Design, Synthesis, and Mechanistic Study of Novel Ciprofloxacin/Thiazole Chalcone Hybrids as Potential Anticancer Agents
Abstract
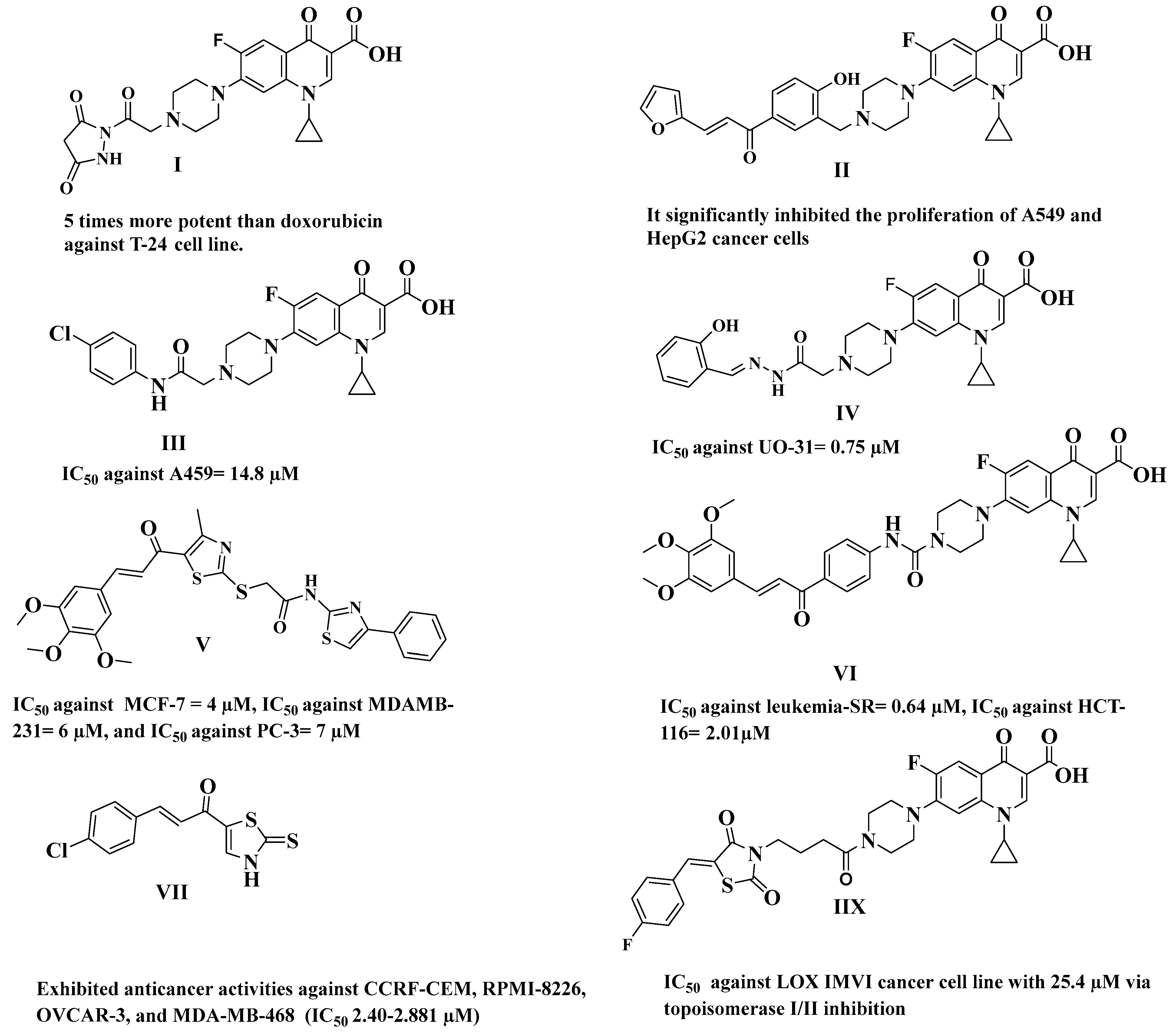
1. Introduction
2. Results and Discussion
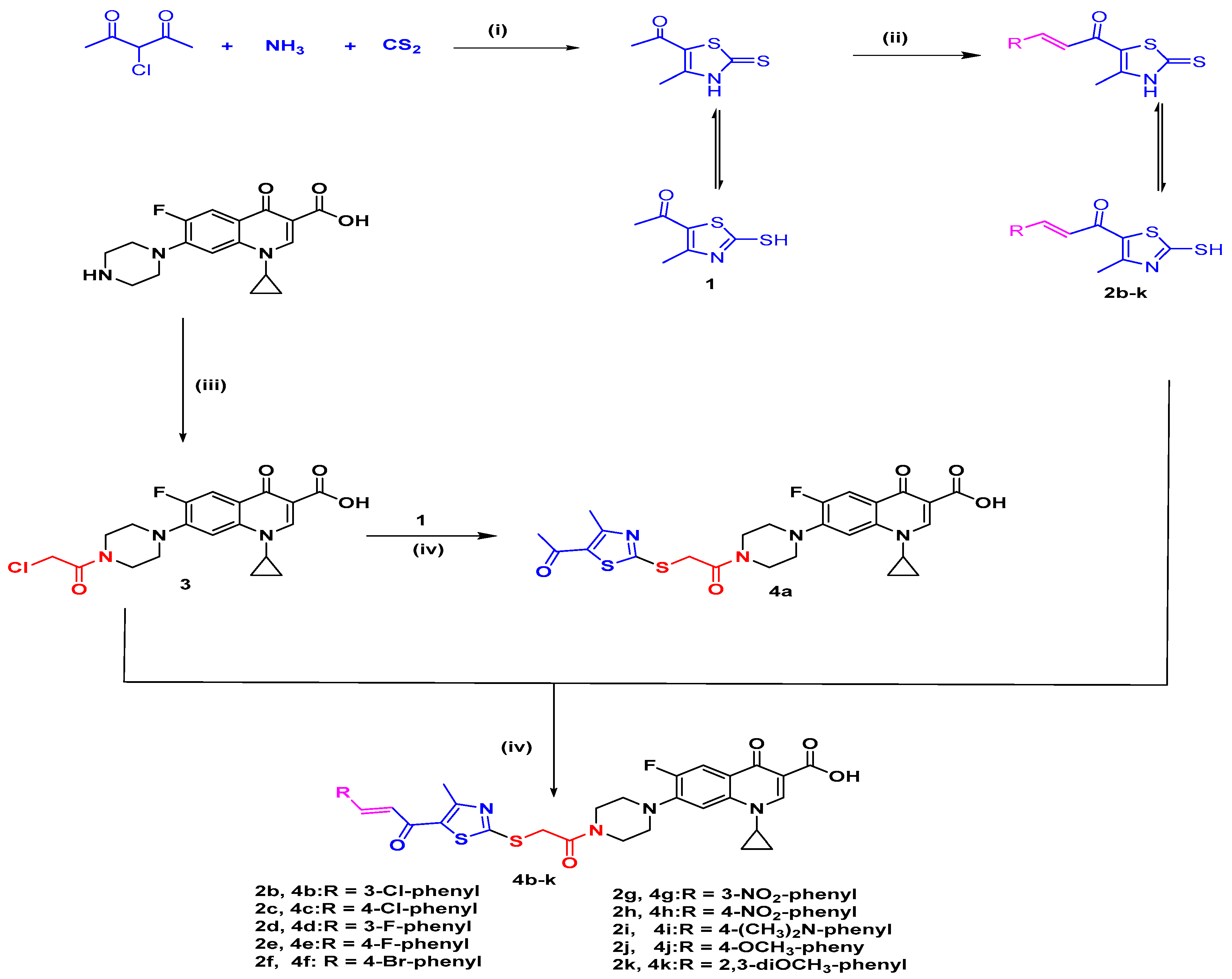
2.1. Chemistry
Synthesis of the Target Compounds 4a–4k
2.2. Biological Investigations
2.2.1. In Vitro Cytotoxicity Screening
One-Dose Anticancer Screening of Compounds 4a–4k (NCI, USA)
Five-Dose Testing for Compounds 4b and 4d
In Vitro Cytotoxic Activity of Compounds 4b and 4d Against Normal Cell Line WI 38
2.2.2. Topoisomerases I/II Inhibition Assay
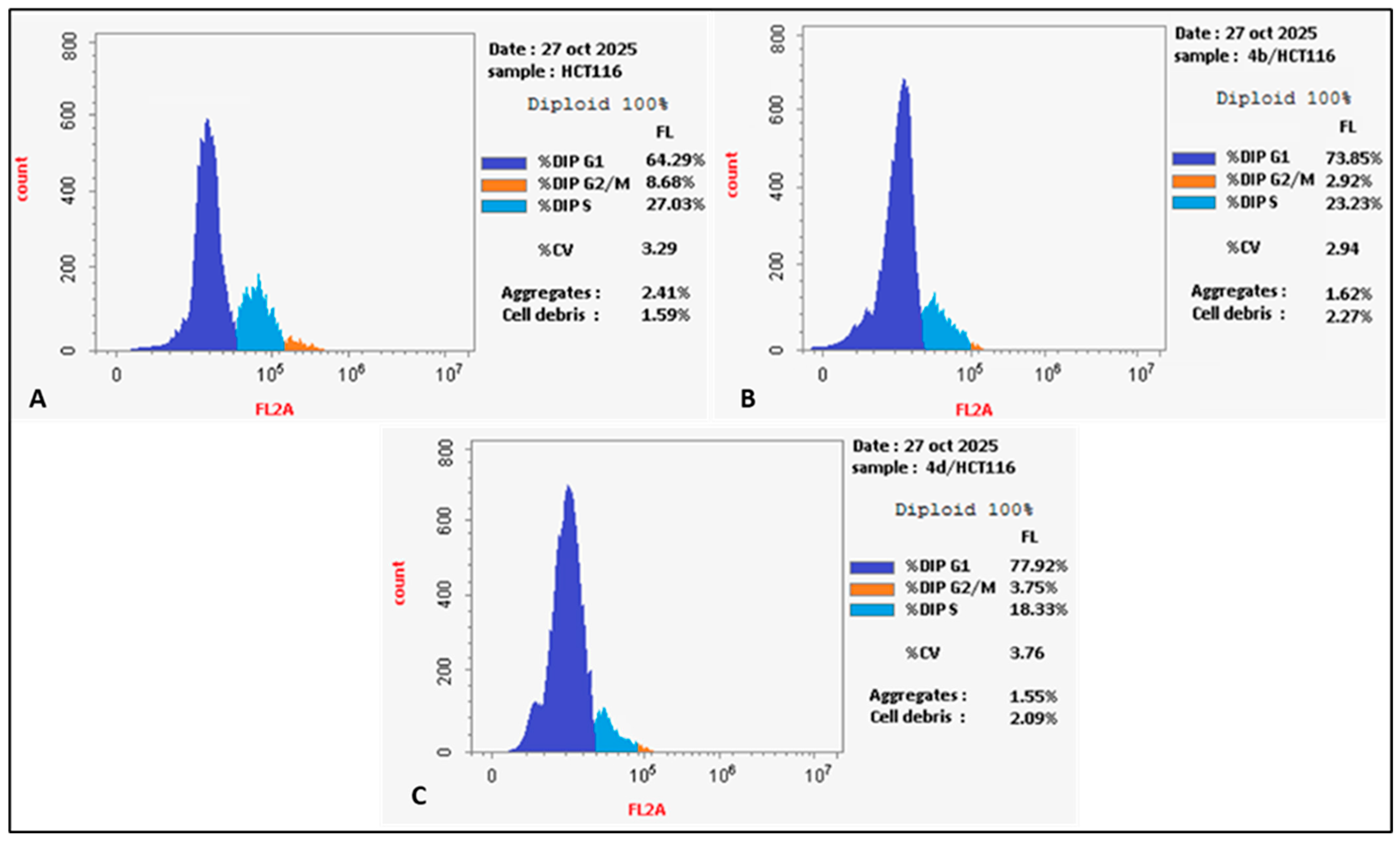
2.2.3. Cell Cycle Analysis
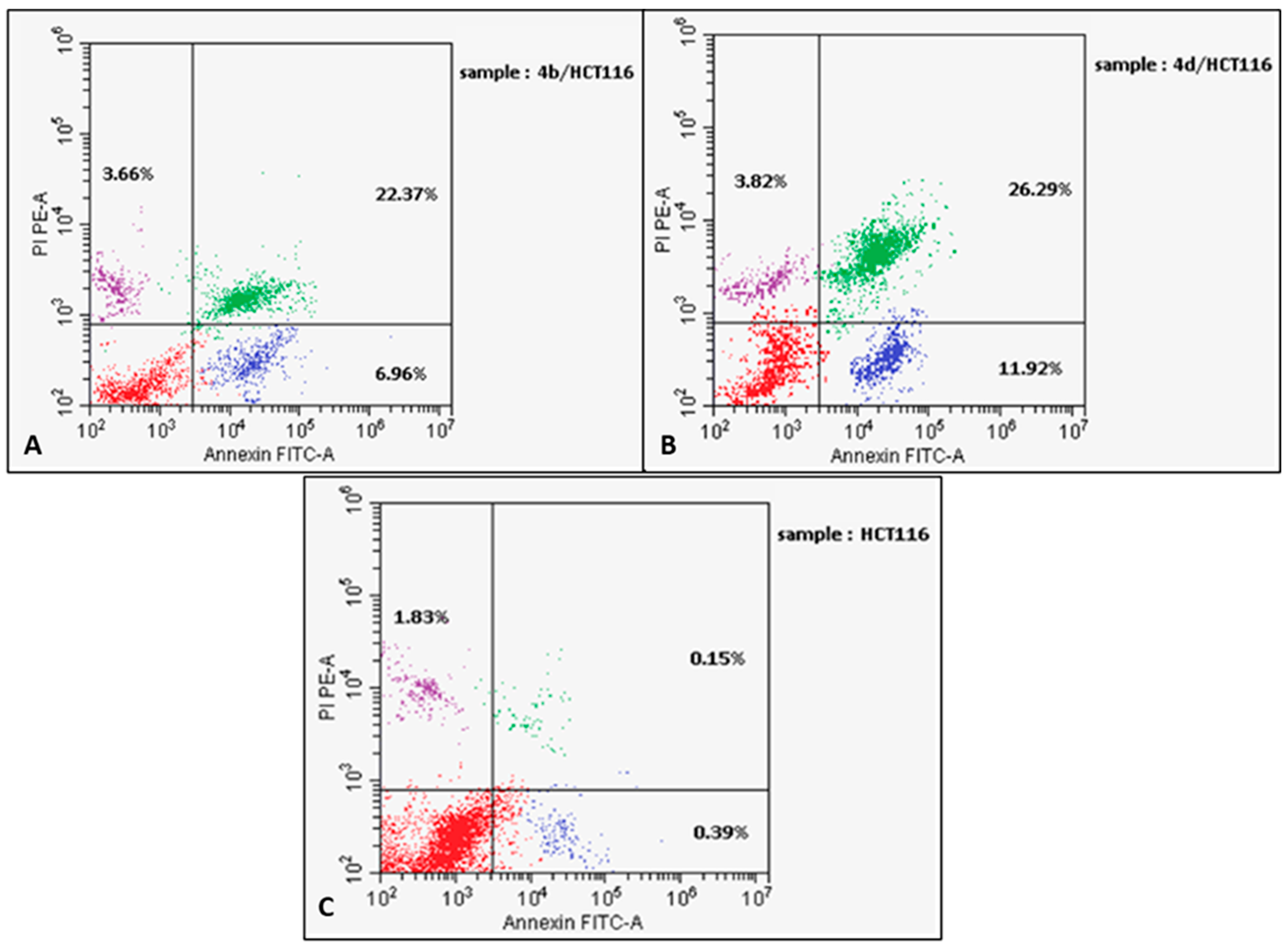
2.2.4. Apoptosis Assay
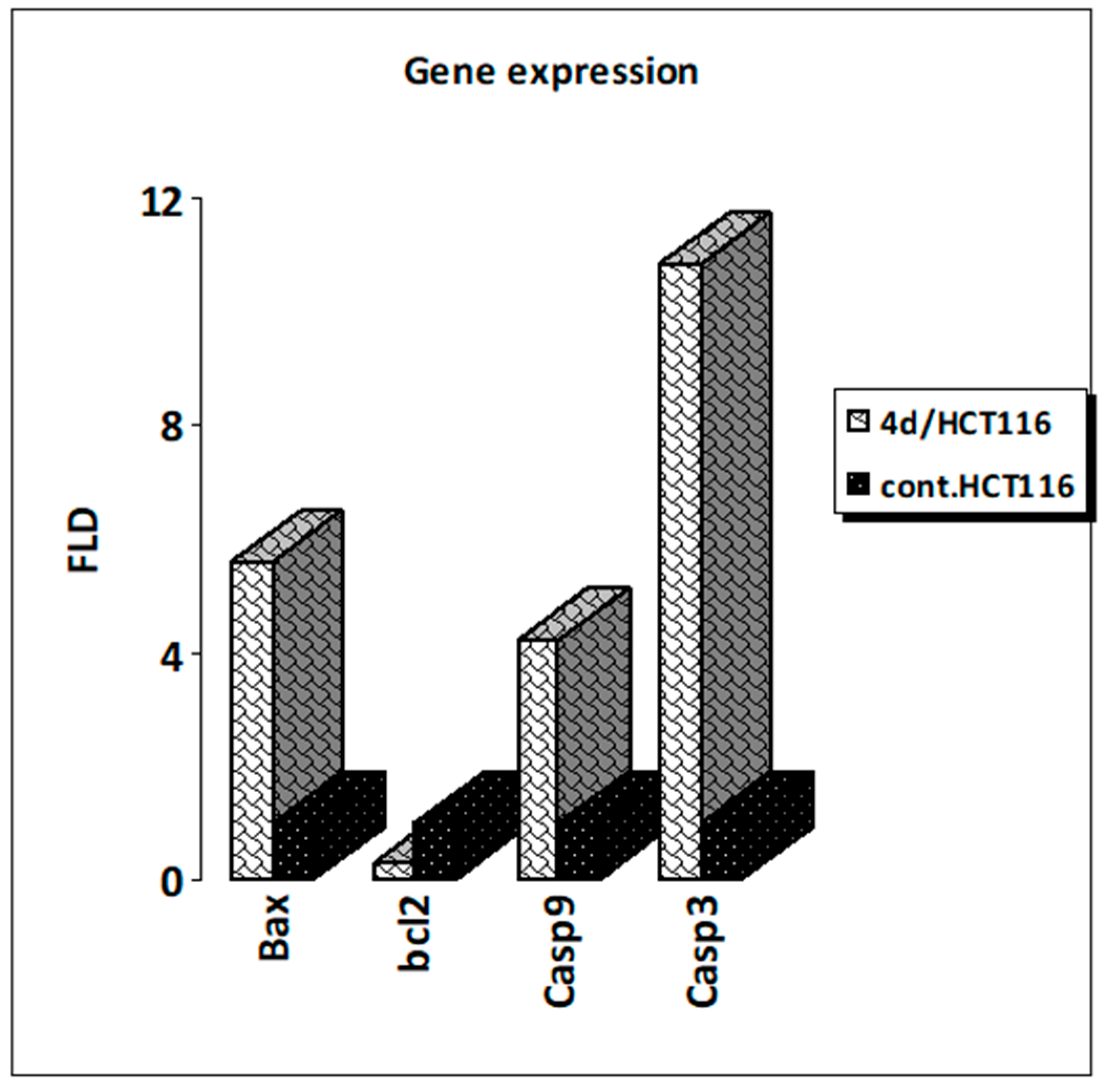
2.2.5. Effects of Compound 4d on Relative Gene Expression Levels of Caspase-3, Caspase-9, Bax, and Bcl-2
2.2.6. In Silico Studies
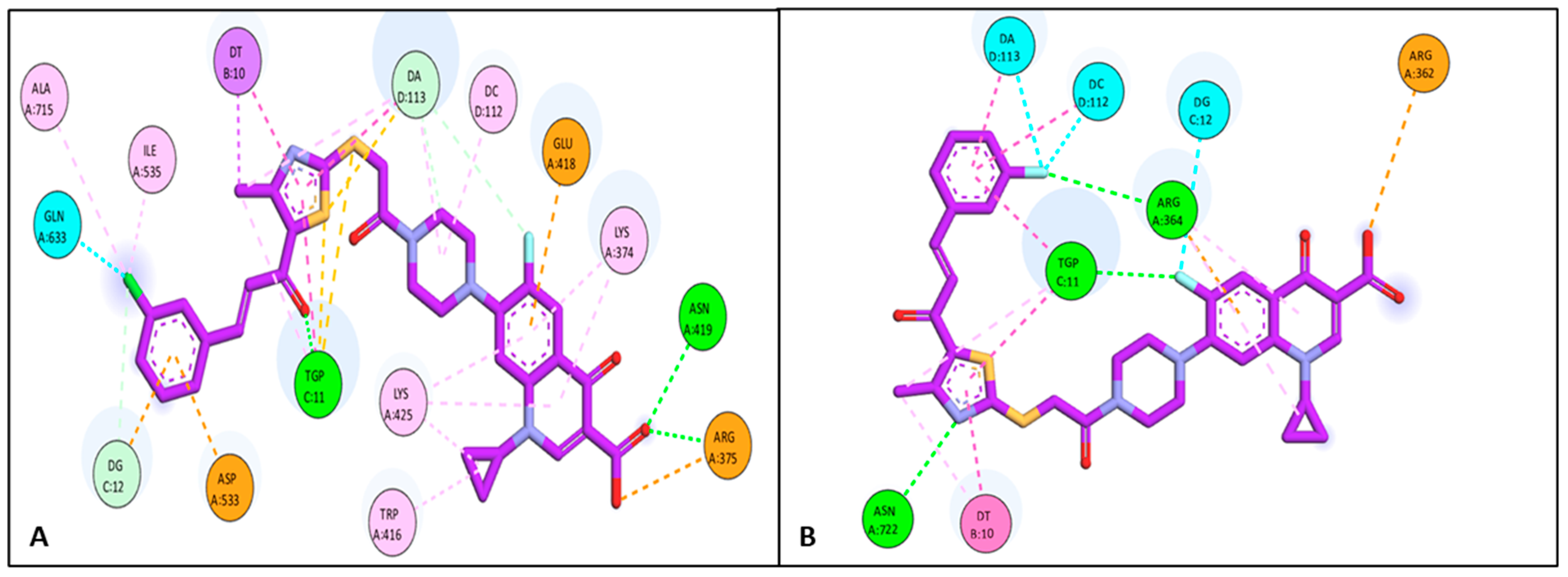
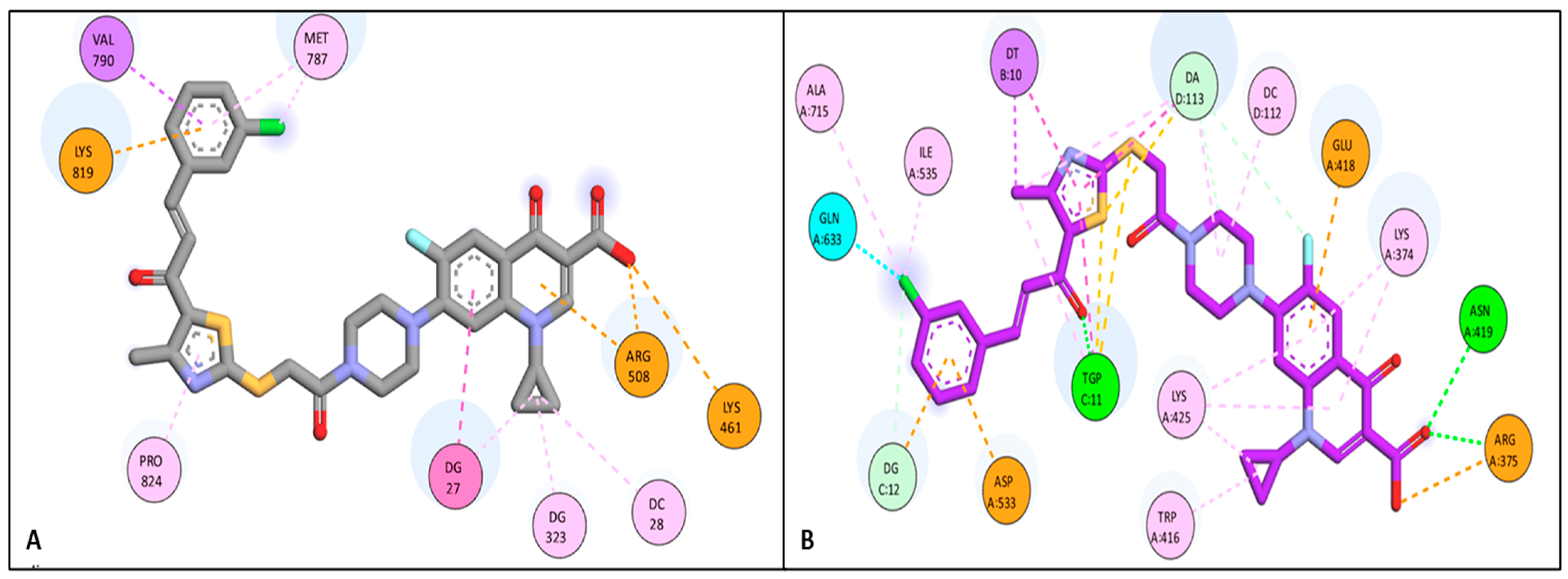
Docking Studies
Physicochemical and Pharmacokinetic Prediction
3. Experimental Section
3.1. Chemistry
3.1.1. General Method of Synthesis of the Target Compounds 4a–4k
7-(4-(2-((5-Acetyl-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4a)
(E)-7-(4-(2-((5-(3-(3-Chlorophenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4b)
(E)-7-(4-(2-((5-(3-(4-Chlorophenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4c)
(E)-1-Cyclopropyl-6-fluoro-7-(4-(2-((5-(3-(3-fluorophenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4d)
(E)-1-Cyclopropyl-6-fluoro-7-(4-(2-((5-(3-(4-fluorophenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4e)
(E)-7-(4-(2-((5-(3-(4-Bromophenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4f)
(E)-1-Cyclopropyl-6-fluoro-7-(4-(2-((4-methyl-5-(3-(3-nitrophenyl)acryloyl)thiazol-2-yl)thio)acetyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4g)
(E)-1-Cyclopropyl-6-fluoro-7-(4-(2-((4-methyl-5-(3-(4-nitrophenyl) acryloyl)thiazol-2-yl)thio)acetyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4h)
(E)-1-Cyclopropyl-7-(4-(2-((5-(3-(4-(dimethylamino)phenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4i)
(E)-1-Cyclopropyl-6-fluoro-7-(4-(2-((5-(3-(4-methoxyphenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4j)
(E)-1-Cyclopropyl-7-(4-(2-((5-(3-(2,3-dimethoxyphenyl)acryloyl)-4-methylthiazol-2-yl)thio)acetyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (4k)
3.2. Screening of Anticancer Activity in the National Cancer Institute (NCI)
3.3. Evaluation of Topoisomerase I/II Inhibition
3.4. Cell Cycle Analysis and Measurement of Apoptotic Potential
3.5. Effects of Compound 4d on Gene Expression Levels of Caspase-3, Caspase-9, Bax, and Bcl-2 Activation
3.6. In Silico Studies
3.6.1. Docking Study
3.6.2. Prediction of Physicochemical and Pharmacokinetic Properties
4. Structure Activity Relationship (SAR) Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy lifestyle and cancer risk: Modifiable risk factors to prevent cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, M.; Naseem, U.; Nezhadmoghadam, F.; Jatoi, M.A.; Gulliver, T.A.; Tamez-Peña, J.G. Breast cancer risk prediction using machine learning: A systematic review. Front. Oncol. 2024, 14, 1343627. [Google Scholar] [CrossRef]
- Barnard, M.E.; Farland, L.V.; Yan, B.; Wang, J.; Trabert, B.; Doherty, J.A.; Meeks, H.D.; Madsen, M.; Guinto, E.; Collin, L.J.; et al. Endometriosis typology and ovarian cancer risk. Jama 2024, 332, 482–489. [Google Scholar] [CrossRef]
- Hidig, S.M.; Adan, M.M. Understanding the Incidence, Screening, and Burden of Cancer in Somalia: A Literature. Glob. Sci. J. 2024, 12, 572–584. [Google Scholar]
- Al-Kazazz, Z.K.; Jawad, A.H. A Study on the Mortality Rate from Lung Cancer in Polluted Areas in Baghdad Governorate. Front. Health Inform. 2024, 13, 451. [Google Scholar]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, H.A.; Ibrahim, M.K.; Metwaly, A.M.; Belal, A.; Mehany, A.B.M.; El-Gamal, K.M.A.; El-Sharkawy, A.; Elhendawy, M.A.; Radwan, M.M.; Elsohly, M.A.; et al. Design, synthesis, molecular modeling, in vivo studies and anticancer evaluation of quinazolin-4(3H)-one derivatives as potential VEGFR-2 inhibitors and apoptosis inducers. Bioorg. Chem. 2020, 94, 103422. [Google Scholar] [CrossRef]
- Othman, I.M.M.; Alamshany, Z.M.; Tashkandi, N.Y.; Nossier, E.S.; Anwar, M.M.; Radwan, H.A. Chemical synthesis and molecular docking study of new thiazole, thiophene, and thieno[2,3-d]pyrimidine derivatives as potential antiproliferative and antimicrobial agents. J. Mol. Struct. 2022, 1270, 133926. [Google Scholar] [CrossRef]
- Gul, H.I.; Yamali, C.; Sakagami, H.; Angeli, A.; Leitans, J.; Kazaks, A.; Tars, K.; Ozgun, D.O.; Supuran, C.T. New anticancer drug candidates sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg. Chem. 2018, 77, 411–419. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Hashem, H.; Abdelfattah, S.; Hassan, H.M.; Al-Emam, A.; Alqarni, M.; Alotaibi, G.; Radwan, I.T.; Kaur, K.; Rao, D.P.; Bräse, S.; et al. Discovery of a novel 4-pyridyl SLC-0111 analog targeting tumor-associated carbonic anhydrase isoform IX through tail-based design approach with potent anticancer activity. Front. Chem. 2025, 13, 1571646. [Google Scholar] [CrossRef]
- Elmetwally, S.A.; Saied, K.F.; Eissa, I.H.; Elkaeed, E.B. Design, synthesis and anticancer evaluation of thieno[2,3-d]pyrimidine derivatives as dual EGFR/HER2 inhibitors and apoptosis inducers. Bioorg. Chem. 2019, 88, 102944. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Hashem, H.; Hassan, A.; Abdelmagid, W.M.; Habib, A.G.K.; Abdel-Aal, M.A.A.; Elshamsy, A.M.; El Zawily, A.; Radwan, I.T.; Bräse, S.; Abdel-Samea, A.S.; et al. Synthesis of new thiazole-privileged chalcones as tubulin polymerization inhibitors with potential anticancer activities. Pharmaceuticals 2024, 17, 1154. [Google Scholar] [CrossRef]
- Leite, F.F.; de Sousa, N.F.; de Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; de Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer activity of chalcones and its derivatives: Review and in silico studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; Sabet, S.; El-Shorbagy, H.M.; Abdelhamid, I.A.; Ibrahim, S.A. Chalcones: Promising therapeutic agents targeting key players and signaling pathways regulating the hallmarks of cancer. Chem. Biol. Interact. 2023, 369, 110297. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Wei, G.; Zhou, Q.; Gan, X. Synthesis and anticancer activity of chalcone–quinoxalin conjugates. Synth. Commun. 2021, 51, 1363–1372. [Google Scholar] [CrossRef]
- Rodríguez-Silva, C.N.; Prokopczyk, I.M.; Dos Santos, J.L. The medicinal chemistry of chalcones as anti-mycobacterium tuberculosis agents. Mini Rev. Med. Chem. 2022, 22, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Hassan, R.; Elmagzoub, R.M.; Al-Emam, A.; Kossenas, K.; Abdel-Samea, A.S.; Khalifa, H.O.; Akocak, S.; Bräse, S.; Hashem, H. From Infection to Tumor: Exploring the Therapeutic Potential of Ciprofloxacin Derivatives as Anticancer Agents. Pharmaceuticals 2025, 18, 72. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Abosella, L.; Hassan, A.; Elakesh, E.O.; Bräse, S.; Abuo-Rahma, G.E.-D.A.; Aziz, H.A. Design, Synthesis, and In Silico Studies of New Norfloxacin Analogues with Broad Spectrum Antibacterial Activity via Topoisomerase II Inhibition. Pharmaceuticals 2025, 18, 545. [Google Scholar] [CrossRef]
- Al-Taweel, S.; Al-Saraireh, Y.; Al-Trawneh, S.; Alshahateet, S.; Al- Tarawneh, R.; Ayed, N.; Alkhojah, M.; Al-Khaboori, W.; Zereini, W.; Al-Qaralleh, O. Synthesis and biological evaluation of ciprofloxacin–1,2,3-triazole hybrids as antitumor, antibacterial, and antioxidant agents. Heliyon 2023, 9, e22592. [Google Scholar] [CrossRef]
- Adly, M.E.; Taher, A.T.; Ahmed, F.M.; Mahmoud, A.M.; Salem, M.A.; El-Masry, R.M. New series of fluoroquinolone derivatives as potential anticancer Agents: Design, Synthesis, in vitro biological Evaluation, and Topoisomerase II Inhibition. Bioorganic Chem. 2025, 156, 108163. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Abdel-Rahman, I.M.; Ali, F.E.; Bekhit, A.A.; Elhamadany, E.Y.; Zhao, Q.L.; Cui, Z.G.; Fathy, M. Dual topoisomerase I/II inhibition-induced apoptosis and necro-apoptosis in cancer cells by a novel ciprofloxacin derivative via RIPK1/RIPK3/MLKL activation. Molecules 2022, 27, 7993. [Google Scholar] [CrossRef]
- Idowu, T.; Schweizer, F. Ubiquitous nature of fluoroquinolones: The oscillation between antibacterial and anticancer activities. Antibiotics 2017, 6, 26. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Park, S.-E.; Abuo-Rahma, G.E.-D.A.A.; Sayed, M.A.; Kwon, Y. Novel N-4-piperazinyl-ciprofloxacin-chalcone hybrids: Synthesis, physicochemical properties, anticancer and topoisomerase I and II inhibitory activity. Eur. J. Med. Chem. 2013, 69, 427–438. [Google Scholar] [CrossRef]
- Abdel-Rahman, I.M.; Mustafa, M.; Mohamed, S.A.; Yahia, R.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Hayallah, A.M. Novel Mannich bases of ciprofloxacin with improved physicochemical properties, antibacterial, anticancer activities and caspase-3 mediated apoptosis. Bioorg. Chem. 2021, 107, 104629. [Google Scholar] [CrossRef]
- Ahadi, H.; Shokrzadeh, M.; Hosseini-khah, Z.; Ghassemi barghi, N.; Ghasemian, M.; Emadi, E.; Zargari, M.; Razzaghi-Asl, N.; Emami, S. Synthesis and biological assessment of ciprofloxacin-derived 1,3,4-thiadiazoles as anticancer agents. Bioorganic Chem. 2020, 105, 104383. [Google Scholar] [CrossRef]
- Swedan, H.K.; Kassab, A.E.; Gedawy, E.M.; Elmeligie, S.E. Design, synthesis, and biological evaluation of novel ciprofloxacin derivatives as potential anticancer agents targeting topoisomerase II enzyme. J. Enzym. Inhib. Med. Chem. 2023, 38, 118–137. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Abd El-Hafeez, A.A.; Abbas, S.H.; Abdelhafez, E.S.M.; Abuo-Rahma, G.E.D.A. New antiproliferative 7-(4-(N-substituted carbamoylmethyl) piperazin-1-yl) derivatives of ciprofloxacin induce cell cycle arrest at G2/M phase. Bioorg. Med. Chem. 2016, 24, 4636–4646. [Google Scholar] [CrossRef]
- Kassab, A.E.; Gedawy, E.M. Novel ciprofloxacin hybrids using biology oriented drug synthesis (BIODS) approach: Anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis, topoisomerase II inhibition, and antibacterial activity. Eur. J. Med. Chem. 2018, 150, 403–418. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Elshamsy, A.M.; Ali, T.F.S.; Youssif, B.G.M.; Bräse, S.; Abdel-Aziz, M.; El-Koussi, N.A. Design, synthesis, in silico studies, and apoptotic antiproliferative activity of novel thiazole-2-acetamide derivatives as tubulin polymerization inhibitors. Front. Chem. 2025, 13, 1565699. [Google Scholar] [CrossRef]
- Aziz, H.A.; El-Saghier, A.M.; Badr, M.; Elsadek, B.E.M.; Abuo-Rahma, G.E.-D.A.; Shoman, M.E. Design, synthesis and mechanistic study of N-4-Piperazinyl Butyryl Thiazolidinedione derivatives of ciprofloxacin with Anticancer Activity via Topoisomerase I/II inhibition. Sci. Rep. 2024, 14, 24101. [Google Scholar] [CrossRef]
- Ali, D.M.E.; Aziz, H.A.; Bräse, S.; Al Bahir, A.; Alkhammash, A.; Abuo-Rahma, G.E.-D.A.; Elshamsy, A.M.; Hashem, H.; Abdelmagid, W.M. Unveiling the anticancer potential of a new ciprofloxacin-chalcone hybrid as an inhibitor of topoisomerases i & ii and apoptotic inducer. Molecules 2024, 29, 5382. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Algarni, H.; Alsayari, A.; Bin Muhsinah, A.; Kheder, N.A.; Almarhoon, Z.M.; Al-aizari, F.A. Synthesis, x-ray analysis, biological evaluation and molecular docking study of new thiazoline derivatives. Molecules 2019, 24, 1654. [Google Scholar] [CrossRef]
- Mohammed, H.H.H.; Abdelhafez, E.-S.M.N.; Abbas, S.H.; Moustafa, G.A.I.; Hauk, G.; Berger, J.M.; Mitarai, S.; Arai, M.; Abd El-Baky, R.M.; Abuo-Rahma, G.E.-D.A. Design, synthesis and molecular docking of new N-4-piperazinyl ciprofloxacin-triazole hybrids with potential antimicrobial activity. Bioorg. Chem. 2019, 88, 102952. [Google Scholar] [CrossRef]
- Badran, M.M.; Abbas, S.H.; Fujita, M.; Abdel-Aziz, M. Harnessing pyrimidine as a building block for histone deacetylase inhibitors. Arch. Pharm. 2023, 356, e2300208. [Google Scholar] [CrossRef]
- Kaina, B. DNA damage-triggered apoptosis: Critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem. Pharmacol. 2003, 66, 1547–1554. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Hashem, H.; Maher, S.A.; Enaili, S.S.; Alkhammash, A.; Bräse, S.; Aziz, H.A. Design, Synthesis, Anticancer Screening, and Mechanistic Study of Spiro-N-(4-sulfamoyl-phenyl)-1,3,4-thiadiazole-2-carboxamide Derivatives. Int. J. Mol. Sci. 2025, 26, 863. [Google Scholar] [CrossRef]
- Moyer, A.; Tanaka, K.; Cheng, E.H. Apoptosis in cancer biology and therapy. Annu. Rev. Pathol. Mech. Dis. 2025, 20, 303–328. [Google Scholar] [CrossRef]
- Kleczka, A.; Kubina, R.; Dzik, R.; Jasik, K.; Stojko, J.; Cholewa, K.; Kabała-Dzik, A. Caffeic acid phenethyl ester (cape) induced apoptosis in serous ovarian cancer ov7 cells by deregulation of BCL2/BAX genes. Molecules 2020, 25, 3514. [Google Scholar] [CrossRef]
- Naguib, Y.W.; Alhaj-Suliman, S.O.; Wafa, E.I.; Saha, S.; Ebeid, K.; Mohammed, H.H.H.; Abdel-Rahman, S.A.; Abuo-Rahma, G.E.-D.A.; Geary, S.M.; Salem, A.K. Ciprofloxacin derivative-loaded nanoparticles synergize with paclitaxel against type ii human endometrial cancer. Small 2024, 20, e2302931. [Google Scholar] [CrossRef]
- Roshdi, M.; Mohamed, M.F.; Beshr, E.A.; Aziz, H.A.; Gebril, S.M.; Bräse, S.; Mohassab, A.M. Design, Synthesis, In Silico Docking, Multitarget Bioevaluation and Molecular Dynamic Simulation of Novel Pyrazolo [3, 4-d] Pyrimidinone Derivatives as Potential In Vitro and In Vivo Anti-Inflammatory Agents. Pharmaceuticals 2025, 18, 1326. [Google Scholar] [CrossRef]
- Shi, W.; Marcus, S.L.; Lowary, T.L. Cytotoxicity and topoisomerase I/II inhibition of glycosylated 2-phenyl-indoles, 2-phenyl-benzo[b]thiophenes and 2-phenyl-benzo[b]furans. Bioorg. Med. Chem. 2011, 19, 603–612. [Google Scholar] [CrossRef] [PubMed]
- El-Saghier, A.M.; Abosella, L.; Hamed, A.M.; Elakesh, E.O.; Abuo-Rahma, G.E.D.A.; Abdellattif, M.H.; Aziz, H.A. New norfloxacin analogues based on N-4-piperazinyl-(3-arylidene/alkylidene acrylonitrile) moieties: Design, synthesis, antibacterial evaluation, and in silico studies. Monatshefte Für Chem.-Chem. Mon. 2025, 1–18. [Google Scholar] [CrossRef]




| Compounds | Compounds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Lines | 4a | 4b | 4c | 4d | 4e | 4f | 4g | 4h | 4i | 4j | 4k | |
| Leukemia | CCRF-CEM | 96.2 | 49.7 | 101.9 | 17.1 | 104.3 | 105.5 | 74.5 | 45.7 | 90.6 | 110.5 | 72.0 |
| HL-60(TB) | 102.2 | −99.1 | 92.9 | −99.8 | 107.2 | 83.3 | 80.5 | 80.9 | 89.5 | 101.4 | −99.4 | |
| K-562 | 122.8 | −20.5 | 93.1 | 56.1 | 98.2 | 96.2 | 29.3 | 28.9 | 61.3 | 98.3 | 133.2 | |
| MOLT-4 | 102.1 | 66.1 | 102.7 | 0.79 | 105.3 | 96.2 | 86.4 | 62.7 | 89.2 | 99.7 | 80.4 | |
| RPMI-8226 | 132.6 | −99.5 | 7.7 | −99.9 | 29.1 | 31.5 | 9.9 | 16.3 | 4.7 | 105.7 | −99.7 | |
| SR | NT | NT | 35.32 | NT | 55.6 | 56.5 | 11.6 | 23.8 | 64.5 | 88.9 | NT | |
| Non-Small-Cell Lung Cancer | A549/ATCC | 103.6 | 82.4 | 115.2 | 65.8 | 104.4 | 106.7 | 90.3 | 99.7 | 102.9 | 105.0 | 106.2 |
| EKVX | 104.2 | 83.9 | 102.8 | 70.2 | 107.8 | 107.4 | 110.8 | 112.7 | 110.0 | 102.7 | 96.9 | |
| HOP-62 | 95.6 | −34.0 | 122.5 | −55.3 | 107.9 | 94.3 | 94.5 | 88.3 | 105.3 | 98.3 | −20.2 | |
| HOP-92 | 105.4 | 101.1 | 119.3 | 91.9 | 98.0 | 114.7 | 68.4 | 126.0 | 91.7 | 104.8 | 122.0 | |
| NCI-H226 | 116.9 | 80.9 | 106.6 | 70.9 | 98.3 | 107.4 | 101.5 | 87.9 | 100.3 | 101.8 | 102.0 | |
| NCI-H23 | 106.2 | 54.0 | 96.5 | 45.6 | 100.9 | 101.9 | 87.8 | 73.2 | 76.2 | 101.7 | 87.9 | |
| NCI-H322M | 105.5 | 100.9 | NT | 95.7 | NT | NT | NT | NT | NT | NT | 114.8 | |
| NCI-H460 | 103.6 | 76.7 | 105.9 | 14.8 | 108.2 | 107.4 | 105.7 | 99.4 | 107.5 | 106.2 | 73.1 | |
| NCI-H522 | 98.9 | 98.5 | 95.9 | 88.2 | 98.2 | 104.9 | 72.3 | 27.9 | 78.5 | 99.0 | 103.7 | |
| Colon Cancer | COLO 205 | 118.4 | 113.8 | 106.6 | 101.9 | 118.6 | 112.4 | 90.5 | 95.9 | 116.9 | 106.2 | 125.9 |
| HCC-2998 | 96.6 | 25.6 | 104.2 | −29.0 | 106.6 | 115.1 | 50.3 | 71.9 | 44.4 | 100.2 | 76.8 | |
| HCT-116 | 112.6 | 2.8 | 61.0 | −77.8 | 51.2 | 94.3 | −73.5 | 33.8 | 2.6 | 102.6 | −77.9 | |
| HCT-15 | 102.2 | 26.9 | 102.3 | −41.7 | 96.8 | 105.9 | 71.7 | 62.3 | 83.2 | 104.3 | 64.5 | |
| HT29 | 104.5 | 24.4 | 108.4 | 3.7 | 115.1 | 116.6 | 37.2 | 76.1 | 47.9 | 108.5 | 82.6 | |
| KM12 | 102.4 | −46.3 | 104.4 | −68.8 | 97.3 | 107.7 | 21.7 | 54.4 | 79.4 | 111.7 | 4.4 | |
| SW-620 | 112.8 | 53.3 | 112.8 | 3.2 | 126.2 | 115.7 | 69.8 | 78.2 | 116.7 | 122.8 | 90.7 | |
| CNS Cancer | SF-268 | 102.8 | 76.1 | 179.7 | 25.7 | 110.7 | 116.2 | 171.7 | 81.1 | 140.4 | 147.7 | 108.7 |
| SF-295 | 94.2 | 93.3 | 101.8 | 93.1 | 98.8 | 108.1 | 110.0 | 116.2 | 91.4 | 100.7 | 97.4 | |
| SF-539 | 86.4 | 22.1 | 97.7 | −79.6 | 99.0 | 89.9 | 64.5 | 67.9 | 74.4 | 97.5 | 85.2 | |
| SNB-19 | 102.4 | 54.7 | 100.1 | 12.2 | 100.8 | 93.8 | 87.7 | 72.2 | 88.6 | 103.1 | 87.2 | |
| SNB-75 | 79.2 | 102.2 | 95.6 | 49.1 | 102.6 | 65.7 | 95.7 | 91.2 | 104.5 | 86.2 | 100.1 | |
| U251 | 113.7 | 41.9 | 110.8 | −7.6 | 113.6 | 98.4 | 50.7 | 59.9 | 48.05 | 107.2 | 102.7 | |
| Melanoma | LOX IMVI | 106.3 | 36.9 | 80.9 | −63.4 | 91.2 | 90.5 | 34.2 | 28.3 | 24.6 | 97.9 | 103.5 |
| MALME-3M | 104.9 | 143.6 | NT | 106.8 | NT | NT | NT | NT | NT | NT | 115.2 | |
| M14 | 103.9 | 48.7 | 98.7 | −15.3 | 97.8 | 108.1 | 83.3 | 70.5 | 75.9 | 109.1 | 75.1 | |
| MDA-MB-435 | 99.4 | −6.2 | 102.0 | −3.7 | 103.6 | 106.6 | 71.9 | 88.4 | 26.6 | 107.8 | 53.2 | |
| SK-MEL-2 | 97.4 | 60.8 | 99.4 | −15.4 | 103.7 | 104.6 | 68.9 | 87.7 | 85.5 | 103.6 | 93.4 | |
| SK-MEL-28 | 96.7 | 84.1 | 100.3 | 77.5 | 115.8 | 113.7 | 80.8 | 60.0 | 95.9 | 113.9 | 100.4 | |
| SK-MEL-5 | 89.0 | 50.3 | 100.3 | 45.1 | 99.9 | 100.9 | 81.7 | 83.5 | 89.8 | 99.7 | 83.0 | |
| UACC-257 | 102.4 | 77.9 | 116.7 | 53.9 | 106.1 | 117.8 | 99.8 | 82.6 | 107.8 | 114.4 | 101.6 | |
| UACC-62 | 103.9 | 76.3 | 94.9 | 33.1 | 99.6 | 105.3 | 71.9 | 59.0 | 79.9 | 111.2 | 101.5 | |
| Ovarian Cancer | IGROV1 | 115.9 | 73.9 | NT | 44.6 | NT | NT | NT | NT | NT | NT | 101.3 |
| OVCAR-3 | 98.8 | 13.9 | 187.9 | −10.9 | 116.2 | 170.4 | 163.4 | 49.4 | 172.1 | 174.4 | 84.5 | |
| OVCAR-4 | 95.1 | 106.1 | 107.4 | 94.5 | 102.5 | 102.3 | 103.1 | 89.2 | 102.4 | 100.9 | 117.5 | |
| OVCAR-5 | NT | NT | 100.3 | NT | 119.2 | 110.6 | 119.9 | 121.1 | 102.5 | 110.0 | NT | |
| OVCAR-8 | 105.2 | 46.1 | 76.3 | 35.2 | 105.1 | 75.0 | 69.2 | 68.9 | 76.5 | 94.6 | 87.6 | |
| NCI/ADR-RES | 108.4 | 111.7 | 98.9 | 110.2 | 103.7 | 107.3 | 102.4 | 88.8 | 97.1 | 109.3 | 112.5 | |
| SK-OV-3 | 100.7 | 46.3 | 129.2 | 5.0 | 114.1 | 109.5 | 120.6 | 118.9 | 119.3 | 114.8 | 76.7 | |
| Renal Cancer | 786−0 | 96.2 | 74.3 | 96.2 | 41.9 | 88.3 | 93.6 | 71.2 | 87. 6 | 59.2 | 88.6 | 96.9 |
| A498 | 103.7 | 36.0 | 119.4 | −54.1 | 105.9 | 113.7 | 129.4 | 124.8 | 126.3 | 117.3 | 90.9 | |
| ACHN | 98.3 | 99.1 | 96.4 | 94.4 | 98.9 | 107.2 | 110.2 | 96.7 | 97.7 | 97.4 | 104.8 | |
| CAKI-1 | 99.2 | 102.5 | 100.1 | 79.8 | 97.4 | 101.4 | 92.6 | 115.5 | 81.7 | 94.9 | 106.6 | |
| RXF 393 | 91.7 | −8.1 | 108.9 | −25.9 | 95.9 | 106.6 | 92.9 | 89.5 | 90.5 | 99.3 | 86.3 | |
| SN12C | 98.0 | 51.0 | 96.7 | 27.7 | 99.5 | 101.4 | 65.4 | 78.3 | 81.1 | 96.2 | 87.5 | |
| TK-10 | 97.9 | 108.2 | 105.8 | 97.5 | 102.6 | 107.8 | 109.2 | 115.6 | 91.8 | 101.7 | 105.0 | |
| Prostate Cancer | PC-3 | 98.8 | 74.1 | 100.3 | 53.4 | 96.6 | 102.5 | 112.1 | 85.9 | 96.7 | 107.4 | 93.5 |
| DU-145 | 95.3 | 94.6 | 177.6 | 73.7 | 105.3 | 113.4 | 108.4 | 85.3 | 147.4 | 112.9 | 98.0 | |
| Breast Cancer | MCF7 | 94.2 | 6.5 | 52.9 | −57.6 | 70.6 | 70.8 | 11.6 | 7.2 | 17.3 | 93.3 | −20.8 |
| MDA-MB231/ATCC | NT | NT | 100.1 | NT | 101.1 | 98.6 | 92.4 | 94.5 | 73.1 | 94.8 | NT | |
| HS 578T | 87.4 | 61.5 | 104.6 | 36.2 | 105.4 | 99.6 | 99.1 | 99.6 | 81.4 | 96.4 | 92.8 | |
| BT-549 | 98.3 | −62.2 | 85.9 | −88.5 | 101.8 | 106.6 | 66.9 | 83.9 | 12.9 | 101.5 | 63.3 | |
| T-47D | NT | NT | 116.3 | NT | 95.6 | 89.3 | 84.1 | 64.1 | 88.2 | 94.7 | NT | |
| MDA-MB-468 | 110.9 | −12.9 | 109.6 | −11.1 | 105.9 | 109.2 | 92.8 | 85.2 | 87.6 | 106.7 | 48.5 | |
| Mean growth percentage | 102.2 | 49.6 | 102.6 | 20.2 | 100.2 | 101.8 | 80.0 | 77.6 | 83.9 | 105.1 | 77.9 | |
| Cell Line | Compound | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4b | 4d | ||||||||||||
| IC50 | MG-MID a | SI c | GI50 | TGI | LC50 | IC50 | MG-MID a | SI c | GI50 | TGI | LC50 | ||
| Leukemia | CCRF-CEM | 11.7 | 7.62 | 9.22 | 6.9 | 31.6 | 100.0 | 4.37 | 2.9 | 24.6 | 2.88 | 4.3 | 100.0 |
| HL-60(TB) | 3.7 | 2.6 | 11.2 | 34.7 | 2.9 | 1.78 | 3.8 | 10.96 | |||||
| K-562 | 4.9 | 4.2 | 100.0 | 100.0 | 2.4 | 1.58 | 8.7 | 9.12 | |||||
| MOLT-4 | 21.9 | 12.9 | 26.9 | 56.2 | 4.8 | 3.4 | 0.34 | 53.7 | |||||
| RPMI-8226 | 0.3 | 0.2 | 0.3 | 0.6 | 0.34 | 0.19 | 2.63 | 0.6 | |||||
| SR | 3.2 | 1.8 | 4.1 | 9.1 | 2.3 | 1.4 | 100.0 | 5.3 | |||||
| Non-Small-Cell Lung Cancer | A549/ATCC | 100.0 | 96.35 | 0.73 | 100.0 | 100.0 | 100.0 | 100.0 | 86.5 | 0.81 | 100.0 | 7.94 | 100.0 |
| EKVX | NT | ND | ND | 100.0 | 5. 9 | 1.7 | 100.0 | 100.0 | |||||
| HOP-62 | 100.0 | 4.3 | 100.0 | 100.0 | 72.4 | 7.4 | 100.0 | 100.0 | |||||
| HOP-92 | 100.0 | 44.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| NCI-H226 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| NCI-H23 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| NCI-H322M | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| NCI-H460 | 70.8 | 58.9 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| NCI-H522 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| Colon Cancer | COLO 205 | 6.2 | 32.16 | 2.18 | 4.3 | 100.0 | 100.0 | 9.3 | 25.3 | 2.8 | 4.9 | 19.95 | 100.0 |
| HCC-2998 | 2.2 | 1.2 | 2.9 | ND | 8.5 | 1.4 | 0.42 | 100.0 | |||||
| HCT-116 | 0.3 | 0.2 | ND | ND | 0.34 | 0.21 | 100.0 | ND | |||||
| HCT-15 | 100.0 | ND | 100.0 | 100.0 | 53.7 | 33.9 | 100.0 | 100.0 | |||||
| HT29 | 100.0 | 100.0 | 100.0 | 100.0 | 100 | 100.0 | 1.91 | 100 | |||||
| KM12 | 1.3 | 0.9 | 3.0 | 9.3 | 0.69 | 0.52 | 97.7 | 6.92 | |||||
| SW-620 | 15.1 | 10.5 | 40.7 | 100.0 | 4.7 | 3.7 | 100.0 | 100.0 | |||||
| CNS Cancer | SF-268 | 53.7 | 69.63 | 1.01 | 22.4 | 93.3 | 100.0 | 100.0 | 72.0 | 0.97 | 27.5 | 100.0 | 100.0 |
| SF-295 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 8.7 | 100.0 | |||||
| SF-539 | 15.1 | 3.7 | 61.7 | 100.0 | 4.1 | 2.0 | 100.0 | 100.0 | |||||
| SNB-19 | 49.0 | 22.4 | 100.0 | 100.0 | 28.2 | 6.9 | 100.0 | 100.0 | |||||
| SNB-75 | 100.0 | 28.8 | 77.6 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| U251 | 100.0 | 34.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| Melanoma | LOX IMVI | 3.0 | 74.08 | 0.95 | 2.4 | 100.0 | 100.0 | 4.1 | 79.00 | 0.89 | 2.8 | 100.0 | 100.0 |
| MALME-3M | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| M14 | 69.2 | 27.5 | 100.0 | 100.0 | 100.0 | 12.0 | 100.0 | 100 | |||||
| MDA-MB-435 | 7.4 | 3.5 | 52.5 | 100.0 | 6.9 | 3.7 | 100.0 | 100.0 | |||||
| SK-MEL-2 | 100.0 | 46.8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| SK-MEL-28 | 87.1 | 26.9 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| SK-MEL-5 | 100.0 | 21.9 | 100.0 | 100.0 | 100.0 | 42.7 | 100.0 | 100.0 | |||||
| UACC-257 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 46.8 | 100.0 | 100.0 | |||||
| UACC-62 | 100.0 | 20.9 | 100.0 | 100.0 | 100.0 | 25.7 | 100.0 | 100.0 | |||||
| Ovarian Cancer | IGROV1 | 100.0 | 91.67 | 0.77 | 100.0 | 100.0 | 100.0 | 100.0 | 100 | 0.70 | 100.0 | 100.0 | 100.0 |
| OVCAR-3 | 41.7 | 22.9 | 52.5 | 100.0 | 100.0 | 13.5 | 100.0 | 100.0 | |||||
| OVCAR-4 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| OVCAR-5 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| OVCAR-8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| NCI/ADR-RES | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| SK-OV-3 | 100.0 | ND | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| Renal Cancer | 786−0 | 100.0 | 47.7 | 1.5 | 100.0 | 100.0 | 100.0 | 100.0 | 48.2 | 1.5 | 100 | 100.0 | 100.0 |
| A498 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| ACHN | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| CAKI-1 | 100.0 | 87.1 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| RXF 393 | ND | ND | ND | ND | ND | ND | ND | ND | |||||
| SN12C | 100.0 | 44.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| TK-10 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| UO-31 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| PC | PC-3 | 100.0 | 100.0 | 0.70 | 55.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.70 | 51.3 | 100.0 | 100.0 |
| DU-145 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| Breast Cancer | MCF7 | 0.60 | 67.8 | 1.04 | 0.4 | 3.5 | 100.0 | 0.69 | 71.1 | 0.99 | 0.49 | 100.0 | 100.0 |
| MDA-MB-231/ATCC | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||
| HS 578T | 70.8 | 18.6 | 75.9 | 100.0 | 100.0 | 100.0 | 28.2 | 100.0 | |||||
| BT-549 | 35.5 | 15.8 | 36.3 | 85.1 | 25.7 | 5.4 | 100.0 | 100.0 | |||||
| T-47D | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 77.6 | 100.0 | |||||
| MDA-MB-468 | 100.0 | 3.9 | 15.5 | 100.0 | 100.0 | 4.1 | 100.0 | ||||||
| Full-panel MG-MID b | 70.3 | 70.2 | |||||||||||
| Compound | In Vitro Cytotoxicity (IC50) |
|---|---|
| WI 38 Normal Cell Line (µM ± SEM) | |
| 4b | 26.8 ± 0.92 |
| 4d | 41.2 ± 1.42 |
| Doxorubicin | 19.8 ± 0.68 |
| Compound | Topoisomerase I % Inhibition | Topoisomerase II % Inhibition |
|---|---|---|
| 4b | 77.3% inhibition (10.05 ± 0.39 ng/mL) | 73.4% inhibition (1.196 ± 0.046 ng/mL) |
| 4d | 72.3% inhibition (12.25 ± 0.47 ng/mL) | 51.9% inhibition (2.164 ± 0.08 ng/mL) |
| Control | 0% inhibition (44.15 ± 1.71 ng/mL) | 0% inhibition (4.496 ± 0.17 ng/mL) |
| Compound | DNA Content | ||
|---|---|---|---|
| G0-G1 Phase | S Phase | G2/M Phase | |
| 4b/HCT116 | 73.85% | 23.23% | 2.92% |
| 4d/HCT116 | 77.92% | 18.33% | 3.75% |
| DMSO/HCT116 | 64.29% | 27.03% | 8.68% |
| Compound | Apoptosis | Necrosis | ||
|---|---|---|---|---|
| Total | Early | Late | ||
| 4b/HCT116 | 29.33% | 6.96% | 22.37% | 3.66% |
| 4d/HCT116 | 38.21% | 11.92% | 26.29% | 3.82% |
| DMSO/HCT116 | 0.54% | 0.39% | 0.15% | 1.83% |
| Compound | Target | Binding Affinity (Kcal/mol) | Amino Acid Residue/DNA Nucleotide Base | Types of Interaction |
|---|---|---|---|---|
| 4b | Topo I | −11.65 | LYS 374 | Pi-Alkyl |
| ALA 715 | Pi-Alkyl | |||
| LYS 425 | Pi-Alkyl | |||
| DC 112 | Pi-Alkyl | |||
| TRP 416 | Pi-Alkyl | |||
| ILE 535 | Pi-Alkyl | |||
| TGP 11 | H-bond | |||
| ASN 419 | H-bond | |||
| DA 113 | Pi-sulfur | |||
| ARG 375 | Pi-anion | |||
| GLU 418 | Pi-anion | |||
| DG 12 | Pi-anion | |||
| ASP 533 | Pi-anion | |||
| GLN 633 | Halogen interaction | |||
| 4d | Topo I | −10.96 | DA 113 | Halogen interaction |
| DC 112 | Halogen interaction | |||
| DG 12 | Halogen interaction | |||
| ARG 362 | Attractive interaction | |||
| TGP 11 | H-bond | |||
| ARG 364 | H-bond | |||
| ASN 722 | H-bond | |||
| DT 10 | Pi-Pi | |||
| Topotecan | Topo I | −10.26 | LYS 532 | H-bond |
| ASP 533 | H-bond | |||
| ARG 364 | H-bond | |||
| DT 8 | Pi-Pi | |||
| TGP 11 | Pi-Pi | |||
| DA 113 | Pi-Pi | |||
| DC 112 | Pi-Pi | |||
| Glu 356 | Attractive interaction |
| Compound | Target | Binding Affinity (Kcal/mol) | Amino Acid Residue/DNA Nucleotide Base | Types of Interaction |
|---|---|---|---|---|
| 4b | Topo I | −8.16 | LYS 461 | Attractive charge |
| ARG 508 | Pi-cation | |||
| LYS 819 | Attractive charge | |||
| VAL 790 | Pi-Sigma | |||
| PRO 824 | Pi-Alkyl | |||
| MET 787 | Pi-Alkyl | |||
| DG 323 | Pi-Alkyl | |||
| DC 28 | Pi-Alkyl | |||
| DG 27 | Pi-Pi | |||
| 4d | Topo I | −8.39 | PRO 824 | Pi-Alkyl |
| VAL 790 | Pi-Alkyl | |||
| ALA 821 | Pi-Alkyl | |||
| MET 787 | Pi-Sulfur | |||
| ARG 508 | Pi-cation | |||
| LYS 461 | Pi-cation | |||
| DC 22 | H-bond | |||
| DG 27 | H-bond | |||
| DC 28 | H-bond | |||
| Etoposide | Topo I | −8.61 | DC 28 | H-bond |
| DG 27 | Pi-Pi | |||
| DG 23 | Pi-Pi | |||
| ARG 508 | Pi-cation | |||
| PRO 824 | Pi-Alkyl | |||
| MET 787 | Pi-Alkyl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, H.; Elshamsy, A.M.; Rabea, S.M.; Marzouk, A.A.; Bräse, S.; Hetta, H.F.; Alkhammash, A.; Alotaibi, G.; Farhan, H.M.; Aziz, H.A. Design, Synthesis, and Mechanistic Study of Novel Ciprofloxacin/Thiazole Chalcone Hybrids as Potential Anticancer Agents. Pharmaceuticals 2025, 18, 1700. https://doi.org/10.3390/ph18111700
Hashem H, Elshamsy AM, Rabea SM, Marzouk AA, Bräse S, Hetta HF, Alkhammash A, Alotaibi G, Farhan HM, Aziz HA. Design, Synthesis, and Mechanistic Study of Novel Ciprofloxacin/Thiazole Chalcone Hybrids as Potential Anticancer Agents. Pharmaceuticals. 2025; 18(11):1700. https://doi.org/10.3390/ph18111700
Chicago/Turabian StyleHashem, Hamada, Ali M. Elshamsy, Safwat M. Rabea, Adel A. Marzouk, Stefan Bräse, Helal F. Hetta, Abdullah Alkhammash, Ghallab Alotaibi, Hadeer M. Farhan, and Hossameldin A. Aziz. 2025. "Design, Synthesis, and Mechanistic Study of Novel Ciprofloxacin/Thiazole Chalcone Hybrids as Potential Anticancer Agents" Pharmaceuticals 18, no. 11: 1700. https://doi.org/10.3390/ph18111700
APA StyleHashem, H., Elshamsy, A. M., Rabea, S. M., Marzouk, A. A., Bräse, S., Hetta, H. F., Alkhammash, A., Alotaibi, G., Farhan, H. M., & Aziz, H. A. (2025). Design, Synthesis, and Mechanistic Study of Novel Ciprofloxacin/Thiazole Chalcone Hybrids as Potential Anticancer Agents. Pharmaceuticals, 18(11), 1700. https://doi.org/10.3390/ph18111700








