Cardiovascular Effects, Phytochemistry, Drug Interactions, and Safety Profile of Foeniculum vulgare Mill. (Fennel): A Comprehensive Review
Abstract
1. Introduction
2. Methodology of Research
3. Botanical Description of Foeniculum vulgare Mill.
4. Phytochemistry of Foeniculum vulgare Mill.
4.1. Volatile and Non-Volatile Compounds
4.2. Phenolic Compounds
4.2.1. Flavonoids
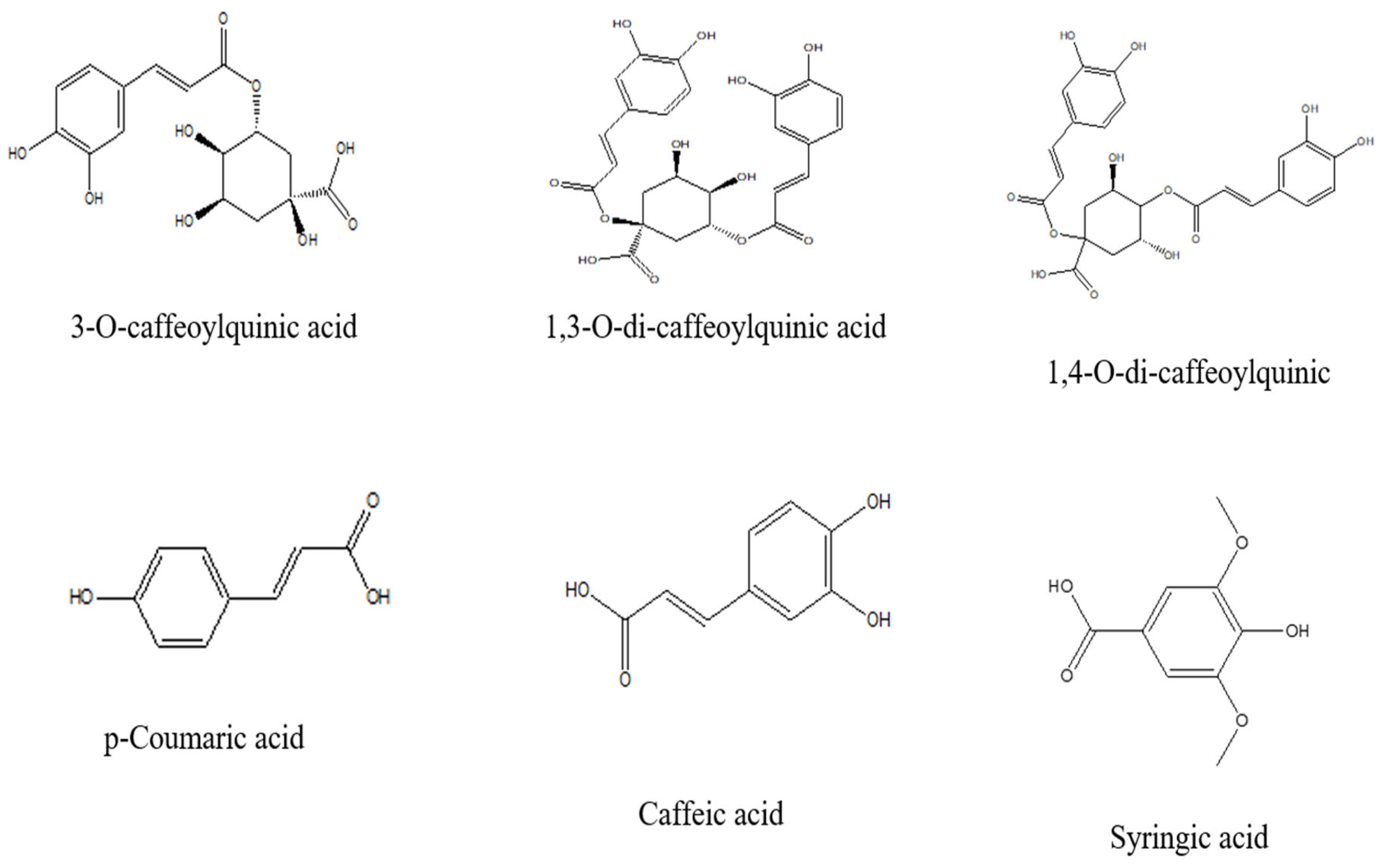
4.2.2. Phenolic Acids
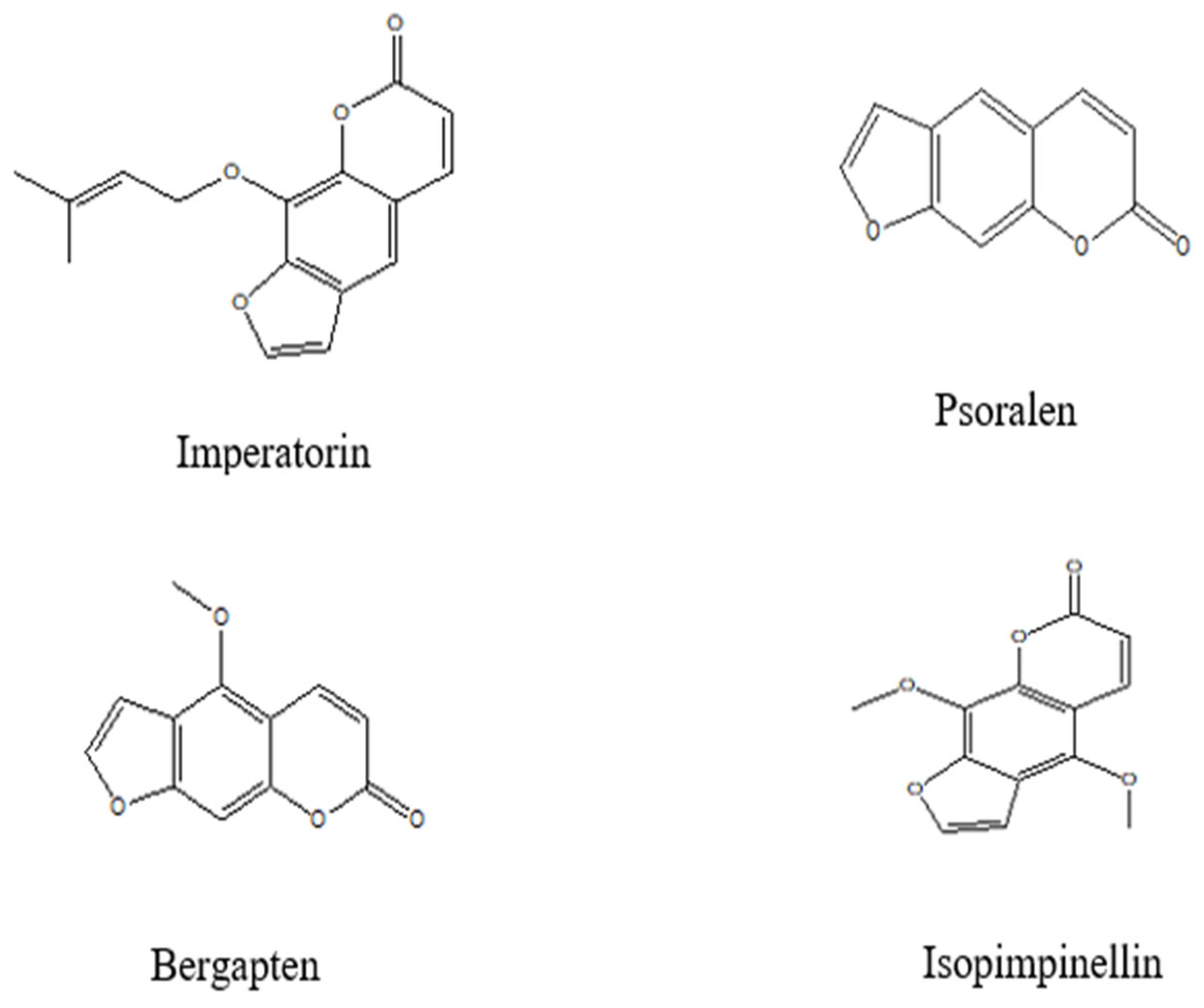
4.2.3. Coumarins
5. Cardiovascular Activities of Foeniculum vulgare Mill.
5.1. Hypotensive Activity
5.2. Antihypertensive Activity
5.3. Diuretic Activity
5.4. Vasorelaxant Activity
5.5. Cardioprotective Activity
5.6. Anticoagulant and Antithrombotic Activities
5.7. Anti-Inflammatory Activity
5.8. Hypolipidemic Activity
5.9. Antioxidant Activity
6. Safety Profile of Foeniculum vulgare Mill.
7. Interactions Between Foeniculum vulgare Mill. and Cardiovascular Drugs
8. Limitations and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| AKT | Protein Kinase B |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| CAT | Catalase |
| COX1/2 | Cyclooxygenase-1/2 |
| CPK | Creatine Phosphokinase |
| CREB | cAMP Response Element-Binding Protein |
| cGMP | Cyclic Guanosine Monophosphate |
| CRP | C-Reactive Protein |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| FV | Foeniculum vulagre Mill. |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| GPx | Glutathione Peroxidase |
| GSK-3β | Glycogen Synthase Kinase-3 Beta |
| GST | Glutathione S-Transferase |
| HepG2 | Human Liver Cancer Cell Line G2 |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl-Coenzyme A |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode Array Detection |
| IFN-γ | Interferon-Gamma |
| IKB-α | Inhibitor of Nuclear Factor Kappa-B Alpha |
| IL-1β | Interleukin-1 Beta |
| IL-4 | Interleukin-4 |
| IncRNA MIAT | Long Non-Coding RNA Myocardial Infarction Associated Transcript |
| iNOS | Inducible Nitric Oxide Synthase |
| JNK1 | c-Jun N-terminal Kinase 1 |
| LDH | Lactate Dehydrogenase |
| L-NAME | N-G-Nitro-L-Arginine Methyl Ester |
| MAPK | Mitogen-Activated Protein Kinase |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NOD-Like Receptor Pyrin Domain Containing 3 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| PMA | Phorbol 12-Myristate 13-Acetate |
| SOD | Superoxide Dismutase |
| SREBP-2 | Sterol Regulatory Element-Binding Protein 2 |
| TNF-α | Tumor Necrosis Factor Alpha |
References
- WHO. Cardiovascular Diseases. 2021. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 3 June 2024).
- WHR. Confronting the World’s Number One Killer; World Heart Federation. 2023. Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 4 June 2024).
- Moon, K.Z.; Rahman, M.H.; Alam, M.J.; Hossain, M.A.; Hwang, S.; Kang, S.; Moon, S.; Park, M.N.; Ahn, C.-H.; Kim, B. Unraveling the interplay between cardiovascular diseases and alcohol use disorder: A bioinformatics and network-based exploration of shared molecular pathways and key biomarkers validation via western blot analysis. Comput. Biol. Chem. 2025, 115, 108338. [Google Scholar] [CrossRef]
- Covani, M.; Niccoli, G.; Fujimoto, D.; Scalamera, R.; Vergallo, R.; Porto, I.; McNulty, I.; Lee, H.; Kakuta, T.; Jang, I.-K. Plaque vulnerability and cardiovascular risk factor burden in acute coronary syndrome: An optical coherence tomography analysis. J. Am. Coll. Cardiol. 2025, 86, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Majeed, F.; Taleb, A.; Zubair, H.M.; Shumzaid, M.; Farooq, M.A.; Baig, M.M.F.A.; Abbas, M.; Saeed, M.; Changxing, L. A Review of Medicinal Plants in Cardiovascular Disorders: Benefits and Risks. Am. J. Chin. Med. 2020, 48, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H. A review of medicinal plants used in therapy of cardiovascular diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 572–591. [Google Scholar]
- Kheirkhah, A.H.; Kavianpour, M.; Ababzadeh, S. Herbal medicine: A potent booster for stem cell therapy in cardiovascular diseases. Adv. Tradit. Med. 2025, 25, 395–410. [Google Scholar] [CrossRef]
- Celiński, R.; Krzemińska, B.; Grzywa-Celińska, A.; Szewczyk, G.; Szewczyk, K.D.S. A Review on the Potential Use of Medicinal Plants from the Apiaceae and the Rosaceae Families in Cardiovascular Diseases—Experimental Evidence and Traditional Applications. Appl. Sci. 2024, 14, 3728. [Google Scholar] [CrossRef]
- Muckensturm, B.; Foechterlen, D.; Reduron, J.-P.; Danton, P.; Hildenbrand, M. Phytochemical and chemotaxonomic studies of Foeniculum vulgare. Biochem. Syst. Ecol. 1997, 25, 353–358. [Google Scholar] [CrossRef]
- Choi, E.-M.; Hwang, J.-K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef]
- Zakernezhad, F.; Barati, M.; Sanadgol, N.; Movahedi, M.; Majd, A.; Golab, F. Assessing the Association Between Fennel (Foeniculum vulgare) Extract and Serum Lipid Profile and Leptin Receptor Expression. Basic Clin. Neurosci. J. 2021; in press. [Google Scholar]
- Zahi, A.; Driouech, M.; Hakkou, Z.; Mansouri, F.; El Hajji, F.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Legssyer, A. Vasorelaxant effect of fennel seeds (Foeniculum vulgare Mill.) extracts on rat mesenteric arteries: Assessment of phytochemical profiling and antioxidant potential. Fitoterapia 2025, 181, 106359. [Google Scholar] [CrossRef]
- Natarajan, P.; Grace, J. Effect of Mill. on Isoproterenol induced myocardial infarction in Foeniculum vulgare albino rats. Asian J. Pharm. Pharmacol. 2019, 5, 259–265. [Google Scholar]
- Rahimi, R.; Ardekani, M.R.S. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin. J. Integr. Med. 2013, 19, 73–79. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Septina, E.; Yetti, R.D.; Rivai, H. Overview of Traditional Use, Phytochemical, and Pharmacological Activities of Chinese Petai (Leucaena leucocephala). Int. J. Pharm. Sci. Med. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Ali, S.A. Medicinal flowers of Pakistan. Int. J. Adv. Res 2016, 4, 1313–1341. [Google Scholar]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Foeniculum vulgare-A review. IOSR J. Pharm. 2018, 8, 81–96. [Google Scholar]
- Khandelwal, K.R. Practical Pharmacognosy; Pragati Books Pvt. Ltd.: Pune, India, 2008. [Google Scholar]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Esteban, J.; Sanz, J. Comparison of the volatile composition of wild fennel samples (Foeniculum vulgare Mill.) from Central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Anubhuti, P.; Rahul, S.; Kant, K.C. Standardization of fennel (Foeniculum vulgare), its oleoresin and marketed ayurvedic dosage forms. Int. J. Pharm Sci Drug Res 2011, 3, 265–269. [Google Scholar]
- Telci, I.; Demirtas, I.; Sahin, A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30, 126–130. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; Abd ElRazik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Damayanti, A.; Setyawan, E. Essential oil extraction of fennel seed (Foeniculum vulgare) using steam distillation. Int. J. Sci. Eng. 2012, 3, 12–14. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Shi, M.; Liu, C.; Kang, W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci. Hum. Wellness 2019, 8, 67–72. [Google Scholar] [CrossRef]
- Abdullah, B.H.; Abbas, I.S.; Jasiem, T.M. Phytochemical study and evaluation of Iraqi fennel seed oil as antibacterial of urinary tract infection. Indian J. Forensic Med. Toxicol. 2020, 14, 842–846. [Google Scholar]
- Díaz-Maroto, M.C.; Díaz-Maroto Hidalgo, I.J.; Sánchez-Palomo, E.; Pérez-Coello, M.S. Volatile components and key odorants of Fennel (Foeniculum vulgare Mill.) and Thyme (Thymus vulgaris L.) oil extracts obtained by simultaneous distillation− extraction and supercritical fluid extraction. J. Agric. Food Chem. 2005, 53, 5385–5389. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Kujundžić, S.; Soković, M.; Couladis, M. Essential oil composition and antifungal activity of Foeniculum vulgare Mill. obtained by different distillation conditions. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 368–371. [Google Scholar] [CrossRef]
- Mehra, N.; Tamta, G.; Nand, V. Phytochemical screening and in vitro antioxidant assays in Foeniculum vulgare Mill.(Fennel) seeds collected from Tarai region in the Uttarakhand. Indian J. Nat. Prod. Resour. IJNPR Former. Nat. Prod. Radiance NPR 2022, 13, 213–222. [Google Scholar]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening the essential oil composition of wild Sicilian fennel. Biochem. Syst. Ecol. 2010, 38, 213–223. [Google Scholar] [CrossRef]
- Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants 2022, 11, 2318. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Schmeda-Hirschmann, G.; Burillo, J.; Codina, C. Bioguided isolation and identification of the nonvolatile antioxidant compounds from fennel (Foeniculum vulgare Mill.) waste. J. Agric. Food Chem. 2004, 52, 1890–1897. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.I.; Aboutabl, E.-s.A.; Makled, Y.A.; El-Khrisy, E.-D.; Osman, A.F. Secondary metabolites and pharmacology of Foeniculum vulgare Mill. Subsp. Piperitum. Rev. Latinoam. Química 2010, 38, 103–112. [Google Scholar]
- Yang, I.J.; Lee, D.U.; Shin, H.M. Anti-inflammatory and antioxidant effects of coumarins isolated from Foeniculum vulgare in lipopolysaccharide-stimulated macrophages and 12-O-tetradecanoylphorbol-13-acetate-stimulated mice. Immunopharmacol. Immunotoxicol. 2015, 37, 308–317. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Agrawal, S.S.; Srivastava, S.; Saxena, R. Oculohypotensive effects of Foeniculum vulgare in experimental models of glaucoma. Indian J. Physiol. Pharmacol. 2008, 52, 77–83. [Google Scholar]
- Abdul-Ghani, A.-S.; Amin, R. The vascular action of aqueous extracts of Foeniculum vulgare leaves. J. Ethnopharmacol. 1988, 24, 213–218. [Google Scholar] [CrossRef]
- Bardai, S.E.; Lyoussi, B.; Wibo, M.; Morel, N. Pharmacological evidence of hypotensive activity of Marrubium vulgare and Foeniculum vulgare in spontaneously hypertensive rat. Clin. Exp. Hypertens. 2001, 23, 329–343. [Google Scholar] [CrossRef]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef]
- Swaminathan, A.; Sridhara, S.R.C.; Sinha, S.; Nagarajan, S.; Balaguru, U.M.; Siamwala, J.H.; Rajendran, S.; Saran, U.; Chatterjee, S. Nitrites Derived From Foneiculum Vulgare (Fennel) Seeds Promotes Vascular Functions. J. Food Sci. 2012, 77, H273–H279. [Google Scholar] [CrossRef]
- Tettey, C.; Yang, I.; Ocloo, A.; Shin, H. Vasorelaxant and Anti-Inflammatory Activities of the Methylene Chloride Fraction of F oeniculum vulgare Fruit Extract. J. Food Biochem. 2015, 39, 55–63. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Maity, N.; Nema, N.K.; Bhadra, S.; Saha, B.P.; Mukherjee, P.K. Angiotensin converting enzyme inhibition activity of fennel and coriander oils from India. Nat. Prod. Commun. 2013, 8, 1934578X1300800531. [Google Scholar] [CrossRef]
- Abu-Zaiton, A.; Alu’datt, M.; Wafa, M. Evaluating the effect of Foeniculum vulgare extract on enzymes related with blood pressure and diabetes (in vitro study). Int. J. Adv. Chem. Eng. Biol. Sci. 2015, 2, 77–80. [Google Scholar]
- Han, A.Y.; Lee, H.S.; Seol, G.H. Foeniculum vulgare Mill. increases cytosolic Ca2+ concentration and inhibits store-operated Ca2+ entry in vascular endothelial cells. Biomed. Pharmacother. 2016, 84, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Rani, S. Formulation and Evaluation of Nanoemulsion of the Phenolic Content of Foeniculum vulgare for Antidepressant and Antihypertensive Potentiality. Indian J. Pharm. Educ. Res. 2023, 57, s660–s666. [Google Scholar] [CrossRef]
- Seo, J.W.; Habiba, S.U.; Munni, Y.A.; Choi, H.J.; Aktar, A.; Mazumder, K.; Nah, D.-Y.; Yang, I.-J.; Moon, I.S. Protective Effects of Anethole in Foeniculum vulgare Mill. Seed Ethanol Extract on Hypoxia/Reoxygenation Injury in H9C2 Heart Myoblast Cells. Antioxidants 2024, 13, 1161. [Google Scholar] [CrossRef]
- Tanira, M.; Shah, A.; Mohsin, A.; Ageel, A.; Qureshi, S. Pharmacological and toxicological investigations on Foeniculum vulgare dried fruit extract in experimental animals. Phytother. Res. 1996, 10, 33–36. [Google Scholar] [CrossRef]
- Jemal, A. Evaluation of the Diuretic Activity of Aqueous and 80% Methanol Extracts of Foeniculum vulgare Mill (Apiaceae) Leaf in Rats. Masters’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Mansouri, E.; Kooti, W.; Bazvand, M.; Boroon, M.G.; Amirzargar, A.; Afrisham, R.; Afzalzadeh, M.R.; Ashtary-Larky, D.; Jalali, N. The effect of hydro-alcoholic extract of Foeniculum vulgare Mill on leukocytes and hematological tests in male rats. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e18396. [Google Scholar] [CrossRef]
- Lutviani, S.; Anisa, I.N.; Soemardji, A.A. Anti-Inflammatory and Mucolytic Activity Test of Ethanol Extract Fennel Leaf (Foeniculum vulgare Mill.). In Proceedings of the Conference on Natural Resources and Life Sciences 2022 (NRLS-BIO 2022), Online, 24–25 August 2022; Atlantis Press: Dordrecht, The Netherlands, 2023; pp. 133–137. [Google Scholar]
- Tanveer, M.; Shehzad, A.; Butt, M.; Shahid, M. Pharmacodynamics of Foeniculum vulgare against ulcer, inflammation, hepatotoxicity and nephrotoxicity. J. Anim. Plant Sci. 2021, 31, 841–853. [Google Scholar] [CrossRef]
- Panthong, S.; Itharat, A.; Aukkanibut, S.; Intarawan, T. Anti-inflammatory effect and total flavonoid content of the ethanolic seed extracts of three umbelliferae species. Sci. Technol. Asia 2020, 25, 174–181. [Google Scholar]
- Arif, A.; Bhatti, A.; John, P. Therapeutic potential of Foeniculum vulgare mill. Derived selenium nanoparticles in arthritic Balb/c mice. Int. J. Nanomed. 2019, 14, 8561–8572. [Google Scholar] [CrossRef]
- Özbek, H. The Anti-inflammatory Activity of the Foeniculum vulgare L. Essential Oil and Investigation of its Median Lrthal Dose in Rats and Mice. Int. J. Pharmacol. 2005, 50, 329–331. [Google Scholar]
- Özbek, H.; Sever Yilmaz, B. Anti-inflammatory and hypoglycemic activities of alpha-pinene. Acta Pharm. Sci. 2017, 55, 7. [Google Scholar] [CrossRef]
- Rezayat, S.M.; Dehpour, A.-R.; Motamed, S.M.; Yazdanparast, M.; Chamanara, M.; Sahebgharani, M.; Rashidian, A. Foeniculum vulgare essential oil ameliorates acetic acid-induced colitis in rats through the inhibition of NF-kB pathway. Inflammopharmacology 2018, 26, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. UPLC-ESI-QTRAP-MS/MS analysis to quantify bioactive compounds in fennel (foeniculum vulgare mill.) waste with potential anti-inflammatory activity. Metabolites 2022, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Yakut, H.I.; Koyuncu, E.; Cakir, U.; Tayman, C.; Koyuncu, İ.; Taskin Turkmenoglu, T.; Cakir, E.; Ozyazici, A.; Aydogan, S.; Zenciroglu, A. Preventative and therapeutic effects of fennel (Foeniculum vulgare) seed extracts against necrotizing enterocolitis. J. Food Biochem. 2020, 44, e13284. [Google Scholar] [CrossRef]
- Koppula, S.; Alluri, R.; Kopalli, S.R. Foeniculum vulgare Mill. inhibits lipopolysaccharide-induced microglia activation and ameliorates neuroinflammation-mediated behavioral deficits in mice. Asian Pac. J. Trop. Biomed. 2024, 14, 28–39. [Google Scholar] [CrossRef]
- Cherbal, A.; Bouabdallah, M.; Benhalla, M.; Hireche, S.; Desdous, R. Phytochemical screening, phenolic content, and anti-inflammatory effect of Foeniculum vulgare seed extract. Prev. Nutr. Food Sci. 2023, 28, 141. [Google Scholar] [CrossRef]
- Darzi, S.E.; Khazraei, S.P.; Amirghofran, Z. The immunoinhibitory and apoptosis-inducing activities of Foeniculum vulgare on human peripheral blood lymphocytes. Res. Pharm. Sci. 2018, 13, 103–110. [Google Scholar] [CrossRef]
- Lee, H.-S. Anticoagulant properties of compounds derived from Fennel (Foeniculum vulgare Gaertner) fruits. Food Sci. Biotechnol. 2006, 15, 763–767. [Google Scholar]
- Elghazaly, N.A.; Radwan, E.H.; Zaatout, H.H.; Elghazaly, M.M.; El din Allam, N. Beneficial effects of fennel (Foeniculum vulgare) in treating obesity in rats. J. Obes. Manag. 2019, 1, 16–33. [Google Scholar] [CrossRef]
- Naderi, G.A.; Roghani, M.; Esmaeil Jamaat, E.; Zahedi, E.; Sanaeirad, A. The effect of Foeniculum vulgare (Fennel) hydroalcoholic extract on serum lipid profiles and liver enzymes in male rats fed a high cholesterol regimen. J. Basic Clin. Pathophysiol. 2019, 7, 20–27. [Google Scholar]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Tufail, T.; Ain, H.; Khalid, W.; Hanif, A.; Ali, B.; Khan, M.; Iqbal, R.; Alwahibi, M.; ERCİŞLİ, S. Assessment of antioxidant activities of flaxseed (Linum usitatisimum L.) and fennel seed (Foeniculum vulgare Mill.) extracts. Pol. J. Environ. Stud. 2024, 33, 3359–3366. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.D. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Medica 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ouariachi, E.; Lahhit, N.; Bouyanzer, A.; Hammouti, B.; Paolini, J.; Majidi, L.; Desjobert, J.M.; Costa, J. Chemical composition and antioxidant activity of essential oils and solvent extracts of Foeniculum vulgare Mill. from Morocco. J. Chem. Pharm. Res. 2014, 6, 743–748. [Google Scholar]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. from Portugal. Food Chem. Toxicol. 2009, 47, 2458–2464. [Google Scholar] [CrossRef]
- Miguel, M.G.; Cruz, C.; Faleiro, L.; Simões, M.T.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Foeniculum vulgare essential oils: Chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Commun. 2010, 5, 1934578X1000500231. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculum vulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef]
- Singh, A.; Raja, W. Assessment of antioxidant activity of Foeniculum vulgare seed extract using fenton reaction. Res. J. Med. Plants Ayurveda 2020, 1, 1–7. [Google Scholar]
- Khammassi, M.; Abidi, A.; Ochi, N.; Ayadi, A.; Mabrouk, Y.; Amri, I.; De Feo, V.; Sebai, H.; Polito, F. Protective effect of essential oil of Foeniculum vulgare Mill. against bleomycin-induced pulmonary fibrosis and oxidative stress in rat. Phytomed. Plus 2024, 4, 100660. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Cholinergic basis of memory-strengthening effect of Foeniculum vulgare Linn. J. Med. Food 2006, 9, 413–417. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, R.J.; Magalhães, P.J.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Cardiovascular effects of the essential oil of Croton zehntneri leaves and its main constituents, anethole and estragole, in normotensive conscious rats. Life Sci. 2006, 78, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Menezes, I.A.; Barreto, C.M.; Antoniolli, A.R.; Santos, M.R.; de Sousa, D.P. Hypotensive activity of terpenes found in essential oils. Z. Naturforsch. C J. Biosci. 2010, 65, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Martin-Santamaria, S.; Recio, I.; Sanchez-Moreno, C.; de Pascual-Teresa, B.; Rimbach, G.; de Pascual-Teresa, S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef]
- Olszanecki, R.; Bujak-Gizycka, B.; Madej, J.; Suski, M.; Wołkow, P.; Jawień, J.; Korbut, R. Kaempferol, but not resveratrol inhibits angiotensin converting enzyme. J. Physiol. Pharmacol. 2008, 59, 387–392. [Google Scholar]
- Maneesai, P.; Iampanichakul, M.; Potue, P.; Khamseekaew, J.; Tong-Un, T.; Prachaney, P.; Settheetham-Ishida, W.; Pakdeechote, P. Kaempferol Ameliorates Renal Remodelling by Inhibiting the Renin-Angiotensin System Cascade in Hypertensive Rats. J. Food Biochem. 2024, 2024, 8810152. [Google Scholar] [CrossRef]
- Huang, W.Y.; Fu, L.; Li, C.Y.; Xu, L.P.; Zhang, L.X.; Zhang, W.M. Quercetin, hyperin, and chlorogenic acid improve endothelial function by antioxidant, antiinflammatory, and ACE inhibitory effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Amal, Z.; Mounime, K.; Mounia, D.; Abderrahim, Z.; Hassane, M.; Mohamed, B.; Abdelkhaleq, L. The Contribution of the Rat Mesenteric Vascular Bed Model to Phytopharmacology with Computational Studies of the Main Vasorelaxant Phytochemicals. Lett. Drug Des. Discov. 2024, 21, 4184–4204. [Google Scholar] [CrossRef]
- Mahadevaswamy, M.; Suchitha, G.; Pavan, S.; Vivek, H.; Nithya, S.; Chandan, S.; Prasad, S.K.; Keshava Prasad, T.; Ahmad, S.F.; Attia, S.M. Naringin attenuates angiotensin II induced cardiac hypertrophy by inhibiting carbonic anhydrase II. Sci. Rep. 2025, 15, 11789. [Google Scholar]
- Samadi-Noshahr, Z.; Ebrahimzadeh-Bideskan, A.; Hadjzadeh, M.A.; Shafei, M.N.; Salmani, H.; Hosseinian, S.; Khajavi-Rad, A. trans-Anethole attenuated renal injury and reduced expressions of angiotensin II receptor (AT1R) and TGF-β in streptozotocin-induced diabetic rats. Biochimie 2021, 185, 117–127. [Google Scholar] [CrossRef]
- Moser, J.C.; Cechinel-Zanchett, C.C.; Mariano, L.N.B.; Boeing, T.; da Silva, L.M.; de Souza, P. Diuretic, Natriuretic and Ca2+-Sparing Effects Induced by Rosmarinic and Caffeic Acids in Rats. Rev. Bras. Farmacogn. 2020, 30, 588–592. [Google Scholar] [CrossRef]
- Bashir, A.; Mushtaq, M.N.; Younis, W.; Anjum, I. Fenchone, a monoterpene: Toxicity and diuretic profiling in rats. Front. Pharmacol. 2023, 14, 1119360. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.G.; Gasparotto, F.M.; Boffo, M.A.; Lourenço, E.L.B.; Stefanello, M.É.A.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Diuretic and potassium-sparing effect of isoquercitrin—An active flavonoid of Tropaeolum majus L. J. Ethnopharmacol. 2011, 134, 210–215. [Google Scholar] [CrossRef]
- Mariano, L.N.B.; Boeing, T.; da Silva, R.d.C.M.V.d.A.F.; Cechinel-Filho, V.; Niero, R.; da Silva, L.M.; de Souza, P.; Andrade, S.F.d. Preclinical evaluation of the diuretic and saluretic effects of (-)-epicatechin and the result of its combination with standard diuretics. Biomed. Pharmacother. 2018, 107, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Mudnic, I.; Modun, D.; Rastija, V.; Vukovic, J.; Brizic, I.; Katalinic, V.; Kozina, B.; Medic-Saric, M.; Boban, M. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010, 119, 1205–1210. [Google Scholar] [CrossRef]
- Yuan, T.-Y.; Zi-Ran, N.; Di, C.; Yu-Cai, C.; Hui-Fang, Z.; Lian-Hua, F.; Du, G.-H. Vasorelaxant effect of quercetin on cerebral basilar artery in vitro and the underlying mechanisms study. J. Asian Nat. Prod. Res. 2018, 20, 477–487. [Google Scholar] [CrossRef]
- Mahobiya, A.; Singh, T.U.; Rungsung, S.; Kumar, T.; Chandrasekaran, G.; Parida, S.; Kumar, D. Kaempferol-induces vasorelaxation via endothelium-independent pathways in rat isolated pulmonary artery. Pharmacol. Rep. 2018, 70, 863–874. [Google Scholar] [CrossRef]
- Tan, C.S.; Tew, W.Y.; Jingying, C.; Yam, M.F. Vasorelaxant effect of 5,7,4′-Trihydroxyflavanone (Naringenin) via endothelium dependent, potassium and calcium channels in Sprague Dawley rats: Aortic ring model. Chem. Biol. Interact. 2021, 348, 109620. [Google Scholar] [CrossRef]
- Opie, L.H.; Commerford, P.J.; Gersh, B.J.; Pfeffer, M.A. Controversies in ventricular remodelling. Lancet 2006, 367, 356–367. [Google Scholar] [CrossRef]
- Tan, J.; Ma, Z.; Han, L.; Du, R.; Zhao, L.; Wei, X.; Hou, D.; Johnstone, B.H.; Farlow, M.R.; Du, Y. Caffeic acid phenethyl ester possesses potent cardioprotective effects in a rabbit model of acute myocardial ischemia-reperfusion injury. Am. J. Physiol. -Heart Circ. Physiol. 2005, 289, H2265–H2271. [Google Scholar] [CrossRef]
- Aswar, U.; Mahajan, U.; Kandhare, A.; Aswar, M. Ferulic acid ameliorates doxorubicin-induced cardiac toxicity in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 659–668. [Google Scholar] [CrossRef]
- Ding, S.K.; Wang, L.X.; Guo, L.S.; Luo, P.; Du, J.J.; Zhao, Z.L.; Wang, G.G. Syringic acid inhibits apoptosis pathways via downregulation of p38MAPK and JNK signaling pathways in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Mol. Med. Rep. 2017, 16, 2290–2294. [Google Scholar] [CrossRef]
- Shaik, A.H.; Al Omar, S.Y.; Mohammad, A.; Kodidhela, L.D. Combined cardio-protective ability of syringic acid and resveratrol against isoproterenol induced cardio-toxicity in rats via attenuating NF-kB and TNF-α pathways. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Rhana, P.; Barros, G.M.; Santos, V.C.d.O.; Costa, A.D.; Santos, D.M.d.; Fernandes-Braga, W.; Durço, A.O.; Santos, M.R.V.; Roman-Campos, D.; Vasconcelos, C.M.L.d.; et al. S-limonene protects the heart in an experimental model of myocardial infarction induced by isoproterenol: Possible involvement of mitochondrial reactive oxygen species. Eur. J. Pharmacol. 2022, 930, 175134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Li, S.; Mo, L. Cardioprotective effect of quercetin against ischemia/reperfusion injury is mediated through NO system and mitochondrial K-ATP channels. Cell J. (Yakhteh) 2021, 23, 184. [Google Scholar]
- Cong, L.; Su, Y.; Wei, D.; Qian, L.; Xing, D.; Pan, J.; Chen, Y.; Huang, M. Catechin relieves hypoxia/reoxygenation-induced myocardial cell apoptosis via down-regulating lncRNA MIAT. J. Cell. Mol. Med. 2020, 24, 2356–2368. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S. Investigation of the anticoagulant and antithrombotic effects of chlorogenic acid. J. Biochem. Mol. Toxicol. 2017, 31, e21865. [Google Scholar] [CrossRef]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, S. Mechanisms of attenuation of clot formation and acute thromboembolism by syringic acid in mice. J. Funct. Foods 2018, 43, 112–122. [Google Scholar] [CrossRef]
- Pignatelli, P.; Pulcinelli, F.M.; Celestini, A.; Lenti, L.; Ghiselli, A.; Gazzaniga, P.P.; Violi, F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am. J. Clin. Nutr. 2000, 72, 1150–1155. [Google Scholar] [CrossRef]
- Navarro-Núñez, L.; Rivera, J.; Guerrero, J.A.; Martínez, C.; Vicente, V.; Lozano, M. Differential effects of quercetin, apigenin and genistein on signalling pathways of protease-activated receptors PAR1 and PAR4 in platelets. Br. J. Pharmacol. 2009, 158, 1548–1556. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, Q.-h.; Xiong, X.-g.; Wang, Y.; Zhang, Z.-h.; Sun, M.-j.; Lu, X.; Wu, D. Anti-inflammatory effects of p-coumaric acid, a natural compound of Oldenlandia diffusa, on arthritis model rats. Evid. Based Complement. Altern. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Qiu, W.; Shi, Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol. Cell. Toxicol. 2022, 18, 509–519. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Y.; Su, H.; Wu, L.; Huang, Y.; Zhao, L.; Han, B.; Shu, G.; Xiang, M.; Yang, J.-M. Chlorogenic acid methyl ester exerts strong anti-inflammatory effects via inhibiting the COX-2/NLRP3/NF-κB pathway. Food Funct. 2018, 9, 6155–6164. [Google Scholar] [CrossRef]
- Ponte, E.L.; Sousa, P.L.; Rocha, M.V.; Soares, P.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.; Assreuy, A. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol. Rep. 2012, 64, 984–990. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Devshilt, I.; Yun, Q.; Huang, C.; An, L.; Dorjbat, S.; He, X. Fennel main constituent, trans-anethole treatment against LPS-induced acute lung injury by regulation of Th17/Treg function. Mol. Med. Rep. 2018, 18, 1369–1376. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef]
- Ahmad, S.; Beg, Z.H. Hypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspension fed rats. Food Chem. 2013, 138, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, U.; Shobha, I.; Andallu, B. Comparison of aniseeds and coriander seeds for antidiabetic, hypolipidemic and antioxidant activities. Spatula DD 2011, 1, 9–16. [Google Scholar] [CrossRef]
- Dhivya, K.; Vengateswari, G.; Arunthirumeni, M.; Karthi, S.; Senthil-Nathan, S.; Shivakumar, M.S. Bioprospecting of Prosopis juliflora (Sw.) DC seed pod extract effect on antioxidant and immune system of Spodoptera litura (Lepidoptera: Noctuidae). Physiol. Mol. Plant Pathol. 2018, 101, 45–53. [Google Scholar] [CrossRef]
- Araruna, M.E.C.; Alves Júnior, E.B.; de Lima Serafim, C.A.; Pessoa, M.M.B.; de Souza Pessôa, M.L.; Alves, V.P.; Sobral, M.V.; da Silva, M.S.; Alves, A.F.; de Paiva Sousa, M.C. (−)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms. Pharmaceuticals 2024, 18, 18. [Google Scholar] [CrossRef]
- Prasad, J.; Baitharu, I.; Sharma, A.K.; Dutta, R.; Prasad, D.; Singh, S.B. Quercetin Reverses Hypobaric Hypoxia-Induced Hippocampal Neurodegeneration and Improves Memory Function in the Rat. High Alt. Med. Biol. 2013, 14, 383–394. [Google Scholar] [CrossRef]
- Hsu, J.-Y.; Lin, H.-H.; Chyau, C.-C.; Wang, Z.-H.; Chen, J.-H. Aqueous Extract of Pepino Leaves Ameliorates Palmitic Acid-Induced Hepatocellular Lipotoxicity via Inhibition of Endoplasmic Reticulum Stress and Apoptosis. Antioxidants 2021, 10, 903. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Lee, S.-J.; Mun, G.-I.; An, S.-M.; Boo, Y.-C. Evidence for the association of peroxidases with the antioxidant effect of p-coumaric acid in endothelial cells exposed to high glucose plus arachidonic acid. BMB Rep. 2009, 42, 561–567. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, T.-t.; Liang, Y.; Duan, L.-f.; Zhang, Y.-d.; Wang, L.-J.; He, G.-m.; Xiao, H.-t. p-Coumaric Acid Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by MAPK Signaling. Oxidative Med. Cell. Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef]
- Bian, Y.-Y.; Guo, J.; Majeed, H.; Zhu, K.-X.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Ferulic acid renders protection to HEK293 cells against oxidative damage and apoptosis induced by hydrogen peroxide. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 722–729. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev. Res. 2019, 80, 253–261. [Google Scholar] [CrossRef]
- Shah, A.; Qureshi, S.; Ageel, A. Toxicity studies in mice of ethanol extracts of Foeniculum vulgare fruit and Ruta chalepensis aerial parts. J. Ethnopharmacol. 1991, 34, 167–172. [Google Scholar] [CrossRef]
- Kishore, R.N.; Anjaneyulu, N.; Ganesh, M.N.; Sravya, N. Evaluation of anxiolytic activity of ethanolic extract of Foeniculum vulgare in mice model. Int. J. Pharm. Pharm. Sci. 2012, 4, 584–586. [Google Scholar]
- Ostad, S.; Soodi, M.; Shariffzadeh, M.; Khorshidi, N.; Marzban, H. The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study. J. Ethnopharmacol. 2001, 76, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, N.R.; Miller, E.C.; Miller, J.A.; Pitot, H.C. Hepatocarcinogenicity of estragole (1-allyl-4-methoxybenzene) and 1′-hydroxyestragole in the mouse and mutagenicity of 1′-acetoxyestragole in bacteria. J. Natl. Cancer Inst. 1976, 57, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Miller, E.C. The metabolic activation and nucleic acid adducts of naturally-occurring carcinogens: Recent results with ethyl carbamate and the spice flavors safrole and estragole. Br. J. Cancer 1983, 48, 1–15. [Google Scholar] [CrossRef][Green Version]
- Miller, E.C.; Swanson, A.B.; Phillips, D.H.; Fletcher, L.; Liem, A.; Miller, J.A. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983, 43, 1124–1134. [Google Scholar] [PubMed]
- Paini, A.; Punt, A.; Viton, F.; Scholz, G.; Delatour, T.; Marin-Kuan, M.; Schilter, B.; van Bladeren, P.J.; Rietjens, I.M. A physiologically based biodynamic (PBBD) model for estragole DNA binding in rat liver based on in vitro kinetic data and estragole DNA adduct formation in primary hepatocytes. Toxicol. Appl. Pharmacol. 2010, 245, 57–66. [Google Scholar] [CrossRef]
- Ostad, S.; Khakinegad, B.; Sabzevari, O. Evaluation of the teratogenicity of fennel essential oil (FEO) on the rat embryo limb buds culture. Toxicol. Vitr. 2004, 18, 623–627. [Google Scholar] [CrossRef]
- Abraham, S. Anti-genotoxicity of trans-anethole and eugenol in mice. Food Chem. Toxicol. 2001, 39, 493–498. [Google Scholar] [CrossRef]
- Dai, G.; Wang, C.; Tang, W.; Liu, J.; Xue, B. A 90-Day Oral Toxicity Study of the Ethanol Extract from Eupatorium japonicum Thunb and Foeniculum vulgare in Rats. BioMed Res. Int. 2020, 2020, 6859374. [Google Scholar] [CrossRef]
- FDA. U.S. Food and Drug Administration. Fennel, Sweet (Foeniculum vulgare mill. var. Dulce); FDA: Silver Spring, MD, USA, 2025.
- Zisaki, A.; Miskovic, L.; Hatzimanikatis, V. Antihypertensive drugs metabolism: An update to pharmacokinetic profiles and computational approaches. Curr. Pharm. Des. 2015, 21, 806–822. [Google Scholar] [CrossRef]
- Danjuma, M.I.; Mukherjee, I.; Makaronidis, J.; Osula, S. Converging indications of aldosterone antagonists (spironolactone and eplerenone): A narrative review of safety profiles. Curr. Hypertens. Rep. 2014, 16, 414. [Google Scholar] [CrossRef]
- Flockhart, D.A.; Thacker, D.; McDonald, C.; Desta, Z. The Flockhart Cytochrome P450 Drug-Drug Interaction Table. 2021. Available online: https://drug-interactions.medicine.iu.edu/ (accessed on 21 October 2025).
- Akbar, M.; Henderson, C.; Helen Grant, M. Inhibition of cell growth and glutathione depletion by calcium channel blockers. Toxicology 2008, 253, 14–15. [Google Scholar] [CrossRef]
- Mazzari, A.L.; Prieto, J.M. Herbal medicines in Brazil: Pharmacokinetic profile and potential herb-drug interactions. Front. Pharmacol. 2014, 5, 162. [Google Scholar] [CrossRef]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-glycoprotein transport system and cardiovascular drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef]




 Increase;
Increase;  Decrease.
Decrease.
 Increase;
Increase;  Decrease.
Decrease.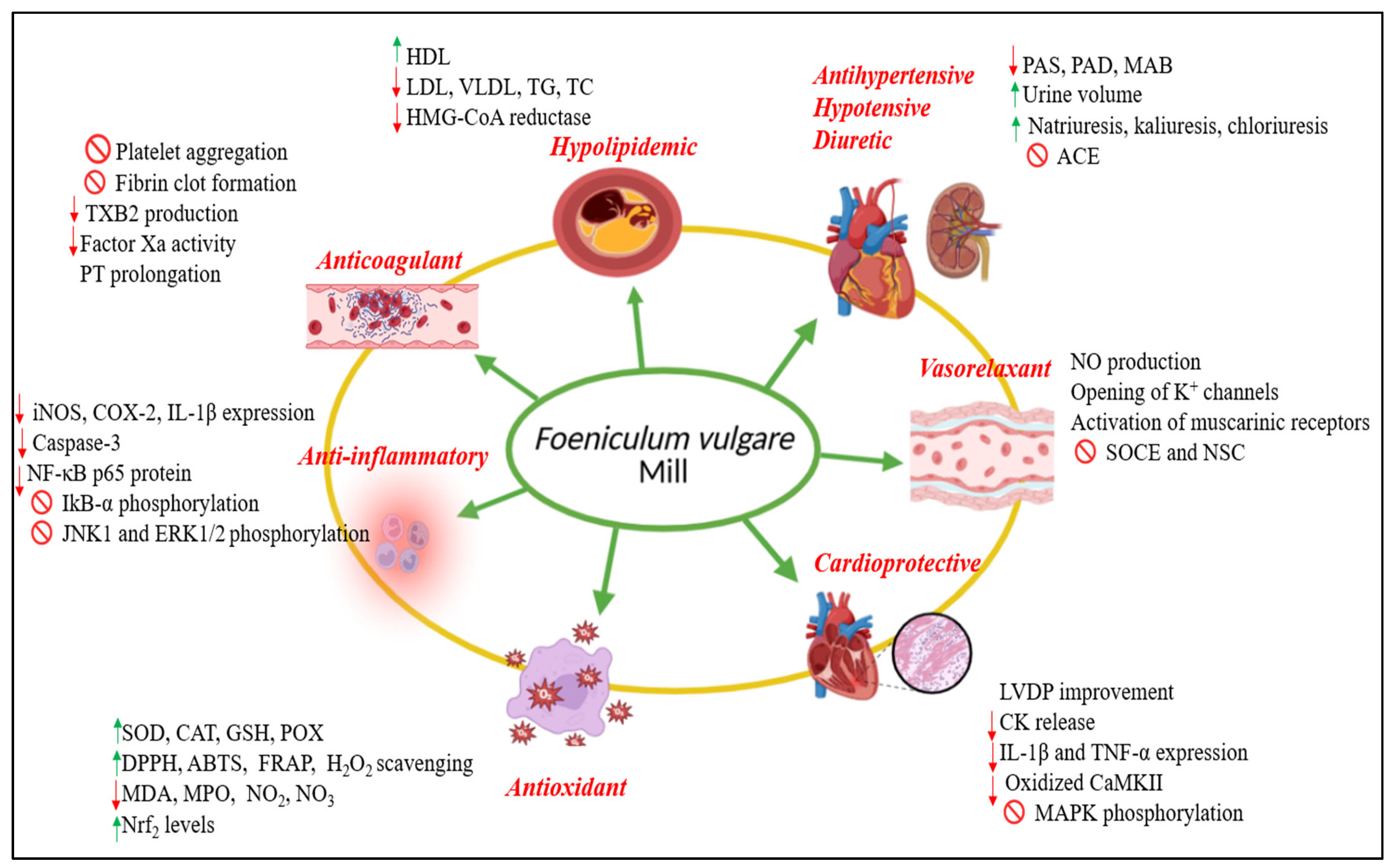
| Fennel’s Parts | Extracts (Compound)/Dose Used | Experimental Model/Assays Type | Cardiovascular Activity | References |
|---|---|---|---|---|
| Seeds | Aqueous extract 0.3, 0.6 and 1.2% (w/v) | Normotensive and glaucoma acute in chronic models of rabbits (In vivo) | Hypotensive effect 17.49, 21.16 and 22.03% decrease in intraocular pressure at 0.3%, 0.6% and 1.2%, respectively. | [38] |
| Leaves | Aqueous extract (12 mg/kg). | Normotensive rats (In vivo) | Hypotensive effect Decrease in MBP in a dose-related manner without affecting the pulse or respiratory rate. | [39] |
| Fruits | Aqueous extract (190 mg/Kg/day) | Spontaneously hypertensive rats (SHR) and normotensive Wistar Kyoto rats (WKY) (In vivo) | Antihypertensive and vasorelaxant effects Reduction in the SBP of SHR but not of WKY. Increasing water, sodium and potassium excretion. NO-dependent vasorelaxation. | [40] |
| Fruits and aerial parts | Essential oil and anethole (1 to 300 µg/mL) | Rat isolated aorta (In vitro) | Vasorelaxant effect Fennel oil or anethole reduced phenylephrine or KCl induced contractions with IC50 ranging from 50 to 147 µg/mL. NO-independent vasorelaxation. | [41] |
| Seeds | Aqueous extract-derived nitrites (0.00069–1.38 µg/g) | Fertilized Chicken eggs (In vitro) | Vasorelaxant effect Significantly increases NO production and elevated the cGMP levels of endothelial cells. | [42] |
| Fruits | Methylene chloride fraction of crude methanolic extract (100–1000 µg/mL) 50,100, and 200 μg/mL (for inflammatory assay) | Isolated rat aortic rings (In vitro) RAW 264.7 macrophage cells (In vitro) | Vasorelaxant effect At 1000 μg/mL, a 69% vasodilatation was observed, which was endothelium-dependent. Anti-inflammatory effect Fennel extract at 100 and 200 µg/mL suppressed the expression of mRNA and proteins of both TNF-α and IL-1β in LPS-activated macrophages. | [43] |
| Seeds | Aqueous extract (0.01, 0.1, and 1) and sequential fractions of fennel (0.001–1 mg/mL) | Rat isolated mesenteric vascular beds (In vitro) | Vasorelaxant effect Fennel extract induced concentration endothelium dependent vasodilatory response with Emax = 81.73 ± 0.36%. The butanolic fraction showed the highest vasorelaxant effect by involvement of the NO/GMPc pathway, potassium channels, and muscarinic receptors. | [12] |
| Fruits | Essential oil and anethole (10–500 µg/mL) | ACE inhibition assay (In vitro) | Antihypertensive effect Both fennel oil and anethole showed significant ACE inhibition, with IC50 values of 40.7 ± 3.5 and 52 ± 5.8 µg/mL, respectively. | [44] |
| Leaves | Methanolic extract of phenolic compounds | ACE inhibition assay (In vitro) | Antihypertensive effect ACE inhibition activity of 50.8% was observed at a phenolic content of 17.04 mg/g. | [45] |
| Seeds | Oil and trans-anethole (0.01%) | Vascular endothelial cells (In vitro) | Vasorelaxant effect Fennel oil and trans-anethole inhibit SOCE in endothelial cells, which may involve the inhibition of NSC channels, IP3-dependent Ca2+ release, and PLC activation. | [46] |
| Aerial part | Nanoemulsion of phenolic compounds (1% and 2% w/v) | Salt-induced hypertensive rat model (In vivo) | Antihypertensive effect Significantly decreased MBP, SBP, DBP and normalized the heart rate in hypertensive rats at both doses. | [47] |
| Fruits | Hydroalcoholic extract (200; 250; and 400 mg/kg) | Isoproterenol (85 mg/kg, s.c) induced myocardial infarction in albino rats (In vivo) | Cardioprotective effect Significant reduction in serum levels of SGOT, SGPT, ALP, LDH, CPK, CRP, glucose, triglycerides, and the LDL/HDL ratio. Significant elevation in glutathione levels in both heart and liver. Myocardial cells regeneration. | [13] |
| Seeds | Ethanolic extract (0–100 µg/mL) Anethole (0–200 µM) | Hypoxia/reoxygenation injury in H9C2 heart myoblast cells (In vitro) | Cardioprotective effect Decrease in ROS generation, DNA double-strand break damage, nuclear condensation, and dissipation of mitochondrial membrane potential induced by hypoxia/reperfusion. | [48] |
| Fruits | Ethanolic extract (500 mg/kg) | Normotensive rats (In vivo) | Diuretic effect A highly significant diuretic effect was observed both at 5 h and 24 h after administration, without any change in sodium and/or potassium excretion. | [49] |
| Leaves | Aqueous and 80% methanol extracts (100, 200, 400 mg/kg) | Normotensive rats (In vivo) | Diuretic effect Both extracts increased urine volume after 24 h, natriuresis, kaliuresis, and chloriuresis at 200 and 400 mg/kg). | [50] |
| Seeds | Hydro-alcoholic extract (250–1000 mg/kg, oral) | Normal Wistar rats (In vivo) | Immunomodulatory effect The extract significantly increased RBC and WBC counts, particularly at a dose of 250 mg/mL, and CT at 500 mg/mL, compared to the control group. | [51] |
| Fruits | Methanolic extract (200 mg/kg, orally) | Carrageenan-induced paw edema, arachidonic acid-induced ear edema, formaldehyde-induced arthritis and type IV allergy (In vivo) | Anti-inflammatory effect Significant inhibition of paw edema (69%), and inhibition of ear edema (~70%). A Significant inhibitory effect on delayed-type hypersensitivity (immunosuppressive effect). Antioxidant effect Increase in SOD and CAT, and decreased levels of TBARS (lipid peroxidation). | [10] |
| Leaves | Ethanol extract (25.75, 51.50, 103, 206, and 412 μg/mL) | Membrane stabilization by induction of a hypotonic solution (In vitro) | Anti-inflammatory effect Significant inhibition of HRBC hemolysis, proportional to extract concentration, with an optimal concentration of 412 µg/mL. | [52] |
| Seeds | Ethanolic extract (CSE) Extraction with CO2 (SFE) | Carrageenan-induced paw edema (In vivo). | Anti-inflammatory effect Paw edema reduction (SFE: 30.43% CSE: 24.54%). | [53] |
| Seeds | Ethanolic extract (100 µg/mL) | RAW264.7 cells (In vitro) | Anti-inflammatory effect Inhibition of NO production: 78.70% ± 6.81% (IC50 = 47.91 µg/mL). Inhibition of TNF-α and IL-6 production: 42.21% ± 0.42% and 63.20% ± 1.04%, respectively. | [54] |
| Seeds | Selenium nanoparticles derived from fennel (5 and 10 mg/kg) | Arthritic Balb/c mice (In vivo). | Anti-inflammatory effect Reduction in paw volume at 5 mg/kg and at 10 mg/kg. Restored cellular morphology and no signs of erosion (5 and 10 mg/kg). | [55] |
| Seeds | Essential oil (0.050 and 0.200 mL/kg | Carrageenan induced rat paw edema (In vivo). | Anti-inflammatory effect Antiedema effect at 0.200 mL/kg (56.78% inhibition). | [56] |
| Seeds | Alpha-pinene (0.05, 0.10, 0.25 and 0.50 mL/kg) | Carrageenan induced rat paw edema (In vivo). | Anti-inflammatory effect Significant decrease in inflammation at 0.50 mL/kg (60.33%). | [57] |
| Fruits | Essential oil (100, 200, and 400 mg/kg) | Acute intestinal colitis induced by acetic acid (In vivo). | Anti-inflammatory effect Reduction of the ulcer index at 200 and 400 mg/kg. Reduction of the expression of TNF-α positive cells at 200 and 400 mg/kg. Reduction of the expression of p-NF-κB p65 protein at 400 mg/kg. | [58] |
| Leaf, bulb, stem, and little stem | Phenolic acids and glycosylated flavonoids (25, 50, 100, and 150 µM) | COX inhibitory fluorometric assay (In vitro). | Anti-inflammatory effect Kaempferol (IC50 = 228.38 ± 16.81 µM), isorhamnetin (IC50 = 94.72 ± 1.22 µM), and quercetin glucuronide (IC50 = 570.83 ± 40 µM) inhibited COX-1 enzymes more effectively than COX-2 enzymes. | [59] |
| Seeds | Aqueous extract (200 mg/kg/day) | Experimental necrotizing enterocolitis in rat (NEC) (In vivo). | Anti-inflammatory effect Significantly reduction in IL-6, TNF-α, and caspase-3 levels. Decrease in bowel injury. | [60] |
| Fruits | Aqueous extract (25, 50, and 100 µg/mL) | LPS-stimulated neuroinflammatory in BV-2 microglial cells (In vitro). | Anti-inflammatory effect Suppressed the expression of iNOS and COX-2 protein levels. NF-κB activation and IκB-α phosphorylation were inhibited in a dose-dependent manner. Fennel extract at 50 and 100 µg/mL significantly suppressed the increased expression of IL-6 and TNF-α. | [61] |
| Seeds | Hydromethanolic extract (50, 150, 200, and 250 µg/mL) | Protein denaturation, protease activity, membrane stabilization, and heat-induced hemolysis in RBC (In vitro). | Anti-inflammatory effect The maximum percentage of protein denaturation inhibition was 35.68 ± 0.40% at 200 µg/mL. The maximum inhibition of RBC hemolysis was 9.67 ± 0.30% at 200 µg/mL. Significantly higher protease inhibitory activity at 150, 200, and 250 µg/mL. | [62] |
| Aerial parts | Hexane, dichloromethane, butanol, and water fractions (0.01 to 200 µg/mL) | Proliferative lymphocytes by the BrdU incorporation assay (In vitro). | Anti-inflammatory effect All fractions suppressed lymphocyte proliferation (dichloromethane fraction was the most potent, with an IC50 of 19.8 µg/mL). The butanol fraction at 100 µg/mL reduced inflammatory cytokine levels, specifically IL-4 and IFN-γ. | [63] |
| Fruits | Essential oil | The washed platelets in rabbits (In vitro). | Anticoagulant effect (+)-Fenchone and estragole at 10 and 5 µg/mL showed significantly high inhibition of collagen-induced platelet aggregation, with (+)-fenchone exhibiting 93.5% and 58.4% inhibition, respectively, and estragole exhibiting 98.7% and 54.6% inhibition, respectively. | [64] |
| Fruits and aerial parts | Essential oil (10, 30, and 100 mg/kg/day); anethole (1, 3, 10, or 30 mg/kg/day) | Guinea pig plasma (In vitro). Acute pulmonary thromboembolism (In vivo). | Anticoagulant and antithrombotic effect Fennel oil and anethole showed the following effects: significant inhibition of arachidonic acid, collagen, ADP, and U46619-induced platelet aggregation (IC50 from 4 to 147 g/mL); prevention of thrombin-induced clot retraction; and protection against collagen-epinephrine-induced paralysis at 30 mg/kg, with 70% and 83% protection, respectively. | [41] |
| Seeds | Fennel powder (300 mg/kg b.w) | Obese male albino rats (In vivo). | Hypolipidemic effect Significant decrease in body weight. Significant decrease in albumin levels and total protein. Significant decrease in TC and TG. Significant increase in HDL-chol and decrease in LDL-chol. Significant decrease in ALT, AST, and ALP, MDA and MPO. | [65] |
| Seeds | Aqueous extract (50,100, and 200 mg/kg. i.p) | Male BALB/c mice fed a high cholesterol (In vivo). | Hypolipidemic effect Significant decrease in TC at 100 mg/kg; triglycerides at 100 and 200 mg/kg, and LDL at 50 and 100 mg/kg. However, HDL enhanced significantly at 100 mg/kg. | [11] |
| Seeds | Hydroalcoholic extract (150 mg/kg b.w for 3 weeks) | Male rats fed a high cholesterol regimen (In vivo). | Hypolipidemic effect Significant reduction in TG, TC, LDL, and elevation in HDL. Significant decrease in ALP and ALT levels. | [66] |
| Seeds | Methanolic extract (100 mg/kg/day) | Swiss albino mice exposed to 2-Gy gamma irradiation (In vivo). DPPH radical scavenging (In vitro). | Antioxidant effect Significant decrease in MDA, Significant increase in SOD and CAT levels. Fennel extract completely inhibited DPPH radicals, showing 100% scavenging activity at a concentration equivalent to 29.64 mg/g of total phenolic compounds in dry matter. | [67] |
| Seeds | Distilled water, ethanolic (80%), and acetonic (80%) | FTC, β-carotene, and ABTS assays (In vitro). | Antioxidant effect A significant antioxidant effect was observed with the distilled water extract (48.35 ± 0.19%), followed by the ethanol (45.10 ± 0.34%) and acetone (28.45 ± 0.11%) extracts in the FTC assay. Distilled water showed the higher protection against β-carotene bleaching (66.63 ± 0.05%), followed by ethanol (66.63 ± 0.05%) and acetone (58.11 ± 0.11%). Acetone extract exhibits the greatest ABTS value (7.28 ± 0.17 mM TE/g), followed by ethanol (5.70 ± 0.27 mM TE/g) and distilled water (4.26 ± 0.028 mM TE/g). | [68] |
| Aerial parts | Essential oil (1000, 750, 500, 250, and 100 ppm) | TBARS assay and micellar model system (In vitro). | Antioxidant effect Strong antioxidant activity that α-tocopherol at all concentrations. Inhibiting the peroxidation of linoleic acid. Reduction of the formation of hydroperoxydienes. | [69] |
| Leaves | Essential oil (1.5 to 24 mg/mL), diethyl ether (40 to 400 mg/mL), and ethyl acetate (28 to 160 mg/mL) | DPPH assay (In vitro). | Antioxidant effect Potential antioxidant activity compared to ascorbic acid was observed for the essential oil (IC50: 900 µg/mL), diethyl ether extract (IC50: 6.2 µg/mL), and ethyl acetate extract (IC50: 1.5 µg/mL). | [70] |
| Leaves | Essential oils (10 µg/mL) | DPPH assay (In vitro). | Antioxidant effect The weak DPPH scavenging ability of the samples may be attributed to their anethole content. | [71] |
| Fruits | Coumarins (30 µM) | DPPH and ABTS free radical scavenging activities (In vitro). | Antioxidant effect Scopoletin (48.34%), 8-methoxypsoralen (51.57%), bergapten (49.89%), and imperatorin (50.73%), significantly inhibited DPPH. Their corresponding ABTS radical scavenging activities were 47.05%, 50.53%, 50.44%, and 50.27%, respectively. | [37] |
| Leaves, stems, shoots, and inflorescences | Methanolic extract (0.15–20 mg/mL) | DPPH scavenging, reducing power, and inhibition of β-carotene bleaching assays (In vitro). | Antioxidant effect DPPH radical scavenging increases with the concentration increase in shoots, leaves, and inflorescences extracts (>50% at 10 mg/mL). Reducing power rose with concentration, reaching excellent levels for shoot, inflorescence, and leaf extracts at 5 mg/mL. Shoot and leaf extracts exhibited the most potent β-carotene bleaching inhibition (>90% at 20 mg/mL). | [72] |
| Aerial parts and fruits | Essential oils 1–24 g/L (DPPH) 100–2000 mg/L (TBARS and hydroxyl radical) 50–250 mg/L (lipoxygenase) | DPPH, TBARS, H2O2 radical scavenging activity, lipoxygenase assays (In vitro). | Antioxidant effect At the highest concentrations (12–24 g/L), the DPPH free radical scavenging capacity is >85%. At the lowest concentrations (100 and 250 mg/L), the fruit oils showed lower activity than the oils obtained from the aerial parts in the TRABS assay. Neither the oils from the aerial parts nor the fruit showed hydroxyl radical scavenging capacity > 50%. A stronger 5-lipoxygenase inhibition was observed for the essential oils tested at 250 mg/L. | [73] |
| Leaves | Essential oils 1–250 µg/mL (ABTS and H2O2) 1–200 µg/mL (Antioxidant Enzymes Activity) | ABTS and H2O2 radicals scavenging assays (In vitro). ROS generation and antioxidant enzymes activity on polymorphonuclear leukocytes (PMN) (In vitro). | Antioxidant effect EO induced 50% reduction in ABTS and H2O2 radicals with IC50 value > 100 µg/mL, respectively. A significant reduction of the ROS levels in PMN treated with 100 and 200 µg/mL of EO. Increase in CAT, SOD, and GPx with increasing EO concentrations. | [74] |
| Seeds | Hydro-methanolic extract (10–100 µg/mL) | H2O2 radical scavenging activity or Fenton reaction (In vitro). | Antioxidant effect H2O2 radical scavenging activity of the extract increased with concentration, reaching 82.64 ± 0.13% inhibition at 100 µg/mL. | [75] |
| Fruits | Essential oil (0.75–10 mg/mL). 100 and 200 mg/kg/day (In vivo) | TAC, DPPH, ABTS, FRAP assays (In vitro). The bleomycin (BLM)-induced pulmonary fibrosis assay (In vivo). | Antioxidant effect TAC assay showed good antioxidant potential with a value of 7.26 ± 0.34 mg GAE/g FEO. FRAP assay revealed significant reducing power with EC50 = 63.44 ± 2.29 mg/mL. DPPH radical scavenging activity is comparable to that of BHT. ABTS activity was lower than Trolox. Fennel oil has been shown to decrease MDA levels while enhancing the activities of SOD and CAT enzymes. | [76] |
| Seeds | Aqueous extract (AEFv) and butanolic fraction (BFFv) (0.01–1.5 mg/mL) | DPPH, FRAP, and β-carotene bleaching assays (In vitro). | Antioxidant effect DPPH scavenging activity increased with concentration, peaking at 400 μg/mL. BFFv exhibited stronger antioxidant activity than AEFv, with an IC50 of 30.6 ± 0.61 μg/mL. BFFv and AEFv showed greater inhibition activity in the bleaching of β-carotene, with IC50 values of 0.24 ± 0.051 and 0.3 ± 0.047 mg/mL, respectively. The iron chelation assay demonstrated the ability of the AEFv and BFFv to reduce ferric ions to ferrous ions. This effect was proportional to the concentration tested. | [12] |
| Mechanisms of Action | Bioactive Compounds |
|---|---|
| Reduction of mean arterial pressure and heart rate | Estragole, trans-anethole, phenolic compounds |
| Inhibition of angiotensin-converting enzyme (ACE) activity | Trans-anethole, gallic acid, caffeic acid, p-coumaric acid, quercetin |
| Downregulation of angiotensin II receptor (AT1R) and related gene expression | Kaempferol, quercetin, trans-anethole, naringin |
| Enhancement of diuresis and electrolyte excretion (Na+, K+, Cl−, Ca2+) | Fenchone, chlorogenic acid, caffeic acid, isoquercitrin, (–)-epicatechin |
| Activation of the NO/sGC/cGMP vasorelaxant pathway | Caffeic acid, ferulic acid, coumaric acid, quercetin, kaempferol, naringenin |
| Activation of muscarinic receptors and endothelium-dependent relaxation | Phenolic acids, naringin |
| Inhibition of store-operated and voltage-dependent Ca2+ entry | Trans-anethole, essential oil constituents |
| Opening of potassium channels leading to hyperpolarization | Quercetin, kaempferol, naringenin |
| Enhancement of endogenous antioxidant defense (↑ SOD, ↑ CAT, ↑ GPx, ↑ GST, ↓ MDA, ↓ MPO, ↓ ROS) | Trans-anethole, caffeic acid, syringic acid, quercetin, catechin, S-limonene, kaempferol, α-pinene, (–)-fenchone, chlorogenic acid |
| Reduction of oxidative stress, DNA damage, and mitochondrial dysfunction; downregulation of IL-1β, TNF-α, IL-6 | Caffeic acid, quercetin, catechin, anethole, ferulic acid, chlorogenic acid |
| Inhibition of collagen, ADP, and AA-induced platelet aggregation (↓ TXB2, ↓ PGE2); prolongation of PT; inhibition of fibrin formation and factor Xa activity | Trans-anethole, estragole, (+)-fenchone, quercetin, catechin, p-coumaric acid, chlorogenic acid, syringic acid |
| Inhibition of iNOS and COX-2 expression; suppression of NF-κB and MAPK signaling | Trans-anethole, estragole, quercetin, ferulic acid, chlorogenic acid, p-coumaric acid |
| Regulation of lipid metabolism: ↓ LDL, ↓ VLDL oxidation, ↓ SREBP-2 and LDLR expression, ↓ HMG-CoA reductase activity; ↑ HDL formation and LDL clearance | Trans-anethole, ferulic acid, p-coumaric acid, quercetin, catechin, hesperidin, isorhamnetin, limonene |
| Free radical scavenging and metal ion chelation (DPPH, ABTS, hydroxyl, superoxide assays; lipid peroxidation inhibition) | Quercetin, kaempferol, rutin, epicatechin, p-coumaric acid, ferulic acid, chlorogenic acid, syringic acid, scopoletin, bergapten, imperatorin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahi, A.; Rani, A.; Aktary, N.; Rahman, M.; Mekhfi, H.; Ziyyat, A.; Park, M.N.; Legssyer, A.; Kim, B. Cardiovascular Effects, Phytochemistry, Drug Interactions, and Safety Profile of Foeniculum vulgare Mill. (Fennel): A Comprehensive Review. Pharmaceuticals 2025, 18, 1761. https://doi.org/10.3390/ph18111761
Zahi A, Rani A, Aktary N, Rahman M, Mekhfi H, Ziyyat A, Park MN, Legssyer A, Kim B. Cardiovascular Effects, Phytochemistry, Drug Interactions, and Safety Profile of Foeniculum vulgare Mill. (Fennel): A Comprehensive Review. Pharmaceuticals. 2025; 18(11):1761. https://doi.org/10.3390/ph18111761
Chicago/Turabian StyleZahi, Amal, Amama Rani, Nahida Aktary, Muntajin Rahman, Hassane Mekhfi, Abderrahim Ziyyat, Moon Nyeo Park, Abdelkhaleq Legssyer, and Bonglee Kim. 2025. "Cardiovascular Effects, Phytochemistry, Drug Interactions, and Safety Profile of Foeniculum vulgare Mill. (Fennel): A Comprehensive Review" Pharmaceuticals 18, no. 11: 1761. https://doi.org/10.3390/ph18111761
APA StyleZahi, A., Rani, A., Aktary, N., Rahman, M., Mekhfi, H., Ziyyat, A., Park, M. N., Legssyer, A., & Kim, B. (2025). Cardiovascular Effects, Phytochemistry, Drug Interactions, and Safety Profile of Foeniculum vulgare Mill. (Fennel): A Comprehensive Review. Pharmaceuticals, 18(11), 1761. https://doi.org/10.3390/ph18111761










