The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases
Abstract
:1. Introduction
2. Materials and Methods
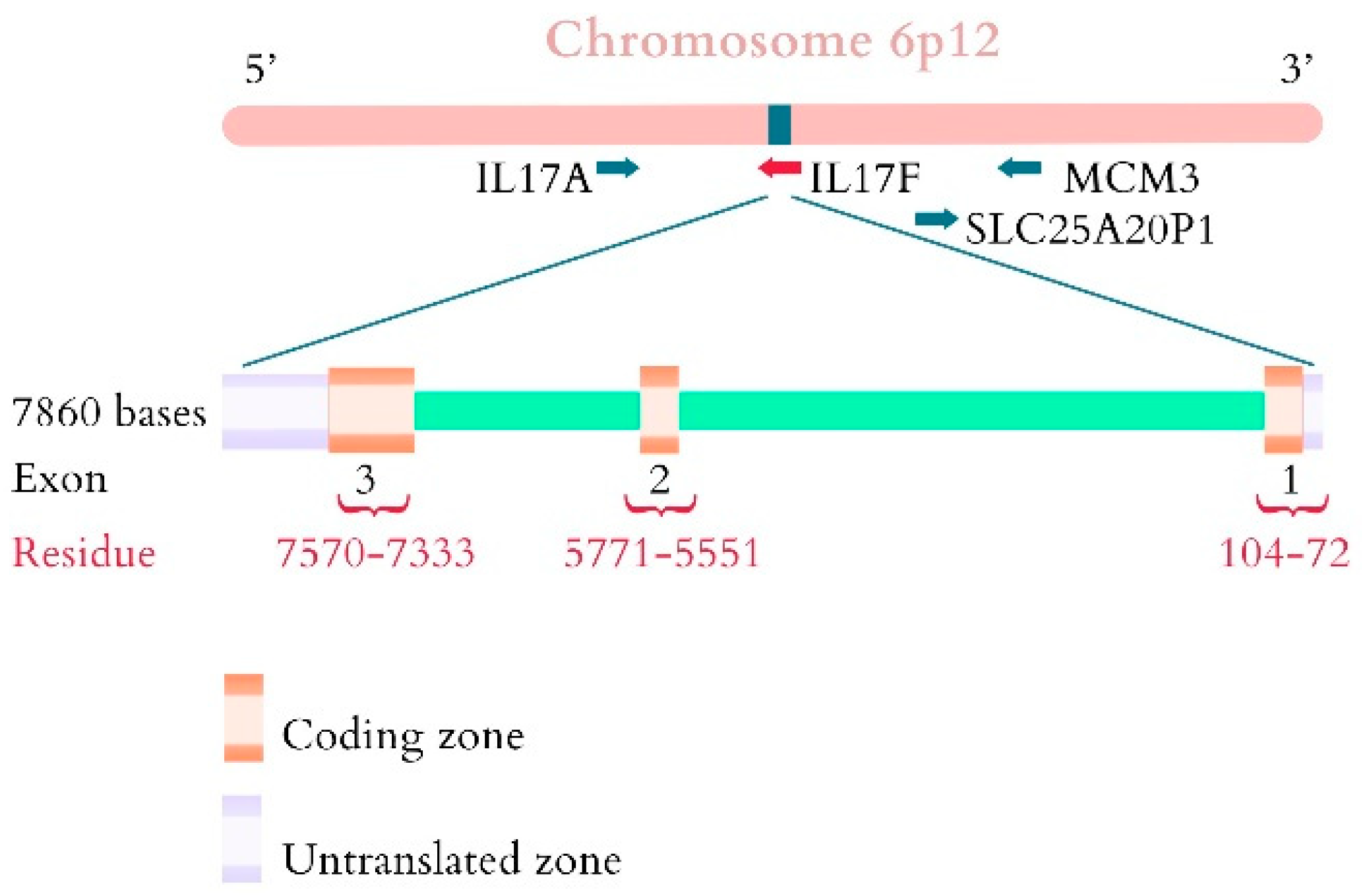
3. Interleukin Family
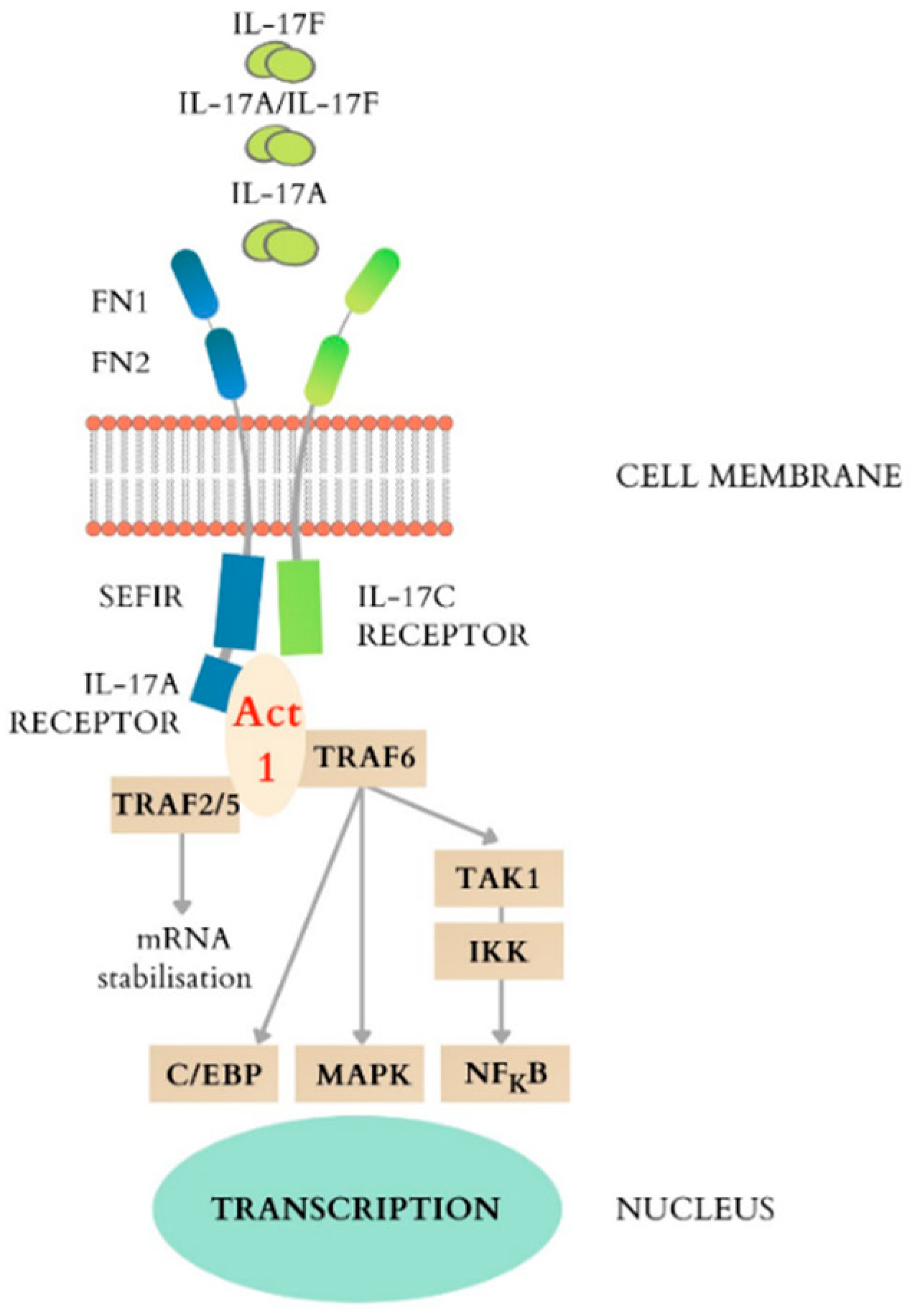
4. The IL-17 Receptor Family
5. The Role of IL-17 in Joint Inflammation and Bone Homeostasis
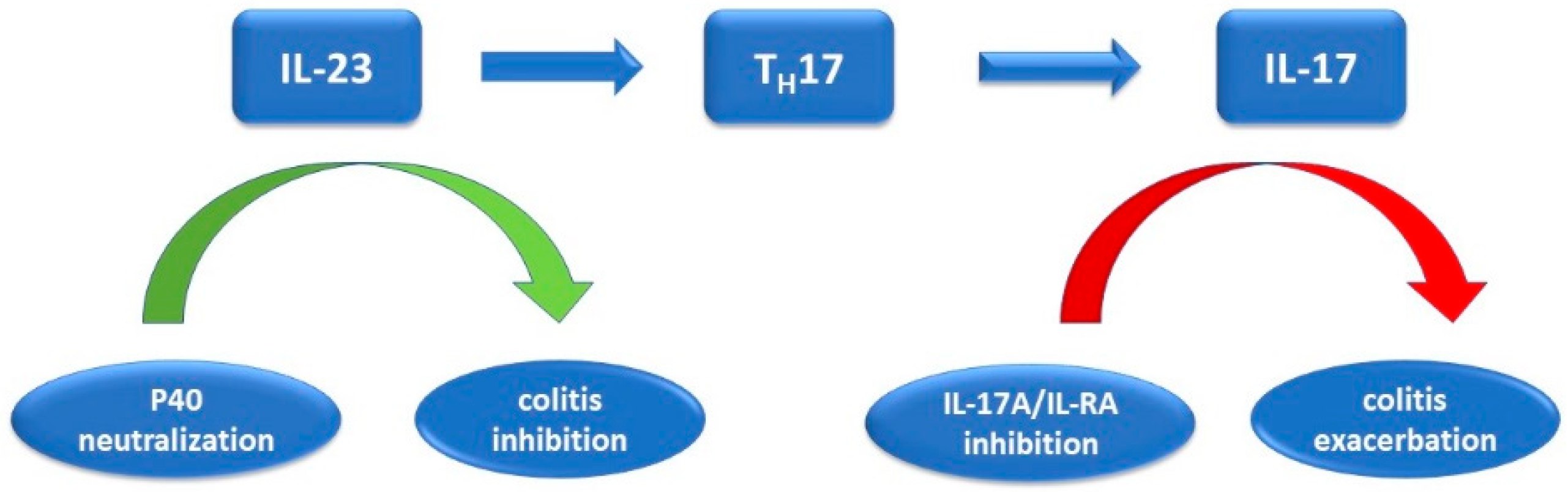
6. The Role of IL-17 in Intestinal Inflammation
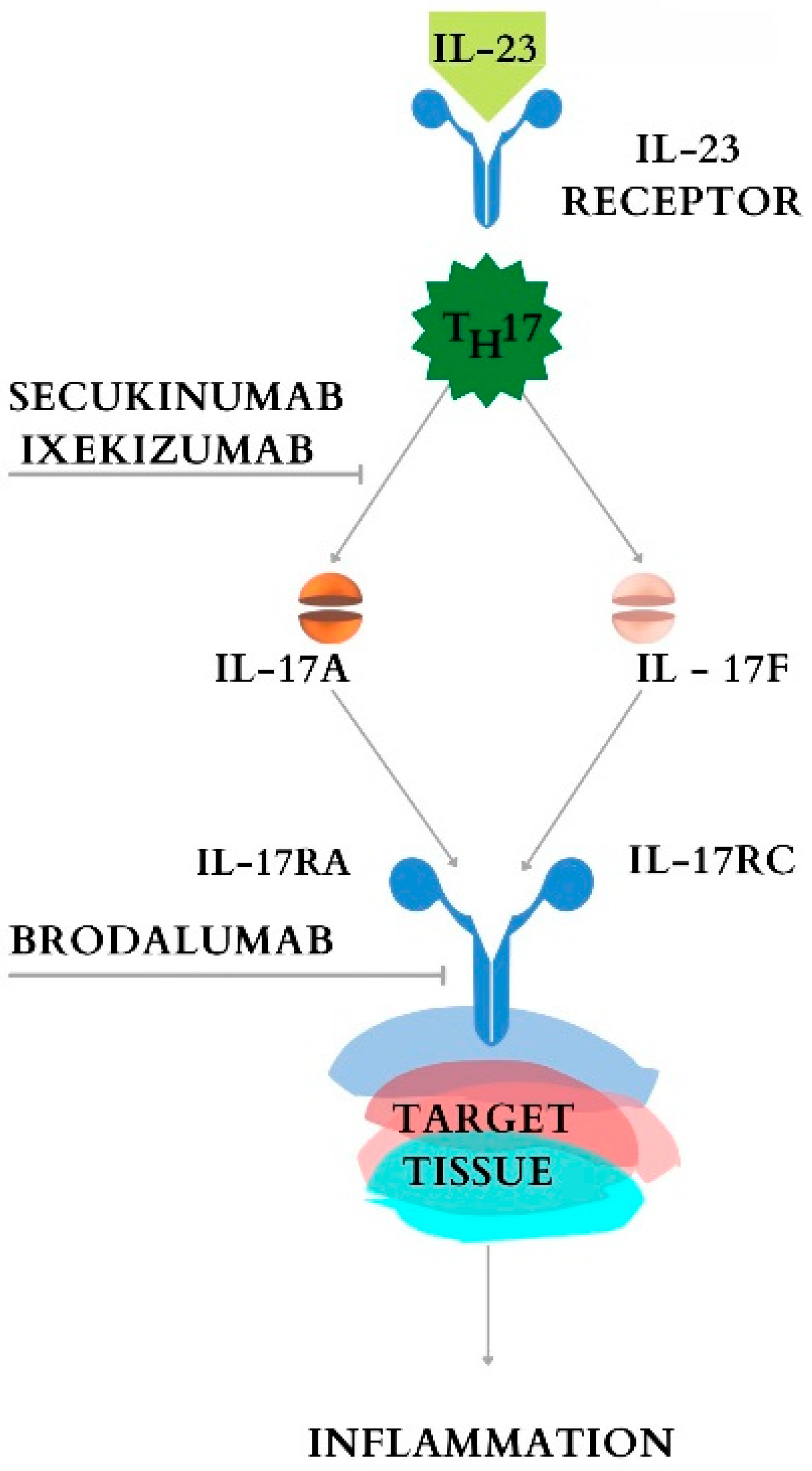
7. IL-17 Inhibitors and Their Role in Inflammatory Bowel Disease
7.1. Secukinumab
7.2. Ixekizumab
7.3. Other IL-17i
7.4. The Contribution of IL-17i to the Modification of the Intestinal Microbiome
8. Practical Recommendations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in dis-ease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Draberova, H.; Janusova, S.; Knizkova, D.; Semberova, T.; Pribikova, M.; Ujevic, A.; Harant, K.; Knapkova, S.; Hrdinka, M.; Fanfani, V.; et al. Systematic analysis of the IL-17 receptor signalosome reveals a robust regulatory feedback loop. EMBO Rep. 2020, 39, e104202. [Google Scholar] [CrossRef] [PubMed]
- Fieldhouse, K.A.; Ukaibe, S.; Crowley, E.L.; Khanna, R.; O’Toole, A.; Gooderham, M.J. Inflammatory bowel disease in patients with psoriasis treated with interleukin-17 inhibitors. Drugs Context 2020, 9, 2020-2-1. [Google Scholar] [CrossRef] [Green Version]
- Conrad, C.; Di Domizio, J.; Mylonas, A.; Belkhodja, C.; Demaria, O.; Navarini, A.A.; Lapointe, A.K.; French, L.E.; Vernez, M.; Gilliet, M. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat. Commun. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Fauny, M.; Moulin, D.; D’Amico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef]
- Brembilla, N.C.; Senra, L.; Boehncke, W.-H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [Green Version]
- Noviello, D.; Mager, R.; Roda, G.; Borroni, R.G.; Fiorino, G.; Vetrano, S. The IL23-IL17 immune axis in the treatment of ulcerative colitis: Successes, defeats, and ongoing challenges. Front. Immunol. 2021, 12, 611256. [Google Scholar] [CrossRef]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R.; Shah, B.; Acero, L.; Wang, Y.-H.; Schluns, K.S.; Broaddus, R.R.; Zhu, Z.; et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008, 205, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, J.; Huang, A.; Stinson, J.; Heldens, S.; Foster, J.; Dowd, P.; Gurney, A.L.; Wood, W.I. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. USA 2000, 97, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Im, E.; Jung, J.; Rhee, S.H. Toll-like receptor 5 engagement induces interleukin-17C expression in intestinal epithelial cells. J. Interferon Cytokine Res. 2012, 32, 583–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, J.M.; Martinez, G.J.; Nallaparaju, K.C.; Chang, S.H.; Wang, Y.H.; Dong, C. Cutting edge: Regulation of intestinal inflammation and barrier function by IL-17C. J. Immunol. 2012, 189, 4226–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimzhanov, A.M.; Yang, X.O.; Dong, C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 2007, 282, 5969–5972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, S.; Sakuraba, A. Distinct roles of interleukin-17 and T helper 17 cells among autoimmune diseases. J. Transl. Autoimmun. 2021, 4, 100104. [Google Scholar] [CrossRef]
- Berry, G.; Dossou, S.; Kashif, C.; Sharifinejad, A.; Azizi, N.; Hamedifar, G.; Sabzvari, A.; Zian, A. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int. Immunopharmacol. 2022, 102, 108402–108403. [Google Scholar] [CrossRef]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014, 2014, 928461. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef]
- Garduño, R.C.; Däbritz, J. New Insights on CD8+ T cells in inflammatory bowel disease and therapeutic Approaches. Front. Immunol. 2021, 12, 738762. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Maloy, K.J.; Kullberg, M.C. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol. 2008, 1, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Ogura, H.; Murakami, M.; Okuyama, Y.; Tsuruoka, M.; Kitabayashi, C.; Kanamoto, M.; Nishihara, M.; Iwakura, Y.; Hirano, T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008, 29, 628–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalom-Barak, T.; Quach, J.; Lotz, M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J. Biol. Chem. 1998, 273, 27467–27473. [Google Scholar] [CrossRef] [Green Version]
- Rouvier, E.; Luciani, M.F.; Mattéi, M.G.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a Herpesvirus Saimiri Gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar] [PubMed]
- Gaffen, S.L. An Overview of IL-17 function and signaling. Cytokine 2008, 43, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Painter, S.L.; Fanslow, W.C.; Ulrich, D.; Macduff, B.M.; Spriggs, M.K.; Armitage, R.J. Cutting edge: Human IL-17: A novel cytokine derived from T cells. J. Immunol. 1995, 155, 5483–5486. [Google Scholar] [PubMed]
- Liu, C.; Qian, W.; Qian, Y.; Giltiay, N.V.; Lu, Y.; Swaidani, S.; Misra, S.; Deng, L.; Chen, Z.J.; Li, X. Act1, a U-Box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2009, 2, ra63. [Google Scholar] [CrossRef] [Green Version]
- Schwandner, R.; Yamaguchi, K.; Cao, Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF) 6 in interleukin 17 signal transduction. J. Exp. Med. 2000, 191, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Qian, Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine 2013, 62, 175–182. [Google Scholar] [CrossRef]
- Sun, D.; Novotny, M.; Bulek, K.; Liu, C.; Li, X.; Hamilton, T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat. Immunol. 2011, 12, 853–860. [Google Scholar] [CrossRef]
- Herjan, T.; Yao, P.; Qian, W.; Li, X.; Liu, C.; Bulek, K.; Sun, D.; Yang, W.P.; Zhu, J.; He, A.; et al. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J. Immunol. Res. 2013, 191, 640–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000, 69, 142–159. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kwan, W.H.; van der Touw, W.; Paz-Artal, E.; Li, M.O.; Heeger, P.S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 2013, 210, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Justa, S.; Brucks, M.; Endres, J.; Fox, D.A.; Zhou, X.; Alnaimat, F.; Whitaker, B.; Wheeler, J.C.; Jones, B.H.; et al. Interleukin (IL)-17A, F and AF in inflammation: A study in collagen-induced arthritis and rheumatoid arthritis: IL-17 subtypes in inflammatory arthritis. Clin. Exp. Immunol. 2014, 177, 652–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.D.; Lee, D.M.; Kolbinger, F.; Antoni, C. Effect of IL-17A blockade with Secukinumab in autoimmune diseases. Ann. Rheum. Dis. 2013, 72 (Suppl. S2), ii116–ii123. [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.S.H.; Jadon, D.R. Th17 cell responses in spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 777–796. [Google Scholar] [CrossRef]
- Jandus, C.; Bioley, G.; Rivals, J.-P.; Dudler, J.; Speiser, D.; Romero, P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008, 58, 2307–2317. [Google Scholar] [CrossRef]
- Blanco, F.J.; Möricke, R.; Dokoupilova, E.; Codding, C.; Neal, J.; Andersson, M.; Rohrer, S.; Richards, H. Secukinumab in active rheumatoid arthritis: A phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017, 69, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Appel, H.; Maier, R.; Wu, P.; Scheer, R.; Hempfing, A.; Kayser, R.; Thiel, A.; Radbruch, A.; Loddenkemper, C.; Sieper, J. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 2011, 13, R95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.; Ciurtin, C.; Ismajli, M.; Leandro, M.; Sengupta, R.; Machado, P.M. Biologics for treating axial spondyloarthritis. Expert Opin. Biol. Ther. 2018, 18, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, L.A.; Ilieș, M.; Vesa Ștefan, C.; Seicean, R.; Din, S.; Iuga, C.A.; Seicean, A. Serum interleukin (IL)-23 and IL-17 profile in inflammatory bowel disease (IBD) patients could differentiate between severe and non-severe disease. J. Pers. Med. 2021, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Rosca, E.; Tit, D.M.; Mutiu, G.; Bungau, S.G.; Pop, O.L. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom. J. Morphol. Embriol. 2015, 56, 1103–1109. [Google Scholar]
- Mirsattari, D.; Seyyedmajidi, M.; Zojaji, H.; Haghazali, M.; Orimi, P.G.; Shoushtarizadeh, T.; Almasi, S. The relation between the level of interleukin-23 with duration and severity of ulcerative colitis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 49–53. [Google Scholar]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Jiang, W.; Su, J.; Zhang, X.; Cheng, X.; Zhou, J.; Shi, R.; Zhang, H. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 2014, 63, 943–950. [Google Scholar] [CrossRef]
- Gheita, T.A.; El Gazzar, I.I.; El-Fishawy, H.S.; Aboul-Ezz, M.A.; Kenawy, S.A. Involvement of IL-23 in enteropathic arthritis patients with inflammatory bowel disease: Preliminary results. Gastroenterol. Hepatol. 2012, 33, 49–53. [Google Scholar] [CrossRef]
- Fuss, I.J.; Becker, C.; Yang, Z.; Groden, C.; Hornung, R.L.; Heller, F.; Neurath, M.F.; Strober, W.; Mannon, P.J. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 P40 monoclonal antibody. Inflamm. Bowel Dis. 2006, 12, 9–15. [Google Scholar] [CrossRef]
- McGovern, D.; Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 2007, 56, 1333–1336. [Google Scholar] [CrossRef] [Green Version]
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J. A genome-wide association study identifies IL-23R as an inflammatory bowel disease gene. Science 2006, 314, 1461–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einarsdottir, E.; Koskinen, L.L.E.; Dukes, E.; Kainu, K.; Suomela, S.; Lappalainen, M.; Ziberna, F.; Korponay-Szabo, I.R.; Kurppa, K.; Kaukinen, K.; et al. IL23R in the Swedish, Finnish, Hungarian and Italian populations: Association with IBD and psoriasis, and linkage to celiac disease. BMC Med. Genet. 2009, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Saieva, L.; Peralta, S.; Giardina, A.; Cannizzaro, A.; Sireci, G.; De Leo, G.; Alessandro, R.; et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 2015, 74, 1739–1747. [Google Scholar] [CrossRef]
- Zeng, B.; Shi, S.; Ashworth, G.; Dong, C.; Liu, J.; Xing, F. ILC3 Function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 2019, 10, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allocca, M.; Furfaro, F.; Fiorino, G.; Gilardi, D.; D’Alessio, S.; Danese, S. Can IL-23 be a good target for ulcerative colitis? Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Ogawa, A.; Andoh, A.; Araki, Y.; Bamba, T.; Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 2004, 110, 55–62. [Google Scholar] [CrossRef]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Däbritz, J.; Gerner, P.; Enninger, A.; Claßen, M.; Radke, M. Inflammatory bowel disease in childhood and adolescence. Dtsch. Arztebl. Int. 2017, 114, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Negrut, N.; Khan, S.A.; Bungau, S.; Zaha, D.C.; Anca, C.A.R.; Bratu, O.; Diaconu, C.C.; Ionita-Radu, F. Diagnostic challenges in gastrointestinal infections. Rom. J. Mil. Med. 2020, 123, 83–90. [Google Scholar]
- Petitpain, N.; D’Amico, F.; Yelehe-Okouma, M.; Jouzeau, J.-Y.; Netter, P.; Peyrin-Biroulet, L.; Gillet, P. IL-17 inhibitors and inflammatory bowel diseases: A postmarketing study in Vigibase. Clin. Pharmacol. Ther. 2021, 110, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Cosentyx. Prescribing Information; Novartis Pharmaceutical Corporation: Basel, Switzerland. Available online: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/cosentyx.pdf (accessed on 1 January 2022).
- Hohenberger, M.; Cardwell, L.A.; Oussedik, E.; Feldman, S.R. Interleukin-17 inhibition: Role in psoriasis and inflammatory bowel disease. J. Dermatolog. Treat. 2018, 29, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis-Results of two phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W.; Higgins, P.D.R.; Wehkamp, J.; Feagan, B.G.; Yao, M.D.; Karczewski, M.; et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef] [Green Version]
- Gómez, A.O.; Velázquez, L.M.; Sanchez, L.B.; Sánchez, I.P.; Rabasco, E.P.; Monsalve, G.; Ferrández, A.G.; Sepulcre, J. Inflammatory bowel disease new-onset during Secukinumab therapy: Real-world data from a tertiary center. Rev. Esp. Enferm. Dig. 2021, 113, 858–859. [Google Scholar] [CrossRef]
- Wright, S.; Aloo, A.; Strunk, A.; Garg, A. Real-world risk of new-onset inflammatory bowel disease among patients with psoriasis exposed to interleukin 17 inhibitors. J. Am. Acad. Dermatol. 2020, 83, 382–387. [Google Scholar] [CrossRef]
- Yamada, A.; Wang, J.; Komaki, Y.; Komaki, F.; Micic, D.; Sakuraba, A. Systematic review with meta-analysis: Risk of new onset IBD with the use of anti-interleukin-17 agents. Aliment. Pharmacol. Ther. 2019, 50, 373–385. [Google Scholar] [CrossRef]
- Wang, J.; Bhatia, A.; Krugliak Cleveland, N.; Gupta, N.; Dalal, S.; Rubin, D.T.; Sakuraba, A. Rapid onset of inflammatory bowel disease after receiving Secukinumab infusion. ACG Case Rep. J. 2018, 5, e56. [Google Scholar] [CrossRef]
- Schreiber, S.; Colombel, J.F.; Feagan, B.G.; Reich, K.; Deodhar, A.A.; McInnes, I.B.; Porter, B.; Das Gupta, A.; Pricop, L.; Fox, T. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with Secukinumab: A retrospective analysis of pooled data from 21 clinical trials. Ann. Rheum. Dis. 2019, 78, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Burisch, J.; Eigner, W.; Schreiber, S.; Aletaha, D.; Weninger, W.; Trauner, M.; Reinisch, W.; Narula, N. Risk for development of inflammatory bowel disease under inhibition of interleukin 17: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0233781. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Hardin, D.S.; Abreu, M.T.; Sartor, B.; Xu, R.; Solotkin, W.; Bachelez, K.; Colombel, H. Incidence of inflammatory bowel disease among Ixekizumab-treated patients with moderate-to-severe plaque psoriasis and psoriatic arthritis: Data from 8 Clinical Trials. J. Am. Acad. Dermatol. 2017, 76, AB412. [Google Scholar]
- Smith, M.K.; Pai, J.; Panaccione, R.; Ferraz, J.G.; Jijon, J.G. Crohn’s-like disease in a patient exposed to anti-Interleukin-17 blockade (Ixekizumab) for the treatment of chronic plaque psoriasis: A case report. BMC Gastroenterol. 2019, 19, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Heijde, D.; Wei, C.-C.; Dougados, J.; Mease, M.; Deodhar, P.; Maksymowych, A.; Van Den Bosch, W.P.; Sieper, F.; Tomita, J.; Landewé, T.; et al. Carlier on Behalf of the COAST-V Study Group* Ixekizumab, an Interleukin-17A Antagonist in the Treatment of Ankylosing Spondylitis or Radiographic Axial Spondyloarthritis in Patients Previously Untreated with Biological Disease-Modifying Anti-Rheumatic Drugs (COAST-V): 16 Week Results of a Phase 3 Randomised, Double-Blind, Active-Controlled and Placebo-Controlled Trial. Lancet 2018, 392, 2441–2451. [Google Scholar]
- Strober, B.; Leonardi, C.; Papp, K.A.; Mrowietz, U.; Ohtsuki, M.; Bissonnette, R.; Ferris, L.K.; Paul, C.; Lebwohl, M.; Braun, D.K.; et al. Short- and long-term safety outcomes with Ixekizumab from 7 clinical trials in psoriasis: Etanercept comparisons and integrated Data. J. Am. Acad. Dermatol. 2017, 76, 432–440.e17. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.A.; Leonardi, C.; Menter, A.; Ortonne, J.P.; Krueger, J.G.; Kricorian, G.; Aras, G.; Li, J.; Russell, C.B.; Thompson, E.H.Z.; et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 2012, 366, 1181–1189. [Google Scholar] [CrossRef]
- Targan, S.R.; Feagan, B.; Vermeire, S.; Panaccione, R.; Melmed, G.Y.; Landers, C.; Li, D.; Russell, C.; Newmark, R.; Zhang, N.; et al. A Randomized, double-blind, placebo-controlled phase 2 study of Brodalumab in patients with moderate-to-severe Crohn’s disease. Am. J. Gastroenterol. 2016, 111, 1599–1607. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Kavanaugh, A.; Merola, J.F.; Schett, G.; Scher, J.U.; Warren, R.B.; Assudani, D.; Kumke, T.; Ink, B.; McInnes, I.B.; et al. Dual neutralization of IL-17A and IL-17F with bimekizumab in patients with active PsA: Results from a 48-week phase 2b, randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheumatol. 2018, 70 (Suppl. S10). Available online: https://acrabstracts.org/abstract/dual-neutralization-of-il-17a-and-il-17f-with-bimekizumab-in-patients-with-active-psa-results-from-a-48-week-phase-2b-randomized-double%e2%80%91blind-placebo-controlled-dose-ranging-study/ (accessed on 25 April 2022).
- Orrell, K.A.; Murphrey, M.; Kelm, R.C.; Lee, H.H.; Pease, D.R.; Laumann, A.E.; West, D.P.; Nardone, B. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors Secukinumab and Ixekizumab: Postmarketing analysis from the RADAR (“Research on Adverse Drug Events and Reports”) Program. J. Am. Acad. Dermatol. 2018, 79, 777–778. [Google Scholar] [CrossRef]
- Mohy-ud-din, N.; Carleton, N.; El-Hachem, S.; Baki, H.A.; Syed, A.; Dulai, P.; Kochhar, G. 731 De Novo Inflammatory Bowel Disease after Secukinumab Use: A Population Based Analysis: 731. Am. J. Gastroenterol. 2019, 114, S431. [Google Scholar] [CrossRef]
- Jancin, B. Here Comes Bimekizumab, the Newest IL-17 Inhibitor. Available online: https://www.mdedge.com/edermatologynews/article/158562/psoriatic-arthritis/here-comes-bimekizumab-newest-Il-17-inhibitor (accessed on 1 January 2020).
- Egeberg, A.; Thyssen, J.P.; Burisch, J.; Colombel, J.-F. Incidence and Risk of Inflammatory Bowel Disease in Patients with Psoriasis-A Nationwide 20-Year Cohort Study. J. Investig. Dermatol. 2019, 139, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Maronese, C.A.; Zelin, E.; Moltrasio, C.; Genovese, G.; Marzano, A.V. Genetic screening in new onset inflammatory bowel disease during anti-interleukin 17 therapy: Unmet needs and call for action. Expert Opin. Biol. Ther. 2021, 21, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Manasson, L.; Wallach, D.S.; Guggino, G.; Stapylton, M.; Badri, M.B.; Solomon, G.; Reddy, S.M.; Aksenov, C.R.; Jones, A.A.; Girija, D.R.; et al. Interleukin-17 Inhibition in Spondyloarthritis Is Associated with Subclinical Gut Microbiome Perturbations and a Distinctive Interleukin-25-Driven Intestinal Inflammation. Arthritis Rheumatol. 2020, 72, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, C.; Gaitanis, G.; Katsanos, K.H.; Christodoulou, D.K.; Tsianos, E.; Bassukas, I.D. Psoriasis and inflammatory bowel disease: Links and risks. Psoriasis 2016, 6, 73–92. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Davidson, L.; van den Reek, J.M.P.A.; Bruno, M.; van Hunsel, F.; Herings, R.M.C.; Matzaraki, V.; Boahen, C.K.; Kumar, V.; Groenewoud, H.M.M.; van de Veerdonk, F.L.; et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple in-dependent sources. Lancet Reg. Health Eur. 2022, 13, 100266. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Review Article: Faecal Calprotectin and Histologic Remission in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2020, 51, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Lepage, P.; Häsler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef]

| Identification of Studies via Databases and Registers for the Review | |
|---|---|
| Identification | Key words with “AND” operator “IL-17 inhibitors” AND ”inflammatory intestinal disease” |
| Consulted databases Web of Science, PubMed, Scopus | |
| Criteria for inclusion Review or original article, relevant for the topic and thematic | |
| Criteria for exclusion Abstract paper, articles where full text was not available, not relevant to the topic or thematic | |
| Records identified 170 | |
| Articles after duplicate removal (duplicates = 28) 142 | |
| Full-text analysis Appliance of inclusion and exclusioncriteria 106 records (eliminated 36 articles) | |
| Final bibliographical sources Verification and agreement on article 94 relevance and quality | |
| Study and Patients | Results | Observations | Reference |
|---|---|---|---|
| >56 million persons health records from Explorys * (IBM New York) Tracking period: 10 years (1999–2019) | At 6 months, the incidence of IBD: 0.16% among patients with Ps exposed to any IL-17i, 0.24% among those exposed to SEC alone, 0.11% among those unexposed. At first year: the incidence of IBD 0.27% for the patients who received IL-17i, 0.32% for those being treated with SEC only, 0.19% for those who did not receive treatment with IL-17i. | The incidence of IBD is low, and the risk appears similar to that in unexposed patients. | [68] |
| 38 (RCTs **) including 16,690 patients Tracking period: 60 weeks | 12 cases of new onset IBD were reported in five studies, whereas no cases were reported with placebo | De novo IBD was rare | [69] |
| 21 RCT of 7355 patients with Ps, PsA, AS treated with SEC Tracking period: 5 years | Of the 5181 patients with Ps, 68 were diagnosed with IBD; of the 1380 with PsA, 8 cases of IBD were diagnosed, and of the 794 patients with AS, 13 developed IBD. | The IBD was present in 1.7% | [71] |
| 66 studies of 14,390 patients exposed to induction and 19,380 patients exposed to induction and/or maintenance treatment Tracking period: 2010–2018 | During induction, 11 cases of IBD were reported, 33 cases diagnosed during entire treatment period | The risk for onset of IBD in patients treated with IL-17i is not elevated | [72] |
| 38 RCTs with a total 12,614 patients treated with IL-17i and 4076 treated with placebo. The RCTs included 8 studies of BRO (4588 patients), 8 of IXE (4485 patients), and 22 of SEC (7617 patients). | 12 new cases of IBD (4 on IXE, 8 on SEC) | Incidence: 2.4 cases of new onset IBD/1000 patient-years. Statistically there was no difference in the risk of developing new onset IBD with IL-17i compared with placebo. | [14] |
| Collected clinical cases of de novo IBD reported in the studies on digestive paradoxical effects after treatment with IL-17i. Tracking period: up to 2020. | 21 patients with IBD (19 after SEC and 2 after IXE). | The treatment with BRO, IXE, SEC, is associated with a low incidence of IBD in patients with rheumatological and dermatological autoimmune diseases in both clinical trials and clinical practice (2.4/1000 patient-years) | [6] |
| Published articles identifies 21 publications on the onset of IBD after treatment with IL-17Ai (SEC and IXE). Tracking period: 2018–2019 | 27 patients diagnosed with Ps, PsA, and AS after treatment with | IL-17i have proven efficacy for the treatment of Ps and PsA with a strong safety profile. | [4] |
| Study and Patients | Results | Conclusion | Reference |
|---|---|---|---|
| >5 million patients with IBD treated with SEC and IXE from RADAR * and NMEDW. Tracking period: 2 years (2015–2017) for SEC IXE: 1 year and 7 months (2016–2017) for IXE | Cases with new IBD from reviewed database | AE reported after administration of SEC and IXE to the FAERS ** were insignificant. The PRR *** = 4.65 | [81] |
| 62 million electronic health records were analyzed from Explorys (IBM New York) ****. A number of 2870 patients were included | 3.2% patients develop IBD after treatment with SEC; risk in population was 0.74% | The risk for de-novo IBD is higher compared to general population. The patients were obese and younger. | [82] |
| 235,038 patients with Ps and non-Ps from the general population, all without a history of CD or UC at baseline Tracking period: 20 years | IBD appear in <1% of patients with Ps, but the biologic classes were not differentiated | Patients receiving biologic therapy did not have a higher risk of developing IBD compared with the control group (biologic treatement was not separated/differentiated by drug class) | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țiburcă, L.; Bembea, M.; Zaha, D.C.; Jurca, A.D.; Vesa, C.M.; Rațiu, I.A.; Jurca, C.M. The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Curr. Issues Mol. Biol. 2022, 44, 1851-1866. https://doi.org/10.3390/cimb44050127
Țiburcă L, Bembea M, Zaha DC, Jurca AD, Vesa CM, Rațiu IA, Jurca CM. The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Current Issues in Molecular Biology. 2022; 44(5):1851-1866. https://doi.org/10.3390/cimb44050127
Chicago/Turabian StyleȚiburcă, Laura, Marius Bembea, Dana Carmen Zaha, Alexandru Daniel Jurca, Cosmin Mihai Vesa, Ioana Adela Rațiu, and Claudia Maria Jurca. 2022. "The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases" Current Issues in Molecular Biology 44, no. 5: 1851-1866. https://doi.org/10.3390/cimb44050127
APA StyleȚiburcă, L., Bembea, M., Zaha, D. C., Jurca, A. D., Vesa, C. M., Rațiu, I. A., & Jurca, C. M. (2022). The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Current Issues in Molecular Biology, 44(5), 1851-1866. https://doi.org/10.3390/cimb44050127









