, and even infant formula [110,111,112]. This suggests that these substances can easily enter the food chain, and ingestion may be the primary route of exposure [113,114]. Research has shown that the ingestion of various PAEs can lead to different health problems, such as reproductive, hepatic, cardiac, and neurodevelopmental disorders [115,116,117,118].
Although little research exists on how phthalates directly affect the intestinal system, these harmful substances are commonly found in contaminated food and water, making it highly likely that they negatively impact the gastrointestinal tract and gut microbiota.
Exposure of female CD-1 mice to phthalates at doses ranging from 0.2 to 200 mg/kg for 10–14 days caused colonic damage and inflammation. This was due to the dysregulation of the tight junction gene (Zo-3), cell cycle regulatory gene (Ccnb1), and cytokine levels (sICAM-1 and TNF-α) [119]. Additionally, Xiong et al. (2020) and Fu et al. (2021) observed elevated serum lipopolysaccharides (LPS) levels in mice exposed to PAEs, indicating epithelial barrier disruption and intestinal permeability [117,120]. Similarly, Deng et al. (2020) also reported reduced serum diaminoxidase (DAO) activity in CD-1 mice exposed to PAEs, which is an important indicator of impaired intestinal function [96]. Lastly, recent evidence suggests that the gut microbiota, due to its complex interaction with the intestinal epithelium and barrier, may play a significant role in influencing the local and systemic toxicity of these molecules [121].
It is widely acknowledged that PAEs and other plasticizers, including Bisphenol A, have the potential to negatively impact human health. In light of this, the European Food Safety Authority (EFSA) has recently advised lowering the acceptable daily intake (TDI) of such substances to safer levels [122,123].
Micro- and nanoplastics can also act as vectors for toxic heavy metals or other pollutants that can be released [124] into the environment and lead to health risks [38].
One example is chromium (Cr), which has a greater potential to adsorb on microplastics than other heavy metals. Microplastics can carry 19–7970 ng of Cr per g of microplastics [124]. When Cr (IV) enters the body, it causes DNA damage in various tissues at high acute doses or with chronic oral exposure [125]. To study the effect of the ingestion of adsorbed Cr on microplastics, ~150 μ PE, PP, PVC, and PS-MP contaminated with Cr at concentrations commensurate with water Cr-MP levels were prepared [124]. Using an in vitro method to model the entire digestive system, the researchers found that Cr (IV) availability was high for PLA in the stomach, small intestine, and large intestine. However, the risk quotients for adults and children calculated from the bioavailability did not raise concerns about the carcinogenicity.
3.2. MNP Gut Immunological Impact
The immune system present in the intestine is continuously exposed to external antigens, which are derived from food and non-pathogenic micro-organisms that need to be tolerated immunologically. However, the intestinal immune system also needs to be prepared to respond to pathogenic micro-organisms and external toxins. This balance is maintained by the equilibrium between pro- and anti-inflammatory stimuli, which involves innate lymphocytes, myeloid cells, and T- and B-lymphocytes residing in the lamina propria of the gut epithelium and draining in the mesenteric lymph nodes [126].
After being exposed to MNPs, immune cells trigger a significant modulation at the transcriptional level, affecting enzyme levels and cytokine release. Several studies, both on invertebrates and vertebrates, revealed an immune-toxic effect caused by nano- and microplastics on the intestinal immune system.
Exposure to PS nanoparticles has been found to cause higher hemocyte counts in Daphnia magna, while also decreasing the total antioxidant capacity and increasing DNA damage in mussels [127,128]. Amino-modified PS nanoparticles, on the other hand, have been shown to induce hemocyte changes in mussels, depending on the duration of exposure [129,130]. Additionally, exposure to PS microbeads or nanoparticles has been found to increase the production of oxygen reactive and nitrogen species, result in higher hemocyte mortality, and modify several enzymes related to the immune system, such as acid phosphatase, alkaline phosphatase, lysozyme, and phenoloxidase, depending on the duration and dose of exposure [131,132,133,134,135]. Studies have also shown that PS nanoparticles cause more damage than PS microparticles [134,135].
Studies on vertebrates have revealed some interesting findings. Exposure to PS nanoparticles led to a dose-dependent increase in myeloperoxidase activity and the release of neutrophil extracellular traps in fathead minnows Pimephales promelas. Similarly, polycarbonate microplastics dose-dependently disrupted neutrophil functions [136]. Exposure to PE microparticles in carp impaired the activity of the complement system and immunity-related enzymes [3]. Furthermore, in zebrafish, exposure to PE and PS particles reduced the liver transcript levels of two immune genes, leukotriene B4 receptor (ltb4r) and interferon-induced transmembrane protein (ifitm1) [86]. Furthermore, microplastics in the gastrointestinal tract have been found to upregulate the expression of T-cell receptors β and δ (TCRβ and TCRδ) and IgM in the spleen of Scyliorhinus canicula [137].
Lehner et al. (2020) developed a 3D in vitro intestinal model comprising human intestinal epithelial cell lines Caco-2 and HT29-MTX-E12 to study the effects of ingested MPs such as the 50-MP polymer of 500 μm representing tire wear and polyolefins at the concentration of 823.5–1380.0 μg/cm2. Although the results showed some changes in the levels of inflammatory cytokines (IL-8, TNFα, and IL-1β) and barrier integrity, these changes were not significant [99]. In contrast, other forms of MPs, polypropylene MPs (50–500 µm), have been shown to induce immune responses by triggering the production of proinflammatory cytokines such as IL-6 and TNF α in a size- and concentration-dependent manner [100].
A study on mice models has shown that exposure to PE microparticles can cause changes in the levels of certain proteins such as IL1α and granulocyte colony-stimulating factor (G-CSF) in the blood, a decrease in the count of regulatory T-lymphocytes, and an increase in the proportion of Th17 cells in the spleen [95]. In this study, it was found that high concentrations (600 μg/day) of PE-MPs (10–150 μm) caused inflammatory reactions by increasing the expression of Toll-like receptor 4 (TLR4), Activator Protein 1 (AP-1), and Interferon regulatory factor 5 (IRF5). The exposure to MP also led to a significant increase in the serum level of IL-1α and a decrease in Th17 and Treg cells in CD4+ T cells [95]. Additionally, PE microplastic exposure (40–48 μm per dosing volume of 200 μL/day for 90 days) can lead to an increase in the number of blood neutrophils and immunoglobulin IgA levels in female mice and an alteration of spleen lymphocytes in both dams and offspring [101].
While there is evidence of the effects of MNPs on the immune system, most studies have focused solely on the innate immune response, and the impact of MNPs on the adaptive immune response remains unclear.
A recent study [138] found that there is a connection between microplastics (MPs) in feces and inflammatory bowel disease (IBD). The study discovered that the fecal concentration of MPs in IBD patients was significantly higher (41.8 items/g dm) than in healthy individuals (28.0 items/g dm), including 15 different types of MPs. Among the MPs found, polyethylene terephthalate (22.3–34.0%) and polyamide (8.9–12.4%) were the most dominant types. The researchers observed that the primary shapes of the detected MPs were sheets and fibers [138]. Additionally, the study showed that there is a positive correlation between the concentration of MPs and the activity level of IBD, suggesting that MP exposure may be related to the disease process, or that IBD could promote the retention of MPs.
Indeed, further recent evidence in mouse models confirms these suspicions. It was observed that PS-NPs aggravate inflammation and intestinal injury in mice with chronic colitis [102]. Specifically, mice subjected to sodium dextran sulfate (DSS) exposures were subsequently fed via gastric tube with water containing 100 nm polystyrene nanospheres (PS-NPs, at concentrations of 1 mg/kg, 5 mg/kg, and 25 mg/kg) for 28 consecutive days. The results showed that PS-NPs exacerbated intestinal inflammation by activating the MAPK signaling pathway and also aggravated inflammation and oxidative stress in mice with chronic colitis.
These findings show that the intestinal immune system is altered by exposure to microplastics; however, further studies, especially in species more closely related to humans, are warranted.
3.3. MNP Effects on Gut Microbiota
The human gut is home to numerous communities of micro-organisms, collectively referred to as the “gut microbiota”. This microbiota comprises over 250 species of viruses, fungi, bacteria, and archaea, and is a dynamic system that changes over the course of a human’s life. The relationship between the gut microbiota and the host is mutually beneficial, as the former plays a crucial role in several physiological and pathological pathways of human life [139]. Human gut microbiota is primarily composed of five bacterial phyla: Firmicutes (60% to 80%), Bacteroidetes (20% to 40%), Verrucomicrobia, Actinobacteria, and a lesser extent of Proteobacteria; and one Archea phyla, the Euryarchaeota [139]. The gut microbiota is a crucial component of the gut ecosystem that plays a vital role in human health. It helps in the formation and maturation of immunity, acts as a barrier against pathogens, facilitates the absorption of nutrients and drugs, and regulates metabolic intake [140]. When there is an imbalance in the gut microbiota, it can lead to various gastrointestinal and extraintestinal disorders [140]. As a result, several therapeutic approaches, such as fecal microbiota transplantation [141], are increasingly being investigated for the treatment of microbiome-based disorders.
There is a lack of data concerning the effects of MNPs on the gut microbiota in humans. However, studies conducted on mammals have shown that both short- and long-term exposure to MNPs can cause modifications in microbial communities. Dysbiosis, or an imbalance in the gut microbiota, is a common finding in murine mole studies, with reduced alpha- and beta-diversity, and a loss of resilience. This can lead to frequent outbreaks of pathogens and metabolic disorders, both locally and systemically [93,95]. Particularly, at the phylum level, exposure to PS particles caused changes in the abundance of Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria. At the genus level, variations in the abundance of Staphylococcus, Clostridium, and Bacteroides were observed when compared to animals that were not exposed to PS particles. In addition, up to 15 types of bacteria were affected by exposure to MPs, in particular, Bifidobacterium, Prevotella, Veillonella, Actinobacteria, and Ruminococcus.
Discrepancies were found regarding the abundance of Proteobacteria. In 2019, Lu et al. reported a decrease or increase in its abundance, while Jin et al. discovered a relative reduction in the abundance of Proteobacteria after PS microparticle exposure [93,94].
Conversely, in the same year, Luo et al. (2019) discovered that the Actinobacteria abundance increased while the abundance of Proteobacteria and Firmicutes remained unchanged in mice that were exposed to PS-MPs (0.5 µm and 5 µm) at a concentration of 100 µg/L and 1000 µg/L during their gestation and lactation period [103].
On the other hand, in 2021, Jiang et al. (2021) noted, following a 33-day period of the ingestion of 0.1 mg/kg MPs (5 µm), a shift in the relative abundance of bacterial taxa in mice models [104]. Specifically, there was a significant increase in the presence of Proteobacteria, while Bacteroides and Marvinbryantia exhibited a marked decrease. Additionally, Bifidobacterium also exhibited an increase. Qiao et al. confirmed mice exposed to PS-MNPs (70 nm, 5 μm in diameter) at a concentration between 2 mg and 0.2 mg kg−1 for 28 days experienced an increase in the relative abundance of Proteobacteria and Verrucomicrobia, while the major short-chain fatty acid (SCFA)-producing genera decreased in abundance [105].
In a study conducted by Liu et al. in 2022 [106], female mice were exposed to polyethylene terephthalate (PET) microplastics (ranging from 2 μm to 631 μm) at a concentration of 500 mg/kg for a period of 28 days. The study reported a decrease in the abundance of Bacteroidetes and an increase in the abundance of Firmicutes, which was accompanied by an increase in the abundance of Lactobacillus and a decrease in the abundance of Parabacteroides.
Two different studies focusing on PE microplastic exposure in mice, respectively, found an increase in the abundance of Firmicutes and Melainabacteria phyla and Staphylococcus genera, with a decrease in the abundance of Bacteroidetes phylum and Parabacteroides genera [95], and an increase in the abundance of the Actinobacteria phylum and Lactobacillus, Adlercreutzia, Butyricimonas, and Parabacteroides genera [96].
It is important to note that exposure to MNPs has been shown to reduce the abundance of bacteria that are known to promote tight junction functions. This reduction may have additional indirect toxic effects due to the dysbiosis of the gut microbiota [105].
Although plastic particles are inert to biodegradation due to their hydrophobic nature, high molecular weight, and long polymer chain, some micro-organisms ingest these polymers and convert them into environmentally friendly carbon compounds [142,143,144].
Polymer biodegradation is a process that occurs due to micro-organisms present in three domains of life, namely, Bacteria, Archaea, and Eukarya. Among the different kingdoms, fungi and bacteria are the most vital players in biodegradation processes in natural environments. The effectiveness of micro-organisms in degrading a specific type of plastic depends on the environmental conditions and the plastic typologies [145,146]. Arthrobacter, Bacillus, Micrococcus, Pseudomonas, Corynebacterium, Streptomyces, and Nocardia are the most commonly studied bacteria for their ability to degrade various types of plastics [147,148,149]. Besides free-living micro-organisms in the environment, the gut microbiota is an important driver of MNPs degradation, with most of the attention focused on insects and their larvae [150]. Indeed, several studies showed that MPs biodegradation does not occur after antibiotic treatment in mealworms, thus suggesting a crucial role played by the gut microbiota [151].
However, little is known about the microbial degradation capacity in mammals, probably due to the lack of appropriate high-resolution analytical methods to quantify small MPs and NPs and chemical intermediates in animal and human feces. Similarly, research regarding the microbial degradation of MPs and the human gut microbiota is still scarce; however, numerous plastic-degrading bacteria described in insects or larvae are part of the core of human gut microbiota, particularly, several potentially pathogenic Proteobacteria, such as Enterobacteriaceae, Enterococcaceae, Listeria, Pseudomonas, and Klebsiella, but also Lactococcus [152,153].
4. Conclusions
Annually, the global production of plastic waste amounts to millions of tons, a considerable quantity which disintegrates and accumulates in the form of minute particles that pollute and disseminate throughout terrestrial environments. Ingestion is a prevalent means of exposure of animals and humans to micro- and nanoplastics that can accumulate in the intestinal system to a degree and in a manner that remain incompletely understood.
Studies conducted in vitro on human cell lines have shown conflicting results regarding the toxicity of MNPs on the intestinal system. The discrepancies could be due to the different dosages of particles used in each study. Additionally, the various treatment periods and particle concentrations employed could also contribute to the conflicting nature of the findings. Furthermore, the studies cited only assess the short-term effects of MNPs on different endpoints, while possible long-term effects remain unexplored.
In contrast, studies conducted on mammals suggest that MNPs may have adverse effects in terms of intestinal cells toxicity, immunotoxicity, and dysbiosis. Nonetheless, the use of various study designs generates a degree of unclearness, and the absence of a definitive classification system for plastic waste based on parameters such as size, shape, and physical and chemical properties further complicates the issue. Additionally, the toxicological studies cited in this context do not account for the impact of realistic environmental exposure, nor do they consider the possible interactions between plastics and other pollutants.
Thus, we understand how these studies are not yet robust enough to determine their intestinal toxicity on mammals and humans with any degree of certainty. To gain a better understanding of the impact of MNPs ingestion on human gut health, it is essential to introduce validated and shared analytical methods. These arrangements will allow animal and cell studies to understand toxicological effects and will allow reference values to be generated to assess dietary intake and help stratify dietary risk. Observational and biomarker-based studies, on the other hand, will be able to help us unravel the real adverse effects of these particles on human gut health.
In conclusion, further studies and analytical methodologies are needed to characterize the real toxicological effects of MPNs on the intestinal human system and the precise role of the gut microbiota as a potential key player in this context.
Author Contributions
Conceptualization, C.C. and F.D.V.; methodology, C.C.; software, C.C.; validation, M.P and C.C.; formal analysis, C.C.; investigation, C.C.; resources, C.C. and F.D.V.; data curation, C.C.; writing—original draft preparation, C.C. and F.D.V.; writing—review and editing, C.C.; visualization, M.P.; supervision, G.C.; project administration, C.C and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hodge, P.; Hellwich, K.-H.; Hiorns, R.C.; Jones, R.G.; Kahovec, J.; Luscombe, C.K.; Purbrick, M.D.; Wilks, E.S. A Concise Guide to Polymer Nomenclature for Authors of Papers and Reports in Polymer Science and Technology (IUPAC Technical Report). Pure Appl. Chem. 2020, 92, 797–813. [Google Scholar] [CrossRef]
- Singh Jadaun, J.; Bansal, S.; Sonthalia, A.; Rai, A.K.; Singh, S.P. Biodegradation of Plastics for Sustainable Environment. Bioresour. Technol. 2022, 347, 126697. [Google Scholar] [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus Carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide Distribution and Abundance of Microplastic: How Dire Is the Situation? Waste Manag. Res. J. A Sustain. Circ. Econ. 2018, 36, 873–897. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Naegelen, I. Mikroplastik Als Gesundheitsgefahr Für Den Menschen. Biol. Unserer Zeit 2019, 49, 241–242. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–23. [Google Scholar]
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.B.C.; Gonçalves, R.V.; Lima, G.D.A.; Novaes, R.D. The Emerging Risk of Microplastics and Nanoplastics on the Microstructure and Function of Reproductive Organs in Mammals: A Systematic Review of Preclinical Evidence. Life Sci. 2022, 295, 120404. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic Sources, Formation, Toxicity and Remediation: A Review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the Soil Environment: Occurrence, Risks, Interactions and Fate—A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, Transport, and Accumulation of Different Types of Plastic Litter in Aquatic Environments: A Review Study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-Review of Microplastics in the Atmosphere and Their Risks to Humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and Potential Risk Assessment of Suspended Atmospheric Microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic Fallout in Different Indoor Environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne Microplastics Using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Anal. Health Toxicol. 2020, 35, e2020004. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The Emerging Risk of Exposure to Nano(Micro)Plastics on Endocrine Disturbance and Reproductive Toxicity: From a Hypothetical Scenario to a Global Public Health Challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Lim, X. Microplastics Are Everywhere—But Are They Harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- Wagner, S.; Reemtsma, T. Things We Know and Don’t Know about Nanoplastic in the Environment. Nat. Nanotechnol. 2019, 14, 300–301. [Google Scholar] [CrossRef]
- Guerrera, M.C.; Aragona, M.; Porcino, C.; Fazio, F.; Laurà, R.; Levanti, M.; Montalbano, G.; Germanà, G.; Abbate, F.; Germanà, A. Micro and Nano Plastics Distribution in Fish as Model Organisms: Histopathology, Blood Response and Bioaccumulation in Different Organs. Appl. Sci. 2021, 11, 5768. [Google Scholar] [CrossRef]
- Sökmen, T.Ö.; Sulukan, E.; Türkoğlu, M.; Baran, A.; Özkaraca, M.; Ceyhun, S.B. Polystyrene Nanoplastics (20 Nm) Are Able to Bioaccumulate and Cause Oxidative DNA Damages in the Brain Tissue of Zebrafish Embryo (Danio Rerio). Neurotoxicology 2020, 77, 51–59. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine Environment Microfiber Contamination: Global Patterns and the Diversity of Microparticle Origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic Microfibers in the Marine Environment: A Review on Their Occurrence in Seawater and Sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Woods, M.N.; Stack, M.E.; Fields, D.M.; Shaw, S.D.; Matrai, P.A. Microplastic Fiber Uptake, Ingestion, and Egestion Rates in the Blue Mussel (Mytilus Edulis). Mar. Pollut. Bull. 2018, 137, 638–645. [Google Scholar] [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic Fibers—Underestimated Threat to Aquatic Organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef]
- Banerjee, A.; Shelver, W.L. Micro- and Nanoplastic Induced Cellular Toxicity in Mammals: A Review. Sci. Total Environ. 2021, 755, 142518. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Ershov, D.; Islam, M.A.; Kämpfer, A.M.; Maslowska, K.A.; van der Gucht, J.; Alink, G.M.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M. Role of Membrane Disturbance and Oxidative Stress in the Mode of Action Underlying the Toxicity of Differently Charged Polystyrene Nanoparticles. RSC Adv. 2014, 4, 19321–19330. [Google Scholar] [CrossRef]
- Feng, L.-J.; Li, J.-W.; Xu, E.G.; Sun, X.-D.; Zhu, F.-P.; Ding, Z.; Tian, H.; Dong, S.-S.; Xia, P.-F.; Yuan, X.-Z. Short-Term Exposure to Positively Charged Polystyrene Nanoparticles Causes Oxidative Stress and Membrane Destruction in Cyanobacteria. Environ. Sci. Nano 2019, 6, 3072–3079. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 5577–5591. [Google Scholar] [CrossRef]
- Hollóczki, O.; Gehrke, S. Can Nanoplastics Alter Cell Membranes? ChemPhysChem 2020, 21, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Salvati, A.; Boya, P. Lysosome-Dependent Cell Death and Deregulated Autophagy Induced by Amine-Modified Polystyrene Nanoparticles. Open Biol. 2018, 8, 170271. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Valiyaveettil, S.; Tang, B. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public. Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Rubio, L.; Marcos, R.; Hernández, A. Potential Adverse Health Effects of Ingested Micro- and Nanoplastics on Humans. Lessons Learned from in Vivo and in Vitro Mammalian Models. J. Toxicol. Environ. Health Part B 2020, 23, 51–68. [Google Scholar] [CrossRef]
- Zheng, T.; Yuan, D.; Liu, C. Molecular Toxicity of Nanoplastics Involving in Oxidative Stress and Desoxyribonucleic Acid Damage. J. Mol. Recognit. 2019, 32, e2804. [Google Scholar] [CrossRef]
- Liu, X.; Tian, X.; Xu, X.; Lu, J. Design of a Phosphinate-Based Bioluminescent Probe for Superoxide Radical Anion Imaging in Living Cells. Luminescence 2018, 33, 1101–1106. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential Toxicity of Polystyrene Microplastic Particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and Nanoplastics—Current State of Knowledge with the Focus on Oral Uptake and Toxicity. Nanoscale Adv. 2020, 2, 4350–4367. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Synthetic Particles as Contaminants in German Beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, N.; Wang, M. Effects of Microplastics on Marine Copepods. Ecotoxicol. Environ. Saf. 2021, 217, 112243. [Google Scholar] [CrossRef]
- Hasan, J.; Islam, S.M.M.; Alam, M.S.; Johnson, D.; Belton, B.; Hossain, M.A.R.; Shahjahan, M. Presence of Microplastics in Two Common Dried Marine Fish Species from Bangladesh. Mar. Pollut. Bull. 2022, 176, 113430. [Google Scholar] [CrossRef]
- Ahmadi, A.; Moore, F.; Keshavarzi, B.; Soltani, N.; Sorooshian, A. Potentially Toxic Elements and Microplastics in Muscle Tissues of Different Marine Species from the Persian Gulf: Levels, Associated Risks, and Trophic Transfer. Mar. Pollut. Bull. 2022, 175, 113283. [Google Scholar] [CrossRef]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in Drinking Water Treatment—Current Knowledge and Research Needs. Sci. Total Environ. 2019, 667, 730–740. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Drinking Water: A Systematic Review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, L.W.D.; van der Zande, M.; Koelmans, A.A.; Hoogenboom, R.L.A.P.; Peters, R.J.B.; Groot, M.J.; Peijnenburg, A.A.C.M.; Weesepoel, Y.J.A. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods 2020, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and Fate of Dietary Nanoparticles and Microparticles in the Gastrointestinal Tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Coméra, C.; Cartier, C.; Gaultier, E.; Catrice, O.; Panouille, Q.; El Hamdi, S.; Tirez, K.; Nelissen, I.; Théodorou, V.; Houdeau, E. Jejunal Villus Absorption and Paracellular Tight Junction Permeability Are Major Routes for Early Intestinal Uptake of Food-Grade TiO2 Particles: An in Vivo and Ex Vivo Study in Mice. Part. Fibre Toxicol. 2020, 17, 26. [Google Scholar] [CrossRef]
- Smith, M.; Thomas, N.; Jenkins, P.; Miller, N.; Cremaschi, D.; Porta, C. Selective Transport of Microparticles across Peyer’s Patch Follicle-associated M Cells from Mice and Rats. Exp. Physiol. 1995, 80, 735–743. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro- and Nano-Plastics and Human Health. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 343–366. [Google Scholar]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Ma, C.; Li, L.; Chen, Q.; Lee, J.-S.; Gong, J.; Shi, H. Application of Internal Persistent Fluorescent Fibers in Tracking Microplastics in Vivo Processes in Aquatic Organisms. J. Hazard. Mater. 2021, 401, 123336. [Google Scholar] [CrossRef]
- Volkheimer, G. Hematogenous Dissemination of Ingested Polyvinyl Chloride Particles. Ann. N. Y. Acad. Sci. 1975, 246, 164–171. [Google Scholar] [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Tromp, P.; Helsper, J.P.F.G.; van der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. Translocation of Differently Sized and Charged Polystyrene Nanoparticles in in Vitro Intestinal Cell Models of Increasing Complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, Y.; Wang, Y.; Zhang, D.; Pan, X. Transfer of Micro(Nano)Plastics in Animals: A Mini-Review and Future Research Recommendation. J. Hazard. Mater. Adv. 2022, 7, 100101. [Google Scholar] [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Helsdingen, R.; van der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. In Vitro Gastrointestinal Digestion Increases the Translocation of Polystyrene Nanoparticles in an in Vitro Intestinal Co-Culture Model. Nanotoxicology 2015, 9, 886–894. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic Particles Cause Intestinal Damage and Other Adverse Effects in Zebrafish Danio Rerio and Nematode Caenorhabditis Elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Zhang, M.; Ding, G.; Sun, J.; Du, M.; Liu, Q.; Cong, Y.; Jin, F.; Zhang, W.; et al. The Uptake and Elimination of Polystyrene Microplastics by the Brine Shrimp, Artemia Parthenogenetica, and Its Impact on Its Feeding Behavior and Intestinal Histology. Chemosphere 2019, 234, 123–131. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological Effects of Polystyrene Microplastics on Earthworm (Eisenia Fetida). Environ. Pollut. 2020, 259, 113896. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-N.; Wen, B.; Zhu, J.-G.; Zhang, Y.-S.; Gao, J.-Z.; Chen, Z.-Z. Exposure to Microplastics Impairs Digestive Performance, Stimulates Immune Response and Induces Microbiota Dysbiosis in the Gut of Juvenile Guppy (Poecilia Reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef]
- Gu, H.; Wang, S.; Wang, X.; Yu, X.; Hu, M.; Huang, W.; Wang, Y. Nanoplastics Impair the Intestinal Health of the Juvenile Large Yellow Croaker Larimichthys Crocea. J. Hazard. Mater. 2020, 397, 122773. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-M.; Byeon, E.; Jeong, H.; Kim, M.-S.; Chen, Q.; Lee, J.-S. Different Effects of Nano- and Microplastics on Oxidative Status and Gut Microbiota in the Marine Medaka Oryzias Melastigma. J. Hazard. Mater. 2021, 405, 124207. [Google Scholar] [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galbán-Malagón, C. Microplastic Ingestion Cause Intestinal Lesions in the Intertidal Fish Girella Laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal Alterations in European Sea Bass Dicentrarchus Labrax (Linnaeus, 1758) Exposed to Microplastics: Preliminary Results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics Induce Transcriptional Changes, Immune Response and Behavioral Alterations in Adult Zebrafish. Sci. Rep. 2019, 9, 15775. [Google Scholar] [CrossRef]
- Abdelkhaliq, A.; van der Zande, M.; Punt, A.; Helsdingen, R.; Boeren, S.; Vervoort, J.J.M.; Rietjens, I.M.C.M.; Bouwmeester, H. Impact of Nanoparticle Surface Functionalization on the Protein Corona and Cellular Adhesion, Uptake and Transport. J. Nanobiotechnology 2018, 16, 70. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models in Vitro. Toxicol. Vitr. 2019, 61, 104610. [Google Scholar] [CrossRef]
- Huang, W.; Yin, H.; Yang, Y.; Jin, L.; Lu, G.; Dang, Z. Influence of the Co-Exposure of Microplastics and Tetrabromobisphenol A on Human Gut: Simulation in Vitro with Human Cell Caco-2 and Gut Microbiota. Sci. Total Environ. 2021, 778, 146264. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Mattioda, V.; Benedetti, V.; Tessarolo, C.; Oberto, F.; Favole, A.; Gallo, M.; Martelli, W.; Crescio, M.I.; Berio, E.; Masoero, L.; et al. Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines. Biomolecules 2023, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene Microplastics Affect the Distribution of Gut Microbiota and Inflammation Development in Mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics Release Phthalate Esters and Cause Aggravated Adverse Effects in the Mouse Gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Jung, W.H.; Choi, K.J.; Koh, B.; Kim, K.Y. A Comparative Systematic Analysis of The Influence of Microplastics on Colon Cells, Mouse and Colon Organoids. Tissue Eng. Regen. Med. 2023, 20, 49–58. [Google Scholar] [CrossRef]
- Lehner, R.; Wohlleben, W.; Septiadi, D.; Landsiedel, R.; Petri-Fink, A.; Rothen-Rutishauser, B. A Novel 3D Intestine Barrier Model to Study the Immune Response upon Exposure to Microplastics. Arch. Toxicol. 2020, 94, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An Assessment of the Toxicity of Polypropylene Microplastics in Human Derived Cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Park, E.-J.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the next Generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Ma, J.; Wan, Y.; Song, L.; Wang, L.; Wang, H.; Li, Y.; Huang, D. Polystyrene Nanobeads Exacerbate Chronic Colitis in Mice Involving in Oxidative Stress and Hepatic Lipid Metabolism. Part. Fibre Toxicol. 2023, 20, 49. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Jiang, P.; Yuan, G.; Jiang, B.; Zhang, J.; Wang, Y.; Lv, H.; Zhang, Z.; Wu, J.; Wu, Q.; Li, L. Effects of Microplastics (MPs) and Tributyltin (TBT) Alone and in Combination on Bile Acids and Gut Microbiota Crosstalk in Mice. Ecotoxicol. Environ. Saf. 2021, 220, 112345. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lv, M.; Sun, A.; Ding, J.; Wang, Y.; Chang, X.; Chen, L. Exposure to Microplastics Reduces the Bioaccumulation of Sulfamethoxazole but Enhances Its Effects on Gut Microbiota and the Antibiotic Resistome of Mice. Chemosphere 2022, 294, 133810. [Google Scholar] [CrossRef]
- Montano, D. Chemical and Biological Work-Related Risks across Occupations in Europe: A Review. J. Occup. Med. Toxicol. 2014, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Lyche, L. Chapter 48—Phthalates. In Reproductive and Developmental Toxicology; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 637–655. ISBN 9780123820327. [Google Scholar] [CrossRef]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human Health Impacts of Exposure to Phthalate Plasticizers: An Overview of Reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef]
- Cirillo, T.; Latini, G.; Castaldi, M.A.; Dipaola, L.; Fasano, E.; Esposito, F.; Scognamiglio, G.; Francesco, F.D.; Cobellis, L. Exposure to Di-2-Ethylhexyl Phthalate, Di-N-Butyl Phthalate and Bisphenol A through Infant Formulas. J. Agric. Food Chem. 2015, 63, 3303–3310. [Google Scholar] [CrossRef]
- Kıralan, S.S.; Toptancı, İ.; Öncül Abacıgil, T.; Ramadan, M.F. Phthalates Levels in Olive Oils and Olive Pomace Oils Marketed in Turkey. Food Addit. Contam. Part A 2020, 37, 1332–1338. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and Diet: A Review of the Food Monitoring and Epidemiology Data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Guo, J.-L.; Xue, J.; Bai, C.-L.; Guo, Y. Phthalate Metabolites: Characterization, Toxicities, Global Distribution, and Exposure Assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef]
- Polinski, K.J.; Dabelea, D.; Hamman, R.F.; Adgate, J.L.; Calafat, A.M.; Ye, X.; Starling, A.P. Distribution and Predictors of Urinary Concentrations of Phthalate Metabolites and Phenols among Pregnant Women in the Healthy Start Study. Environ. Res. 2018, 162, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, Q.; Tian, P.; Wang, L.; Li, X.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Gut Microbiota Dysbiosis Might Be Responsible to Different Toxicity Caused by Di-(2-Ethylhexyl) Phthalate Exposure in Murine Rodents. Environ. Pollut. 2020, 261, 114164. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, Z.; Zheng, Z.; Han, J.; Yang, W.; Lu, R.; Lin, W.; Zheng, Y.; Nie, D.; Chen, G. DEHP Induce Cholesterol Imbalance via Disturbing Bile Acid Metabolism by Altering the Composition of Gut Microbiota in Rats. Chemosphere 2021, 263, 127959. [Google Scholar] [CrossRef]
- Fu, X.; Han, H.; Li, Y.; Xu, B.; Dai, W.; Zhang, Y.; Zhou, F.; Ma, H.; Pei, X. Di-(2-ethylhexyl) Phthalate Exposure Induces Female Reproductive Toxicity and Alters the Intestinal Microbiota Community Structure and Fecal Metabolite Profile in Mice. Environ. Toxicol. 2021, 36, 1226–1242. [Google Scholar] [CrossRef]
- Lei, M.; Menon, R.; Manteiga, S.; Alden, N.; Hunt, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Chiu, K.; Bashir, S.T.; Nowak, R.A.; Mei, W.; Flaws, J.A. Subacute Exposure to Di-Isononyl Phthalate Alters the Morphology, Endocrine Function, and Immune System in the Colon of Adult Female Mice. Sci. Rep. 2020, 10, 18788. [Google Scholar] [CrossRef]
- Xiong, Z.E.; Zeng, Y.; Zhou, J.; Shu, R.; Xie, X.; Fu, Z. Exposure to Dibutyl Phthalate Impairs Lipid Metabolism and Causes Inflammation via Disturbing Microbiota-Related Gut—Liver Axis. Acta Biochim. Biophys. Sin. 2020, 52, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.P.; Saravanan, C. An Insight into the Critical Role of Gut Microbiota in Triggering the Phthalate-Induced Toxicity and Its Mitigation Using Probiotics. Sci. Total Environ. 2023, 904, 166889. [Google Scholar] [CrossRef]
- Dekant, W. Grouping of Phthalates for Risk Characterization of Human Exposures. Toxicol. Lett. 2020, 330, 1–6. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Birnbaum, L.S.; Collins, T.J.; Heindel, J.; Hunt, P.A.; Iguchi, T.; Kortenkamp, A.; Myers, J.P.; Vom Saal, F.S.; Sonnenschein, C.; et al. European Medicines Agency Conflicts With the European Food Safety Authority (EFSA) on Bisphenol A Regulation. J. Endocr. Soc. 2023, 7, bvad107. [Google Scholar] [CrossRef]
- Liao, Y.; Yang, J. Microplastic Serves as a Potential Vector for Cr in an In-Vitro Human Digestive Model. Sci. Total Environ. 2020, 703, 134805. [Google Scholar] [CrossRef] [PubMed]
- Nickens, K.P.; Patierno, S.R.; Ceryak, S. Chromium Genotoxicity: A Double-Edged Sword. Chem. Biol. Interact. 2010, 188, 276–288. [Google Scholar] [CrossRef]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut Permeability and Mucosal Inflammation: Bad, Good or Context Dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.E.; Brunner, F.S.; Plaistow, S.J. Temperature and Clone-Dependent Effects of Microplastics on Immunity and Life History in Daphnia Magna. Environ. Pollut. 2019, 255, 113178. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of Nanoplastics on Mytilus Galloprovincialis after Individual and Combined Exposure with Carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Auguste, M.; Balbi, T.; Ciacci, C.; Canonico, B.; Papa, S.; Borello, A.; Vezzulli, L.; Canesi, L. Shift in Immune Parameters After Repeated Exposure to Nanoplastics in the Marine Bivalve Mytilus. Front. Immunol. 2020, 11, 503705. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Balbi, T.; Pallavicini, A.; Vezzulli, L.; Canesi, L. Impact of Nanoplastics on Hemolymph Immune Parameters and Microbiota Composition in Mytilus Galloprovincialis. Mar. Environ. Res. 2020, 159, 105017. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of Marine Mussels Mytilus Spp. to Polystyrene Microplastics: Toxicity and Influence on Fluoranthene Bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Murano, C.; Agnisola, C.; Caramiello, D.; Castellano, I.; Casotti, R.; Corsi, I.; Palumbo, A. How Sea Urchins Face Microplastics: Uptake, Tissue Distribution and Immune System Response. Environ. Pollut. 2020, 264, 114685. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of Microplastics on the Innate Immunity and Intestinal Microflora of Juvenile Eriocheir Sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef]
- Shi, W.; Han, Y.; Sun, S.; Tang, Y.; Zhou, W.; Du, X.; Liu, G. Immunotoxicities of Microplastics and Sertraline, Alone and in Combination, to a Bivalve Species: Size-Dependent Interaction and Potential Toxication Mechanism. J. Hazard. Mater. 2020, 396, 122603. [Google Scholar] [CrossRef]
- Tang, Y.; Rong, J.; Guan, X.; Zha, S.; Shi, W.; Han, Y.; Du, X.; Wu, F.; Huang, W.; Liu, G. Immunotoxicity of Microplastics and Two Persistent Organic Pollutants Alone or in Combination to a Bivalve Species. Environ. Pollut. 2020, 258, 113845. [Google Scholar] [CrossRef]
- Greven, A.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus Canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of Plastics and Plastic-Degrading Bacteria in Cold Marine Habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef]
- Yeom, S.-J.; Le, T.-K.; Yun, C.-H. P450-Driven Plastic-Degrading Synthetic Bacteria. Trends Biotechnol. 2022, 40, 166–179. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. Phylogenetic Distribution of Plastic-Degrading Microorganisms. mSystems 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- García-Depraect, O.; Bordel, S.; Lebrero, R.; Santos-Beneit, F.; Börner, R.A.; Börner, T.; Muñoz, R. Inspired by Nature: Microbial Production, Degradation and Valorization of Biodegradable Bioplastics for Life-Cycle-Engineered Products. Biotechnol. Adv. 2021, 53, 107772. [Google Scholar] [CrossRef]
- Maity, W.; Maity, S.; Bera, S.; Roy, A. Emerging Roles of PETase and MHETase in the Biodegradation of Plastic Wastes. Appl. Biochem. Biotechnol. 2021, 193, 2699–2716. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic Biodegradation: Frontline Microbes and Their Enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef]
- Jacquin, J.; Cheng, J.; Odobel, C.; Pandin, C.; Conan, P.; Pujo-Pay, M.; Barbe, V.; Meistertzheim, A.-L.; Ghiglione, J.-F. Microbial Ecotoxicology of Marine Plastic Debris: A Review on Colonization and Biodegradation by the “Plastisphere”. Front. Microbiol. 2019, 10, 424560. [Google Scholar] [CrossRef]
- Lear, G.; Kingsbury, J.M.; Franchini, S.; Gambarini, V.; Maday, S.D.M.; Wallbank, J.A.; Weaver, L.; Pantos, O. Plastics and the Microbiome: Impacts and Solutions. Environ. Microbiome 2021, 16, 2. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of Polyethylene Microplastic Particles by the Fungus Aspergillus Flavus from the Guts of Wax Moth Galleria Mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Plaza Oñate, F.; Le Chatelier, E.; Almeida, M.; Cervino, A.C.L.; Gauthier, F.; Magoulès, F.; Ehrlich, S.D.; Pichaud, M. MSPminer: Abundance-Based Reconstitution of Microbial Pan-Genomes from Shotgun Metagenomic Data. Bioinformatics 2019, 35, 1544–1552. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).