1. Introduction
Stroke is the second most common cause of death after ischemic heart disease and a leading cause of disability worldwide. It is considered one of the most prevalent and devastating diseases affecting humanity today [
1]. According to the Global Burden of Disease Report, the number of patients diagnosed with stroke has been steadily increasing in recent years, leading to significant economic burdens, especially in low- and middle-income countries [
2]. Statistics show that nearly 6.5 million people died from stroke in 2013, and it is estimated that by 2030, stroke will cause 12 million deaths [
3]. Therefore, elucidating the mechanisms underlying ischemic brain injury is of paramount importance. In this study, we explored the biological processes involved in an attempt to identify potential therapeutic targets and to provide some preliminary research basis for the development of effective treatments.
Pyroptosis is a novel form of programmed cell death triggered by the activation of inflammatory Caspase-1 by various inflammasomes. This process mediates the action of the Gasdermin (GSDM) D protein, leading to cell lysis and the release of cytoplasmic contents and pro-inflammatory mediators, including IL-1β and IL-18, ultimately resulting in an excessive inflammatory response [
4,
5]. Specifically, NLR family pyrin domain containing 3 (NLRP3) is considered one of the primary inflammasomes, highly expressed in the brain due to its crucial role in recognizing cellular damage and initiating inflammatory cascades, ultimately leading to cell death [
6]. Some studies indicate that the NLRP3 inflammasome plays a crucial role in the occurrence of brain ischemia/reperfusion (I/R) injury [
7]. Pyroptosis serves as an effective inducer of the pro-inflammatory pathway in IS, primarily distributed in the ischemic penumbra [
8]. Recent studies suggest that pyroptosis may be associated with brain I/R injury, involving processes such as free radical generation, inflammation, autophagy, and mitochondrial dysfunction. According to reports, intravenous immunoglobulin therapy targeting Caspase-1 can inhibit the activity of the NLRP3 inflammasome, thereby exerting neuroprotective effects in experimental stroke models [
9]. Another study indicates that a deficiency of the NLRP3 gene can protect mice from ischemic injury [
10,
11].
HLJD is a traditional Chinese medicinal formula known for its heat-clearing and detoxifying properties. Its composition includes
Coptidis chinesis Franch (Huang-Lian),
Scutellaria baicalensis Georgi (Huang-Qin),
Phellodendron amurense Rupr (Huang-Bo), and
Gardenia jasminoides Ellis (Zhi-Zi), in a ratio of 3:2:2:3. Emerging studies suggest that HLJD and its constituents and extracts possess pharmacological effects against inflammation, infectious diseases, neurological disorders, and certain cardiovascular diseases [
12,
13,
14]. Substantial evidence has established the correlation between HLJD and inflammatory injury [
15,
16]. However, due to the absence of appropriate methodologies and the multi-target, multi-pathway nature of traditional Chinese medicine, there is an abundance of information that complicates the extraction of pivotal insights. The pharmacological mechanisms underlying HLJD’s effects on IS remain incompletely understood.
This study employs a multi-omics approach to unravel the intricate interplay of pharmacodynamics, phenotype correlations, drug targets, and specific mechanisms. It seeks to elucidate whether HLJD can alleviate IS by modulating pyroptosis, aiming to uncover potential target molecules and pivotal pathways. This research endeavors to provide profound scientific insights into the mechanisms through which HLJD exerts therapeutic effects in IS.
3. Discussion
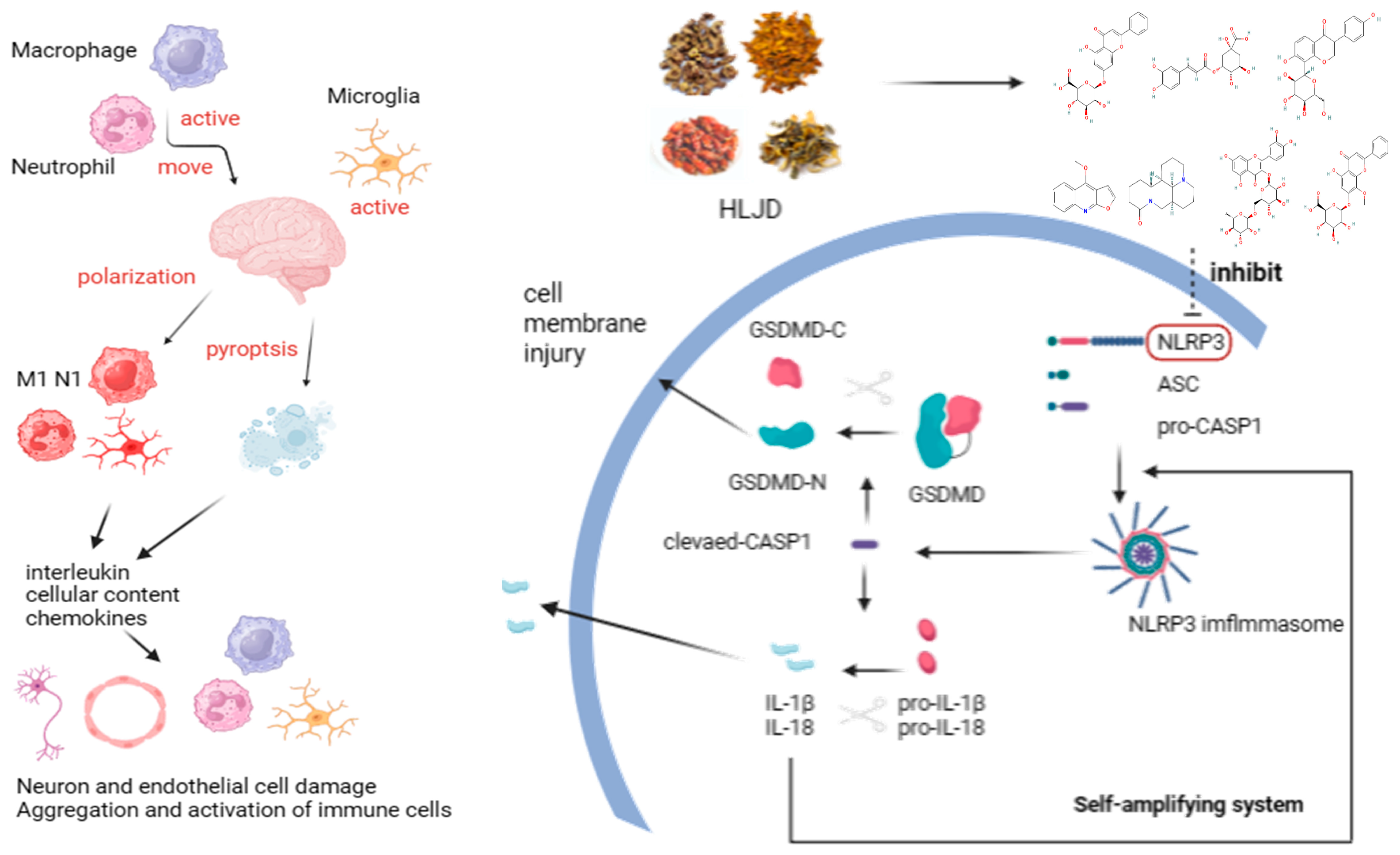
IS poses a significant health risk with increasing incidence rates and a lack of effective clinical treatments, leading to substantial economic burdens. TCM attributes the pathogenesis of IS to the concept of “fire-heat” causing internal damage. Inflammation and inflammation-induced pyroptosis are critical factors in IS pathology, aligning closely with the TCM understanding of fire–heat mechanisms. In this study, we investigated the therapeutic effects and potential mechanisms of HLJD, a classic TCM formula known for its heat-clearing and detoxifying properties, in treating IS. Utilizing a multi-omics approach, we comprehensively explored how HLJD modulates pyroptosis to exert its protective effects against IS.
For a long time, TCM has been employed in treating neurological disorders [
17]. Research has demonstrated that Buyang Huanwu Decoction can improve IS by regulating APRT and PED1B [
18], while Tongqiao Huoxue Decoction can reduce IS damage by inhibiting ferroptosis through promoting ACSL4 ubiquitination [
19].
The inflammatory response plays a key role in IS injury [
20]. After the onset of IS, microglial cells in the brain are the first to become activated, leading to neuroinflammation and damage to the BBB. This activation results in the release of chemokines and cytokines. Peripheral innate immune cells, such as macrophages and neutrophils, are then recruited by these chemokines and enter the brain through the compromised BBB, causing further inflammatory damage.
Currently, substantial evidence indicates that inflammasome activation and pyroptosis play critical roles in the pathogenesis of IS [
21,
22]. In recent years, there has been an increasing number of studies focusing on the effects of TCM on these processes [
23,
24].
In previous studies, most attention has been focused on cell types within the brain, such as microglia, neurons, and endothelial cells [
25,
26,
27,
28]. Research on TCM has largely concentrated on the overall effects of complex formulas, often overlooking the specific small molecular compounds and the precise targets with which they interact [
29,
30,
31,
32].
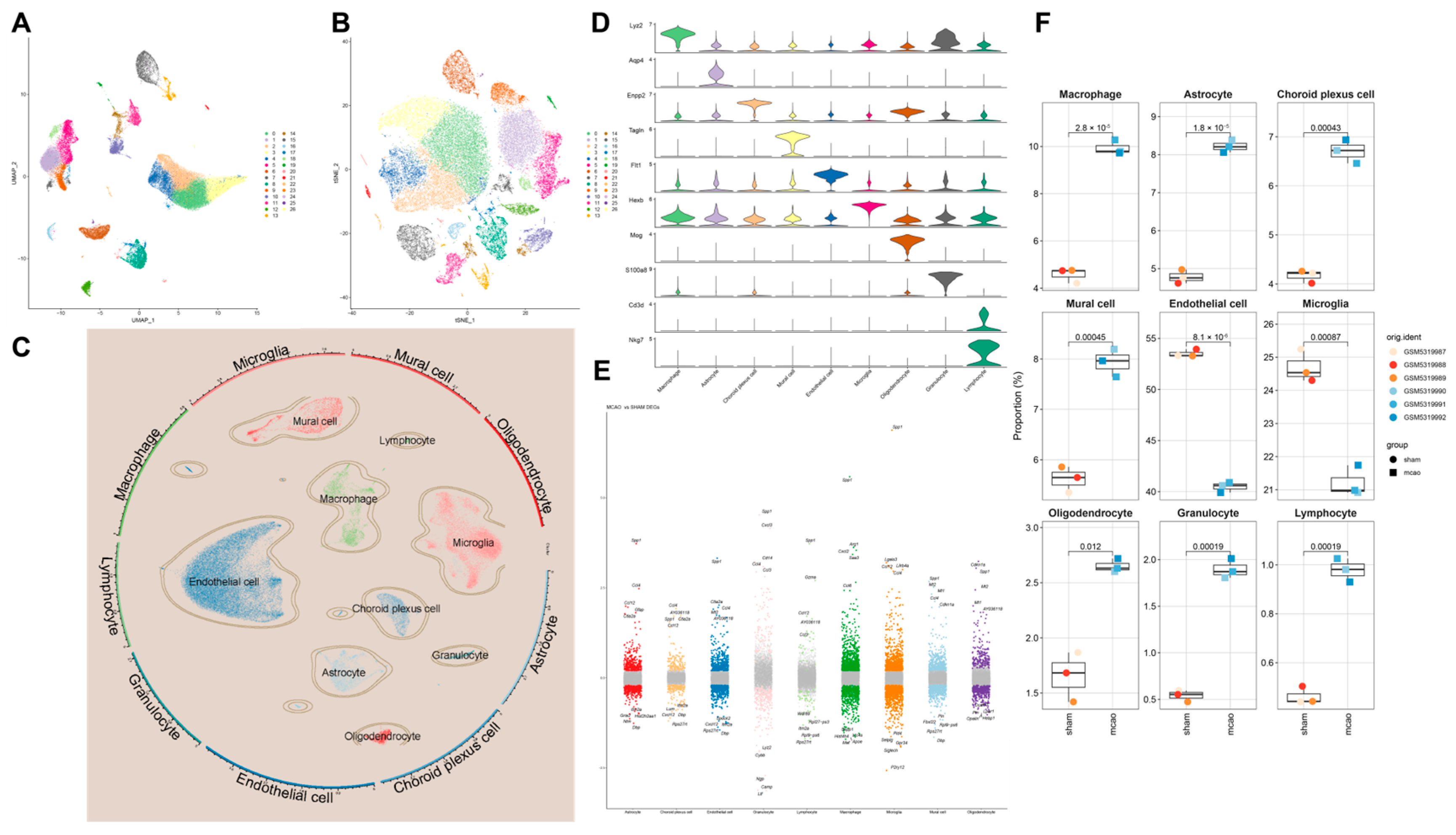
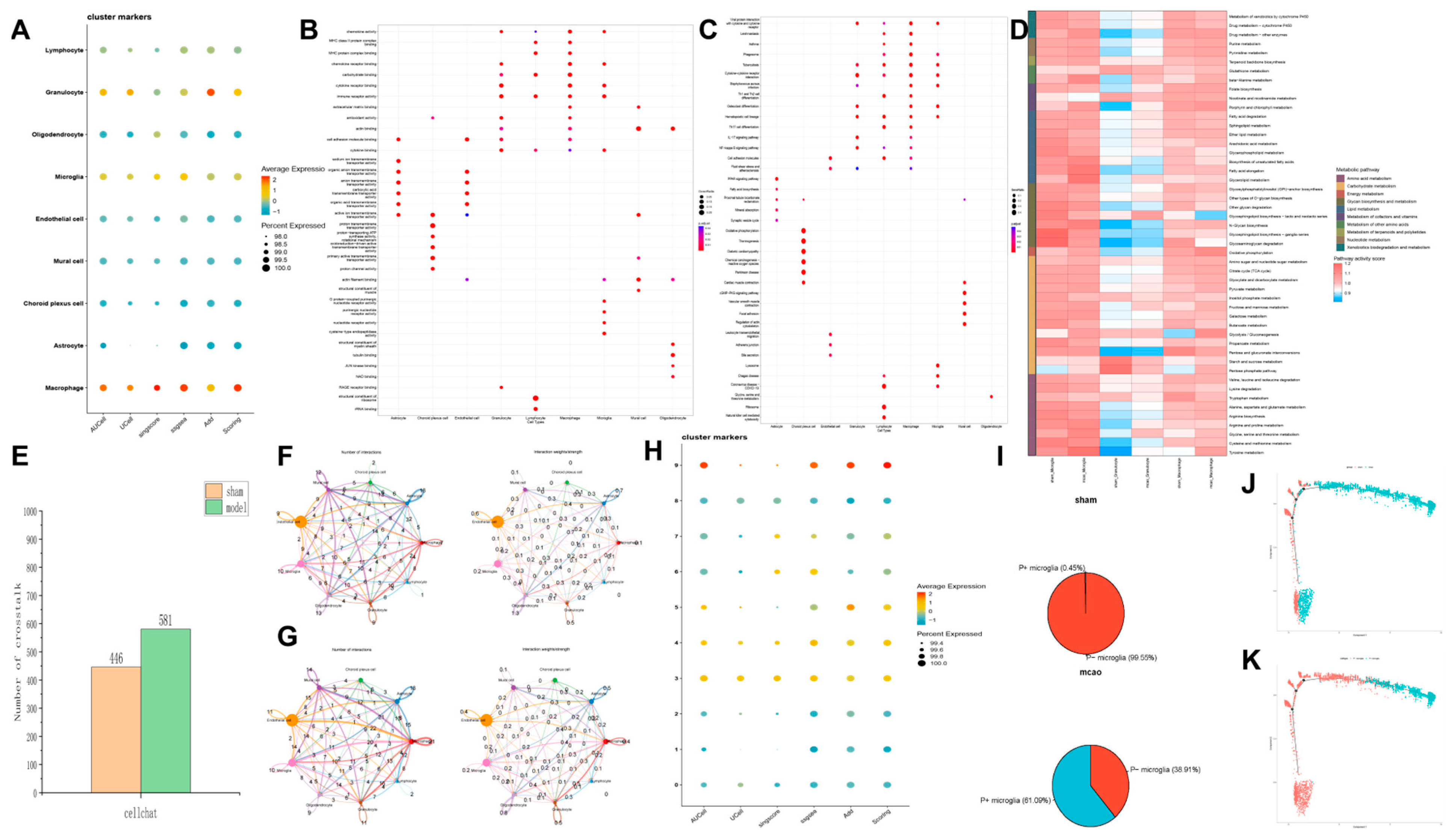
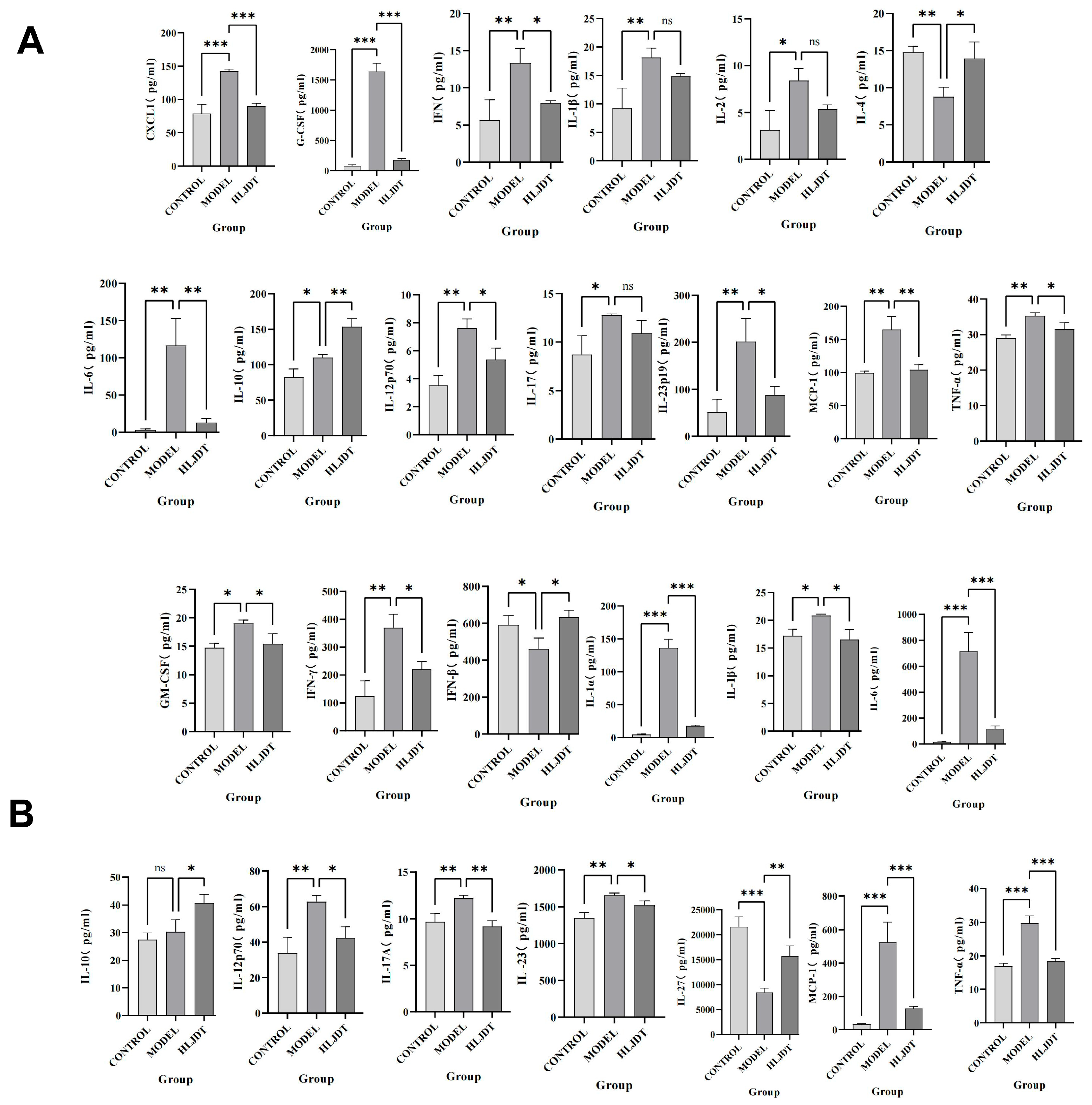
However, in the disease process, the infiltration of peripheral immune cells (such as neutrophils and macrophages) into the central nervous system is also a critical factor in neuroinflammation and neurodamage. Additionally, inflammasomes are highly expressed in immune cells, making them key cell types in the process of pyroptosis. During the acute phase of IS, the primary activators and executors of immune responses are innate immune cells, including macrophages, neutrophils, microglia, and natural killer cells. CXCL1 and G-CSF facilitate neutrophil infiltration and promote their conversion to a pro-inflammatory phenotype. GM-CSF and IFN-γ attract and activate macrophages, leading to inflammatory damage. IL-1α, IL-1β, IL-6, IL-12p70, IL-17, IL-17A, IL-23, IL-23p19, MCP-1, and TNF-α are involved in various innate immune pathways and are closely related to inflammatory damage caused by monocytes, macrophages, neutrophils, and NK cells. Inhibiting their expression can reduce their activation and subsequent inflammatory damage to endothelial cells, neurons, and other cell types. Although IL-2 has a weaker association with innate immunity, it can enhance the cytotoxic effects of killer T cells, contributing to inflammatory damage. However, this effect is less pronounced in the acute phase of IS due to the delayed response of adaptive immunity compared to innate immunity. IFN-β, IL-4, and IL-10 can modulate monocytes toward an M2 macrophage phenotype, promoting protective and reparative effects. IL-27 does not directly repair or inhibit inflammation but can induce IL-10 production by stimulating T cells to express IL-12R, indirectly reducing neural and endothelial damage. These findings indicate that post-IS, the levels of pro-inflammatory cytokines in the brain are significantly elevated, and cytokines and chemokines that activate inflammatory cells, such as neutrophils and macrophages, also show increased levels. This suggests that the central–peripheral inflammatory microenvironment is activated toward a pro-inflammatory state following stroke. HLJD treatment not only suppresses pro-inflammatory cytokines and related chemokines but also increases anti-inflammatory cytokines like IL-10, promoting the polarization of macrophages, microglia, and neutrophils toward an anti-inflammatory phenotype (M2 and N2, respectively). This reduces inflammatory damage, promotes angiogenesis, and aids in the phagocytosis of debris, preventing further cellular damage. Consequently, HLJD modifies the inflammatory microenvironment and exerts a protective effect. Additionally, based on our single-cell results, pyroptosis is mainly activated in macrophages, neutrophils, and microglia. HLJD may reduce pyroptosis by decreasing the infiltration and inflammatory activation of these cells, thereby mitigating brain damage.
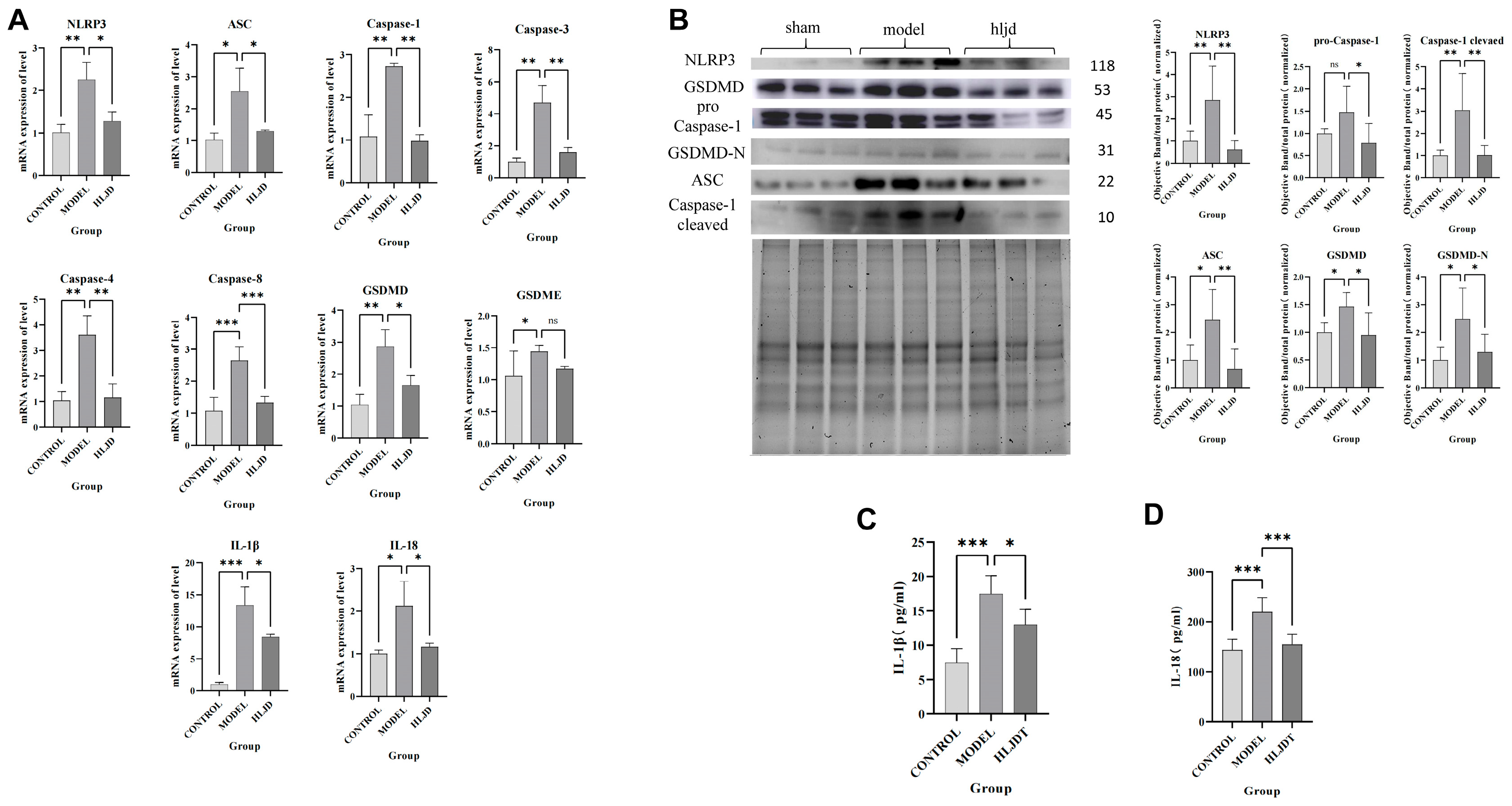
Therefore, using scRNA-seq analysis, we assessed the status of pyroptosis at the cellular level and identified that macrophages, neutrophils, and microglia are the main executors of pyroptosis during the acute phase of IS. Inhibiting pyroptosis-related pathways in these cells could potentially reduce neuroinflammation and provide neuroprotective effects. Interestingly, by comparing mRNA and protein expression levels, we observed that after HLJD treatment, both GSDMD and Caspase-1 RNA levels showed a downward trend, as did full-length GSDMD, GSDMD-N, pro-Caspase-1, and Cleaved Caspase-1 protein levels. Therefore, we propose that HLJD may reduce protein expression not only by inhibiting NLRP3 and the self-amplifying classical pyroptosis pathway but also by suppressing the translation of related targets. This effect is particularly evident in the reduction of full-length GSDMD and pro-Caspase-1 levels.
In this study, we employed a multi-omics approach, systematically progressing from pharmacodynamics and phenotypes to specific cells and small molecules/targets, to elucidate the mechanism by which HLJDT treats IS through the regulation of pyroptosis. By interpreting these findings from both macro and micro perspectives, we provide new insights into the pathological mechanisms of IS and identify potential therapeutic compounds. Furthermore, this methodology can serve as a model for investigating the relationship between traditional Chinese medicine formulas and diseases in future research.
However, our study has some limitations. In the animal experiments, we did not use NLRP3 inflammasome agonists or transgenic animals to conduct phenotype rescue experiments. In addition, due to the lack of commercial GSDMD-N or immunofluorescent antibody against GSDMD-N, we could not further validate the cell types that produce these pyroptosis phenotypes. These aspects require further analysis and exploration.
4. Materials and Methods
4.1. Materials and Reagents Used in the Experiment
Coptidis chinesis Franch (Huang-Lian) (220114004), Scutellaria baicalensis Georgi (Huang-Qin) (221128007), Phellodendron amurense Rupr (Huang-Bo) (220224001), and Gardenia jasminoides Ellis (Zhi-Zi) (220907001) were purchased from Beijing Qiancao Chinese Medicine Decoction Pieces Co., Ltd., (Beijing, China).
Chrysin-7-O-Glucuronide (TQ0287), Neochlorogenic Acid (T6S1538), Puerarin (T2815), Matrine (T2870), Dictamine (T5746), Rutin (T0795), and Wogonoside (T3318) were purchased from Target Mol (Shanghai, China). The NLRP3 protein (ABN-H00114548-P01-25 ug) was purchased from Abnova. The Series S Sensor Chip CM5 (29104988) was purchased from Cytiva (UCDP, Uppsala, Sweden). The primers were purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd., (Beijing, China). The compounds used in the manuscript are all above 99% pure.
TGX Stain-Free FastCast Kit (1610183) was purchased from BIO-RAD (Hercules, CA, USA). The antibodies against pro Caspase-1 + p10 + p12 (ab179515), GSDMD (ab219800), and NLRP3 (ab263899) were purchased from Abcam (Cambridge, UK). The Apoptosis-associated speck-like protein containing a CARD (ASC) antibody (67824S) was purchased from CST (Cell Signaling Technology) (Boston, MA, USA). The IL-1β (SEA563Mu) and IL-18 (SEA064Mu) ELISA kits were purchased from Cloud-Clone Corp (Wuhan, China). Proteins by Flow Cytometry Mouse LEGEND plex MU Proinflam Chemokine Panel (13-plex) (740150) was purchased from BioLegend (San Diego, CA, USA). Quantitative Measurement of 13 Mouse (FAM-INF-1-96 (96 tests)) was purchased from RayBiotech (Atlanta, GA, USA).
4.2. Preparation of HLJD
Weigh 150 g of Coptidis chinensis (Huang-Lian), 100 g of Scutellaria baicalensis (Huang-Qin), 100 g of Phellodendron amurense (Huang-Bo), and 150 g of Gardenia jasminoides (Zhi-Zi). Mix the four medicinal herbs thoroughly and divide them evenly into filtration bags. Add 5000 mL of water and let it soak for 1 h. Then, heat the mixture until it is boiling. Once boiling, reduce the heat to simmer and maintain a boil. Filter the mixture while hot using a 100-mesh filtration bag.
Add another 4000 mL of water and repeat the simmering process as before. Combine the liquids from both boilings and concentrate them. Divide the concentrated mixture into respective containers and dry it using a vacuum freeze dryer to obtain freeze-dried powder. Weigh the freeze-dried powder and calculate the extraction yield. The extraction rate of HLJD is 148.2 g/500 g = 29.64%
Daily dosage of HLJD: According to the calculation of a 60 kg adult, the dosage of raw drug is 30 g, that is, 30 g × 29.64%/60 kg × 12 = 1.7784 g/kg = 0.017784 g/10 g = 0.018 g/10 g daily. Calculate the volume of administration below. The gavage volume in mice is typically 0.2 mL/10 g. Dosing concentration = dosing volume/gavage volume, i.e., (0.018 g/10 g)/(0.2 mL/10 g) = 0.09 g/mL = 9 g/100 mL per mouse. Low dose: weigh 4.5 g HLJD and add animal drinking water to 100 mL. Medium dose: weigh 9 g of HLJD and add drinking water to 100 mL. High dosage: weigh 18 g HLJD and animal drinking water to 100 mL.
4.3. Design of the Animal Experiments
Eight-week-old male C57BL/6 J mice of specific pathogen-free grade, weighing 23 ± 2 g, were provided by Beijing Sibeifu Experimental Animal Technology Co., Ltd. The experimental animals were housed in a clean room at Beijing University of Chinese Medicine, with a 12-h light/dark cycle maintained at 25 ± 1 °C and 55 ± 10% humidity. The environment was free from noise interference.
This study was approved and supervised by the Animal Ethics Committee of Beijing University of Chinese Medicine, ensuring animal welfare throughout the experimental design and procedures. Ethics approval number BUCM-2024011901-1036.
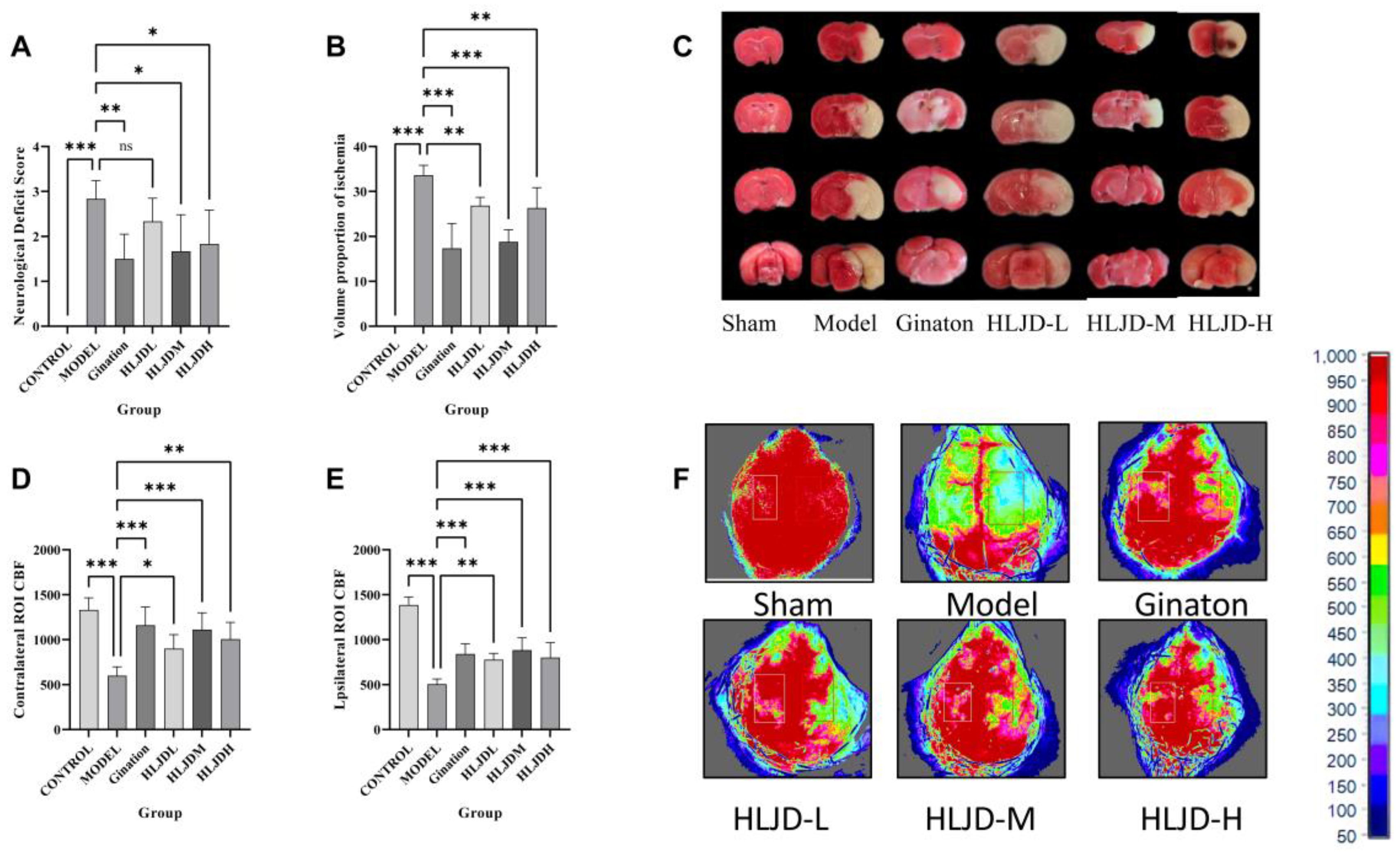
In total, 48 male C57BL/6 J mice, aged 8 weeks and weighing 23 ± 2 g, were randomly divided into 6 groups using a random number table method, with 8 mice per group. The groups were as follows: sham surgery group, model group, Ginaton group, low-dose HLJD group, medium-dose HLJD group, and high-dose HLJD group.
After one week of acclimatization, the mice were administered intragastrically. The sham surgery and model groups received physiological saline. The Ginaton group received Ginaton solution prepared at a concentration of 2.16 mg/mL. The HLJD groups were dosed based on an adult equivalent of 60 kg: the low-dose group received a solution prepared from 15 g of freeze-dried powder per day, the medium-dose group received a solution from 30 g of freeze-dried powder per day, and the high-dose group received a solution from 60 g of freeze-dried powder per day.
In this study, we aimed to investigate the therapeutic mechanism of HLJD on acute ischemic stroke in mice. All groups received a dosage of 0.2 mL/10 g body weight/day for five consecutive days. On the sixth day, after the final dose administration, the transient middle cerebral artery occlusion (tMCAO) model was induced.
4.3.1. Neurobehavioral Score
Starting from the insertion of the embolus into the middle cerebral artery, a 24-h countdown commenced. After this period, mice were removed from their housing cages and placed on an unobstructed flat experimental platform for Longa 5-point neurological behavioral assessment. This scale evaluates the severity of neurological damage, with higher scores indicating greater impairment.
4.3.2. TTC
Starting from the insertion of the embolus into the middle cerebral artery, a 24-h countdown was initiated. After this period, the mice were euthanized, and their brain tissues were harvested for TTC staining.
4.3.3. Detection of Cerebral Blood Flow (CBF)
Mice were anesthetized with isoflurane and fixed on a stereotaxic device. The skin on the top of the head was routinely disinfected, and afterward the scalp was cut. The meninges were then separated, and the skull between the coronal suture and the hermitage was fully exposed. After that, the cortical CBF was evaluated by laser speckle flow imaging.
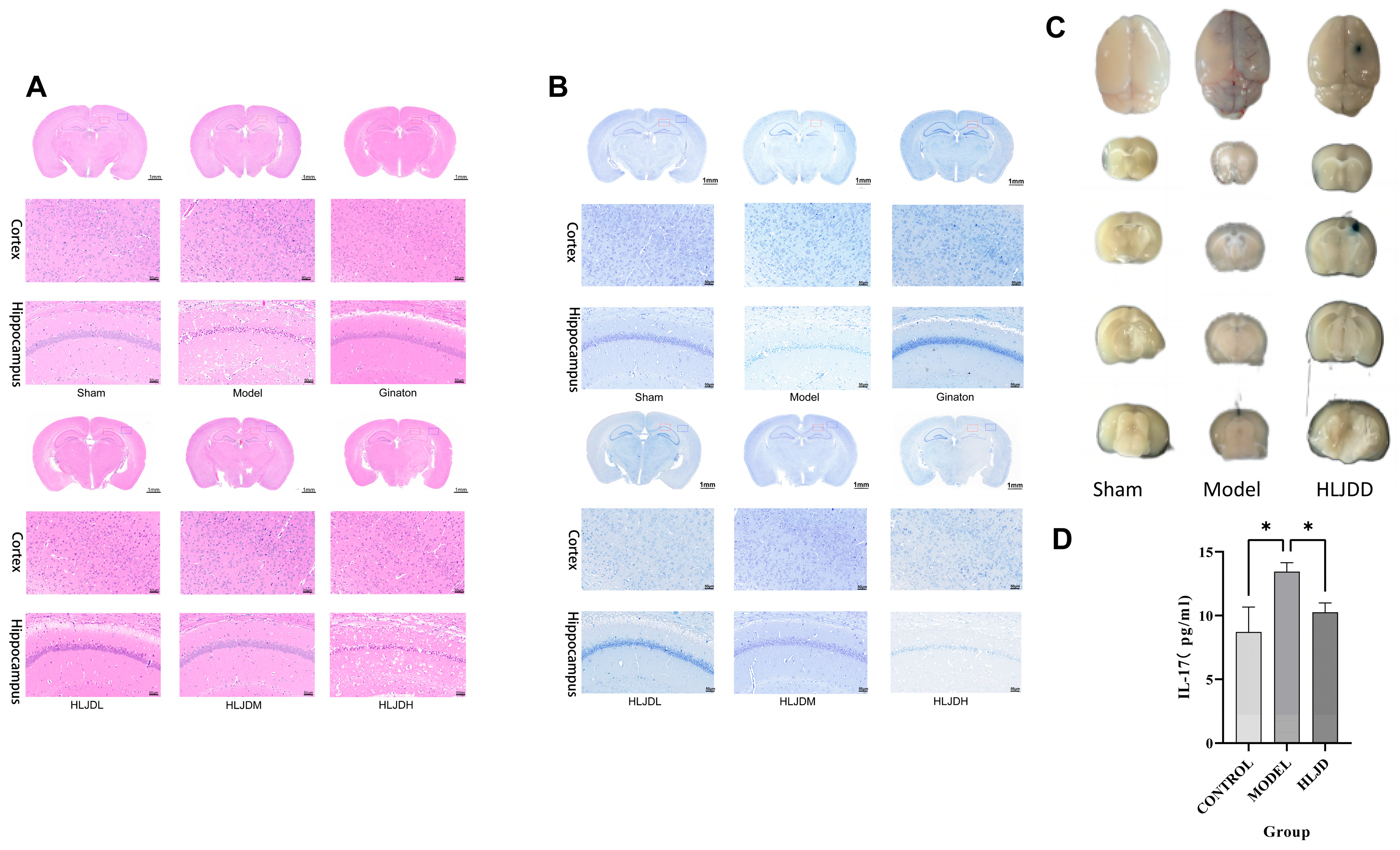
4.3.4. HE and Nissl Staining
The mouse brain tissues were fixed overnight in 4% paraformaldehyde and subsequently embedded in paraffin wax. Following embedding, the brain sections were stained using HE and Nissl staining solution.
4.3.5. EB
To assess blood–brain barrier (BBB) permeability following tMCAO, transient middle cerebral artery occlusion modeling, EB staining was utilized. A 2% EB solution was prepared and injected into the tail vein of the mice 1 h prior to tissue collection. After 1 h of circulation, the mice were anesthetized and perfused transcardially with saline. The brains and brain tissue sections were photographed. The brain tissue was then homogenized, incubated at 54 °C for 2 h, and centrifuged. The optical density of the supernatant for each sample was measured at a wavelength of 620 nm.
4.4. Bulk RNA-Seq Data Preparation
Using the keywords “MCAO”, “ischemic stroke”, and “stroke”, datasets GSE116878 [
33] and GSE227186 [
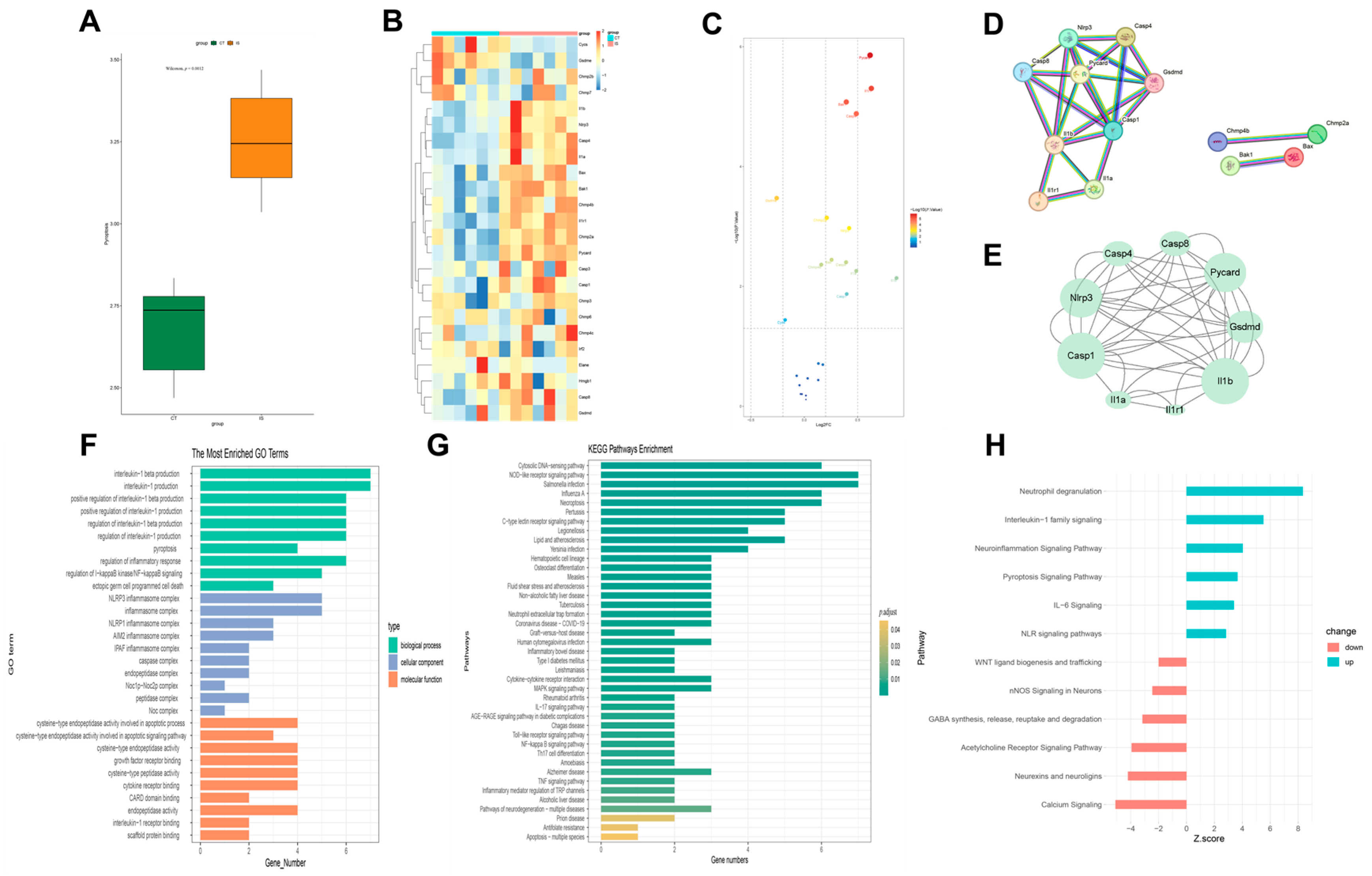
34] were retrieved from the GEO database, selecting “mouse” as the species. Data processing was conducted using R version 4.1.3. The limma package was employed for within-dataset normalization to reduce batch effects related to sequencing depth and gene attributes, and gene expression levels were log2-transformed. Genes with |LOGFC| ≥ 0.15 were selected as target genes and considered differentially expressed genes (DEGs).
4.4.1. Single Sample Gene Set Enrichment Analysis (ssGSEA)
Using the GSEA database and supported by a literature review, a pyroptosis gene set was obtained. The expression levels of these genes in each sample were scored using the ssGSEA method from the GSVA package. The differences between the control group and the ischemic stroke group were analyzed to observe if the target gene set showed overall differential expression between the two groups.
4.4.2. Protein–Protein Interaction (PPI) Network Analysis and Enrichment Analysis
The obtained differentially expressed genes were imported into the STRING database with the species set to mouse and the minimum required interaction score set to 0.900 (highest confidence). The interaction results file was then imported into Cytoscape 3.9.1 for visualization analysis. The MCODE and CytoNCA plugins were used to perform topological analysis of the PPI network to identify the core interaction network. Using R and relevant R packages, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses and plotting were performed.
4.4.3. Ingenuity Pathway Analysis (IPA)
IPA is a powerful tool for analyzing the direct interaction information between proteins, genes, compounds, cells, tissues, drugs, and diseases. The data analysis and interpretation in IPA are based on the comprehensive, manually curated, and corrected QIAGEN® Knowledge Base. Utilizing robust algorithms backed by this database, IPA can uncover upstream and downstream regulatory factors, interaction relationships, mechanisms, functions, and pathways corresponding to the analyzed molecular data. This allows for an in-depth understanding of the reasons behind changes in the analyzed datasets. In this study, IPA was employed to conduct a canonical pathway analysis of the core regulatory gene set.
4.5. scRNA-Seq Data Preparation
Using the keywords “MCAO”, “ischemic stroke”, and “stroke”, we performed a search in the GEO database, selecting “mouse” as the species. From this search, we downloaded the dataset GSE174574 [
35]. scRNA-seq data processing and analysis
To ensure the reliability of analysis, we utilized the Seurat package for processing and analyzing single-cell data. The following criteria were applied for gene filtering and the exclusion of low-quality cells:
1. Cells with detected gene counts >200 and <2500 were retained;
2. Cells with mitochondrial gene content ≤ 10% were retained;
3. Genes expressed in at least three cells were retained.
Additionally, we employed the CellCycle Scoring function in Seurat to assess the expression of cell cycle-related genes in single-cell data.
Following annotation, the Seurat FindAllMarkers function was employed to calculate DEGs across different cell types. Using the Wilcoxon rank-sum test, each cell type was compared against all other cells to identify DEGs specific to each cell type. Genes with p < 0.05 and |LOGFC| ≥ 0.25 were considered statistically significant.
Additionally, we conducted inter-group comparisons of the same cell type to identify DEGs specific to the same cell type in the context of cerebral I/R injury.
4.5.1. scRNA-Seq Enrichment Analysis
Using five single-cell scoring methods—AUCell, UCell, singscore, ssGSEA, and Add—we performed enrichment scoring for the target gene set, resulting in a total enrichment score. Furthermore, we performed GO and KEGG enrichment analyses on the DEGs identified earlier, using appropriate R packages.
Additionally, we employed a method based on the “Metabolic landscape of the tumor microenvironment at single-cell resolution” [
36] to analyze metabolic conditions at the single-cell level.
Cell Communication Analysis
To systematically analyze cell–cell communication networks at the molecular level, we utilized CellChat to investigate ligand–receptor interactions between cells. The minimum number of interacting cells was set to 10.
Cell Trajectory Analysis
To understand the differentiation trajectories of cells, we utilized Monocle2 software (2.26.0) for analysis. To arrange cells along this inferred trajectory, we designated a starting point for the differentiation pathway. Finally, using the orderCells function, we sequentially positioned all cells along the established trajectory. This methodology allows us to visualize and analyze the progression of cellular differentiation within the dataset.
Cell Subpopulation Reanalysis
Target cell populations were extracted, subjected to dimensionality reduction, and re-clustered for further analysis. This allowed for a detailed examination of the specific cellular subsets of interest, providing deeper insights into their characteristics and functions.
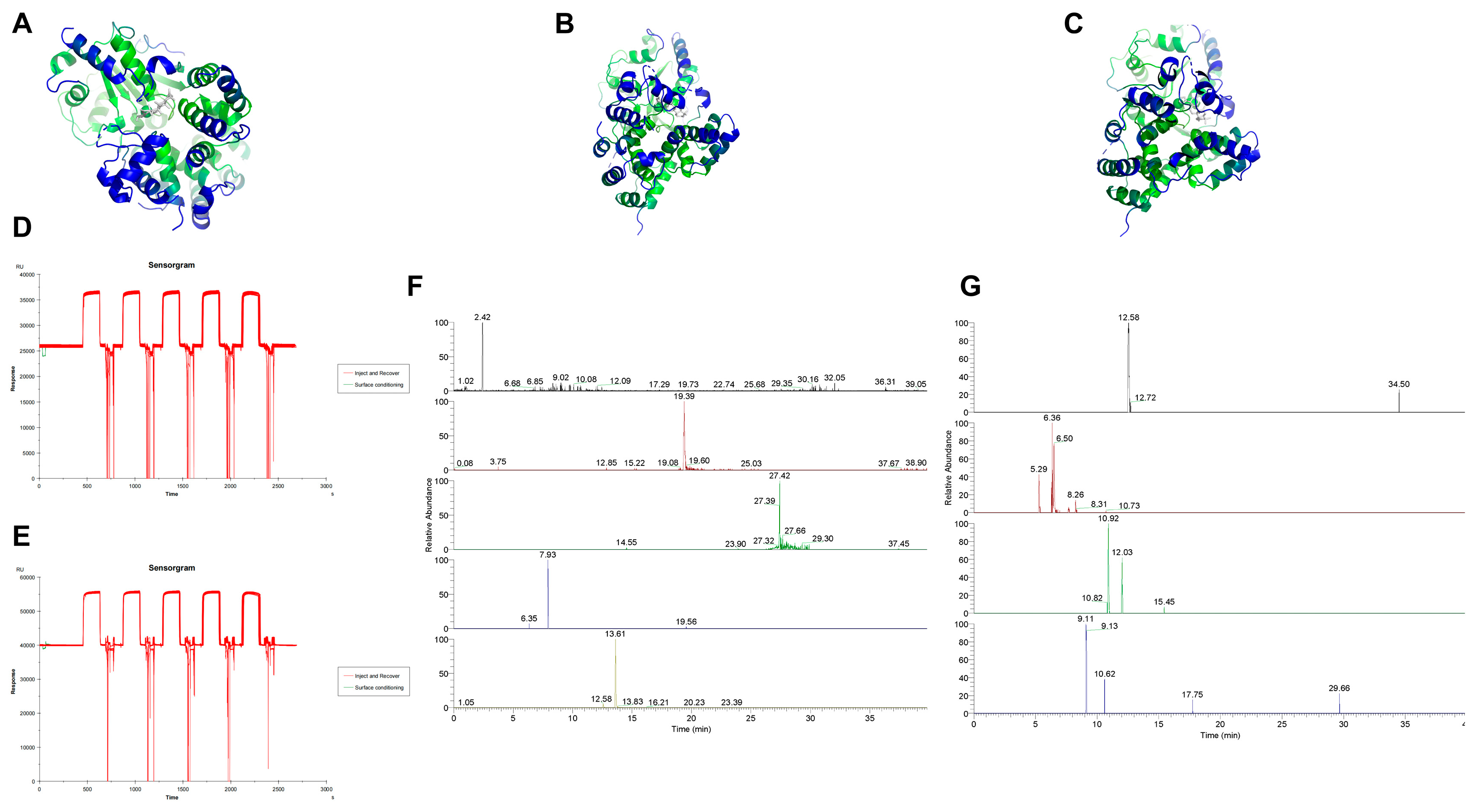
4.6. Molecular Docking
To identify potential active compounds in Huanglian Jiedu Decoction, we utilized a combination of database searches and literature review. The databases employed for this search included TCMSP, TCMID, DCABM-TCM, HERB, and SYMMAP. By analyzing these data and cross-referencing with the existing literature, we identified pyroptosis-related genes that are significant in IS and may serve as potential targets.
Small molecule structures were downloaded from the PubChem database, while protein structures were obtained from the PDB database. After downloading the respective structures, we performed preprocessing steps. For the molecular docking studies, we utilized AutoDock software (4.2.6). Post-docking, we used PyMOL for visualizing the docking results.
4.7. SPR Affinity Analysis
To prepare the drug samples, we utilized the previously prepared lyophilized powder. This powder was dissolved in ultrapure water to achieve a concentration of 100 mg/mL. The solution was then centrifuged at 12,000 rpm for 10 min at 4 °C. Following centrifugation, the supernatant was collected and filtered through a 0.22 μm membrane to produce the final drug sample.
For the protein-loaded chips, we employed a CM5 chip coupled with NLRP3 protein. Prior to initiating the procedure, a buffer solution was prepared by diluting 10× HBS-EP+ buffer with deionized water to a 1× concentration, resulting in a total volume of 500 mL. This mixture was thoroughly blended and placed in the buffer bottle for use.
Once the materials were prepared and the sample injection speed and duration were set on the instrument, the drug sample was injected. The baseline was allowed to stabilize before executing the inject and recover procedure, which involved target fishing and sample collection. After the procedure, the collected samples were stored at −20 °C for future use.
4.8. LC-MS Analysis
To identify small molecules that specifically bind to NLRP3, the solutions binding to both the blank chip and the NLRP3 protein chip were combined. The mixed solutions underwent ultrasonic treatment and were filtered through a 0.22 μm membrane before performing LC-MS analysis.
Chromatography Conditions: Column: ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.8 μm); Mobile Phase: Acetonitrile solution (A), 0.1% formic acid aqueous solution (B); Gradient Elution:0–6 min: 95% B; 6–24 min: 75% to 65% B; 24–32 min: 65% to 5% B; 32–34 min: 5% B; 34–35 min: 5% to 95% B; 35–40 min: 95% B; Flow Rate: 0.3 mL/min; Column Temperature: 35 °C; Injection Volume: 3 μL.
Mass Spectrometry Conditions: Ionization Mode: Positive and negative ion modes; Ion Source: Heated Electrospray Ionization; Ion Source Temperature: 400 °C; Ionization Source Voltage: 3.5 kV (positive ion mode), 3 kV (negative ion mode); Capillary Temperature: 320 °C; S-Lens Voltage: 55 V; Sheath Gas: High purity nitrogen (>99.99%), flow rate 35 arb; Auxiliary Gas: High purity nitrogen, flow rate 10 arb; Scan Mode: Full MS/dd-MS2; Full MS Resolution: 70,000; dd-MS2 Resolution: 17,500; Scan Range: m/z 100–1500 Da; Collision Energies: 10, 30, 50 eV.
Post-acquisition, data processing was conducted using Thermo Scientific Xcalibur software (OPTON-30967). The results obtained from the blank chip binding served as the background, which was subtracted from the NLRP3-binding data to isolate small molecules specifically binding to NLRP3.
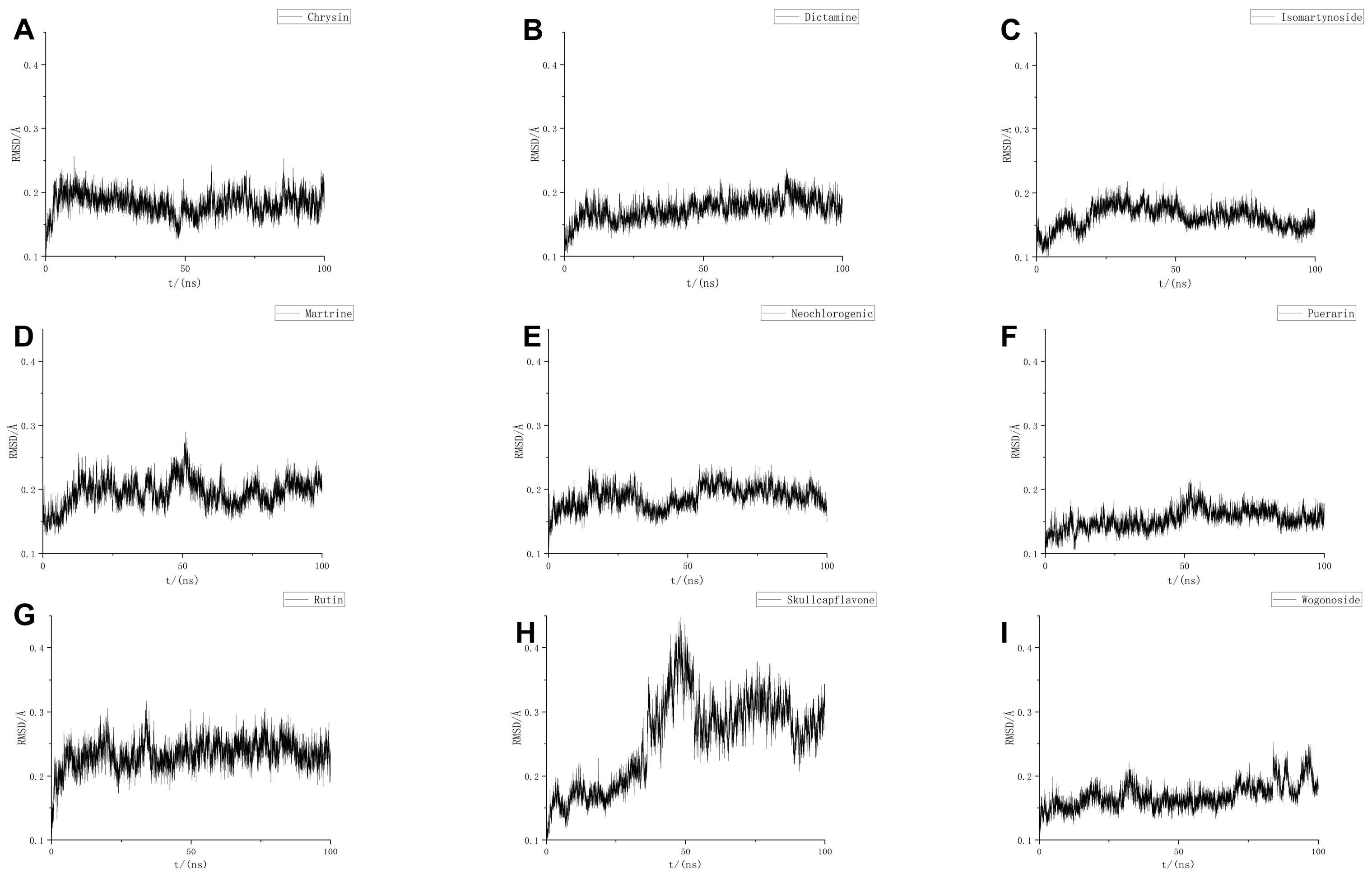
4.9. MD
MD simulations were performed using GROMACS (GMX-2024 GPU-CUDA), employing the CHARMM36 force field and the TIP3P water model. The system was confined within a cubic box with a size of 1.2 nm. Solvent molecules were added using the SPC216 water model. To neutralize the system, Na+/Cl− ion pairs were added to balance the charge.
To minimize potential collisions between the protein and small molecules, energy minimization was carried out using the steepest descent method to achieve an optimal potential energy state. The system was then equilibrated under isothermal conditions at 300 K, followed by isothermal–isobaric equilibration with Parrinello–Rahman pressure coupling at 1 bar for 1000 ps, using a time step of 2 fs. Coordinates and energy of the system were saved every 10 ps.
Following equilibration, each system underwent a 100 ns simulation. The resulting molecular trajectories were then corrected and evaluated.
To further refine the results from the MD simulations, binding free energy calculations were performed to quantify the affinity between the protein and small molecule ligands. The Molecular Mechanics Poisson–Boltzmann Surface Area (MMPBSA) method was employed to predict the stability of the ligand–receptor complex post-MD simulation.
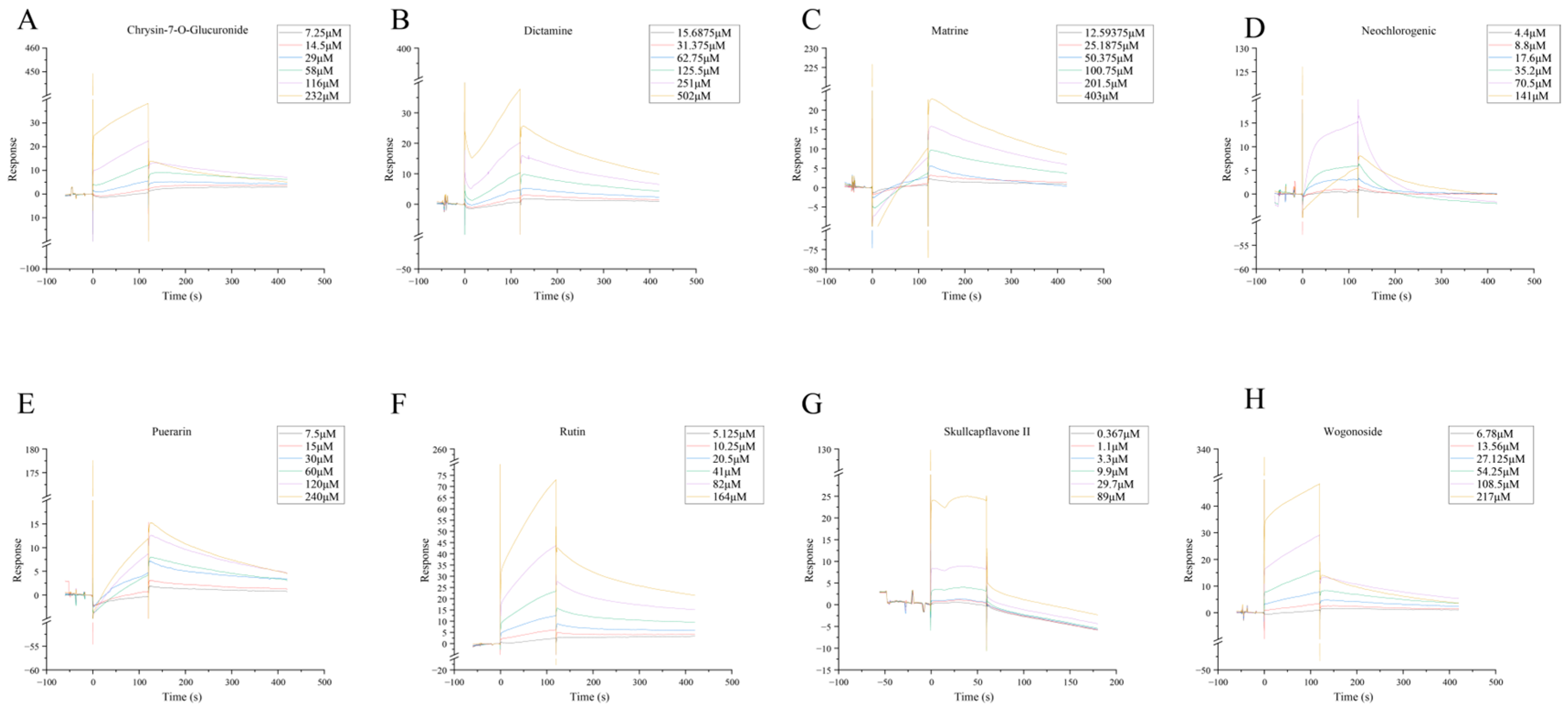
4.10. SPR Molecular Fishing
To investigate the interactions between target proteins and candidate small molecules from HLJD, protein chips were prepared. The test compounds were first dissolved and then diluted to a series of concentrations using appropriate buffer solutions. These diluted solutions were then injected into the flow cells of the chip.
If an interaction between the tested compounds and the target protein occurs, it will be as evident as a binding and dissociation curve. The binding affinity constants, Ka (association rate constant) and Kd (dissociation rate constant), as well as the equilibrium dissociation constant KD, were calculated using Biacore T200 software (CY25775). The equilibrium dissociation constant KD is determined by the ratio Kd/Ka and represents the affinity between the candidate small molecules and their respective target proteins from Huanglian Jiedu Decoction.
4.11. RT–qPCR
Approximately 50 mg of brain tissue was weighed and homogenized for total RNA extraction. The same method was used to extract RNA from cells. Following RNA extraction, reverse transcription was performed to generate cDNA templates. RT–qPCR was then conducted using fluorescent probes. The expression levels of the genes of interest were normalized to β-actin, and the relative gene expression was determined using the 2−ΔΔCt method.
4.12. WB
WB experiments were conducted using the Bio-Rad TGX Stain-Free FastCast Kit (Hercules, CA, USA). The total protein obtained from the kit served as the reference for subsequent analyses. The following primary antibodies were used: NLRP3 (1:1000), ASC (1:1000), pro Caspase-1 + p10 + p12 (1:1000), and GSDMD (1:1000). The relative protein expression was confirmed by total protein quantification. Protein detection and analysis were performed using Image Lab software (SOFT-LIT-170-9690-ILSPC-V-6-1).
4.13. ELISA
The levels of IL-1β and IL-18 in the ischemic cortex of the brain were detected using the ELISA kit for rats following the manufacturer’s instructions. Briefly, the mice were anesthetized and sacrificed, the ischemic cortex was dissected and lysed, the homogenate was centrifuged at 12,000 rpm at 4 °C for 10 min, and then the supernatant was collected for ELISA.
4.14. Cytokine/Chemokine Measurement
We measured the levels of cytokines and chemokines in serum and brain. The levels of multiple inflammatory factors were detected by the cytometric bead array system. CXCL1, G-CSG, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-17, IL-23p19, MCP-1, and TNF-α were detected in blood. We determined GM-CSF, IFN-β, IFN-γ, IL-1α, IL-1β, IL-6, IL-10, IL-12p70, IL-17A, IL-23, IL-27, MCP-1, and TNF-α.