Abstract
Angelica sinensis is a “low-temperature and long-day” perennial plant that produces bioactive compounds such as phthalides, organic acids, and polysaccharides for various types of clinical agents, including those with cardio-cerebrovascular, hepatoprotective, and immunomodulatory effects. To date, the regulatory mechanism of flowering under the photoperiod has been revealed, while the regulatory network of flowering genes during vernalization, especially in the role of lncRNAs, has yet to be identified. Here, lncRNAs associated with flowering were identified based on the full-length transcriptomic analysis of A. sinensis at vernalization and freezing temperatures, and the coexpressed mRNAs of lncRNAs were validated by qRT-PCR. We obtained a total of 2327 lncRNAs after assessing the protein-coding potential of coexpressed mRNAs, with 607 lncRNAs aligned against the TAIR database of model plant Arabidopsis, 345 lncRNAs identified, and 272 lncRNAs characterized on the SwissProt database. Based on the biological functions of coexpressed mRNAs, the 272 lncRNAs were divided into six categories: (1) chromatin, DNA/RNA and protein modification; (2) flowering; (3) stress response; (4) metabolism; (5) bio-signaling; and (6) energy and transport. The differential expression levels of representatively coexpressed mRNAs were almost consistent with the flowering of A. sinensis. It can be concluded that the flowering of A. sinensis is positively or negatively regulated by lncRNAs, which provides new insights into the regulation mechanism of the flowering of A. sinensis.
1. Introduction
Angelica sinensis (Oliv.) Diels is a “low-temperature and long-day” perennial plant that is native to Gansu Province, Northwest China [1,2]. The roots have been used as a traditional Chinese medicine for over 2000 years [3,4]. Currently, the roots are also applied in cardio-cerebrovascular, hepatoprotective, and immunomodulatory clinical agents, largely relying on bioactive compounds such as phthalides, organic acids, and polysaccharides [5,6,7].
Recently, the planting area of A. sinensis has exceeded 40,000 ha to satisfy the increasing demand; however, a higher rate (>40%) of early bolting and flowering (EBF) in commercial cultivation increases the lignified rate of roots and decreases the yield accordingly [8,9]. In order to inhibit the EBF, effective measures that have been taken include selecting excellent germplasm resources with a lower rate of EBF [8], controlling the seedling size (0.4 to 0.6 cm) to delay the transition from vegetative to reproductive growth [10], storing seedlings below freezing temperatures (−3 to −10 °C) to avoid vernalization (0 to 10 °C) [2,11,12], and shading the plants with sunshade nets (40% to 60%) to avoid the long-day conditions during the adult stages [13].
Regarding the regulatory mechanism of flowering in A. sinensis, key genes and the regulatory network during the photoperiod have been identified and mapped based on transcriptomic analysis. Specifically, 13 genes associated with the photoperiod, vernalization, sucrose, and GA pathways were identified from plants at the vegetative stage compared with the EBF stage [14]; 38 genes associated with the photoperiod, carbohydrates, hormone signaling, and floral development were identified from different development stages [15]; and 40 genes associated with the photoperiod, sucrose, GA, and floral development were identified from the EBF compared with Un-EBF [16]. In summary, key genes such as FLC, SOC1, FT, PHYA, AP1, and GA2OX1 were identified during the transition from the vegetative to the reproductive stage [14,15,16]. To date, physiological changes in the levels of carbohydrates, proteins, and hormones during vernalization have been investigated [17,18], while the regulatory network of flowering has yet to be identified.
Generally, ncRNAs include two categories, housekeeping and regulatory ncRNAs, and the latter can be further divided into sRNAs (i.e., miRNA, siRNA, and piRNA) and lncRNAs (>200 nucleotides) [19]. Both miRNAs and lncRNAs can influence plant developmental processes and stress responses [20], with the former being negative regulators functioning as specificity determinants, or guides, within complexes that promote the degradation of mRNA targets, and the latter acting either as precursors of miRNAs or endogenous target mimics (TMs), which mimic the real targets of miRNAs, thus rendering the corresponding miRNAs ineffective [21]. For example, previous studies on resistance against leaf rust in wheat found that 50 miRNAs and 1178 lncRNAs were identified and 49 lncRNAs were found to be the targets for miRNAs, with 1 lncRNA acting as a precursor of 2 miRNAs, and 3 lncRNAs acting as TMs [22].
Extensive investigations have demonstrated that lncRNAs regulate their downstream targets’ expression through the changing of epigenetic modification at the level of transcription and post-transcription by interacting with DNA, RNA, and proteins; thus, they are involved in various biological processes [23,24,25]. In this study, lncRNAs associated with flowering were identified based on the transcriptomic analysis of A. sinensis seedlings treated at vernalization and freezing temperatures (avoiding vernalization). We found that 272 lncRNAs directly or indirectly participate in regulating the flowering of A. sinensis and stress responses.
2. Materials and Methods
2.1. Plant Material
The seedlings (root–shoulder diameter 0.4–0.5 cm; Figure S1) of Angelica sinensis (cultivar Mingui 1) were stored at 0 (vernalization temperature) and −3 °C (freezing temperature), respectively. After storage at 0 °C for 14 (T1) and 60 days (T2), as well as at −3 °C for 125 days (T3), the shoot apical meristem (SAM) was cut from the root shoulder of the seedlings for transcriptomic analysis and qRT-PCR validation. Three biological replicates were performed for each treatment of T1, T2, and T3. Herein, the treatment of T1, T2, and T3 represents uncompleted, completed, and avoided vernalization, respectively, based on the EBF rate (Figure S2) when the stored seedlings were cultivated and grown in a long-day condition.
2.2. Full-Length Isoform Sequencing and Analysis
Total RNA of the SAM samples was extracted using Trizol reagent (Omega Bio-Tek, Norcross, GA, USA). The integrity of the RNA was determined using an Agilent 2100 Bioanalyzer (Agilent Technol., California, CA, USA) and agarose gel electrophoresis, and the purity and concentration of the RNA were determined using a microspectrophotometer (NanoDrop Technol., Wilmington, DE, USA). The high-quality RNAs were sequenced on a Pacific Biosciences Sequel platform (Gene Denovo Biotechnology Co., Ltd., Guangzhou, China). Raw reads of cDNA library were analyzed using a SMRT Link (V8.0.0) [26]. Briefly, high-quality CCS were extracted from the subreads BAM file; the integrity of transcripts (full-length sequences) was judged based on whether CCS reads contained primers (5′ and 3’) and polyAs; then, FLNC reads were generated by removing primers, barcodes, and polyAs; finally, FLNC reads were assembled to obtain the entire isoform [27].
2.3. Analysis of Long Noncoding RNAs (lncRNAs)
Isoforms that were not annotated against the four databases—NR, Swiss-Prot, Kyoto KEGG, and KOG—were used for the analysis of lncRNAs. The isoform that was assessed as a noncoding transcript by both CNCI and CPC software was finally confirmed as a lncRNA [28,29].
2.4. Characterization of LncRNAs
To date, the genome of A. sinensis has not been sequenced. Thus, the lncRNA analysis of A. sinensis was performed via a BLAST search with an E-value cut-off of ≤1 × 10−5 against the known lncRNAs from the TAIR database (https://www.arabidopsis.org accessed on 30 March 2022) [30]. The function of lncRNAs was annotated based on their coexpression mRNAs [31,32,33]. Herein, the biological functions of the coexpressed mRNAs were searched on the UniProt database (https://www.uniprot.org accessed on 30 March 2022).
2.5. qRT-PCR Validation
Based on the coding sequences (CDS) of coexpressed mRNA of lncRNA, 49 primer sequences of representatively coexpressed mRNAs (Table 1) were designed using the NCBI primer-blast tool. First-strand cDNA synthesis and qRT-PCR reaction were carried out using SuperRealPreMix Plus (FP205; Tiangen Biotech., Beijing, China) according to the manufacturer’s instructions; specifically, the cDNA was synthesized successively with one cycle (95 °C, 15 min) and 40 cycles (95 °C, 10 s; 60 °C, 20 s; and 72 °C, 30 s), and the qRT-PCR reaction was incubated successively at 95 °C for 15 s, 60 °C for 1 min and 95 °C for 1 s. The Actin (ACT) gene was used as a reference control gene with forward: TGGTATTGTGCTGGATTCTGGT and reverse: TGAGATCACCACCAGCAAGG (amplicon size 109 bp) [34]. Herein, the cycle threshold (Ct) values and standard curves of the ACT gene at different volumes (0.25, 0.5, 1.0, 1.5, 2.0, and 3.0 μL) was built to correct the gene expression level (Figure S3 and Figure S4), and the expression levels of the 49 candidate genes and their standard deviations for every variant were added to the Supplementary Materials (Table S1). The REL of coexpressed mRNAs was calculated using the 2−△△Ct method [35] according to the following formula.
△CtTest gene = CtTest gene − CtReference gene
△CtTarget gene = CtTarget gene − CtReference gene
−△△Ct(T2 vs. T1) = −(△CtT2 − △CtT1)
−△△Ct(T3 vs. T1) = −(△CtT3 − △CtT1)

Table 1.
Primer sequences used in qRT-PCR validation.
Relative expression level (REL) = 2−△△Ct.
2.6. Statistical Analysis
In order to obtain the precise estimation of PCR efficiency, each experiment for qRT-PCR validation was performed with three biological replicates, along with three technical replicates [36]. A t-test in SPSS 22.0 was performed for independent experiments, with p < 0.05 as the basis for statistical differences.
3. Results
3.1. LncRNAs Analysis
In total, 2327 lncRNAs were obtained after assessing the protein-coding potential of coexpressed mRNAs based on the two software programs, CNCI and CPC (Figure 1A), with 607 genes aligned against the known lncRNAs from the TAIR database of model plant Arabidopsis (Figure 1B), and 345 lncRNAs with coexpressed mRNAs of A. sinensis identified (Figure 1C) based on the SwissProt database. Based on the biological functions, the 272 characterized lncRNAs (Figure 1D) were divided into six categories: chromatin, DNA/RNA and protein modification (29); flowering (36); stress response (24); metabolism (117); biosignaling (23), and energy and transport (43) (Figure 1E). The base sequences of the 272 lncRNAs are shown in Table S2.
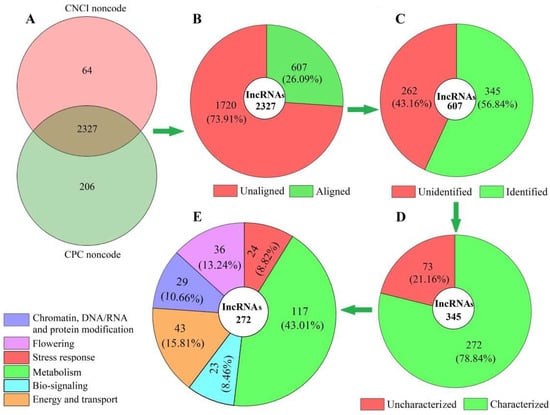
Figure 1.
Distribution and classification of lncRNAs in Angelica sinensis during vernalization, based on the biological functions of coexpressed mRNAs. Abbreviations: CNCI, coding–noncoding index; CPC, coding potential calculator. Images (A), (B), (C), (D) and (E) represent total, aligned, identified, characterized and classified lncRNAs, respectively.
3.2. LncRNAs Linked with Chromatin, DNA/RNA and Protein Modification, as well as Expression Levels of Their Coexpressed mRNAs
Based on the biological functions of coexpressed mRNAs, 29 lncRNAs were linked with chromatin (HMGB2 and HMGB3), DNA/RNA (At1g05910, RID2 and At4g26600) and protein modification (H2AV, At2g28720, HTR2, HOS15, REF6, SKP1A, ASK21, SRK2G, SRK2H, DET1, BOPAt4g295601, At1g45180, At3g50840, At2g36630, At3g47890, UBP7, DER2.1, GRP3, MDH9.13, At3g24715, At3g16560, At3g62260, ESMD1, and At1g27930) (Table 2). The expression levels of 14 representative coexpressed mRNAs were confirmed by qRT-PCR, with 3 mRNAs (HMGB2, HMGB3 and At1g05910) showing down-regulation at T2 versus (vs.) T1, and 11 mRNAs showing lower levels at T2 vs. T1 than T3 vs. T1, with the exception of 2 mRNAs (H2AV and ASK21) (Figure 2).

Table 2.
Twenty-nine lncRNAs linked with chromatin, DNA/RNA, and protein modification.
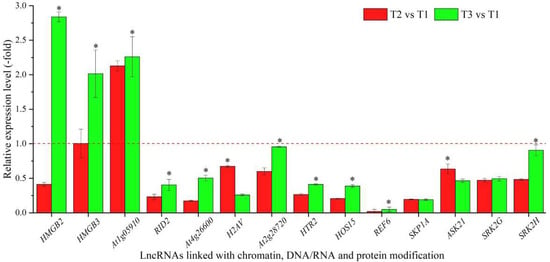
Figure 2.
The expression levels of coexpressed mRNAs of lncRNAs linked with chromatin, DNA/RNA and protein modification in A. sinensis at T2 vs. T1 and T3 vs. T1, as determined by qRT-PCR. T1, T2, and T3 represent uncompleted, completed, and avoided vernalization, respectively. The “*” represents a significant difference at p < 0.05 level between T2 vs. T1 and T3 vs. T2 for the same gene.
3.3. LncRNAs Linked with Flowering and Expression Levels of Their Coexpressed mRNAs
In total, 36 lncRNAs were linked with flowering based on the biological functions of their coexpressed mRNAs, with 12 lncRNAs directly associated with flowering, namely SRR1, PHL, PHYA, AGL62, AGL79, ATJ3, BBX29, CLE13, CLE44, MXC17.10, At1g06515, and BHLH30 (Table 3), and 24 lncRNAs indirectly associated with flowering, such as cell division, embryo development, and cell wall organization (Table S3). The expression levels of six representative coexpressed mRNAs (SRR1, PHL, PHYA, AGL62, AGL79, and ATJ3) were confirmed by qRT-PCR, with all six mRNAs showing down-regulation at T2 vs. T1 and T3 vs. T1, and five mRNAs showing lower levels at T2 vs. T1 than T3 vs. T1, with the exception of the gene AGL62 (Figure 3).

Table 3.
Twelve lncRNAs directly linked with flowering.
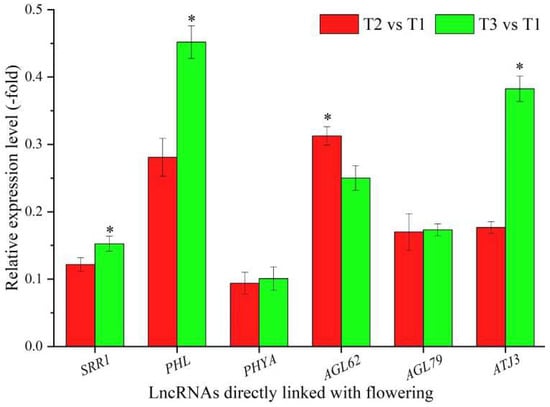
Figure 3.
The expression levels of coexpressed mRNAs of lncRNAs directly linked with flowering in A. sinensis at T2 vs. T1 and T3 vs. T1, as determined by qRT-PCR. The “*” represents a significant difference at p < 0.05 level between T2 vs. T1 and T3 vs. T2 for the same gene.
3.4. LncRNAs Linked with Stress Response and Expression Levels of Their Coexpressed mRNAs
In total, 24 lncRNAs were linked with the stress response based on the biological functions of their coexpressed mRNAs, with 14 lncRNAs associated with the temperature response, namely ACBP6, ENO2, ADH1, CSP2, RH20, RH52, RH53, RAB18, XERO1, MED14, HSP17.8, HSP70-3, HSP70-10, and HSP90-3 (Table 4), and 10 lncRNAs associated with other stresses responses, such as water, salt, and oxidative stress (Table S4). The expression levels of eight representative coexpressed mRNAs involved in the temperature response were confirmed by qRT-PCR, with four mRNAs (ACBP6, ENO2, CSP2, and HSP90-3) showing up-regulation at T3 vs. T1, and three mRNAs (ACBP6, ENO2, and CSP2) showing lower levels at T2 vs. T1 than T3 vs. T1 (Figure 4).

Table 4.
Fourteen lncRNAs directly linked with temperature response.
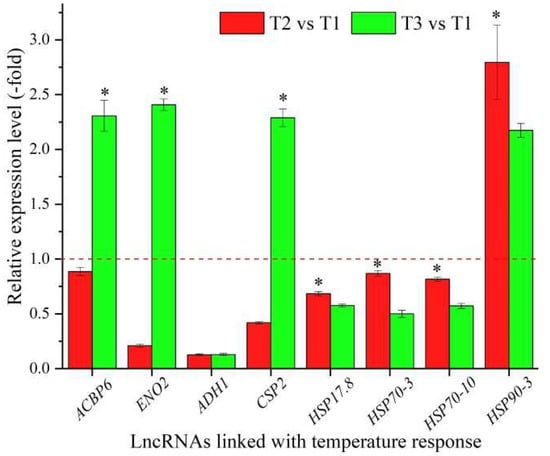
Figure 4.
The expression levels of coexpressed mRNAs of lncRNAs linked with temperature response in A. sinensis at T2 vs. T1 and T3 vs. T1, as determined by qRT-PCR. The “*” represents a significant difference at p < 0.05 level between T2 vs. T1 and T3 vs. T2 for the same gene.
3.5. LncRNAs Linked with Metabolism and Expression Levels of Their Coexpressed mRNAs
In total, 117 lncRNAs were linked with metabolism based on the biological functions of their coexpressed mRNAs, with 19 lncRNAs associated with carbohydrate metabolism, namely CSY4, FBA3, GAPC1, GAPCP2, At3g52990, PGM2, USP, GLCNAC1PUT2, UXS2, XYLA, GALS1, OFUT31, At5g67460, RSW3, At1g59950, At5g25970, UGT76E7, At1g26850, and BAM1 (Table 5), and 98 lncRNAs associated with other types of metabolism, such as nucleotide, protein, and lipid metabolism (Table S5). The expression levels of five representative coexpressed mRNAs (CSY4, GAPCP2, At3g52990, PGM2, and BAM1) involved in carbohydrate metabolism were confirmed by qRT-PCR, with all five mRNAs showing down-regulation at T3 vs. T1, and three mRNAs showing higher levels at T2 vs. T1 than T3 vs. T1, with the exception of the two genes GAPCP2 and BAM1 (Figure 5).

Table 5.
Nineteen lncRNAs directly linked with carbohydrate metabolism.
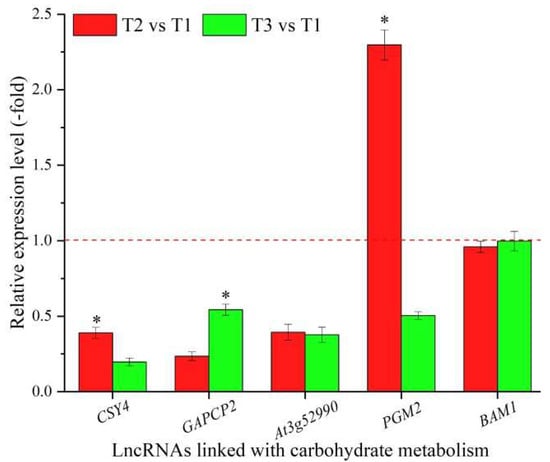
Figure 5.
The expression levels of coexpressed mRNAs of lncRNAs linked with carbohydrate metabolism in A. sinensis at T2 vs. T1 and T3 vs. T1, as determined by qRT-PCR. The “*” represents a significant difference at p < 0.05 level between T2 vs. T1 and T3 vs. T2 for the same gene.
3.6. LncRNAs Linked with Biosignaling and Expression Levels of Their Coexpressed mRNAs
In total, 23 lncRNAs were linked with metabolism based on the biological functions of their coexpressed mRNAs, with 13 lncRNAs associated with hormone signaling, namely ARF1, CUL1, T4L20.330, SOFL4, SOFL5, GRF2, GRF11, ERF3, CIPK20, SF1, AGD9, TIFY4B, and At2g34810 (Table 6), and 10 lncRNAs associated with other types of signaling, such as protein kinase, phosphatidylinositol-mediated, and cell surface receptor signaling (Table S6). The expression levels of eight representative coexpressed mRNAs associated with hormone signaling were confirmed by qRT-PCR, with three mRNAs (ARF1, CUL1, and GRF11) showing up-regulation at T2 vs. T1 and T3 vs. T1, and four mRNAs (ARF1, SOFL4, GRF2, and ERF3) showing lower levels at T2 vs. T1 than T3 vs. T1 (Figure 6).

Table 6.
Thirteen lncRNAs directly linked with hormone signaling.
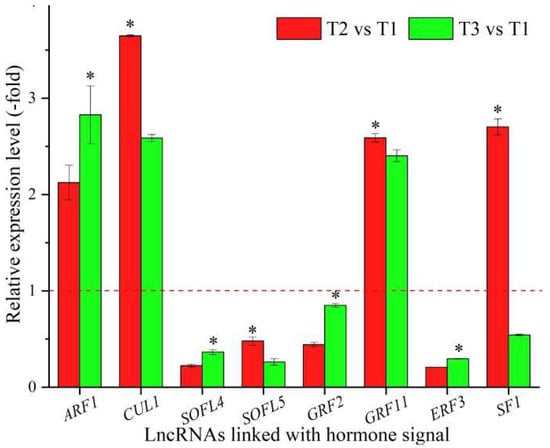
Figure 6.
The expression levels of coexpressed mRNAs of lncRNAs linked with biosignaling in A. sinensis at T2 vs. T1 and T3 vs. T1, as determined by qRT-PCR. The “*” represents a significant difference at p < 0.05 level between T2 vs. T1 and T3 vs. T2 for the same gene.
3.7. LncRNAs Linked with Energy and Transport
In all, 43 lncRNAs were found to be linked with energy and transport based on the biological functions of their coexpressed mRNAs, with 5 lncRNAs associated with energy, such as PURU1, ndhB1, and NDB1, and 38 lncRNAs associated with transport, such as At5g11230, GPT1 and SMXL5 (Table 7). The expression levels of eight representative coexpressed mRNAs (PURU1, MES16, GPT1, ABCF5, SECA2, VPS26A, CML19, and NHX6) were confirmed by qRT-PCR, with three mRNAs (PURU, GPT1, and VPS26A) showing up-regulation at T2 vs. T1 and T3 vs. T1, and six mRNAs (PURU1, GPT1, ABCF5, SECA2, VPS26A, and CML19) showing higher levels at T2 vs. T1 than T3 vs. T1, with the exception of the two genes MES16 and NHX6 (Figure 7).

Table 7.
Forty-three lncRNAs linked with energy and transport.
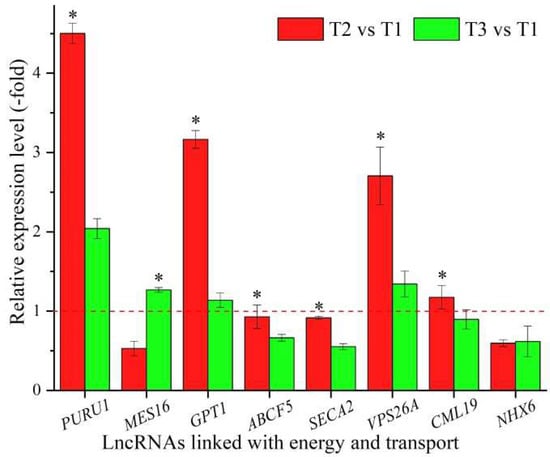
Figure 7.
The expression levels of coexpressed mRNAs of lncRNAs linked with energy and transport in A. sinensis at T2 vs. T1 and T3 vs. T1, as determined by qRT-PCR. The “*” represents a significant difference at p < 0.05 level between T2 vs. T1 and T3 vs. T2 for the same gene.
4. Discussion
Vernalization is a process considered to be an epigenetic switch whereby flowering is promoted by prolonged exposure to cold (0 to 10 °C); meanwhile, it can be lost at high temperatures or avoided below freezing temperatures [37,38]. Epigenetic regulation involves diverse molecular mechanisms including chromatin remodeling, DNA methylation, histone modification, histone variants, and ncRNAs [39]. Studies on Brassica rapa found that 127 differentially expressed lncRNAs were coexpressed with 128 differentially expressed genes, indicating that lncRNAs played an important role during vernalization [40]. In this study, 272 characterized lncRNAs were identified from A. sinensis and divided into six categories, namely (1) chromatin, DNA/RNA, and protein modification; (2) flowering; (3) stress response; (4) metabolism; (5) biosignaling; and (6) energy and transport, based on their coexpressed mRNAs.
FLC is a MADS-box transcriptional regulator that acts as a potent repressor of flowering [38]. In Arabidopsis, the epigenetic silencing of the floral repressor gene FLC is a well-characterized key event of vernalization [41]. In this study, 29 lncRNAs linked with chromatin, DNA/RNA, and protein modification were identified in A. sinensis during vernalization. For the chromatin modification, two coexpressed mRNAs, HMGB2 and HMGB3, are involved in binding preferentially double-stranded DNA and up-regulated in response to cold stress [42]. For the DNA/RNA modification, At1g05910 is involved in DNA demethylation and the negative regulation of chromatin silencing [43]; RID2 is involved in rRNA methyltransferase activity [44]; and At4g26600 is involved in RNA methylation [45]. For protein modification, 24 lncRNAs were involved and the roles of nine coexpressed mRNAs were represented as follows. H2AV plays a central role in regulating transcription, repairing DNA, replicating DNA, and stabilizing the nucleus chromosome [46]; At2g28720 and HTR2 are involved in compacting DNA into chromatin [47,48]; HOS15 promotes the deacetylation of histone H4 [49]; REF6 is involved in demethylating ‘Lys-27’ of histone H3, regulating flowering time by repressing FLC expression and interacting with the NF-Y complex to regulate SOC1 [50,51]; SKP1A and ASK21 are involved in ubiquitination and form an SCF E3 ubiquitin ligase complex together with CUL1, RBX1, and an F-box protein [52,53]; and SRK2G and SRK2H are involved in protein phosphorylation [54,55]. In previous studies, HMGB2 and HMGB3 were up-regulated in response to cold stress but down-regulated in response to drought and salt stresses [42]; H2AV, At2g28720 and HTR2 exhibited a high level in response to osmotic and drought stresses [56]; HOS15 was found to act as a repressor of cold stress-regulated gene expression and played a role in gene regulation for plant acclimation and tolerance to cold stress [49]; SKP1A and ASK21 were overexpressed in the host stress response [57]; and SRK2G and SRK2H were found to be positive regulators in stress responses such as drought, salt, and cold [54,55,58]. Currently, although most of the lncRNAs have been reported to be involved in the stress response in many plants, their roles in the regulation of flowering time have been studied in model plants [23]. Previous studies have found that FLC in Arabidopsis is epigenetically regulated by lncRNAs COOLAIR and COLDAIR [59,60,61]. In this study, a lower expression level was noted for almost all coexpressed mRNAs of lncRNAs involved in chromatin, DNA/RNA, and protein modification at vernalization (T2, 0 °C) compared with freezing temperature (T3, −3 °C), as well as down-regulation at T2, indicating that these lncRNAs participate in epigenetic silencing by transferring euchromatin to heterochromatin and confer early bolting and flowering of A. sinensis. Representatively, the down-regulation of mRNAs HMGB2 and HMGB3 during vernalization transfers the heterochromatin to the euchromatin [42], which generates the ability of flowering; in contrast, their up-regulation at freezing temperatures inhibits flowering by keeping the heterochromatin, which indicates that their coexpressed lncRNAs play positive roles in regulating the flowering of A. sinensis. The down-regulation of mRNA REF6 below 0 °C weakens the demethylation of histone H3 and delays flowering time by inducing FLC expression [50,51]; meanwhile, there is an increased expression level with decreased temperatures, which indicates that this coexpressed lncRNA plays a negative role in regulating the flowering of A. sinensis.
Flower formation occurs at the SAM and is a complex morphological event that is required not only for the circadian clock to measure the passage of time but also the regulation of meristem identity genes [42]. For the 6 representative coexpressed mRNAs of the 12 lncRNAs directly linked with flowering, SRR1 is involved in a circadian clock input pathway and regulation of the expression of clock-regulated genes such as CCA1 and TOC1 [62]; PHL is involved in the circadian rhythm and the regulation of the timing of transition [63]; PHYA is involved in the regulation of flowering time and expression of its own gene as negative feedback [64]; AGL62 is required for promoting the nuclear proliferation of early endosperm [65]; AGL79 is involved in positively regulating the transition of the meristem from the vegetative to reproductive phase [66]; and ATJ3 plays a continuous role in plant development, such as in photoperiodism, flowering, and positive regulation of flower development [67]. Previous studies found that mRNAs (e.g., miR156, miR169 and miR172) play a crucial role in developmental processes in rice, wheat, and maize, especially in the formation of the floral meristem, with miR172 controlling AP2-like genes [23,68,69]. Studies on the flowering of Chenopodium quinoa found that pivotal flowering homologs, including photoreceptor genes PHYA and CRY1, as well as genes associated with florigen-encoding genes (FT and TWIN SISTER of FT) and circadian clock-related genes (ELF3, LHY, and HY5), were specifically affected by night-break and competed with the positive- and negative-flowering lncRNAs [70]. In this study, down-regulation of all the coexpressed mRNAs involved in circadian clock and meristem identity genes was observed, which can be considered acceptable and reasonable, because these mRNAs are often highly expressed at the plant development stage (at photoperiod), while their expression levels were examined during vernalization. In addition, increased expression with decreased temperatures indicates that their coexpressed lncRNAs play negative roles in regulating the flowering of A. sinensis.
The expression of numerous lncRNAs has been demonstrated to be significantly affected by various stresses [23,30]. During vernalization, plants have to face and adapt to low temperature [38]. To date, extensive studies have reported that lncRNAs participate in defense responses associated with plant immunity and adaptation to the environment [22]. Heat-responsive lncRNAs have been found to be differentially expressed in Brassica juncea, and cold-responsive lncRNAs have been identified in grape and Arabidopsis [71,72,73]. lncRNAs could regulate HSP family genes (HSP82 and HSP83) in response to heat stress in Populus x canadensis Moench, and HSP18.1 in response to Cd stress Betula platyphylla [74,75]. For the eight representative coexpressed mRNAs of the 14 lncRNAs linked with the temperature response, ACBP6 confers resistance to cold and freezing [76]; ENO2 acts as a positive regulator in response to cold stress [77]; ADH1 is required for survival and acclimation in response to abiotic stresses (e.g., cold, salt, and drought) [78,79]; CSP2 contributes to the enhancement of cold and freezing tolerance [80]; and HSP17.8, HSP70-3, HSP70-10, and HSP90-3 play vital roles in adapting to biotic and abiotic stresses [81,82]. In this study, increased expression for cold-tolerated mRNAs (ACBP6, ENO2, ADH1, and CSP2), and decreased expression for heat-tolerated mRNAs (HSP17.8, HSP70-3, HSP70-10, and HSP90-3), were observed with decreased temperatures, which indicates that their coexpressed lncRNAs play positive roles in adapting to low temperatures.
For vernalization to occur, sources of energy (sugars) and carbohydrate metabolism are required [37]. In recent years, the roles of lncRNAs in regulating metabolism in cancer, insulin, and chicken have been reported [83,84,85], while, in plants, studies are still limited. For the five representative coexpressed mRNAs of the 19 lncRNAs linked with carbohydrate metabolism, CSY4 is involved in the synthesis of socitrate from oxaloacetate [86]; GAPCP2 plays a critical role in glycolysis and exhibits up-regulation under drought stress [87,88]; At3g52990 is involved in the synthesis of pyruvate from D-glyceraldehyde 3-phosphate [89]; PGM2 participates in the synthesis of glucose [90]; and BAM1 is required for starch breakdown [91]. In this study, the differential expression of these coexpressed mRNAs regulating sucrose and starch metabolism provided energy for the morphogenesis of seedlings and adaptation to low temperatures during vernalization. Representatively, the decreased expression of metabolite-synthesized mRNAs (CSY4, At3g52990, and PGM2), and increased expression of energy-produced mRNA GAPCP2 and metabolite-degraded mRNA BAM1, were observed with decreased temperatures, which indicates that their coexpressed lncRNAs play positive roles in carbohydrate metabolism.
Endogenous hormones such as gibberellin, auxin, cytokinin, brassinosteroid and abscisic acid can either inhibit or promote flowering [37]. In previous studies, a pre-miRNA of miR393 was identified in Brassica rapa during vernalization, and the overexpression of an miR393-resistant form of TIR1 (mTIR1) could enhance auxin sensitivity, thus leading to pleiotropic effects on plant development [92]. For the 8 representative coexpressed mRNAs of the 13 lncRNAs linked with hormone signaling, ARF1 is involved in the recruitment of COPI and GDAP1 to membranes and various auxin-dependent developmental processes [93]; CUL1 participates in forming a SCF complex together with SKP1, RBX1, and a F-box protein and is involved in floral organ development, the auxin signaling pathway and ethylene signaling [94]; SOFL4 and SOFL5 are involved in cytokinin-mediated development [95]; GRF2 and GRF11 participate in the brassinosteroid (BR)-mediated signaling pathway [96,97]; ERF3 is found to be differentially expressed in response to stresses and also regulates other ERFs [98]; and SF1 is required for development and is involved in the alternative splicing of FLM pre-mRNA [99]. In this study, the differential expression of these mRNAs involved in hormone signaling also played certain roles in regulating the flower-bud differentiation of seedlings and cold tolerance during vernalization. In previous studies, GRF2 and ERF3 were found to be down-regulated, while GRF11 was up-regulated in response to cold stress [100,101]; here, contrary findings for mRNAs GRF2 and GRF11 were observed with temperatures decreased, which indicate that their coexpressed lncRNAs may play negative roles in hormone signaling. The interaction between SF1 and FLM pre-mRNA controls flowering time in response to temperature [102]; here, decreased expression of mRNA SF1 was observed with decreased temperatures, which indicates that this coexpressed lncRNA may play a positive role in hormone signaling.
Energy generation and the transport of energy, nutrients and metabolites play essential roles in growth and development and stress tolerance [103]. For the 8 representative coexpressed mRNAs of the 43 lncRNAs linked with energy and transport, PURU1 is involved in photorespiration and participates in preventing the excessive accumulation of 5-formyl tetrahydrofolate [104]; MES16 is involved in chlorophyll breakdown by its demethylation [105]; GPT1 is involved in NADPH generation through a series of processes including Glc6P transport, starch biosynthesis, fatty acid biosynthesis, and oxidative pentose phosphate [106]; ABCF5 belongs to the ABC transporter superfamily and is involved in protein transport [107]; SECA2 is involved in protein export coupling ATP hydrolysis [108]; VPS26A plays a role in vesicular protein sorting and is essential in endosome-to-Golgi retrograde transport [109]; CML19 is a potential calcium sensor that binds calcium and is involved in the early response to stress [110]; and NHX6 is involved in trafficking to the vacuole and exchanging the low-affinity electroneutral Na(+) or K(+)/H(+) [111]. In this study, the differential expression of these coexpressed mRNAs associated with energy and transport provided energy, nutrients, and metabolites for A. sinensis seedlings to obtain the capacity for vernalization and, meanwhile, to adapt to low temperatures.
Based on the above functional analysis of lncRNAs identified in this study, flowering pathways proposed in the previous literature [14,15,16] and a general model of stress-responsive regulation by regulatory lncRNAs [23], a model of vernalization-induced bolting and flowering by regulatory lncRNAs in A. sinensis is proposed (Figure 8). Briefly, the vernalization of seedlings firstly triggers the differential expression of lncRNAs; then, the lncRNAs either act as a precursor of miRNAs or as a miRNA target mimic, which further binds their related targets; then, the binding of targets regulates the expression of their downstream mRNAs that are involved in various biological processes, including the temperature response, flowering pathways (i.e., epigenetic modification, flowering induction, carbohydrate metabolism, and hormone signaling), as well as energy and transport; finally, these biological processes promote the transition of the meristem from the vegetative phase to the bolting and flowering of A. sinensis.
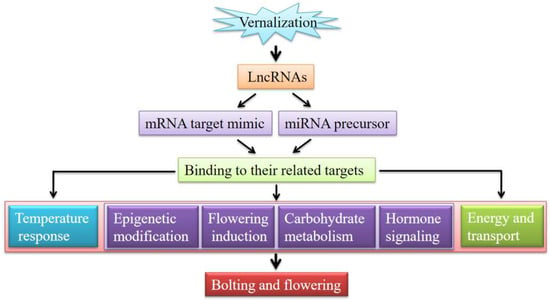
Figure 8.
A proposed model of vernalization-induced bolting and flowering by regulatory lncRNAs in A. sinensis.
5. Conclusions
From the above observations, we found that the lncRNAs positively or negatively regulated the expression of their downstream mRNAs through epigenetic changes at the level of transcription and post-transcription for the flowering of A. sinensis during vernalization. This coexpressed mRNA analysis of lncRNAs focused on five pathways, namely (1) chromatin, DNA/RNA, and protein modification; (2) floral development; (3) temperature response; (4) carbohydrate metabolism; and (5) hormone signaling. While several candidate lncRNAs were identified, their causative roles require further investigations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb44050128/s1.
Author Contributions
X.L.: data curation, formal analysis, validation, and writing—original draft preparation; M.L. (Mimi Luo): formal analysis and validation; M.L. (Mengfei Li): conceptualization, project administration, supervision, and writing—review and editing; J.W.: funding acquisition and resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Key Laboratory of Aridland Crop Science/Gansu Agricultural University (GSCS-2021-Z03), National Natural Science Foundation of China (32160083), China Agriculture Research System of MOF and MARA (CARS-21), and Assurance Project of Ecological Planting and Quality of Daodi Herbs (202103003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets are available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA789039 (release date on 13 January 2022).
Conflicts of Interest
All the authors declare no conflicts of interest.
Abbreviations
| AP1 | APETALA 1 |
| CCS | Circular consensus sequence |
| CNCI | Coding–Noncoding Index |
| COLDAIR | COLD-ASSISTED INTRONIC NON-CODING RNA |
| COOLAIR | COLD-INDUCED LONG ANTISENSE INTRAGENIC RNAs |
| CPC | Coding-Potential Calculator |
| FLC | FLOWERING LOCUS C |
| FLM | FLOWERING LOCUS M |
| FLNC | Full-length nonchimeric |
| FT | FLOWERING LOCUS T |
| GA | Gibberellin |
| GA2OX1 | Gibberellin 2-β-dioxygenase 1 |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KOG | euKaryotic orthologous groups of proteins |
| lncRNAs | Long noncoding RNAs |
| NCBI | National Center for Biotechnology Information |
| ncRNAs | Noncoding RNAs |
| NR | NCBI nonredundant protein |
| PHYA | PHYOCHROME A |
| REL | Relative expression level |
| SOC1 | SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 |
| sRNAs | Small RNAs |
| TAIR | The Arabidopsis Information Resource |
References
- Zhang, H.Y.; Bi, W.G.; Yu, Y.; Liao, W.B. Angelica sinensis (Oliv.) Diels in China: Distribution, cultivation, utilization and variation. Genet. Resour. Crop Evol. 2012, 59, 607–613. [Google Scholar] [CrossRef]
- Wang, W.J. Analysis and control of early bolting characteristic of Angelica sinensis. J. Northwest Univ. 1977, 7, 32–39. [Google Scholar]
- Committee for the Pharmacopoeia of PR China. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2015; p. 133. [Google Scholar]
- Hook, I.L. Danggui to Angelica sinensis root: Are potential benefits to European women lost in translation? A review. J. Ethnopharmacol. 2014, 152, 1–13. [Google Scholar] [CrossRef]
- Upton, R. American Herbal Pharmacopoeia and Therapeutic Compendium: Dang Gui Root-Angelica sinensis (Oliv.); American Herbal Pharmacopoeia: Scotts Valley, CA, USA, 2003; pp. 1–41. [Google Scholar]
- Ma, J.P.; Guo, Z.-B.; Jin, L.; Li, Y.D. Phytochemical progress made in investigations of Angelica sinensis (Oliv.) Diels. Chin. J. Nat. Med. 2015, 13, 241–249. [Google Scholar] [CrossRef]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef]
- Huang, L.Q.; Jin, L. Suitable Technology for Production and Processing of Angelica sinensis; China Pharmaceutical Science and Technology Press: Beijing, China, 2018; pp. 1–14. [Google Scholar]
- Li, M.L.; Cui, X.W.; Jin, L.; Li, M.F.; Wei, J.H. Bolting reduces ferulic acid and flavonoid biosynthesis and induces root lignification in Angelica sinensis. Plant Physiol. Biochem. 2022, 170, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Qiu, D.Y.; Chen, Y. Effect of root diameter on early bolting rate and yield in seedling of Angelica sinensis. Chin. Tradit. Herb. Drugs 2007, 38, 1386–1389. [Google Scholar]
- Wang, W.J. Technology and principle of seedling frozen storage of Angelica sinensis. J. Chin. Med. Mat. 1979, 3, 1–4. [Google Scholar]
- Jia, Z.; Di, S.Q.; Zhao, F.Y.; Li, S.X.; Wang, S.C. Effects of different low overwintering temperatures on Angelica vernalization and premature bolting. Agr. Sci. Tech. 2018, 19, 55–62. [Google Scholar]
- Yao, L. Effect of shading during the nursery of Angelica sinensis on bolting rate and economic characters. Gansu Agr. Sci. Tech. 2005, 10, 54–55. [Google Scholar]
- Yu, G.; Zhou, Y.; Yu, J.J.; Hu, X.Q.; Tang, Y.; Yan, H.; Duan, J.A. Transcriptome and digital gene expression analysis unravels the novel mechanism of early flowering in Angelica sinensis. Sci. Rep. 2019, 9, 10035. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.L.; Zhu, T.T.; Zhang, X.N.; Li, M.F.; Wei, J.H. Integrated transcriptomics and metabolites at different growth stagesreveals the regulation mechanism of bolting and flowering of Angelica sinensis. Plant Biol. 2021, 23, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Li, J.; Wei, J.H.; Paré, P.W. Transcriptional controls for early bolting and flowering in Angelica sinensis. Plants 2021, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.H.; Huang, P. Effects of vernalization treatment on physiological character of Angelica sinensis seedlings. J. Gansu Agric. Univ. 1998, 33, 240–243. [Google Scholar]
- Chen, H.G.; Du, T.; Zhu, T.T.; Gao, S.F.; Chai, L.; He, W.W. Study on physiological mechanisms in frozen storage to reduce early bolting of Angelica sinensis. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Tech. 2014, 16, 203–206. [Google Scholar]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la différence: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011, 12, 221. [Google Scholar] [CrossRef]
- Carrington, J.C.; Ambros, V. Role of microRNAs in plant and animal development. Science 2003, 301, 336–338. [Google Scholar] [CrossRef]
- Jain, N.; Sinha, N.; Krishna, H.; Singh, P.K.; Gautam, T.; Prasad, P.; Balyan, H.S.; Gupta, P.K. A study of miRNAs and lncRNAs during Lr28-mediated resistance against leaf rust in wheat (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2020, 112, 101552. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, A.; Madhu; Shumayla; Singh, K.; Upadhyay, S.K. Long non-coding RNAs as emerging regulators of pathogen response in plants. Non Coding RNA 2022, 8, 4. [Google Scholar] [CrossRef]
- Qi, L.; Li, X.; Zhang, S.; An, D. Genetic regulation by non-coding RNAs. Sci. China Ser. C Life Sci. 2006, 49, 201–217. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.P.; Tseng, E.; Salamov, A.; Zhang, J.; Meng, X.; Zhao, Z.; Kang, D.; Underwood, J.; Grigoriev, I.V.; Figueroa, M.; et al. Widespread polycistronic transcripts in fungi revealed by single-molecule mRNA sequencing. PLoS ONE 2015, 10, e0132628. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.L. The TAIR database. Methods Mol. Biol. 2007, 406, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Xiao, H.; Bu, D.C.; Xie, C.Y.; Miao, R.Y.; Luo, H.T.; Zhao, G.G.; Yu, K.T.; Zhao, H.T.; Skogerbø, G.; et al. ncFANs: A web server for functional annotation of long non-coding RNAs. Nucleic Acids Res. 2011, 39, w118–w124. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Rinn, J.L. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015, 22, 5–7. [Google Scholar] [CrossRef]
- Shumayla; Sharma, S.; Taneja, M.; Tyagi, S.; Singh, K.; Upadhyay, S.K. Survey of high throughput RNA-Seq data reveals potential roles for lncRNAs during development and stress response in bread wheat. Front. Plant Sci. 2017, 8, 1019. [Google Scholar] [CrossRef]
- Xu, R.; Xu, J.; Li, Y.C.; Dai, Y.T.; Zhang, S.P.; Wang, G.; Liu, Z.G.; Dong, L.L.; Chen, S.L. Integrated chemical and transcriptomic analyses unveils synthetic characteristics of different medicinal root parts of Angelica sinensis. Chin. Herb. Med. 2020, 12, 19–28. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Svec, D.; Tichopad, A.; Novosadova, V.; Pfaffl, M.W.; Kubista, M. How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments. Biomol. Detect. Quantif. 2015, 3, 9–16. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant physiology. In The Control of Flowering, 5th ed.; Fosket, D.E., Amasino, R., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010; pp. 559–590. [Google Scholar]
- Amasino, R.M. Vernalization and flowering time. Curr. Opin. Biotechnol. 2005, 16, 154–158. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Cao, X.F.; Deng, X. Histone methylation in epigenetic regulation and temperature responses. Curr. Opin. Plant Biol. 2021, 61, 102001. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.K.; Wu, P.; Wang, Q.; Wang, W.L.; Zhang, C.W.; Sun, F.F.; Liu, Z.K.; Li, Y.; Hou, X.L. Comparative transcriptome discovery and elucidation of the mechanism of long noncoding RNAs during vernalization in Brassica rapa. Plant Growth Regul. 2018, 85, 27–39. [Google Scholar] [CrossRef]
- Velanis, C.N.; Goodrich, J. Vernalization and epigenetic inheritance: A game of histones. Curr. Biol. 2017, 27, R1324–R1326. [Google Scholar] [CrossRef]
- Kwak, K.J.; Kim, J.Y.; Kim, Y.O.; Kang, H. Characterization of transgenic Arabidopsis plants overexpressing high mobility group b proteins under high salinity, drought or cold stress. Plant Cell Physiol. 2007, 48, 221–231. [Google Scholar] [CrossRef]
- Theologis, A.; Ecker, J.R.; Palm, C.J.; Federspiel, N.A.; Kaul, S.; White, O.; Alonso, J.; Altafi, H.; Araujo, R.; Bowman, C.L.; et al. Sequence and analysis of chromosome 1 of the plant Arabidopsis thaliana. Nature 2000, 408, 816–820. [Google Scholar] [CrossRef]
- Ohbayashi, I.; Konishi, M.; Ebine, K.; Sugiyama, M. Genetic identification of Arabidopsis RID2 as an essential factor involved in pre-rRNA processing. Plant J. 2011, 67, 49–60. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Krishnakumar, V.; Chan, A.P.; Thibaud-Nissen, F.; Schobel, S.; Town, C.D. Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017, 89, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Z.; Bai, Y.H.; Zhao, L.H.; Dou, X.Y.; Liu, Y.H.; Wang, L.L.; Li, Y.; Li, W.M.; Hui, Y.N.; Huang, X.Y.; et al. H2A.Z represses gene expression by modulating promoter nucleosome structure and enhancer histone modifications in Arabidopsis. Mol. Plant 2017, 10, 1274–1292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Borg, M.; Lorković, Z.J.; Montgomery, S.A.; Osakabe, A.; Yelagandula, R.; Axelsson, E.; Berger, F. The evolution and functional divergence of the histone H2B family in plants. PLoS Genet. 2020, 16, e1008964. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.V.; Kapoor, A.; Zhang, K.L.; Zhu, J.J.; Zhou, T.; Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K. Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature 2007, 447, 735–738. [Google Scholar] [CrossRef]
- Zhu, J.; Jeong, J.C.; Zhu, Y.; Sokolchik, I.; Miyazaki, S.; Zhu, J.K.; Hasegawa, P.M.; Bohnert, H.J.; Shi, H.; Yun, D.J.; et al. Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. USA 2008, 105, 4945–4950. [Google Scholar] [CrossRef]
- Lu, F.L.; Cui, X.; Zhang, S.B.; Jenuwein, T.; Cao, X.F. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat. Genet. 2011, 43, 715–719. [Google Scholar] [CrossRef]
- Hou, X.L.; Zhou, J.N.; Liu, C.; Liu, L.; Shen, L.S.; Yu, H. Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat. Commun. 2014, 5, 4601. [Google Scholar] [CrossRef]
- Zhao, D.Z.; Yu, Q.L.; Chen, M.; Ma, H. The ASK1 gene regulates B function gene expression in cooperation with UFO and LEAFY in Arabidopsis. Development 2001, 128, 2735–2746. [Google Scholar] [CrossRef]
- Liu, F.; Ni, W.; Griffith, M.E.; Huang, Z.; Chang, C.; Peng, W.; Ma, H.; Xie, D. The ASK1 and ASK2 genes are essential for Arabidopsis early development. Plant Cell 2004, 16, 5–20. [Google Scholar] [CrossRef]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef]
- Yan, J.; Wang, P.C.; Wang, B.S.; Hsu, C.C.; Tang, K.; Zhang, H.R.; Hou, Y.J.; Zhao, Y.; Wang, Q.M.; Zhao, C.Z.; et al. The SnRK2 kinases modulate miRNA accumulation in Arabidopsis. PLoS Genet. 2017, 13, e1006753. [Google Scholar] [CrossRef] [PubMed]
- Sura, W.; Kabza, M.; Karlowski, W.M.; Bieluszewski, T.; Kus-Slowinska, M.; Pawełoszek, Ł.; Sadowski, J.; Ziolkowski, P.A. Dual role of the histone variant H2A.Z in transcriptional regulation of stress-response genes. Plant Cell 2017, 29, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Hamorsky, K.T.; Kouokam, J.C.; Jurkiewicz, J.M.; Nelson, B.; Moore, L.J.; Husk, A.S.; Kajiura, H.; Fujiyama, K.; Matoba, N. N-Glycosylation of cholera toxin B subunit in Nicotiana benthamiana: Impacts on host stress response, production yield and vaccine potential. Sci. Rep. 2015, 5, 8003. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Verslues, P.E.; Zhu, J.K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress re-sponses in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.B.; Sung, S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 2011, 331, 76–79. [Google Scholar] [CrossRef]
- Liu, F.; Marquardt, S.; Lister, C.; Swiezewski, S.; Dean, C. Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 2010, 327, 94–97. [Google Scholar] [CrossRef]
- Sun, Q.; Csorba, T.; Skourti-Stathaki, K.; Proudfoot, N.J.; Dean, C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 2013, 340, 619–621. [Google Scholar] [CrossRef]
- Johansson, M.; Staiger, D. SRR1 is essential to repress flowering in non-inductive conditions in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 5811–5822. [Google Scholar] [CrossRef][Green Version]
- Endo, M.; Tanigawa, Y.; Murakami, T.; Araki, T.; Nagatani, A. Phytochrome-dependent late-flowering accelerates flowering through physical interactions with phytochrome B and CONSTANS. Proc. Natl. Acad. Sci. USA 2013, 110, 18017–18022. [Google Scholar] [CrossRef]
- Yang, S.W.; Jang, I.C.; Henriques, R.; Chua, N.H. FAR-RED ELONGATED HYPOCOTYL1 and FHY1-LIKE associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome a signals for inhibition of hypocotyl elongation. Plant Cell 2009, 21, 1341–1359. [Google Scholar] [CrossRef]
- Kang, I.H.; Steffen, J.G.; Portereiko, M.F.; Lloyd, A.; Drews, G.N. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell 2008, 20, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.M.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. miR156/SPL10 modulates lateral root development, branching and leaf morphology in Arabidopsis by silencing AGAMOUS-LIKE 79. Front. Plant Sci. 2017, 8, 2226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.G.; Kroczynska, B.; Miernyk, J.A. The genomic sequence encoding the Arabidopsis thaliana molecular chaperone AtJ3. Plant Physiol. 2000, 122, 291. [Google Scholar] [CrossRef][Green Version]
- Zhu, Q.H.; Helliwell, C.A. Regulation of flowering time and floral patterning by miR172. J. Exp. Bot. 2011, 62, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, H.; Wang, K.; Liu, L.; Wang, S.; Zhao, Y.; Yin, J.; Li, Y. Development-associated microRNAs in grains of wheat (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 140. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, Y.; Wu, X.; Bai, X.; Ye, X.L.; Liu, C.Y.; Wan, Y.; Xiang, D.B.; Li, Q.; Zou, L.; et al. Identification of the specific long-noncoding RNAs involved in night-break mediated flowering retardation in Chenopodium quinoa. BMC Genom. 2021, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Calixto, C.P.G.; Tzioutziou, N.A.; James, A.B.; Hornyik, C.; Guo, W.B.; Zhang, R.X.; Nimmo, H.G.; Brown, J.W.S. Cold-dependent expression and alternative splicing of Arabidopsis long non-coding RNAs. Front. Plant Sci. 2019, 10, 235. [Google Scholar] [CrossRef]
- Wang, P.; Dai, L.; Ai, J.; Wang, Y.; Ren, F. Identification and functional prediction of cold-related long non-coding RNA (lncRNA) in grapevine. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Bhatia, G.; Singh, A.; Verma, D.; Sharma, S.; Singh, K. Genome-wide investigation of regulatory roles of lncRNAs in response to heat and drought stress in Brassica juncea (Indian mustard). Environ. Exp. Bot. 2020, 171, 103922. [Google Scholar] [CrossRef]
- Wen, X.J.; Ding, Y.; Tan, Z.L.; Wang, J.X.; Zhang, D.Y.; Wang, Y.C. Identification and characterization of cadmium stress-related LncRNAs from Betula platyphylla. Plant Sci. 2020, 299, 110601. [Google Scholar] [CrossRef]
- Xu, J.H.; Fang, M.; Li, Z.H.; Zhang, M.N.; Liu, X.Y.; Peng, Y.Y.; Wan, Y.L.; Chen, J.H. Third-generation sequencing reveals lncRNA-regulated HSP genes in the Populus x Canadensis moench heat stress response. Front. Genet. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.F.; Xiao, S.; Chye, M.L. Overexpression of the Arabidopsis 10-kilodalton acyl-coenzyme A-binding protein ACBP6 enhances freezing tolerance. Plant Physiol. 2008, 148, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Guo, Y.; Ohta, M.; Xiong, L.; Stevenson, B.; Zhu, J.K. LOS2, a genetic locus required for cold-responsive gene transcription encodes a bifunctional enolase. EMBO J. 2002, 21, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Ismond, K.P.; Dolferus, R.; de Pauw, M.; Dennis, E.S.; Good, A.G. Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol. 2003, 132, 1292–1302. [Google Scholar] [CrossRef]
- Zhang, F.; Maeder, M.L.; Unger-Wallace, E.; Hoshaw, J.P.; Reyon, D.; Christian, M.; Li, X.; Pierick, C.J.; Dobbs, D.; Peterson, T.; et al. High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc. Natl. Acad. Sci. USA 2010, 107, 12028–12033. [Google Scholar] [CrossRef]
- Sasaki, K.; Kim, M.; Imai, R. Arabidopsis cold shock domain protein 2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem. Biophys. Res. Commun. 2007, 364, 633–638. [Google Scholar] [CrossRef]
- Anaraki, Z.E.; Tafreshi, S.A.H.; Shariati, M. Transient silencing of heat shock proteins showed remarkable roles for HSP70 during adaptation to stress in plants. Environ. Exp. Bot. 2018, 155, 142–157. [Google Scholar] [CrossRef]
- Huang, S.; Monaghan, J.; Zhong, X.H.; Lin, L.; Sun, T.J.; Dong, O.X.; Li, X. HSP90s are required for NLR immune receptor accumulation in Arabidopsis. Plant J. 2014, 79, 427–439. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Q.; Wang, C.Q.; Zhu, L.; Bi, C.; Zhang, S.; Wang, X.; Jin, H. LncRNAs regulate metabolism in cancer. Int. J. Biol. Sci. 2020, 16, 1194–1206. [Google Scholar] [CrossRef]
- Karimi, P.; Bakhtiarizadeh, M.R.; Salehi, A.; Izadnia, H.R. Transcriptome analysis reveals the potential roles of long non-coding RNAs in feed efficiency of chicken. Sci. Rep. 2022, 12, 2558. [Google Scholar] [CrossRef]
- Tello-Flores, V.A.; Beltrán-Anaya, F.O.; Ramírez-Vargas, M.A.; Esteban-Casales, B.E.; Navarro-Tito, N.; Alarcón-Romero, L.d.C.; Luciano-Villa, C.A.; Ramírez, M.; del Moral-Hernández, Ó.; Flores-Alfaro, E. Role of long non-coding RNAs and the molecular mechanisms involved in insulin resistance. Int. J. Mol. Sci. 2021, 22, 7256. [Google Scholar] [CrossRef] [PubMed]
- Slabas, A.R.; Ndimba, B.; Simon, W.J.; Chivasa, S. Proteomic analysis of the Arabidopsis cell wall reveals unexpected proteins with new cellular locations. Biochem. Soc. Trans. 2004, 32, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bertomeu, J.; Cascales-Miñana, B.; Mulet, J.M.; Baroja-Fernández, E.; Pozueta-Romero, J.; Kuhn, J.M.; Segura, J.; Ros, R. Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol. 2009, 151, 541–558. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Z.; Li, F.; Li, X.; Ji, H.; Yang, S. The specific MYB binding sites bound by TaMYB in the GAPCp2/3 promoters are involved in the drought stress response in wheat. BMC Plant Biol. 2019, 19, 366. [Google Scholar] [CrossRef] [PubMed]
- Rius, S.P.; Casati, P.; Iglesias, A.A.; Gomez-Casati, D.F. Characterization of Arabidopsis lines deficient in GAPC-1, a cytosolic NAD-dependent glyceraldehyde-3-phosphate dehydrogenase. Plant Physiol. 2008, 148, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Egli, B.; Kolling, K.; Kohler, C.; Zeeman, S.C.; Streb, S. Loss of cytosolic phosphoglucomutase compromises gametophyte development in Arabidopsis. Plant Physiol. 2010, 154, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.C.; Stettler, M.; Mettler, T.; Vaughan, C.K.; Li, J.; Francisco, P.; Gil, M.; Reinhold, H.; Eicke, S.; Messerli, G.; et al. Beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. Plant Cell 2008, 20, 1040–1058. [Google Scholar] [CrossRef] [PubMed]
- Oono, Y.; Seki, M.; Satou, M.; Iida, K.; Akiyama, K.; Sakurai, T.; Fujita, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Monitoring expression profiles of Arabidopsis genes during cold acclimation and deacclimation using DNA microarrays. Funct. Integr. Genom. 2006, 6, 212–234. [Google Scholar] [CrossRef]
- Tanaka, H.; Nodzylski, T.; Kitakura, S.; Feraru, M.I.; Sasabe, M.; Ishikawa, T.; Kleine-Vehn, J.; Kakimoto, T.; Friml, J. BEX1/ARF1A1C is required for BFA-sensitive recycling of PIN auxin transporters and auxin-mediated development in Ara-bidopsis. Plant Cell Physiol. 2014, 55, 737–749. [Google Scholar] [CrossRef]
- Feng, S.H.; Shen, Y.P.; Sullivan, J.A.; Rubio, V.; Xiong, Y.; Sun, T.P.; Deng, X.W. Arabidopsis CAND1, an unmodified CUL1-interacting protein, is involved in multiple developmental pathways controlled by ubiquitin/proteasome-mediated protein degradation. Plant Cell 2004, 16, 1870–1882. [Google Scholar] [CrossRef]
- Tayengwa, R.; Zhao, J.; Pierce, C.F.; Werner, B.E.; Neff, M.M. Synopsis of the SOFL plant-specific gene family. G3 Genes Genomes Genet. 2018, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Gampala, S.S.; Kim, T.W.; He, J.X.; Tang, W.; Deng, Z.; Bai, M.Y.; Guan, S.; Lalonde, S.; Sun, Y.; Gendron, J.M.; et al. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 2007, 13, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Chen, W.W.; Chen, L.Q.; Qin, C.; Jin, C.W.; Shi, Y.Z.; Zheng, S.J. The 14-3-3 protein general regulatory factor11 (GRF 11) acts downstream of nitric oxide to regulate iron acquisition in Arabidopsis thaliana. New Phytol. 2013, 197, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Matsui, K.; Hiratsu, K.; Shinshi, H.; Ohme-Takagi, M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 2001, 13, 1959–1968. [Google Scholar] [CrossRef]
- Jang, Y.H.; Park, H.Y.; Lee, K.C.; Thu, M.P.; Kim, S.K.; Suh, M.C.; Kang, H.; Kim, J.K. A homolog of splicing factor SF1 is essential for development and is involved in the alternative splicing of pre-mRNA in Arabidopsis thaliana. Plant J. 2014, 78, 591–603. [Google Scholar] [CrossRef]
- Shang, S.; Wu, C.; Huang, C.; Tie, W.; Yan, Y.; Ding, Z.; Xia, Z.; Wang, W.; Peng, M.; Tian, L.; et al. Genome-wide analysis of the GRF family reveals their involvement in abiotic stress response in cassava. Genes 2018, 9, 110. [Google Scholar] [CrossRef]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [CrossRef]
- Lee, K.C.; Chung, K.S.; Lee, H.T.; Park, J.H.; Lee, J.H.; Kim, J.K. Role of Arabidopsis splicing factor SF1 in tempera-ture-responsive alternative splicing of FLM pre-mRNA. Front. Plant Sci. 2020, 11, 596354. [Google Scholar] [CrossRef]
- Johnson, X.; Alric, J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: Metabolic constraints for carbon partitioning between oil and starch. Eukaryot Cell 2013, 12, 776–793. [Google Scholar] [CrossRef]
- Collakova, E.; Goyer, A.; Naponelli, V.; Krassovskaya, I.; Gregory, J.F.; Hanson, A.D.; Shachar-Hill, Y. Arabidopsis 10-formyl tetrahydrofolate deformylases are essential for photorespiration. Plant Cell 2008, 20, 1818–1832. [Google Scholar] [CrossRef]
- Christ, B.; Schelbert, S.; Aubry, S.; Suessenbacher, I.; Mueller, T.; Kraeutler, B.; Hoertensteiner, S. MES16, a member of the methylesterase protein family, specifically demethylates fluorescent chlorophyll catabolites during chlorophyll breakdown in Arabidopsis. Plant Physiol. 2012, 158, 628–641. [Google Scholar] [CrossRef]
- Baune, M.C.; Lansing, H.; Fischer, K.; Meyer, T.; Charton, L.; Linka, N.; von Schaewen, A. The Arabidopsis plastidial glucose-6-phosphate transporter GPT1 is dually targeted to peroxisomes via the endoplasmic reticulum. Plant Cell 2020, 32, 1703–1726. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, R.; Davies, T.G.E.; Coleman, J.O.; Rea, P.A. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 2001, 276, 30231–30244. [Google Scholar] [CrossRef] [PubMed]
- Skalitzky, C.A.; Martin, J.R.; Harwood, J.H.; Beirne, J.J.; Adamczyk, B.J.; Heck, G.R.; Cline, K.; Fernandez, D.E. Plastids contain a second sec translocase system with essential functions. Plant Physiol. 2011, 155, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Asakura, Y.; Imai, M.; Nakahira, Y.; Kotani, Y.; Hashiguchi, Y.; Nakai, Y.; Takafuji, K.; Bédard, J.; Hirabayashi-Ishioka, Y.; et al. A Ycf2-ftshi heteromeric AAA-ATPase complex is required for chloroplast protein import. Plant Cell 2018, 30, 2677–2703. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Day, I.S.; Thomas, T.; Reddy, A.S.N. KIC, a novel Ca2+ binding protein with one EF-hand motif, interacts with a microtubule motor protein and regulates trichome morphogenesis. Plant Cell 2004, 16, 185–200. [Google Scholar] [CrossRef][Green Version]
- Bassil, E.; Ohto, M.A.; Esumi, T.; Tajima, H.; Zhu, Z.; Cagnac, O.; Belmonte, M.; Peleg, Z.; Yamaguchi, T.; Blumwald, E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 2011, 23, 224–239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).