Abstract
Background/Objectives: Most snakebite incidents in Latin America are caused by species of the Bothrops genus. Their venom induces severe local effects, against which antivenom therapy has limited efficacy. Metabolites derived from Coffea arabica have demonstrated anti-inflammatory and anticoagulant properties, suggesting their potential as therapeutic agents to inhibit the local effects induced by B. asper venom. Methods: Three enzymatic assays were performed: inhibition of the procoagulant and amidolytic activities of snake venom serine proteinases (SVSPs); inhibition of the proteolytic activity of snake venom metalloproteinases (SVMPs); and inhibition of the catalytic activity of snake venom phospholipases A2 (PLA2s). Additionally, molecular docking studies were conducted to propose potential inhibitory mechanisms of the metabolites chlorogenic acid, caffeine, and caffeic acid. Results: Green and roasted coffee extracts partially inhibited the enzymatic activity of SVSPs and SVMPs. Notably, the green coffee extract, at a 1:20 ratio, effectively inhibited PLA2 activity. Among the individual metabolites tested, partial inhibition of SVSP and PLA2 activities was observed, whereas no significant inhibition of SVMP proteolytic activity was detected. Chlorogenic acid was the most effective metabolite, significantly prolonging plasma coagulation time and achieving up to 82% inhibition at a concentration of 62.5 μM. Molecular docking analysis revealed interactions between chlorogenic acid and key active site residues of SVSP and PLA2 enzymes from B. asper venom. Conclusions: The roasted coffee extract demonstrated the highest inhibitory effect on venom toxins, potentially due to the formation of bioactive compounds during the Maillard reaction. Molecular modeling suggests that the tested inhibitors may bind to and occupy the substrate-binding clefts of the target enzymes. These findings support further in vivo research to explore the use of plant-derived polyphenols as adjuvant therapies in the treatment of snakebite envenoming.
1. Introduction
Snakebite envenoming is a neglected tropical disease resulting from the injection of a highly specialized toxic secretion into humans. It primarily occurs in rural areas, with more than five million cases reported annually, leading to between 81,000 and 138,000 deaths. However, this number may triple when accounting for amputations and disabling complications [1]. Most cases are concentrated in Asia, Africa, and Latin America [2]. In 2023, 5421 snakebite incidents were reported in Colombia (117 cases per week), including accidents involving both venomous and non-venomous snakes [3].
In Central America and northern South America, most snakebite incidents are caused by snakes of the Bothrops genus (subfamily Crotalinae, family Viperidae), which are distributed from southern Mexico to Argentina. B. asper is responsible for 50–80% of these cases [4]. Envenoming by B. asper produces both local and systemic symptoms, including pain, edema, dermonecrosis, myotoxicity, defibrination, thrombocytopenia, local and systemic hemorrhage, hypotension, and acute renal failure. Additional complications may include soft tissue infections leading to limb amputation, compartment syndrome, central nervous system hemorrhage, acute renal failure, and premature placental abruption [5].
Snake venoms are composed of biologically active molecules, with nearly 90% of their dry weight consisting of protein components responsible for local tissue damage and systemic toxicity, as well as relevant for treatment approaches [1]. The most abundant components of Bothrops asper venom in Colombia include snake venom serine proteinases (SVSPs), snake venom metalloproteinases (SVMPs), and snake venom phospholipases A2 (SVPLAs) [6].
The main therapeutic strategy for snakebite envenoming is the intravenous administration of antivenom, which consists of purified immunoglobulin G obtained from animal plasma. However, several limitations affect the efficacy and accessibility of antivenoms. These include limited availability in rural areas, where most bites occur, requirements for administration by trained healthcare professionals due to the risk of adverse reactions such as anaphylaxis, and their limited efficacy in neutralizing local tissue damage caused by snake venom [7]. Consequently, there is a need to investigate new inhibitory molecules that can complement antivenom therapy. Small-molecule toxin inhibitors are considered promising candidates for developing broad-spectrum snakebite treatments, as they can neutralize venom enzymatic activities [8].
Beyond being one of the most widely consumed stimulating beverages worldwide, coffee has demonstrated important bioactive properties, which have been the focus of research for their pharmacological role in the prevention and treatment of chronic non-communicable diseases, including cancer, cardiovascular diseases, Parkinson’s disease, and Alzheimer’s disease. Coffea arabica has also been traditionally used in folk medicine. A review by [9] compiled traditional uses of coffee beans and other plant parts. In Haiti, leaves and roasted beans are used to treat anemia, edema, and asthenia. In Nicaragua, a bean decoction is used orally to treat fever; in Peru, an aqueous extract of dried beans is used for fatigue; in Cuba, a hot water extract of beans is considered an aphrodisiac; and in Brazil, beverages prepared from C. arabica beans are used to treat flu. Additionally, roasted beans are used in Africa to treat headaches, migraines, malaria, and general weakness [9].
Various biological activities have been demonstrated for C. arabica seed extracts. For example, methanolic extracts have shown the ability to prevent skin photodamage by inhibiting the expression of metalloproteinases-1, -3, and -9, as well as the MAP kinase pathway [10]. C. arabica seed extracts and their main phenolic compounds, such as caffeic and chlorogenic acids, also exhibit antinociceptive and anti-inflammatory effects in a murine gout model, reducing hypernociception, neutrophil migration, and the concentration of pro-inflammatory cytokines [11]. Moreover, anticancer activity has been reported, such as a dose- and time-dependent reduction in the viability of colorectal cancer cell lines [12].
Therefore, this study aims to evaluate the inhibitory potential of green (GCE) and roasted (RCE) coffee bean extracts, as well as major metabolites found in Coffea arabica (including chlorogenic acid, caffeine, and caffeic acid), cultivated in Colombia, against the coagulant, proteolytic, and phospholipase A2 activities of Bothrops asper venom. Both GCE and RCE were analyzed, as it has been demonstrated that the roasting process alters the chemical composition and biological activity of coffee: while some natural phenolic compounds may be lost, other antioxidant compounds, such as Maillard reaction products, are formed [13]. Additionally, this study employs molecular docking techniques to propose potential inhibitory mechanisms between the evaluated secondary metabolites and key venom enzymes from B. asper.
2. Results
2.1. Characterization of Coffea arabica Extracts
The extracts were characterized by HPLC-DAD, which revealed the presence of caffeine at concentrations of 28.8 mg/g (GCE) and 33.7 mg/g (RCE). At the same time, chlorogenic acids (comprising chlorogenic, neochlorogenic, and cryptochlorogenic acids) were 208.55 mg/g (GCE) and 75.78 mg/g (RCE). Consider that caffeoylquinic acids are a bound form of caffeic acid present in green and roasted coffee at high levels [14]. This study also includes caffeic acid as a relevant metabolite.
2.2. Inhibition of Pro-Coagulant Activity of Bothrops asper Venom
None of the tested concentrations of extracts or individual metabolites exhibited intrinsic coagulant activity when added to citrated human plasma. In contrast, the positive control (venom alone) induced coagulation within 20 to 30 s. However, coagulant activity was significantly inhibited by both green coffee extract (GCE) and roasted coffee extract (RCE) at weight/weight (w/w) ratios of 1:20 and 1:40. For GCE, the coagulation times increased to 42.61 ± 0.76 s and 48.46 ± 1.56 s (p < 0.05), while RCE yielded coagulation times of 41.43 ± 3.69 s and 51.58 ± 5.76 s (p < 0.05), respectively (Figure 1A).
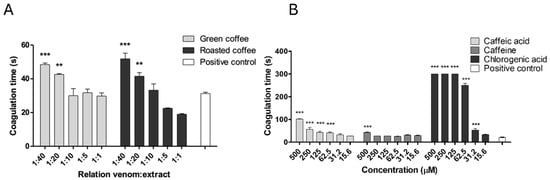
Figure 1.
Inhibition of coagulant activity by C. arabica extracts and metabolites. (A) Green and roasted coffee extracts. (B) Caffeine, caffeic, and chlorogenic acid. Data are represented as the mean of coagulation time ± SEM (n = 4). *** represents statistically significant differences with the positive control with p < 0.001 and ** p < 0.01.
Chlorogenic acid showed the greatest inhibitory effect, prolonging the plasma coagulation time by over five minutes at concentrations between 125 and 500 μM (p < 0.05). Additionally, it partially inhibited B. asper venom-induced coagulant activity at lower concentrations of 31.5 and 62.5 μM (p < 0.05). Caffeic acid also demonstrated a dose-dependent inhibitory effect, with significant differences compared to the positive control observed at concentrations from 62.5 to 500 μM (p < 0.05). In contrast, caffeine only exhibited inhibitory activity at the highest concentration tested (Figure 1B). The effect size for chlorogenic acid, calculated using Cohen’s d, was 4.09, indicating a strong inhibitory effect (Table S1).
2.3. Inhibition of Amidolytic Activity
The inhibition of the amidolytic activity of Bothrops asper venom was evaluated using the colorimetric substrate BApNA (Nα-Benzoyl-DL-arginine 4-nitroanilide), a standard probe for serine proteases. Both extracts demonstrated significant inhibitory effects against the venom amidolytic enzymes at weight-to-weight (w/w) ratios of 1:5, 1:10, and 1:20 (p < 0.05), compared to the positive control (Figure 2A). Notably, Cohen’s d value for roasted coffee extract was 6.06, indicating a strong effect size (Table S6).
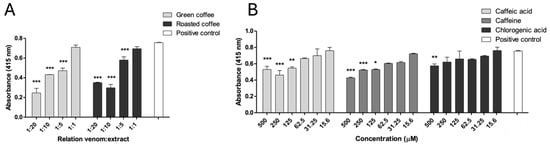
Figure 2.
Inhibition of amidolytic activity of B. asper venom by C. arabica extracts and metabolites. (A) Green and roasted coffee extracts. (B) Caffeine, caffeic, and chlorogenic acid. Data are represented as mean ± SEM (n = 3). *** represents statistically significant differences with the positive control with p < 0.001, ** p < 0.01, and * p < 0.05. Data are represented as mean ± SEM (n = 3), with the subtraction of the negative control to delete background noise and the intrinsic compound absorbance at 415 nm.
Caffeine and caffeic acid partially inhibited this venom activity at concentrations of 125–500 μM (p < 0.05). Chlorogenic acid only displayed a slight inhibition at 500 μM (p < 0.05) (Figure 2B).
2.4. Inhibition of PLA2 Activity
Phospholipase A2 (PLA2) activity of B. asper venom was assessed using the 4-NOBA (4-Nitro-3-octanoyloxy benzoic acid) substrate. Green coffee extract (GCE) showed partial inhibition at a w/w ratio of 1:20, while the other tested ratios (1:1, 1:5, 1:10) and all concentrations of roasted coffee extract (RCE) had no significant inhibitory effect (Figure 3A). All the tested metabolites demonstrated significant inhibition at 500 μM (p < 0.05), with activity reduced by approximately 50%. Additionally, chlorogenic acid partially inhibited 4-NOBA hydrolysis at 250 μM (Figure 3B).
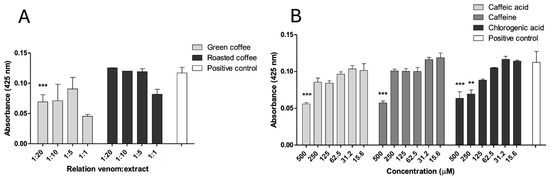
Figure 3.
Inhibition of PLA2 activity of B. asper venom. (A) Green and roasted coffee extracts. (B) Caffeine, caffeic, and chlorogenic acid. Data are represented as mean ± SEM (n = 4), with the subtraction of the negative control to delete background noise and the intrinsic compound absorbance at 425 nm. *** represents statistically significant differences with the positive control with p < 0.001 and ** p < 0.01.
2.5. Inhibition of Proteolytic Activity
Both extracts also partially inhibited the proteolytic activity of B. asper venom on azocasein at all tested w/w ratios (p < 0.05). The highest inhibitory effect was observed with RCE at a ratio of 1:20, resulting in approximately 50% reduction of proteolytic activity (Figure 4A). In contrast, none of the tested metabolites showed significant inhibition of this enzymatic activity (p > 0.05) (Figure 4B).
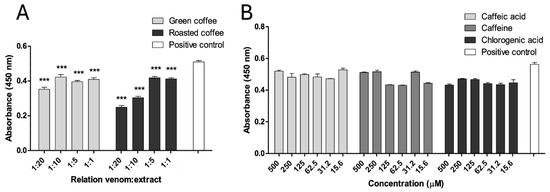
Figure 4.
Inhibition of the proteolytic activity of B. asper venom. (A) Green and roasted coffee extracts. (B) Caffeine, caffeic, and chlorogenic acid. Data are represented as mean ± SEM (n = 4), with the subtraction of the negative control to delete background noise and the intrinsic compound absorbance at 450 nm. *** represents statistically significant differences with the positive control with p < 0.001.
2.6. Structural Modeling and Validation of the SVSP
Molecular modeling generated a three-dimensional structure exhibiting the characteristic features of snake venom serine proteinases (SVSPs), including two β-barrels and four short α-helices, consistent with a trypsin-like fold (Figure 5A). The structural model was validated using PROCHECK (available on https://saves.mbi.ucla.edu/, accessed on 25 March 2025), which revealed that 91.5% of residues were located in the most favored regions of the Ramachandran plot, and 10.2% were in additional allowed regions. No residues were found in generously allowed or disallowed regions (Supplementary Figure S1). The Verify3D analysis showed that 87.83% of the residues scored ≥ 0.1, surpassing the 80% threshold for reliable models (Supplementary Figure S2). The ProSA analysis returned a Z-score of −7.26, within the range expected for proteins of comparable length. Additionally, the energy profile of the model indicated a predominance of residues in favorable (negative) energy regions (Supplementary Figure S3).
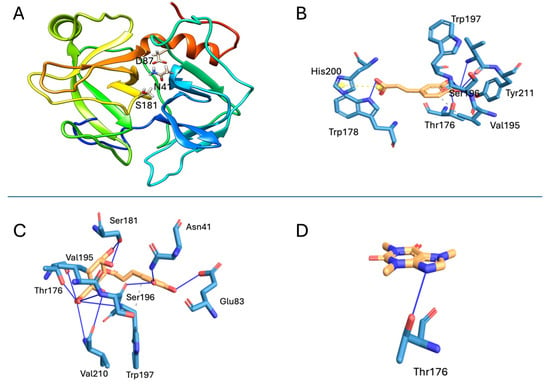
Figure 5.
Molecular docking results. (A) Modeled structure of the SVSP from B. asper venom. The coloring in this view is a N-ter-->C-ter rainbow, with the N terminus being blue and the C terminus red. The catalytic triad of the enzyme is shown in sticks. Interaction of the studied metabolites with the active site residues of the modeled SVSP: (B) caffeic acid, (C) chlorogenic acid, and (D) caffeine. The blue lines represent hydrogen bonds, the yellow lines represent salt bridges, and the gray lines show hydrophobic interactions.
2.7. Molecular Docking
Molecular docking revealed the interactions of the studied metabolites with the modeled SVSP of B. asper (Figure 5). The binding affinities and interaction details are summarized in Table 1. Chlorogenic acid exhibited the strongest predicted interaction, forming hydrogen bonds with Asn41 and Ser181, residues located in the enzyme active site (Figure 5C). Caffeic acid formed fewer hydrogen bonds and did not interact with catalytic residues (Figure 5B). Caffeine showed the weakest affinity, forming only one hydrogen bond with Thr176 (Figure 5D).

Table 1.
Weak interactions and affinities between each compound and the modeled B. asper serine protease.
On the other hand, the molecular docking results for Bothrops asper PLA2 are presented in Figure 6. The tested compounds interacted with amino acid residues located within the enzyme catalytic site or hydrophobic channel. The binding affinities and detailed interaction profiles are summarized in Table 2. Among the metabolites, chlorogenic acid exhibited the most favorable theoretical binding affinity, forming interactions with two key catalytic residues: a salt bridge with His48 and a combination of a salt bridge and Van der Waals interactions with Tyr52 (Figure 6B). In contrast, caffeic acid and caffeine showed equivalent binding affinities. Caffeic acid established a salt bridge with His48, along with additional weak interactions with surrounding residues (Figure 6A), whereas caffeine formed π-stacking interactions with Phe5, a residue located within the hydrophobic channel (Figure 6C).
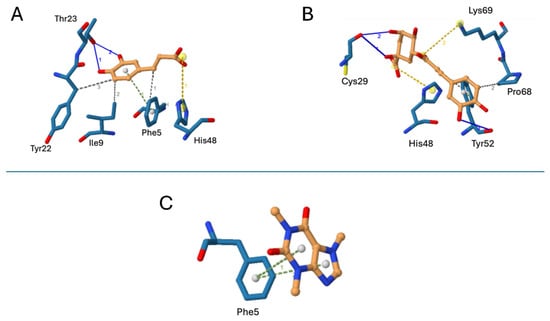
Figure 6.
Molecular docking poses with a PLA2 (blue sticks) from B. asper venom (PDB code 5TFV). Inhibitors are shown in orange sticks. (A) Caffeic acid, (B) chlorogenic acid, and (C) caffeine.The blue lines represent hydrogen bonds, the yellow dotted lines represent salt bridges, the green lines represent π-stacking interactions, and the gray lines indicate hydrophobic interactions.

Table 2.
Weak interactions and affinities between each compound and the B. asper phospholipase A2 (PDB code 5TFV).
3. Discussion
B. asper is the most clinically relevant species in Colombia and other regions. Its venom is mainly composed of proteins and peptides [15], with the most abundant components being enzymes from the groups PLA2s, SVMPs, and SVSPs [6]. Therefore, green and roasted coffee bean extracts from Coffea arabica, along with some of their constituent metabolites, were evaluated for their ability to inhibit the enzymatic and biological activities induced by these toxins.
This study constitutes the first report demonstrating the in vitro inhibitory capacity of green and roasted C. arabica beans against snake-venom-induced biological activities. C. arabica is well known for its richness in bioactive metabolites, including chlorogenic, caffeic, and ferulic acids and catechin, epicatechin, and anthocyanins, as well as alkaloids, such as caffeine and trigonelline. During roasting, additional compounds are formed, such as melanoidins and acrylamide, which may also contribute to its biological activity [16].
C. arabica has exhibited various pharmacological properties, including anti-inflammatory and analgesic effects, which may offer therapeutic potential for snakebite treatment [11]. These findings, together with those presented herein, underscore the potential of this plant as a promising source for the identification of antiophidic compounds.
The proteolytic activity of snake venom is closely associated with its hemorrhagic potential [17]. Accordingly, inhibition of proteolytic enzymes is correlated with a reduction in hemorrhagic effects. In the present study, C. arabica extracts partially inhibited the proteolytic activity of B. asper venom. However, none of the individual compounds tested reduced venom activity at any of the concentrations evaluated. This suggests that the inhibitory effects observed for the extracts may be mediated by other untested molecules or through synergistic interactions among the components. Further studies are needed to explore these possibilities and identify the specific constituents responsible for the observed activity.
On the other hand, PLA2 enzymes in snake venoms contribute to myotoxicity, edema formation, and neurotoxicity, among other effects [18]. In this study, green coffee extract (GCE) exhibited mild inhibitory activity against PLA2, whereas the roasted coffee extract (RCE) showed no such effect. Interestingly, all the pure compounds tested inhibited PLA2 activity at the highest concentration used. The polyphenols and flavonoids present in green coffee are likely responsible for this activity [19]. However, the roasting process alters the composition of these compounds due to the formation of new molecules, such as pyrazines, pyrroles, thiols, and furanones, via the Maillard reaction, potentially affecting bioactivity [20]. Chlorogenic acid, a phenolic ester of cinnamic acid, is considered the main bioactive compound in green coffee beans [21]. In this study, chlorogenic acid inhibited PLA2 activity at concentrations of 250 and 500 µM, resulting in approximately a 50% reduction in activity. Similar results have been reported, including the inhibition of PLA2 and myotoxic activity of Bothrops leucurus venom [22], the neurotoxicity of Crotalus durissus terrificus venom [23], and the myotoxicity of a PLA2 homologue from Bothrops jararacussu venom [24].
Our molecular docking analysis suggests that chlorogenic acid may interact with the catalytic residue His48, which is essential for generating the hydroxyl group that attacks the carbonyl at the sn-2 position of the glycerophospholipid substrate [25]. This interaction could disrupt the enzyme’s catalytic cycle and inhibit its function. Additionally, chlorogenic acid may bind to Lys69, a residue implicated in interfacial recognition and stabilization of substrate binding [26], potentially blocking substrate access. These findings provide a structural basis for the observed inhibitory activity of chlorogenic acid against PLA2 enzymes in snake venom.
The in vitro procoagulant activity of B. asper venom is associated with its in vivo anticoagulant effects, primarily due to the consumption of fibrin and fibrinogen [27]. This effect is largely attributed to SVSPs and, to a lesser extent, SVMPs. These enzymes act on various substrates, including coagulation factors and other proteins [28,29]. In this study, both green and roasted C. arabica extracts partially inhibited the amidolytic, proteolytic, and coagulant activities of B. asper venom. The amidolytic activity is mediated by SVSPs acting on the synthetic substrate BApNA, which is cleaved by proteases with a preference for arginine at the P1 position [30]. Nevertheless, as previously noted, inhibition of amidolytic activity does not always correlate with inhibition of coagulant activity. This was observed in our study, as the concentrations of compounds that prolonged the coagulation time did not coincide with those that inhibited the hydrolytic activity on BApNA. Chlorogenic acid showed the strongest inhibitory effect, significantly increasing coagulation times (by 3- to 15-fold) even at low concentrations (31.6 µM). Similar results have been reported [31], including the chlorogenic-acid-induced inhibition of thrombin, factor V, and factor X. These findings motivated further molecular modeling to investigate the mechanism of action.
Docking simulations suggested that chlorogenic acid may bind to the active site of a thrombin-like SVSP, occupying part of the substrate-binding cleft. The inhibitor potentially forms hydrogen bonds with the catalytic Ser181, which mediates the nucleophilic attack on peptide bonds [32]. This interaction may impair the enzyme catalytic cycle. Additionally, the quinic acid moiety of chlorogenic acid may occupy the S1 subsite, interacting via hydrogen bonding with Thr176 and Trp197, and engaging in van der Waals interactions with Val195 and Trp197. Similar interactions have been reported for chlorogenic acid binding to thrombin [33,34], supporting its capacity to block substrate access and inhibit enzyme activity [32].
This manuscript acknowledges limitations inherent to experimental designs, such as sample size and random sampling, which may affect the study external validity. Moreover, the absence of confounder control and multivariate analysis may limit internal validity. Future in vivo studies are recommended to confirm the therapeutic potential of the studied metabolites.
4. Materials and Methods
4.1. Materials and Chemicals
Venom from Bothrops asper was obtained from adult specimens collected in Magdalena Medio, Urabá, the Pacific Coast, and Chocó (Colombia), and was provided by the Serpentarium of the University of Antioquia. A pooled sample was centrifuged, lyophilized, and stored at −20 °C until use. Solvent removal was conducted using a rotary evaporator, followed by lyophilization.
Green (unroasted) and medium-roasted coffee (Coffea arabica) samples (500 g each) were supplied by Natucafé S.A.S. (Andes, Antioquia, Colombia). Each sample was independently pulverized and extracted for 48 h with a mixture of isopropanol and water (60:40, v/v) at room temperature using a solid-to-liquid ratio of 1:3 (w/v). The extraction included sonication for 30 min at 0, 24, and 48 h. The resulting extract was vacuum filtered, concentrated via rotary evaporation at 40 °C, lyophilized, and stored at −20 °C.
Both extracts were characterized by HPLC-DAD. For this, 100 mg of lyophilized extract were dissolved in 70% ethanol and centrifuged at 13,000 rpm for 10 min, and the supernatant was collected and diluted to 20 mL in a volumetric flask. The final solution was further diluted 1:20 with the mobile phase and injected into an Agilent 1200 Series LC System equipped with an SB-C18 column [35]. Quantification was performed using external standard methodologies [12]. Reference standards—chlorogenic acid (≥95%), caffeine (≥99%), and caffeic acid (≥98%)—were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO).
4.2. Inhibition of Procoagulant Activity of SVSPs
The procoagulant activity assay was performed based on the method described by [36], with minor modifications. The minimum coagulant dose (MCD), defined as the amount of venom that clots citrated human plasma within 20–30 s, was used as a positive control and diluted 1:1 in saline solution. Green coffee extract (GCE) and roasted coffee extract (RCE) were prepared at MCD/extract ratios of 1:1, 1:5, 1:10, 1:20, and 1:40. Chlorogenic acid, caffeine, and caffeic acid were tested at concentrations of 500, 250, 125, 62.5, 31.2, and 15.6 µM. All the treatments were adjusted to 500 µL with saline solution and preincubated at 37 °C for 30 min. Subsequently, 100 µL of each mixture were added to 200 µL of citrated human plasma in quadruplicate. Negative controls consisted of extracts or metabolites with DMSO at the highest evaluated concentration. The coagulation time was monitored for up to 5 min.
4.3. Inhibition of Amidolytic Activity of SVSPs
Amidolytic activity was evaluated using BApNA (N-Succinyl-arginine-p-nitroanilide; Sigma-Aldrich) as the substrate, following the methodology described in [37]. The reaction mixture included 50 µL of Tris-HCl buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl; pH 8.0), 200 µL of BApNA (10 mM), and 10 µL of B. asper venom (2 mg/mL), preincubated with the extracts or metabolites. Extracts were tested at venom/extract ratios of 1:1, 1:5, 1:10, and 1:20, while metabolites were evaluated at the concentrations mentioned above. After 40 min incubation at 37 °C, the absorbance was measured at 415 nm. Positive controls contained venom and substrate only; negative controls included extract or metabolite samples without venom at all the evaluated concentrations.
4.4. Inhibition of Proteolytic Activity of SVMPs
The inhibition of proteolytic activity was assessed using azocasein as the substrate [38]. The minimum proteolytic dose (MPD), defined as the amount of venom producing a 0.5 absorbance increase at 450 nm, was determined as 20 µg of venom. This dose was diluted in 25 mM Tris buffer (150 mM NaCl, 5 mM CaCl2, pH 7.4). GCE, RCE, and the metabolites were tested at the same ratios and concentrations as previously described. After 30 min of preincubation at 37 °C, 100 µL of azocasein (10 mg/mL) were added and incubated for 90 min at 37 °C. The reaction was terminated with 200 µL of 5% trichloroacetic acid, centrifuged at 3000 rpm for 5 min, and 100 µL of the supernatant were mixed with 100 µL of 0.5 M NaOH. The absorbance was read at 450 nm. All the samples were assayed in quadruplicate. As a positive control, an MPD was used, and as a negative control, the extracts and metabolites were evaluated at all concentrations.
4.5. Inhibition of Esterase Activity of SVPLA2s
Esterase activity of phospholipase A2 (PLA2) was assessed using 4-NOBA as a substrate [39], adapted for 96-well plates. Each reaction contained 200 µL of buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl, pH 8.0), 20 µL of 4-NOBA (1 mg/mL), and 20 µL of extract or metabolite solution preincubated with 20 µg of B. asper venom. Reactions were incubated at 37 °C for 60 min, and absorbance was measured at 405 nm using a plate reader (Awareness, Stat Fax 3200, Westport, CT, USA). All the treatments were tested in triplicate. B. asper venom and synthetic substrate 4-NOBA were positive controls. The extracts and metabolites, with DMSO at the highest evaluated concentration, were used as a negative control.
4.6. Molecular Modeling and Docking
Molecular structures of chlorogenic acid, caffeic acid, and caffeine were constructed using GaussView 6 [40]. Geometry optimization was performed using Gaussian 16W [41] at the B3LYP/6-31++G(d,p) level of theory. Molecular docking simulations were conducted using AutoDock Vina 1.2.0 [42] against two targets: a modeled thrombin-like serine protease from B. asper venom and the crystal structure of a PLA2 from B. asper venom (PDB ID: 5TFV), since the evaluated extracts or metabolites showed inhibition upon these toxins.
The SVSP was modeled based on the UniProtKB sequence Q072L6 (VSPL_BOTAS), with the signal and propeptide sequences removed. Structure prediction was carried out using AlphaFold 3 [43]. Further, the stereochemical quality of the modeled protein structure and overall structural geometry were confirmed using Procheck (available on https://saves.mbi.ucla.edu/, accessed on 25 March 2025) [44]. The energy of the residues was determined using ProSA through the ProSA-web service (available on https://prosa.services.came.sbg.ac.at/prosa.php, accessed on 25 March 2025). Finally, Verify 3D software (available on https://saves.mbi.ucla.edu/, accessed on 25 March 2025) was used to determine the compatibility of an atomic model (3D) with its amino acid sequence (1D) by assigning a structural class based on its location and environment (α, β, loop, polar, non-polar, etc.), as well as by comparing the results with suitable database structures.
Proteins were prepared using Maestro 2025-2 with the Protein Preparation Wizard. Hydrogen atoms were added, atomic charges were assigned, and local minimization was applied using the OPLS force field. The grid box was centered on the carbonyl oxygen of Ser181 for the SVSP (X = −3.079, Y = −6.399, Z = 1.810) and the calcium ion for PLA2 (X = 2.279, Y = 15.924, Z = 21.732), with a grid size of 24 Å3 and exhaustiveness set to 20. The ligand poses with the highest binding affinities were selected and analyzed using PLIP 2021 (available on https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index, accessed on 28 March 2025). [45] and UCSF Chimera 1.19 [46].
4.7. Statistical Analysis
Statistical differences between the treatment groups and positive controls were assessed using two-way ANOVA, followed by Bonferroni post hoc tests. Differences were considered statistically significant at p < 0.05. Effect sizes were calculated using Cohen’s d, and all analyses were performed using Jamovi software 2.7.3.
5. Conclusions
This study evaluated the inhibitory potential of Coffea arabica extracts and their metabolites—chlorogenic acid, caffeic acid, and caffeine—against key enzymatic activities of Bothrops asper venom from Colombia. Through a combination of in vitro biochemical assays and molecular modeling, we demonstrated that both green coffee extract (GCE) and roasted coffee extract (RCE) exhibit significant inhibitory effects on serine proteases (SVSPs) and metalloproteinases (SVMPs), which are critical components of venom pathophysiological actions.
Among the individual metabolites, chlorogenic acid exhibited the most robust inhibition, particularly of procoagulant and PLA2 activities, as confirmed by prolonged clotting times. Molecular docking simulations further supported these findings, revealing favorable binding energies and multiple interactions between chlorogenic acid and the catalytic residues of both venom serine protease and PLA2. Caffeic acid also showed moderate inhibition, while caffeine exhibited limited activity.
These results underscore the therapeutic potential of chlorogenic acid and coffee extracts as natural inhibitors of snake venom enzymes. Given their availability, low toxicity, and dual antioxidant and enzyme-inhibitory properties, these compounds represent promising candidates for adjunctive treatment in snakebite envenoming. Future work should include in vivo efficacy testing and pharmacokinetic profiling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18081151/s1, Figure S1: Stereochemical quality of the modeled serine proteinase using PROCHECK; Figure S2: Compatibility of an atomic model (3D) with its amino acid sequence (1D); Figure S3: Energetic architecture of the modeled serine proteinase using the ProSA program; Table S1: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of caffeine, caffeic, and chlorogenic acid on the pro-coagulant activity of B. asper venom; Table S2: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of caffeine, caffeic, and chlorogenic acid on the amidolytic activity of B. asper venom; Table S3: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of caffeine, caffeic, and chlorogenic acid on the phospholipase A2 activity of B. asper venom; Table S4: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of caffeine, caffeic, and chlorogenic acid on the proteolytic activity of B. asper venom; Table S5: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of green and roasted coffee extracts on the pro-coagulant activity of B. asper venom; Table S6: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of green and roasted coffee extracts on the amidolytic activity of B. asper venom; Table S7: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of green and roasted coffee extracts on the phospholipase A2 activity of B. asper venom; Table S8: Mean effect size (Cohen’s d), means, p-value, and 95% confidence intervals for the inhibitory activity of green and roasted coffee extracts on the proteolytic activity of B. asper venom.
Author Contributions
Conceptualization, L.M.P. and E.P.; methodology, L.M.P., I.C.H.-C. and E.P.; software, L.M.P., J.A.P., Y.G.-P. and E.P.; validation L.M.P. and E.P.; formal analysis, L.M.P., I.C.H.-C., J.A.P., Y.G.-P. and E.P.; investigation, L.M.P., J.A.P. and E.P.; resources, L.M.P. and I.C.H.-C.; data curation, L.M.P., J.A.P. and E.P.; writing—original draft preparation, L.M.P. and E.P.; writing—review and editing, L.M.P., I.C.H.-C., J.A.P., Y.G.-P. and E.P.; visualization, L.M.P., I.C.H.-C., J.A.P. and E.P.; supervision, L.M.P.; project administration, I.C.H.-C.; funding acquisition, I.C.H.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Evaluación del potencial de coproductos del café para la generación de materias primas para la industria farmacéutica y alimentaria”, Code 2019-27890, and the University of Antioquia.
Institutional Review Board Statement
This study did not require ethical approval, for not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article and the Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GCE | Green coffee extract |
| RCE | Roasted coffee extract |
| SVMP | Snake venom metalloproteinase |
| SVSP | Snake venom serine proteinase |
| PLA2 | Phospholipase A2 |
References
- Gutiérrez, J.; Calvete, J.; Habib, A.; Harrison, R.; Williams, D.; Warrell, D. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Afroz, A.; Siddiquea, B.; Chowdhury, H.; Jackson, T.; Watt, A. Snakebite Envenoming: A Systematic Review and Meta-Analysis of Global Morbidity and Mortality. PLoS Negl. Trop. Dis. 2024, 18, e0012080. [Google Scholar] [CrossRef] [PubMed]
- Informe de Evento Accidente Ofídico Periodo Epidemiológico XIII de 2023. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/ACCIDENTE%20OFIDICO%20PE%20XIII%202023.pdf (accessed on 15 June 2025).
- Chippaux, J. Incidence and Mortality Due to Snakebite in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005662. [Google Scholar] [CrossRef]
- Erazo, V.; Posso, I.; Ruiz, I.; Castro, F.; Castaño, S.; Delgado, T.; Cañas, C. Viperidae Snake Envenomation from a Highly Complex Hospital in Southwestern Colombia. Heliyon 2024, 10, e26768. [Google Scholar] [CrossRef]
- Mora, D.; Salazar, D.; Pla, D.; Lomonte, B.; Guerrero, J.; Ayerbe, S.; Gibbs, H.; Calvete, J. Venom Variation in Bothrops asper Lineages from North-Western South America. J. Proteom. 2020, 229, 103945. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Isbister, G. Current Research into Snake Antivenoms, Their Mechanisms of Action, and Applications. Biochem. Soc. Trans. 2020, 48, 537–546. [Google Scholar] [CrossRef]
- Xie, C.; Albulescu, L.O.; Bittenbinder, M.A.; Somsen, G.W.; Vonk, F.J.; Casewell, N.R.; Kool, J. Neutralizing Effects of Small Molecule Inhibitors and Metal Chelators on Coagulopathic Viperinae Snake Venom Toxins. Biomedicines 2020, 8, 297. [Google Scholar] [CrossRef]
- Patay, É.; Bencsik, T.; Papp, N. Phytochemical Overview and Medicinal Importance of Coffea Species from the Past Until Now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef]
- Chiang, H.; Lin, T.; Chiu, C.; Chang, C.; Hsu, K.; Fan, P.; Wen, K. Coffea arabica Extract and Its Constituents Prevent Photoaging by Suppressing MMPs Expression and MAP Kinase Pathway. Food Chem. Toxicol. 2011, 49, 309–318. [Google Scholar] [CrossRef]
- Matosinhos, R.; Bezerra, J.; Barros, C.; Ferreira, A.; Coelho, G.; De Paula, C.; Araújo, M.; De Oliveira, D.; Aguiar, R.; Sachs, D.; et al. Coffea arabica Extracts and Their Chemical Constituents in a Murine Model of Gouty Arthritis: How They Modulate Pain and Inflammation. J. Ethnopharmacol. 2022, 284, 114778. [Google Scholar] [CrossRef]
- Villota, H.; Santa, G.; Uribe, D.; Henao, I.; Arroyave, J.; Barrera, C.; Pedroza, J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients 2022, 14, 4880. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.; De Horta, I.; Rosa, L.; Lima, L.; Rosa, J.; Montenegro, J.; Santos, L.; Castro, R.; Freitas, O.; Teodoro, A. Effect of the Roasting Levels of Coffea arabica L. Extracts on Their Potential Antioxidant Capacity and Antiproliferative Activity in Human Prostate Cancer Cells. RSC Adv. 2020, 10, 30115–30126. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 25, 50, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Mora, D.; Lomonte, B.; Pla, D.; Guerrero, J.; Ayerbe, S.; Gutiérrez, J.; Sasa, M.; Calvete, J. Half a Century of Research on Bothrops asper Venom Variation: Biological and Biomedical Implications. Toxicon 2023, 221, 106983. [Google Scholar] [CrossRef]
- Gallardo, J.; Santibáñez, A.; Oropeza, O.; Salazar, R.; Montiel, R.; Cabrera, S.; Gonzales, M.; Cruz, F.; Torrez, N. Chemical and Biological Characterization of Green and Processed Coffee Beans from Coffea arabica Varieties. Molecules 2023, 28, 4685. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J. A Comprehensive View of the Structural and Functional Alterations of Extracellular Matrix by Snake Venom Metalloproteinases (SVMPs): Novel Perspectives on the Pathophysiology of Envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef]
- Tonello, F.; Rigoni, M. Cellular Mechanisms of Action of Snake Phospholipase A2 Toxins. In Snake Venoms; Inagaki, H., Vogel, C.-W., Mukherjee, A.K., Rahmy, T.R., Gopalakrishnakone, P., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 49–65. [Google Scholar] [CrossRef]
- Alam, M.; Alam, M.; Alam, O.; Nargotra, A.; Taneja, S.; Koul, S. Molecular Modeling and Snake Venom Phospholipase A2 Inhibition by Phenolic Compounds: Structure–Activity Relationship. Eur. J. Med. Chem. 2016, 114, 209–219. [Google Scholar] [CrossRef]
- Moreira, M.; Pereira, R.; Dias, D.; Gontijo, V.; Vilela, F.; De Moraes, G.; Giusti, A.; dos Santos, M. Anti-inflammatory Effect of Aqueous Extracts of Roasted and Green Coffea arabica L. J. Funct. Foods 2013, 5, 466–474. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P. The Chemistry of Chlorogenic Acid from Green Coffee and Its Role in Attenuating Obesity and Diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef]
- Silva, D.P.D.; Ferreira, S.S.; Torres-Rêgo, M.; Furtado, A.A.; Yamashita, F.O.; Diniz, E.A.; Vieira, D.S.; Ururahy, M.A.; Silva-Júnior, A.D.; Luna, K.P.; et al. Antiophidic potential of chlorogenic acid and rosmarinic acid against Bothrops leucurus snake venom. Biomed. Pharmacother. 2022, 148, 112766. [Google Scholar] [CrossRef]
- Oliveira, I.; Yoshida, E.; Dini, M.; Paschoal, A.; Cogo, J.; Höfling, M.; Hyslop, S.; Oshima, Y. Evaluation of Protection by Caffeic Acid, Chlorogenic Acid, Quercetin, and Tannic Acid against the In Vitro Neurotoxicity and In Vivo Lethality of Crotalus durissus terrificus (South American Rattlesnake) Venom. Toxins 2021, 13, 801. [Google Scholar] [CrossRef]
- Cardoso, F.; Salvador, G.; Cavalcante, W.; Dal, M.; Fontes, M. BthTX-I, a Phospholipase A2-like Toxin, Is Inhibited by the Plant Cinnamic Acid Derivative: Chlorogenic Acid. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2024, 1872, 140988. [Google Scholar] [CrossRef]
- Castro-Amorim, J.; Novo de Oliveira, A.; Da Silva, S.L.; Soares, A.M.; Mukherjee, A.K.; Ramos, M.J.; Fernandes, P.A. Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction. J. Med. Chem. 2023, 66, 5364–5376. [Google Scholar] [CrossRef] [PubMed]
- Berg, O.G.; Gelb, M.H.; Tsai, M.-D.; Jain, M.K. Interfacial Enzymology: The Secreted Phospholipase A2-Paradigm. Chem. Rev. 2001, 101, 2613–2654. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S. The long road of research on snake venom serine proteinases. Toxicon 2023, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Masood, R.; Ali, I.; Ullah, K.; Ali, H.; Akbar, H.; Betzel, C. Thrombin-like enzymes from snake venom: Structural characterization and mechanism of action. Int. J. Biol. Macromol. 2018, 15, 788811. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef]
- Chase, T., Jr.; Shaw, T.E. Titration of trypsin, plasmin, and thrombin with p-nitrophenyl p′-guanidinobenzoate HCl. Methods Enzymol. 1970, 19, 2027. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S. Investigation of the anticoagulant and antithrombotic effects of chlorogenic acid. J. Biochem. Mol. Toxicol. 2017, 31, e21865. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Y.; Huang, S.; Li, L.; Yuan, Z.; Xu, G. Screening of anti-thrombin active components from Ligusticum chuanxiong by affinity-ultrafiltration coupled with HPLC-Q-Orbitrap-MSn. Phytochemical 2023, 34, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Zhang, Q.; Chen, H.; Xia, Z.; Yang, F. Characterization of phenolic acids binding to thrombin using frontal affinity chromatography and molecular docking. Anal. Methods 2017, 9, 5174–5180. [Google Scholar] [CrossRef]
- Villota, H.; Moreno, M.; Santa, G.; Uribe, D.; Castañeda, I.; Preciado, L.M.; Pedroza, J. Biological Impact of Phenolic Compounds from Coffee on Colorectal Cancer. Pharmaceuticals 2021, 14, 761. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.; Reid, H. Development of simple standard assay procedures for the characterization of snake venom. Bull. WHO 1983, 61, 949–956. [Google Scholar]
- Jänsch, N.; Colin, F.; Schröder, M.; Meyer-Almes, F.-J. Using design of experiment to optimize enzyme activity assays. ChemTexts 2019, 5, 20. [Google Scholar] [CrossRef]
- Wang, W.; Shih, C.; Huang, T. A novel P-I class metalloproteinase with broad substrate-cleaving activity, agkislysin, from Agkistrodon acutus venom. Biochem. Biophys. Res. Commun. 2004, 324, 224–230. [Google Scholar] [CrossRef]
- Holzer, M.; Mackessy, S. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6; Semichem Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Biophys. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Laskowski, R.; MacArthur, M.; Moss, D.; Thornton, J. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Pettersen, E.; Goddard, T.; Huang, C.; Couch, G.; Greenblatt, D.; Meng, E.; Ferrin, T. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comp. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).