Polyphyllin H Reverses Paclitaxel Resistance in Breast Cancer by Binding Membrane Cholesterol to Inhibit Both ABCB1 and ABCC3
Abstract
1. Introduction
2. Results
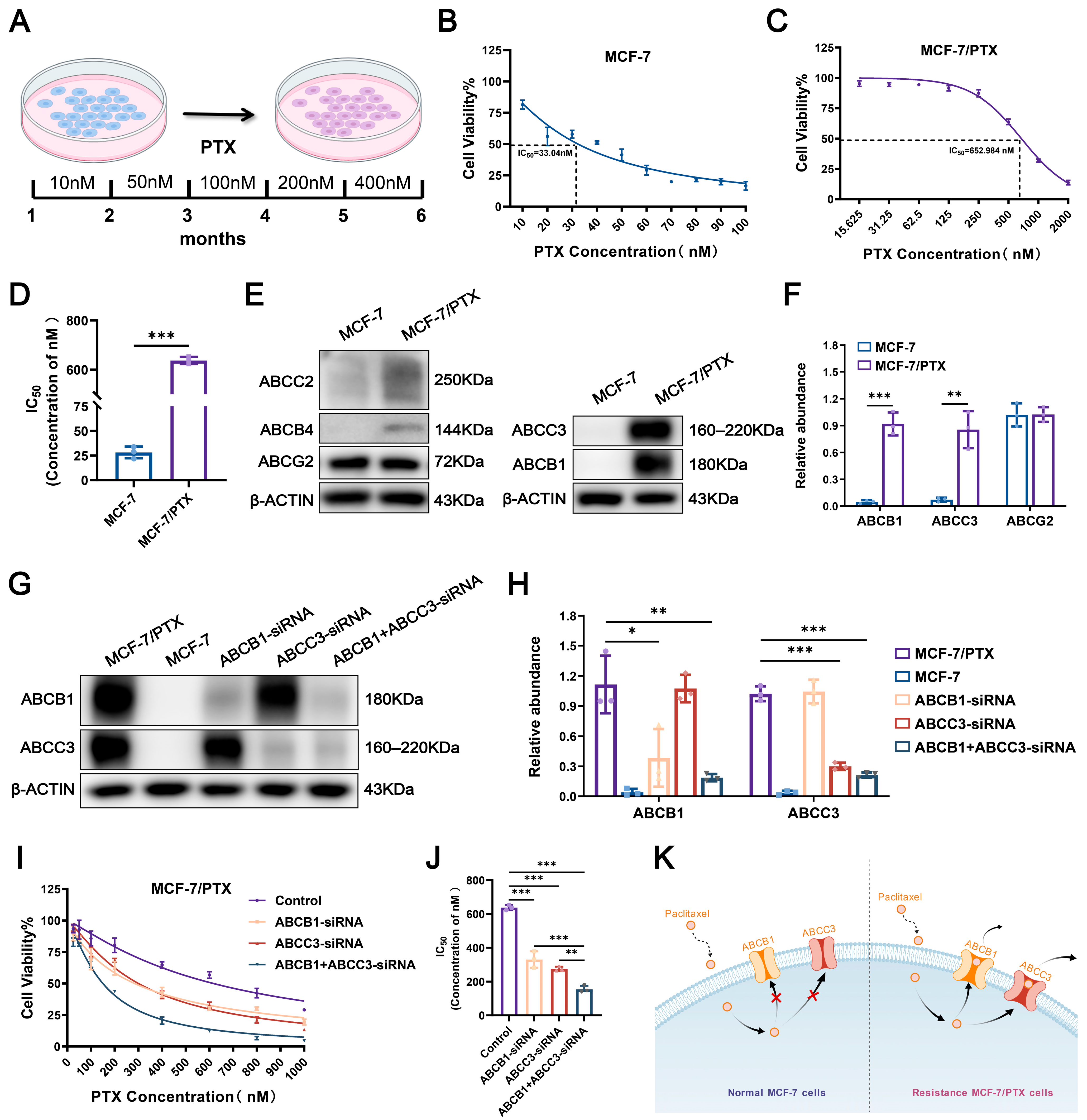
2.1. Establishment of PTX-Resistant MCF-7 Cells
2.2. Activation of Dual-ABC Transporters Leads to PTX Resistance in Tumor Cells
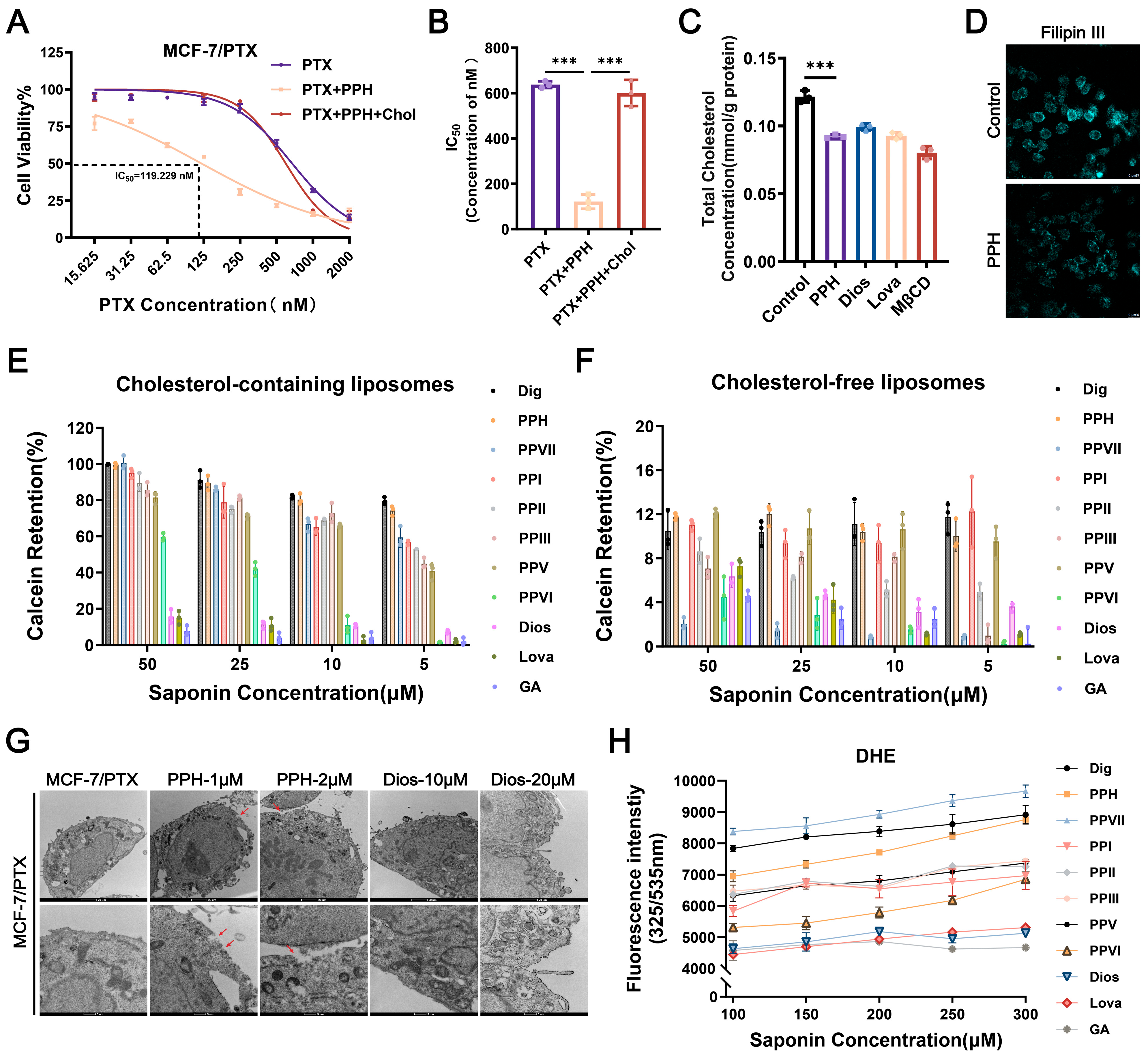
2.3. Cholesterol Accumulation Within Lipid Rafts Contributes to the Dysregulation of ABC Transporters
2.4. PPH Binds with Membrane Cholesterol to Reverse PTX Resistance
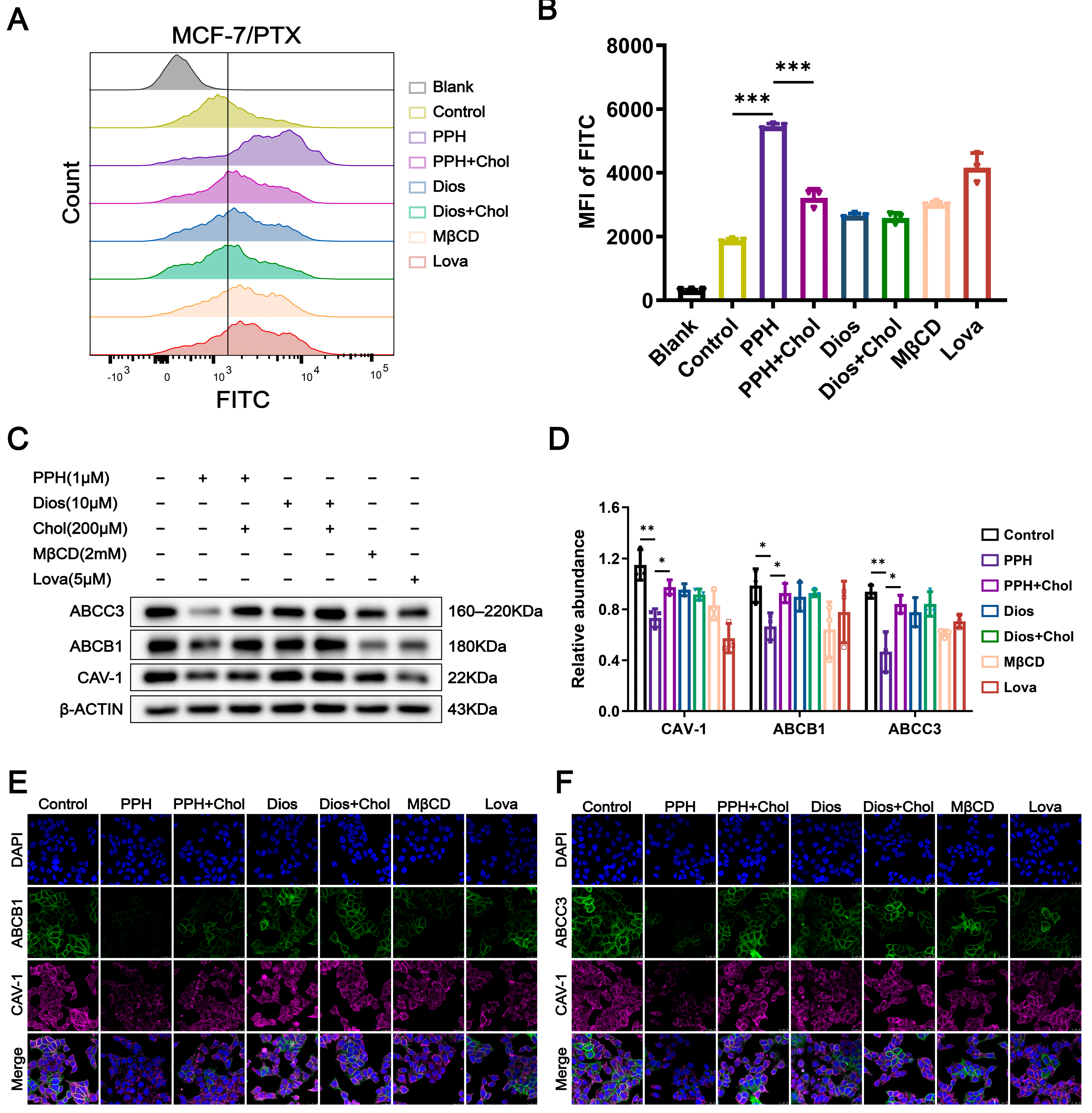
2.5. PPH Targets Cholesterol-Enriched Lipid Rafts to Inhibit Dual-ABC Transporters
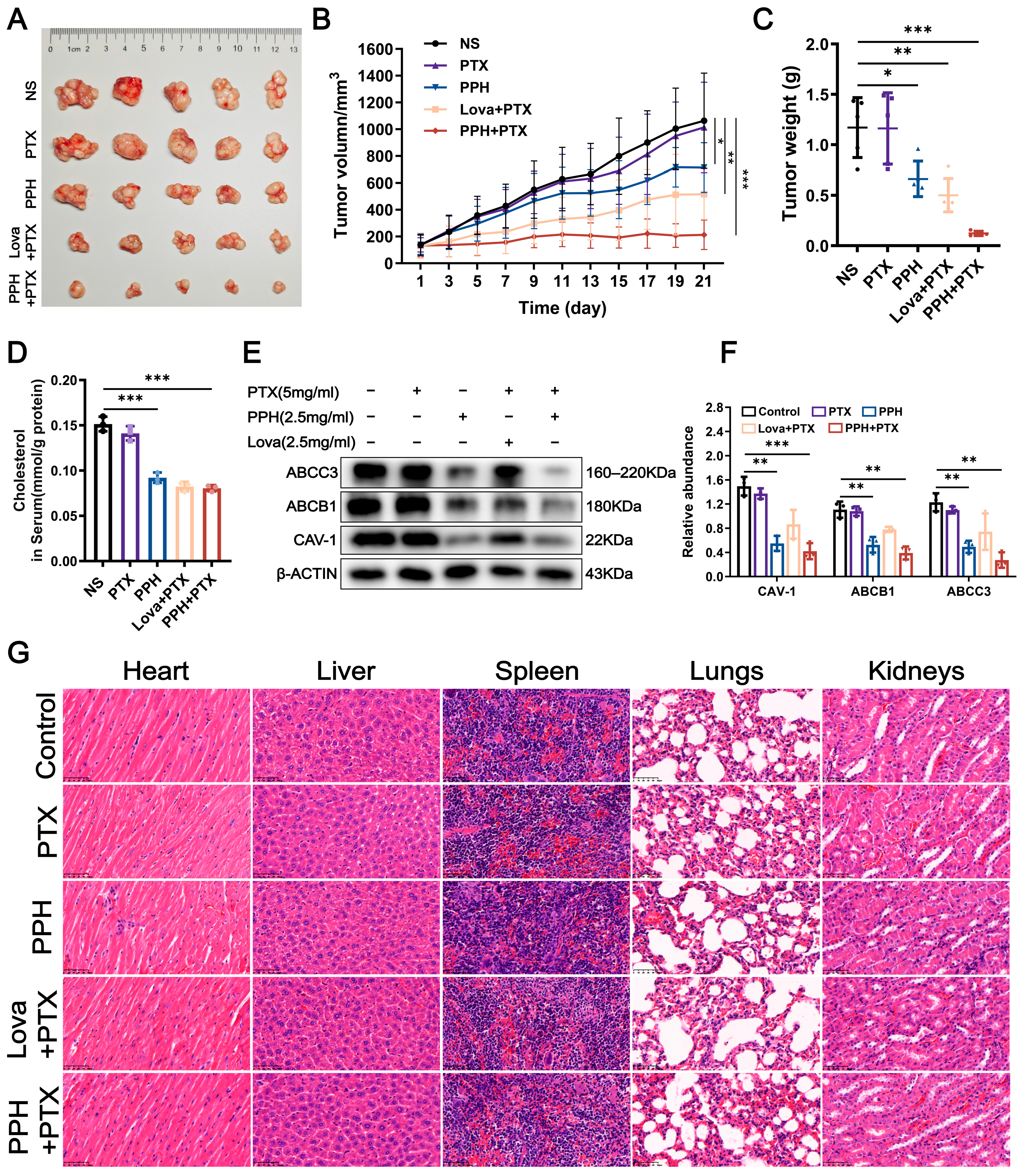
2.6. The In Vivo Evaluation of PPH Reversing PTX Resistance in MCF-7 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Cytotoxicity Assay
4.4. Western Blot
4.5. Silencing of ABCB1 and ABCC3 Expression by siRNA and Its Effects on Cell Growth and Viability
4.6. Immunofluorescent Staining
4.7. Extraction and Isolation of Lipid Rafts
4.8. Filipin III Assays Detect Cell-Membrane Cholesterol Content
4.9. Liposome Preparation and Calcein Encapsulation
4.10. Morphology of Cell Membrane in Transmission Electron Microscope
4.11. Binding Assay of Polyphyllins with DHE
4.12. In Vivo Antitumor Effect
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCK-8 | Cell counting kit-8 |
| ABC | ATP-binding cassette |
| MDR | Multidrug resistance |
| IC50 | Half maximal inhibitory concentration |
| P-gp | P-glycoprotein |
| TC | Total cholesterol |
| CLSM | Confocal laser scanning microscope |
| DOPE | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| PPH | Polyphyllin H |
| PTX | Paclitaxel |
| Dios | Diosgenin |
| Dig | Digitonin |
| GA | Glycyrrhizic acid |
| MβCD | Methyl-β-cyclodextrin |
| Lova | Lovastatin |
| Chol | Cholesterol |
References
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global Patterns and Trends in Breast Cancer Incidence and Mortality across 185 Countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 Cancer Estimates: Data Sources, Methods, and a Snapshot of the Cancer Burden Worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Lee, V.; Albers, K.B.; Prado, C.M.; Alexeeff, S.; Xiao, J.; Shachar, S.S.; Caan, B.J. Body Composition, Adherence to Anthracycline and Taxane-Based Chemotherapy, and Survival After Nonmetastatic Breast Cancer. JAMA Oncol. 2020, 6, 264. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- He, J.; Fortunati, E.; Liu, D.-X.; Li, Y. Pleiotropic Roles of ABC Transporters in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3199. [Google Scholar] [CrossRef]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.-J. Strategies to Overcome Cancer Multidrug Resistance (MDR) through Targeting P-Glycoprotein (ABCB1): An Updated Review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Velázquez, E.D.; Granados-López, A.J.; López, J.A.; Solorio-Alvarado, C.R. Multidrug Resistance Reversed by Maleimide Interactions. A Biological and Synthetic Overview for an Emerging Field. ChemBioChem 2025, 26, e202400640. [Google Scholar] [CrossRef]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R.; et al. Phase II Study of Tariquidar, a Selective P-glycoprotein Inhibitor, in Patients with Chemotherapy-resistant, Advanced Breast Carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a Novel Modulator of P-Glycoprotein, Does Not Improve the Outcome of Older Patients with Newly Diagnosed Acute Myeloid Leukemia: A Randomized, Placebo-Controlled Trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Ruff, P.; Vorobiof, D.A.; Jordaan, J.P.; Demetriou, G.S.; Moodley, S.D.; Nosworthy, A.L.; Werner, I.D.; Raats, J.; Burgess, L.J. A Randomized, Placebo-Controlled, Double-Blind Phase 2 Study of Docetaxel Compared to Docetaxel plus Zosuquidar (LY335979) in Women with Metastatic or Locally Recurrent Breast Cancer Who Have Received One Prior Chemotherapy Regimen. Cancer Chemother. Pharmacol. 2009, 64, 763–768. [Google Scholar] [CrossRef]
- Shukla, S.; Ohnuma, S.V.; Ambudkar, S. Improving Cancer Chemotherapy with Modulators of ABC Drug Transporters. Curr. Drug Targets 2011, 12, 621–630. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, B.; Chen, Z.; Chen, Z.-S. Progress in the Studies on the Molecular Mechanisms Associated with Multidrug Resistance in Cancers. Acta Pharm. Sin. B 2023, 13, 982–997. [Google Scholar] [CrossRef]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of Acquired Paclitaxel Resistance of Breast Cancer Cells and Involvement of ABC Transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef]
- Durmus, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Oral Availability and Brain Penetration of the B-RAFV600E Inhibitor Vemurafenib Can Be Enhanced by the P-Glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) Inhibitor Elacridar. Mol. Pharm. 2012, 9, 3236–3245. [Google Scholar] [CrossRef]
- Stefan, K.; Schmitt, S.M.; Wiese, M. 9-Deazapurines as Broad-Spectrum Inhibitors of the ABC Transport Proteins P-Glycoprotein, Multidrug Resistance-Associated Protein 1, and Breast Cancer Resistance Protein. J. Med. Chem. 2017, 60, 8758–8780. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, N.; Cai, C.; Wang, J.; Lei, Z.; Teng, Q.; Wu, Z.; Cui, Q.; Pan, Y.; Chen, Z. Modulating the Function of ABCB1: In Vitro and in Vivo Characterization of Sitravatinib, a Tyrosine Kinase Inhibitor. Cancer Commun. 2020, 40, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Storch, C.H.; Ehehalt, R.; Haefeli, W.E.; Weiss, J. Localization of the Human Breast Cancer Resistance Protein (BCRP/ABCG2) in Lipid Rafts/Caveolae and Modulation of Its Activity by Cholesterol in Vitro. J. Pharmacol. Exp. Ther. 2007, 323, 257–264. [Google Scholar] [CrossRef]
- Troost, J.; Lindenmaier, H.; Haefeli, W.E.; Weiss, J. Modulation of Cellular Cholesterol Alters P-Glycoprotein Activity in Multidrug-Resistant Cells. Mol. Pharmacol. 2004, 66, 1332–1339. [Google Scholar] [CrossRef]
- Ghetie, M.-A.; Marches, R.; Kufert, S.; Vitetta, E.S. An Anti-CD19 Antibody Inhibits the Interaction between P-Glycoprotein (P-Gp) and CD19, Causes P-Gp to Translocate out of Lipid Rafts, and Chemosensitizes a Multidrug-Resistant (MDR) Lymphoma Cell Line. Blood 2004, 104, 178–183. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of Cell Membrane Lipids in Cancer Drug Resistance: Implications for Drug Transport and Drug Delivery with Nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid Rafts as Signaling Hubs in Cancer Cell Survival/Death and Invasion: Implications in Tumor Progression and Therapy. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef]
- Kopecka, J.; Trouillas, P.; Gašparović, A.Č.; Gazzano, E.; Assaraf, Y.G.; Riganti, C. Phospholipids and Cholesterol: Inducers of Cancer Multidrug Resistance and Therapeutic Targets. Drug Resist. Updates 2020, 49, 100670. [Google Scholar] [CrossRef]
- Bharathiraja, P.; Yadav, P.; Sajid, A.; Ambudkar, S.V.; Prasad, N.R. Natural Medicinal Compounds Target Signal Transduction Pathways to Overcome ABC Drug Efflux Transporter-Mediated Multidrug Resistance in Cancer. Drug Resist. Updates 2023, 71, 101004. [Google Scholar] [CrossRef]
- Hernández-Velázquez, E.D.; Granados-López, A.J.; Araujo-Huitrado, J.G.; Hernández-López, H.; Ortíz-Alvarado, R.; López, J.A.; Solorio-Alvarado, C.R. Synthesis and Biological Evaluation of Strong Cytotoxic Maleimide Derivatives with Potential Multidrug Resistance Reversal Activity in the Breast Cancer Therapy. ChemistrySelect 2025, 10, e202406020. [Google Scholar] [CrossRef]
- Wang, C.-H.; Baskaran, R.; Ng, S.S.-C.; Wang, T.-F.; Li, C.-C.; Ho, T.-J.; Hsieh, D.J.-Y.; Kuo, C.-H.; Chen, M.-C.; Huang, C.-Y. Platycodin D Confers Oxaliplatin Resistance in Colorectal Cancer by Activating the LATS2/YAP1 Axis of the Hippo Signaling Pathway. J. Cancer 2023, 14, 393–402. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, C.; Wu, Z.; Xu, M.; Chen, Z.; Yao, J.; Que, Z.; Tian, J.; Leung, E.L.-H.; Wang, Z. Paris Saponin VII Reverses Resistance to PARP Inhibitors by Regulating Ovarian Cancer Tumor Angiogenesis and Glycolysis through the RORα/ECM1/VEGFR2 Signaling Axis. Int. J. Biol. Sci. 2024, 20, 2454–2475. [Google Scholar] [CrossRef]
- Cui, A.; Liu, H.; Liu, X.; Zhang, M.; Xiao, B.; Wang, B.; Yang, J. Steroidal Saponins: Natural Compounds with the Potential to Reverse Tumor Drug Resistance (Review). Oncol. Lett. 2024, 28, 585. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Li, Z.; Li, S.; Li, J. Advances in the Biosynthesis and Molecular Evolution of Steroidal Saponins in Plants. Int. J. Mol. Sci. 2023, 24, 2620. [Google Scholar] [CrossRef] [PubMed]
- Sudji, I.; Subburaj, Y.; Frenkel, N.; García-Sáez, A.; Wink, M. Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol. Molecules 2015, 20, 20146–20160. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, R.; Chen, R.; Guo, X.; Song, C.; Gao, X.; Zeng, F.; Liu, Q. The Role of Rhizoma Paridis Saponins on Anti-Cancer: The Potential Mechanism and Molecular Targets. Heliyon 2024, 10, e37323. [Google Scholar] [CrossRef]
- Li, J.; Jia, J.; Zhu, W.; Chen, J.; Zheng, Q.; Li, D. Therapeutic Effects on Cancer of the Active Ingredients in Rhizoma Paridis. Front. Pharmacol. 2023, 14, 1095786. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, L.; Hong, C.; Zhang, L.; Lu, L.; Zhao, W.; Ding, Y.; Zhang, T. A Novel Polyphyllin I-Based Liposome Delivery System Sensitizes Hepatic Carcinoma to Doxorubicin via Cholesterol Modulation. J. Drug Deliv. Sci. Technol. 2022, 78, 103925. [Google Scholar] [CrossRef]
- Bergonzini, C.; Gregori, A.; Hagens, T.M.S.; Van Der Noord, V.E.; Van De Water, B.; Zweemer, A.J.M.; Coban, B.; Capula, M.; Mantini, G.; Botto, A.; et al. ABCB1 Overexpression through Locus Amplification Represents an Actionable Target to Combat Paclitaxel Resistance in Pancreatic Cancer Cells. J. Exp. Clin. Cancer Res. 2024, 43, 4. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.-L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) Induction Defines a Common Resistance Mechanism in Paclitaxel- and Olaparib-Resistant Ovarian Cancer Cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Ferro, R.; Lattanzio, R.; Capone, E.; Domenichini, A.; Damiani, V.; Chiorino, G.; Akkaya, B.G.; Linton, K.J.; De Laurenzi, V.; et al. ABCC3 Is a Novel Target for the Treatment of Pancreatic Cancer. Adv. Biol. Regul. 2019, 73, 100634. [Google Scholar] [CrossRef]
- Kool, M.; Van Der Linden, M.; De Haas, M.; Scheffer, G.L.; De Vree, J.M.L.; Smith, A.J.; Jansen, G.; Peters, G.J.; Ponne, N.; Scheper, R.J.; et al. MRP3, an Organic Anion Transporter Able to Transport Anti-Cancer Drugs. Proc. Natl. Acad. Sci. USA 1999, 96, 6914–6919. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Yan, A.; Yang, Y.; Meng, Q.; Sun, L.; Pang, H.; Li, C.; Dong, X.; Cai, L. ABCC3 as a Marker for Multidrug Resistance in Non-Small Cell Lung Cancer. Sci. Rep. 2013, 3, 3120. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Teng, Q.-X.; Yang, Y.; Acharekar, N.; Wang, J.-Q.; He, M.; Yoganathan, S.; Lin, J.; Wang, J.; Chen, Z.-S. MET Inhibitor Tepotinib Antagonizes Multidrug Resistance Mediated by ABCG2 Transporter: In Vitro and in Vivo Study. Acta Pharm. Sin. B 2022, 12, 2609–2618. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP–Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC Multidrug Transporters: Structure, Function and Role in Chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Bacon, N.A.; Larre, I.; Lawag, A.A.; Merritt, C.; Smith, M.; Rosolen, M.; Sollars, V.E. Low Dose HSP90 Inhibition with AUY922 Blunts Rapid Evolution of Metastatic and Drug Resistant Phenotypes Induced by TGF-β and Paclitaxel in A549 Cells. Biomed. Pharmacother. 2020, 129, 110434. [Google Scholar] [CrossRef]
- Tőkés, A.M.; Vári-Kakas, S.; Kulka, J.; Törőcsik, B. Tumor Glucose and Fatty Acid Metabolism in the Context of Anthracycline and Taxane-Based (Neo)Adjuvant Chemotherapy in Breast Carcinomas. Front. Oncol. 2022, 12, 850401. [Google Scholar] [CrossRef]
- Ristovski, M.; Farhat, D.; Bancud, S.E.M.; Lee, J.-Y. Lipid Transporters Beam Signals from Cell Membranes. Membranes 2021, 11, 562. [Google Scholar] [CrossRef]
- Klappe, K.; Hummel, I.; Hoekstra, D.; Kok, J.W. Lipid Dependence of ABC Transporter Localization and Function. Chem. Phys. Lipids 2009, 161, 57–64. [Google Scholar] [CrossRef]
- Abdulla, N.; Vincent, C.T.; Kaur, M. Mechanistic Insights Delineating the Role of Cholesterol in Epithelial Mesenchymal Transition and Drug Resistance in Cancer. Front. Cell Dev. Biol. 2021, 9, 728325. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Weber, C.-C.; Franke, C.; Müller, W.E.; Eckert, G.P. Cholesterol: Coupling between Membrane Microenvironment and ABC Transporter Activity. Biochem. Biophys. Res. Commun. 2007, 354, 216–221. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, Z.; Zhao, M.; Wang, Q.; Qiao, C.; Miao, L.; Ding, X. High Cholesterol in Lipid Rafts Reduces the Sensitivity to EGFR-TKI Therapy in Non-small Cell Lung Cancer. J. Cell. Physiol. 2018, 233, 6722–6732. [Google Scholar] [CrossRef]
- Jin, H.; He, Y.; Zhao, P.; Hu, Y.; Tao, J.; Chen, J.; Huang, Y. Targeting Lipid Metabolism to Overcome EMT-Associated Drug Resistance via Integrin Β3/FAK Pathway and Tumor-Associated Macrophage Repolarization Using Legumain-Activatable Delivery. Theranostics 2019, 9, 265–278. [Google Scholar] [CrossRef]
- Kamau, S.W.; Kr, S. Effect of the Modulation of the Membrane Lipid Composition on the Localization and Function of P-Glycoprotein in MDR1-MDCK Cells. Vitr. Cell. Dev. Biol. Anim. 2005, 41, 207–216. [Google Scholar] [CrossRef][Green Version]
- Yun, U.-J.; Lee, J.-H.; Koo, K.H.; Ye, S.-K.; Kim, S.-Y.; Lee, C.-H.; Kim, Y.-N. Lipid Raft Modulation by Rp1 Reverses Multidrug Resistance via Inactivating MDR-1 and Src Inhibition. Biochem. Pharmacol. 2013, 85, 1441–1453. [Google Scholar] [CrossRef]
- Drozdzik, M.; Drozdzik, M.; Oswald, S. Membrane Carriers and Transporters in Kidney Physiology and Disease. Biomedicines 2021, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- The International Transporter Consortium; Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Linton, K.J. Structure and Function of ABC Transporters. Physiology 2007, 22, 122–130. [Google Scholar] [CrossRef]
- Anderson, H.A.; Hiltbold, E.M.; Roche, P.A. Concentration of MHC Class II Molecules in Lipid Rafts Facilitates Antigen Presentation. Nat. Immunol. 2000, 1, 156–162. [Google Scholar] [CrossRef]
- Anderson, H.A.; Roche, P.A. MHC Class II Association with Lipid Rafts on the Antigen Presenting Cell Surface. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 775–780. [Google Scholar] [CrossRef]
- Croce, C.; Garrido, F.; Dinamarca, S.; Santi-Rocca, J.; Marion, S.; Blanchard, N.; Mayorga, L.S.; Cebrian, I. Efficient Cholesterol Transport in Dendritic Cells Defines Optimal Exogenous Antigen Presentation and Toxoplasma Gondii Proliferation. Front. Cell Dev. Biol. 2022, 10, 837574. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Hong, C.; Jiang, M.; Zou, W.; Ren, Y.; Li, M.; Xue, X.; Xie, X.; Zhang, T.; Ding, Y. Polyphyllin H Reverses Paclitaxel Resistance in Breast Cancer by Binding Membrane Cholesterol to Inhibit Both ABCB1 and ABCC3. Pharmaceuticals 2025, 18, 1699. https://doi.org/10.3390/ph18111699
Ye Z, Hong C, Jiang M, Zou W, Ren Y, Li M, Xue X, Xie X, Zhang T, Ding Y. Polyphyllin H Reverses Paclitaxel Resistance in Breast Cancer by Binding Membrane Cholesterol to Inhibit Both ABCB1 and ABCC3. Pharmaceuticals. 2025; 18(11):1699. https://doi.org/10.3390/ph18111699
Chicago/Turabian StyleYe, Zheng, Chao Hong, Min Jiang, Wenkui Zou, Yaning Ren, Mingfang Li, Xinyue Xue, Xiaoting Xie, Tong Zhang, and Yue Ding. 2025. "Polyphyllin H Reverses Paclitaxel Resistance in Breast Cancer by Binding Membrane Cholesterol to Inhibit Both ABCB1 and ABCC3" Pharmaceuticals 18, no. 11: 1699. https://doi.org/10.3390/ph18111699
APA StyleYe, Z., Hong, C., Jiang, M., Zou, W., Ren, Y., Li, M., Xue, X., Xie, X., Zhang, T., & Ding, Y. (2025). Polyphyllin H Reverses Paclitaxel Resistance in Breast Cancer by Binding Membrane Cholesterol to Inhibit Both ABCB1 and ABCC3. Pharmaceuticals, 18(11), 1699. https://doi.org/10.3390/ph18111699






