Beneficial Effect of Fenofibrate and Silymarin on Hepatic Steatosis and Gene Expression of Lipogenic and Cytochrome P450 Enzymes in Non-Obese Hereditary Hypertriglyceridemic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Gene Expression Assay
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Effects of FF and SLM on Serum and Liver Lipids
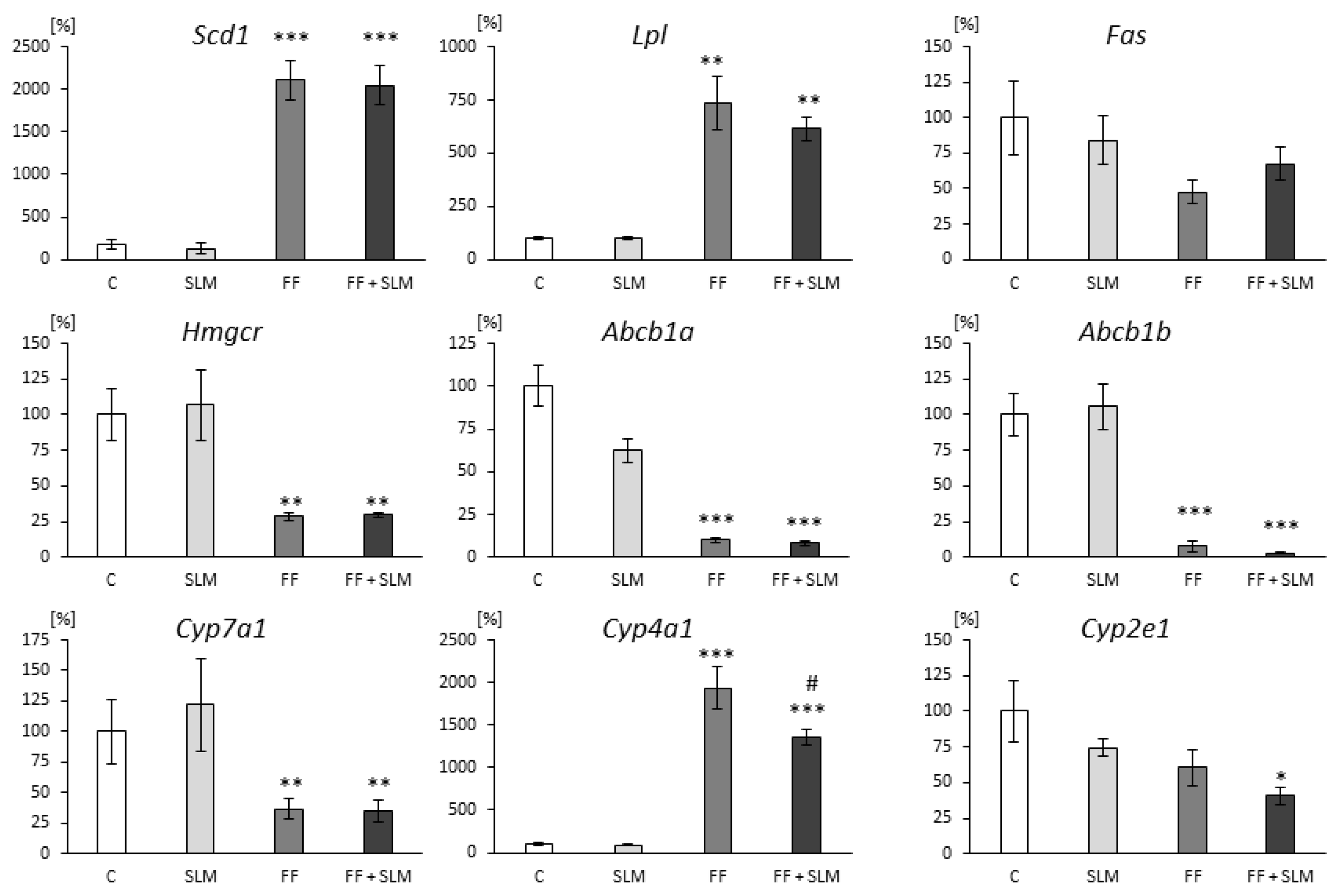
3.2. Effects of FF and SLM on Gene Expression
4. Discussion
4.1. Effects of FF Monotherapy
4.2. Effect of SLM Monotherapy
4.3. Effect of Combination Therapy FF with SLM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCB1a, ABCB1b | ATP-binding cassette transporter sub-family b member 1 |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CYP7A1, CYP4A1, CYP2E1 | cytochrome P450 |
| Fas | fatty acid synthase |
| FF | fenofibrate |
| HHTg | hereditary hypertriglyceridemic |
| HMGCR | 3-hydroxy-3methyl-glutaryl-coenzyme A reductase |
| IL-6 | interleukin 6 |
| LPL | lipoprotein lipase |
| MCP-1 | monocyte chemoattractant protein 1 |
| MUFA | monounsaturated fatty acids |
| NAFLD | nonalcoholic fatty liver disease |
| PPARα | peroxisome proliferator-activated receptor alpha |
| ROS | reactive oxygen species |
| SCD1 | stearoyl-CoA desaturase |
| SHR | spontaneously hypertensive rats |
| SLM | silymarin |
References
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Ioannou, G.N.; Boyko, E.J.; Utzschneider, K.M. Non-Alcoholic Fatty Liver Disease as an Independent Manifestation of the Metabolic Syndrome: Results of a US National Survey in Three Ethnic Groups. J. Gastroenterol. Hepatol. 2013, 28, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.E.; Renquist, B.J. Hepatic Lipid Accumulation: Cause and Consequence of Dysregulated Glucoregulatory Hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the Roles of PPARα in Energy Metabolism and Vascular Homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- McCullough, P.A.; Ahmed, A.B.; Zughaib, M.T.; Glanz, E.D.; di Loreto, M.J. Treatment of Hypertriglyceridemia with Fibric Acid Derivatives: Impact on Lipid Subfractions and Translation into a Reduction in Cardiovascular Events. Rev. Cardiovasc. Med. 2011, 12, 173–185. [Google Scholar] [CrossRef]
- Bajaj, M.; Suraamornkul, S.; Hardies, L.J.; Glass, L.; Musi, N.; DeFronzo, R.A. Effects of Peroxisome Proliferator-Activated Receptor (PPAR)-α and PPAR-γ Agonists on Glucose and Lipid Metabolism in Patients with Type 2 Diabetes Mellitus. Diabetologia 2007, 50, 1723–1731. [Google Scholar] [CrossRef]
- Fernández-Miranda, C.; Pérez-Carreras, M.; Colina, F.; López-Alonso, G.; Vargas, C.; Solís-Herruzo, J.A. A Pilot Trial of Fenofibrate for the Treatment of Non-Alcoholic Fatty Liver Disease. Dig. Liver Dis. 2008, 40, 200–205. [Google Scholar] [CrossRef]
- Fabbrini, E.; Mohammed, B.S.; Korenblat, K.M.; Magkos, F.; McCrea, J.; Patterson, B.W.; Klein, S. Effect of Fenofibrate and Niacin on Intrahepatic Triglyceride Content, Very Low-Density Lipoprotein Kinetics, and Insulin Action in Obese Subjects with Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2010, 95, 2727–2735. [Google Scholar] [CrossRef]
- Oscarsson, J.; Önnerhag, K.; Risérus, U.; Sundén, M.; Johansson, L.; Jansson, P.A.; Moris, L.; Nilsson, P.M.; Eriksson, J.W.; Lind, L. Effects of Free Omega-3 Carboxylic Acids and Fenofibrate on Liver Fat Content in Patients with Hypertriglyceridemia and Non-Alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Lipidol. 2018, 12, 1390–1403. [Google Scholar] [CrossRef]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential Therapeutic Targets. Biochim. Biophys. Acta 2012, 1821, 809–818. [Google Scholar] [CrossRef]
- Waterman, I.J.; Zammit, V.A. Differential Effects of Fenofibrate or Simvastatin Treatment of Rats on Hepatic Microsomal Overt and Latent Diacylglycerol Acyltransferase Activities. Diabetes 2002, 51, 1708–1713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edvardsson, U.; Ljungberg, A.; Lindén, D.; William-Olsson, L.; Peilot-Sjögren, H.; Ahnmark, A.; Oscarsson, J. PPARα Activation Increases Triglyceride Mass and Adipose Differentiation-Related Protein in Hepatocytes. J. Lipid Res. 2006, 47, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, Q.; Xu, C.; Cao, M.; Zhou, X.; Wang, T.; Yu, C.; Jing, F.; Chen, W.; Gao, L.; et al. Peroxisome Proliferator-Activated Receptor α Activation Induces Hepatic Steatosis, Suggesting an Adverse Effect. PLoS ONE 2014, 9, e99245. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Odin, J.A.; Hayashi, P.H.; Chalasani, N.; Fontana, R.J.; Barnhart, H.; Cirulli, E.T.; Kleiner, D.E.; Hoofnagle, J.H. Identification and Characterization of Fenofibrate-Induced Liver Injury. Dig. Dis. Sci. 2017, 62, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Škop, V.; Trnovská, J.; Oliyarnyk, O.; Marková, I.; Malínská, H.; Kazdová, L.; Zídek, V.; Landa, V.; Mlejnek, P.; Šimáková, M.; et al. Hepatotoxic Effects of Fenofibrate in Spontaneously Hypertensive Rats Expressing Human C-Reactive Protein. Physiol. Res. 2016, 65, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.; González-Rubio, M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective Effect of Silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H.J. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Škottová, N.; Kazdová, L.; Oliyarnyk, O.; Večeřa, R.; Sobolová, L.; Ulrichová, J. Phenolics-Rich Extracts from Silybum Marianum and Prunella Vulgaris Reduce a High-Sucrose Diet Induced Oxidative Stress in Hereditary Hypertriglyceridemic Rats. Pharm. Res. 2004, 50, 123–130. [Google Scholar] [CrossRef]
- Poruba, M.; Kazdová, L.; Oliyarnyk, O.; Malinská, H.; Matusková, Z.; Tozzi Di Angelo, I.; Skop, V.; Vecera, R. Improvement Bioavailability of Silymarin Ameliorates Severe Dyslipidemia Associated with Metabolic Syndrome. Xenobiotica 2015, 45, 751–756. [Google Scholar] [CrossRef]
- Ebrahimpour-koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Lower Glycemic Indices and Lipid Profile among Type 2 Diabetes Mellitus Patients Who Received Novel Dose of Silybum Marianum (L.) Gaertn. (Silymarin) Extract Supplement: A Triple-Blinded Randomized Controlled Clinical Trial. Phytomedicine 2018, 44, 39–44. [Google Scholar] [CrossRef]
- Mohammadi, H.; Hadi, A.; Arab, A.; Moradi, S.; Rouhani, M.H. Effects of Silymarin Supplementation on Blood Lipids: A Systematic Review and Meta-Analysis of Clinical Trials. Phytother. Res. 2019, 33, 871–880. [Google Scholar] [CrossRef]
- di Pierro, F.; Callegari, A.; Carotenuto, D.; Tapia, M. Clinical Efficacy, Safety and Tolerability of BIO-C (Micronized Silymarin) as a Galactagogue. Acta Biomed. 2009, 79, 205–210. [Google Scholar]
- Poruba, M.; Matušková, Z.; Kazdová, L.; Oliyarnyk, O.; Malínská, H.; Tozzi, I.; Angelo, D.I.; Večeřa, R. Positive Effects of Different Drug Forms of Silybin in the Treatment of Metabolic Syndrome. Physiol. Res. 2015, 64, 507–512. [Google Scholar] [CrossRef]
- Vrána, A.; Kazdová, L. The Hereditary Hypertriglyceridemic Nonobese Rat: An Experimental Model of Human Hypertriglyceridemia. Transpl. Proc. 1990, 22, 2579. [Google Scholar]
- Zicha, J.; Pecháňová, O.; Čačányiová, S.; Cebová, M.; Kristek, F.; Török, J.; Šimko, F.; Dobešová, Z.; Kuneš, J. Hereditary Hypertriglyceridemic Rat: A Suitable Model of Cardiovascular Disease and Metabolic Syndrome? Physiol. Res. 2006, 55, 49–63. [Google Scholar]
- Škop, V.C.; Malínská, H.; Trnovská, J.; Hüttl, M.; Cahová, M.; Blachnio-Zabielska, A.; Baranowski, M.; Burian, M.; Oliyarnyk, O.; Kazdová, L. Positive Effects of Voluntary Running on Metabolic Syndrome-Related Disorders in Non-Obese Hereditary Hypertriacylglycerolemic Rats. PLoS ONE 2015, 10, e0122768. [Google Scholar] [CrossRef]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global Prevalence, Incidence, and Outcomes of Non-Obese or Lean Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Ibarra-Lara, L.; Sánchez-Aguilar, M.; Sánchez-Mendoza, A.; del Valle-Mondragón, L.; Soria-Castro, E.; Carreón-Torres, E.; Díaz-Díaz, E.; Vázquez-Meza, H.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Fenofibrate Therapy Restores Antioxidant Protection and Improves Myocardial Insulin Resistance in a Rat Model of Metabolic Syndrome and Myocardial Ischemia: The Role of Angiotensin II. Molecules 2016, 22, 31. [Google Scholar] [CrossRef]
- Goel, S.K.; Lalwani, N.D.; Reddy, J.K. Peroxisome Proliferation and Lipid Peroxidation in Rat Liver. Cancer Res. 1986, 46, 1324–1330. [Google Scholar]
- Poruba, M.; Matuskova, Z.; Hüttl, M.; Malinska, H.; Oliyarnyk, O.; Markova, I.; Gurska, S.; Kazdova, L.; Vecera, R. Fenofibrate Decreases Hepatic P-Glycoprotein in a Rat Model of Hereditary Hypertriglyceridemia. Front. Pharm. 2019, 10, 56. [Google Scholar] [CrossRef]
- Yu, X.H.; Zheng, X.L.; Tang, C.K. Peroxisome Proliferator-Activated Receptor α in Lipid Metabolism and Atherosclerosis. Adv. Clin. Chem. 2015, 71, 171–203. [Google Scholar] [PubMed]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα Is Crucial for Whole-Body Fatty Acid Homeostasis and Is Protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Cariello, M.; de Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Oosterveer, M.H.; Grefhorst, A.; van Dijk, T.H.; Havinga, R.; Staels, B.; Kuipers, F.; Groen, A.K.; Reijngoud, D.J. Fenofibrate Simultaneously Induces Hepatic Fatty Acid Oxidation, Synthesis, and Elongation in Mice. J. Biol. Chem. 2009, 284, 34036–34044. [Google Scholar] [CrossRef]
- Kersten, S. Physiological Regulation of Lipoprotein Lipase. Biochim. Biophys. Acta 2014, 1841, 919–933. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.L.; Semenkovich, C.F. Fatty Acid Synthase and Liver Triglyceride Metabolism: Housekeeper or Messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Foucaud-Vignault, M.; Soayfane, Z.; Ménez, C.; Bertrand-Michel, J.; Martin, P.G.P.; Guillou, H.; Collet, X.; Lespine, A. P-Glycoprotein Dysfunction Contributes to Hepatic Steatosis and Obesity in Mice. PLoS ONE 2011, 6, e23614. [Google Scholar] [CrossRef]
- Ehrhardt, M.; Lindenmaier, H.; Burhenne, J.; Haefeli, W.E.; Weiss, J. Influence of Lipid Lowering Fibrates on P-Glycoprotein Activity in Vitro. Biochem. Pharm. 2004, 67, 285–292. [Google Scholar] [CrossRef]
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Roglans, N.; Vázquez-Carrera, M.; Alegret, M.; Novell, F.; Zambón, D.; Ros, E.; Laguna, J.C.; Sánchez, R.M. Fibrates Modify the Expression of Key Factors Involved in Bile-Acid Synthesis and Biliary-Lipid Secretion in Gallstone Patients. Eur. J. Clin. Pharm. 2003, 59, 855–861. [Google Scholar]
- Shen, J.; Arnett, D.K.; Parnell, L.D.; Lai, C.Q.; Straka, R.J.; Hopkins, P.N.; An, P.; Feitosa, M.F.; Ordovás, J.M. The Effect of CYP7A1 Polymorphisms on Lipid Responses to Fenofibrate. J. Cardiovasc. Pharm. 2012, 59, 254–259. [Google Scholar] [CrossRef]
- Srivastava, R.A.K.; Cefalu, A.B.; Srivastava, N.S.; Averna, M. NPC1L1 and ABCG5/8 Induction Explain Synergistic Fecal Cholesterol Excretion in Ob/Ob Mice Co-Treated with PPAR-α and LXR Agonists. Mol. Cell. Biochem. 2020, 473, 247–262. [Google Scholar] [CrossRef]
- Ståhlberg, D.; Reihnér, E.; Ewerth, S.; Einarsson, K.; Angelin, B. Effects of Bezafibrate on Hepatic Cholesterol Metabolism. Eur. J. Clin. Pharm. 1991, 40, S33–S36. [Google Scholar] [CrossRef]
- Orolin, J.; Večeřa, R.; Jung, D.; Meyer, U.A.; Škottová, N.; Anzenbacher, P. Hypolipidemic Effects of Silymarin Are Not Mediated by the Peroxisome Proliferator-Activated Receptor Alpha. Xenobiotica 2008, 37, 725–735. [Google Scholar] [CrossRef]
- Lupp, A.; Karge, E.; Deufel, T.; Oelschläger, H.; Fleck, C. Ciprofibrate, Clofibric Acid and Respective Glycinate Derivatives: Effects of a Four-Week Treatment on Male Lean and Obese Zucker Rats. Arzneimittelforschung 2008, 58, 225–241. [Google Scholar]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk Thistle (Silybum Marianum): A Concise Overview on Its Chemistry, Pharmacological, and Nutraceutical Uses in Liver Diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative Activity of High-Density Lipoprotein (HDL): Mechanistic Insights into Potential Clinical Benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Xiao, F.; Gao, F.; Zhou, S.; Wang, L. The Therapeutic Effects of Silymarin for Patients with Glucose/Lipid Metabolic Dysfunction: A Meta-Analysis. Medicine 2020, 99, e22249. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Ohta, T.; Masutomi, N.; Tsutsui, N.; Sakairi, T.; Mitchell, M.; Milburn, M.V.; Ryals, J.A.; Beebe, K.D.; Guo, L. Untargeted Metabolomic Profiling as an Evaluative Tool of Fenofibrate-Induced Toxicology in Fischer 344 Male Rats. Toxicol. Pathol. 2009, 37, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Vodeničarovová, M. Effects of Low and High Doses of Fenofibrate on Protein, Amino Acid, and Energy Metabolism in Rat. Int. J. Exp. Pathol. 2020, 101, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Edson, K.Z.; Totah, R.A.; Rettie, A.E. Cytochrome P450 ω-Hydroxylases in Inflammation and Cancer. Adv. Pharm. 2015, 74, 223–262. [Google Scholar]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased Expression of Cytochrome P450 2E1 in Nonalcoholic Fatty Liver Disease: Mechanisms and Pathophysiological Role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef]
- Leung, T.M.; Nieto, N. CYP2E1 and Oxidant Stress in Alcoholic and Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2013, 58, 395–398. [Google Scholar] [CrossRef]
- Harjumäki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
| Parameter | C | SLM | FF | FF + SLM |
|---|---|---|---|---|
| Body weight (g) | 414 ± 4 | 406 ± 2 | 370 ± 7 b ** | 386 ± 7 c ** |
| Serum lipids | ||||
| Triglycerides (mmol/L) | 4.95 ± 0.22 | 4.03 ± 0.12 a ** | 1.15 ± 0.04 b *** | 1.16 ± 0.55 c *** |
| Cholesterol (mmol/L) | 1.59 ± 0.05 | 1.60 ± 0.03 | 1.01 ± 0.03 b *** | 1.00 ± 0.03 c *** |
| HDL cholesterol (mmol/L) | 0.43 ± 0.05 | 0.62 ± 0.06 a * | 0.62 ± 0.03 b * | 0.62 ± 0.03 c ** |
| Liver lipids | ||||
| Triglycerides (μmol/g) | 8.92 ± 0.66 | 7.57 ± 0.46 | 2.80 ± 0.10 b *** | 3.13 ± 0.22 c *** |
| Cholesterol (μmol/g) | 6.55 ± 0.18 | 6.57 ± 0.17 | 4.83 ± 0.20 b ** | 4.87 ± 0.18 c ** |
| Lipoperoxidation products in the liver | ||||
| CD (nmol/mg proteins) | 31.5 ± 2.4 | 23.8 ± 2.0 a * | 38.0 ± 2.7 | 38.4 ± 2.2 |
| TBARS (nmol/mg proteins) | 1.69 ± 0.18 | 1.21 ± 0.12 a * | 2.14 ± 0.15 b * | 1.37 ± 0.12 d ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecera, R.; Poruba, M.; Hüttl, M.; Malinska, H.; Oliyarnyk, O.; Markova, I.; Racova, Z.; Soukop, J.; Kazdova, L. Beneficial Effect of Fenofibrate and Silymarin on Hepatic Steatosis and Gene Expression of Lipogenic and Cytochrome P450 Enzymes in Non-Obese Hereditary Hypertriglyceridemic Rats. Curr. Issues Mol. Biol. 2022, 44, 1889-1900. https://doi.org/10.3390/cimb44050129
Vecera R, Poruba M, Hüttl M, Malinska H, Oliyarnyk O, Markova I, Racova Z, Soukop J, Kazdova L. Beneficial Effect of Fenofibrate and Silymarin on Hepatic Steatosis and Gene Expression of Lipogenic and Cytochrome P450 Enzymes in Non-Obese Hereditary Hypertriglyceridemic Rats. Current Issues in Molecular Biology. 2022; 44(5):1889-1900. https://doi.org/10.3390/cimb44050129
Chicago/Turabian StyleVecera, Rostislav, Martin Poruba, Martina Hüttl, Hana Malinska, Olena Oliyarnyk, Irena Markova, Zuzana Racova, Jan Soukop, and Ludmila Kazdova. 2022. "Beneficial Effect of Fenofibrate and Silymarin on Hepatic Steatosis and Gene Expression of Lipogenic and Cytochrome P450 Enzymes in Non-Obese Hereditary Hypertriglyceridemic Rats" Current Issues in Molecular Biology 44, no. 5: 1889-1900. https://doi.org/10.3390/cimb44050129
APA StyleVecera, R., Poruba, M., Hüttl, M., Malinska, H., Oliyarnyk, O., Markova, I., Racova, Z., Soukop, J., & Kazdova, L. (2022). Beneficial Effect of Fenofibrate and Silymarin on Hepatic Steatosis and Gene Expression of Lipogenic and Cytochrome P450 Enzymes in Non-Obese Hereditary Hypertriglyceridemic Rats. Current Issues in Molecular Biology, 44(5), 1889-1900. https://doi.org/10.3390/cimb44050129






