Fluoxetine Disrupts Ovarian Serotonin Signaling and Oocyte Competence in Mice
Abstract
1. Introduction
2. Results
2.1. Short-Term Fluoxetine Administration Drastically Reduces Serum Serotonin Without Affecting Estradiol Levels
2.2. Fluoxetine Administration Does Not Alter Gonadotropin Levels or Estrous Cyclicity
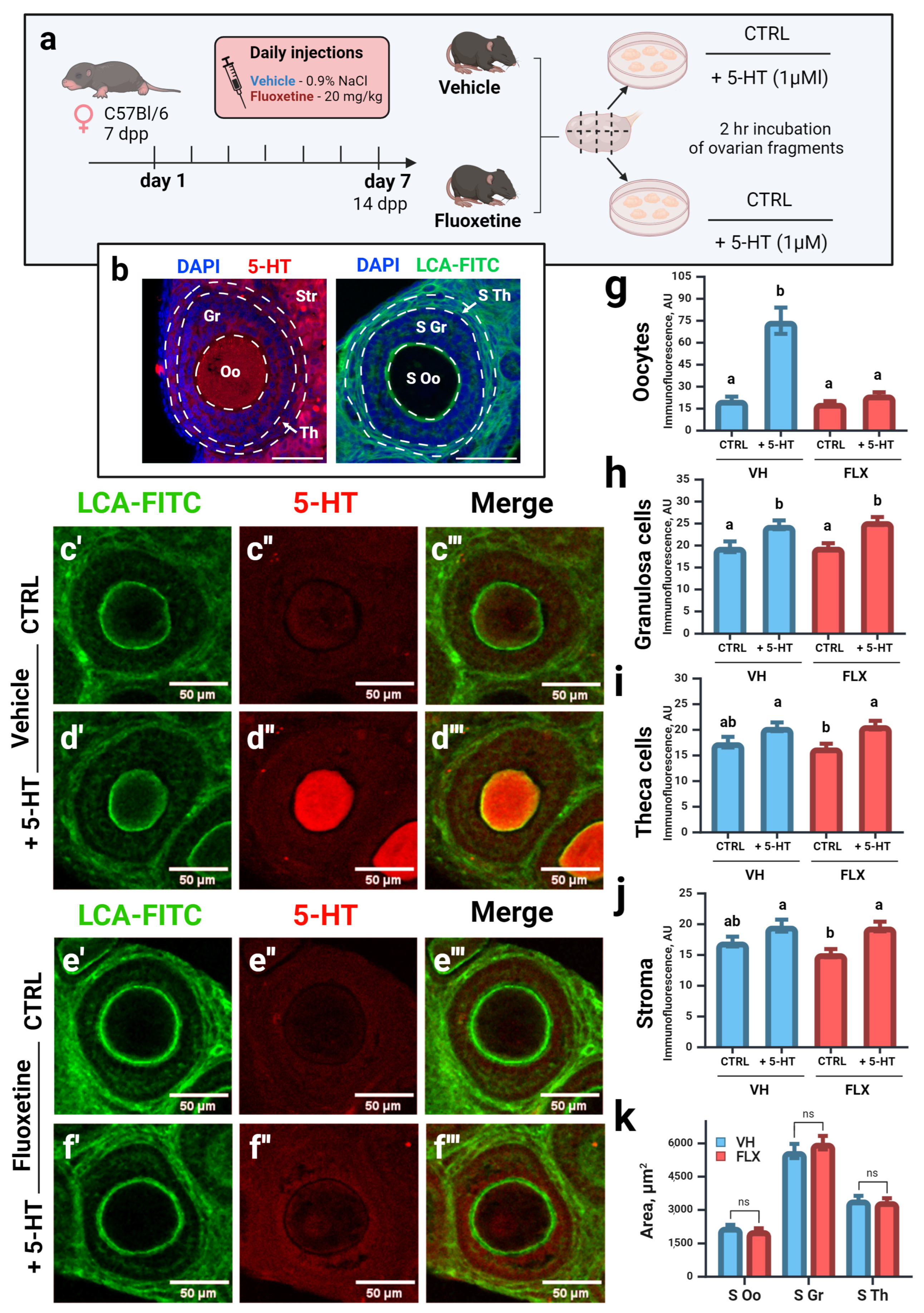
2.3. Fluoxetine Disrupts Serotonin Accumulation in Oocytes of Growing Follicles Without Altering Follicular Morphology
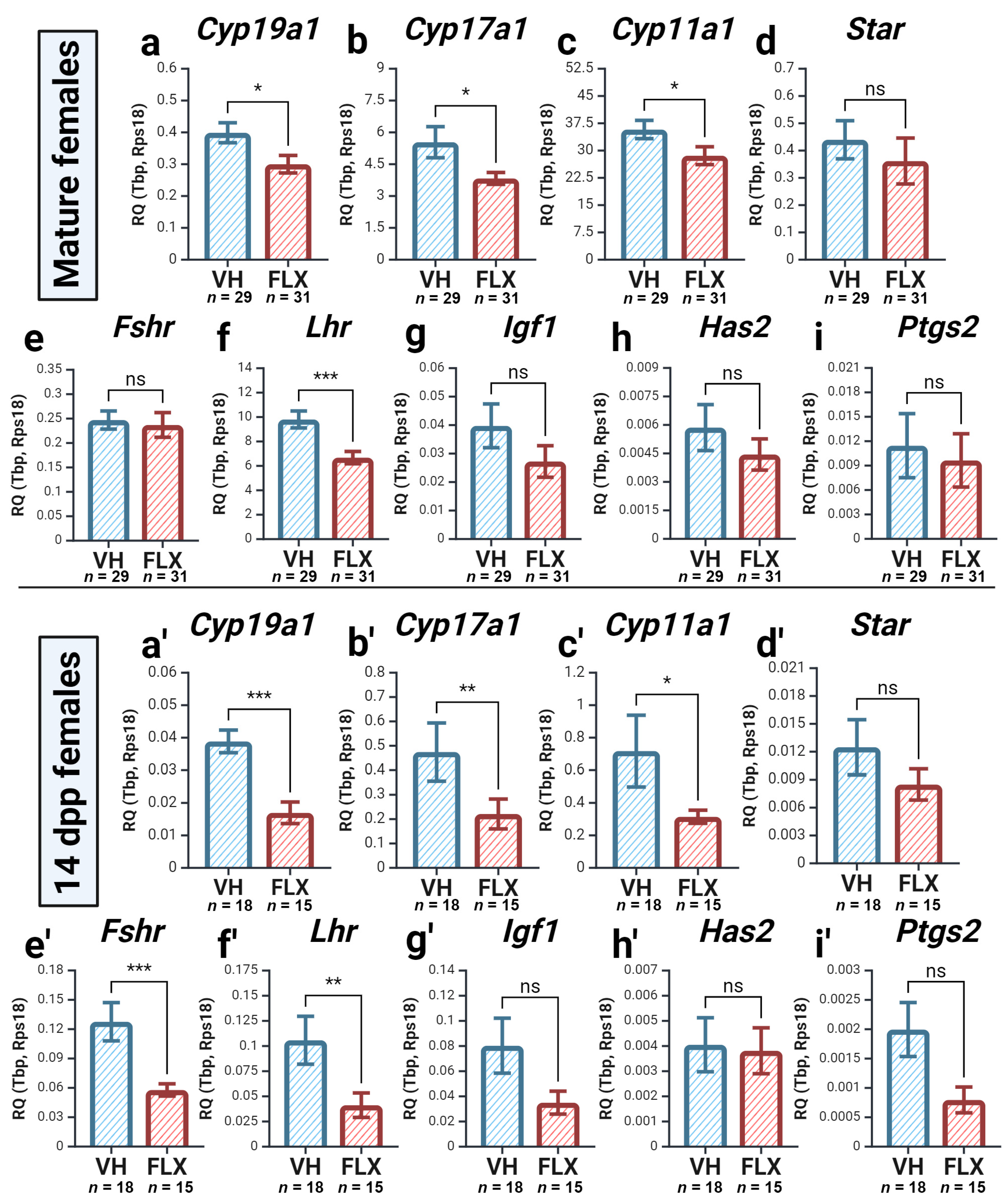
2.4. Fluoxetine Alters the Expression of Key Ovarian Genes
2.4.1. Fluoxetine Downregulates the Expression of Key Oocyte-Secreted Factors and Sert
2.4.2. Fluoxetine Alters the Expression of Genes for Steroidogenesis and Gonadotropin Response
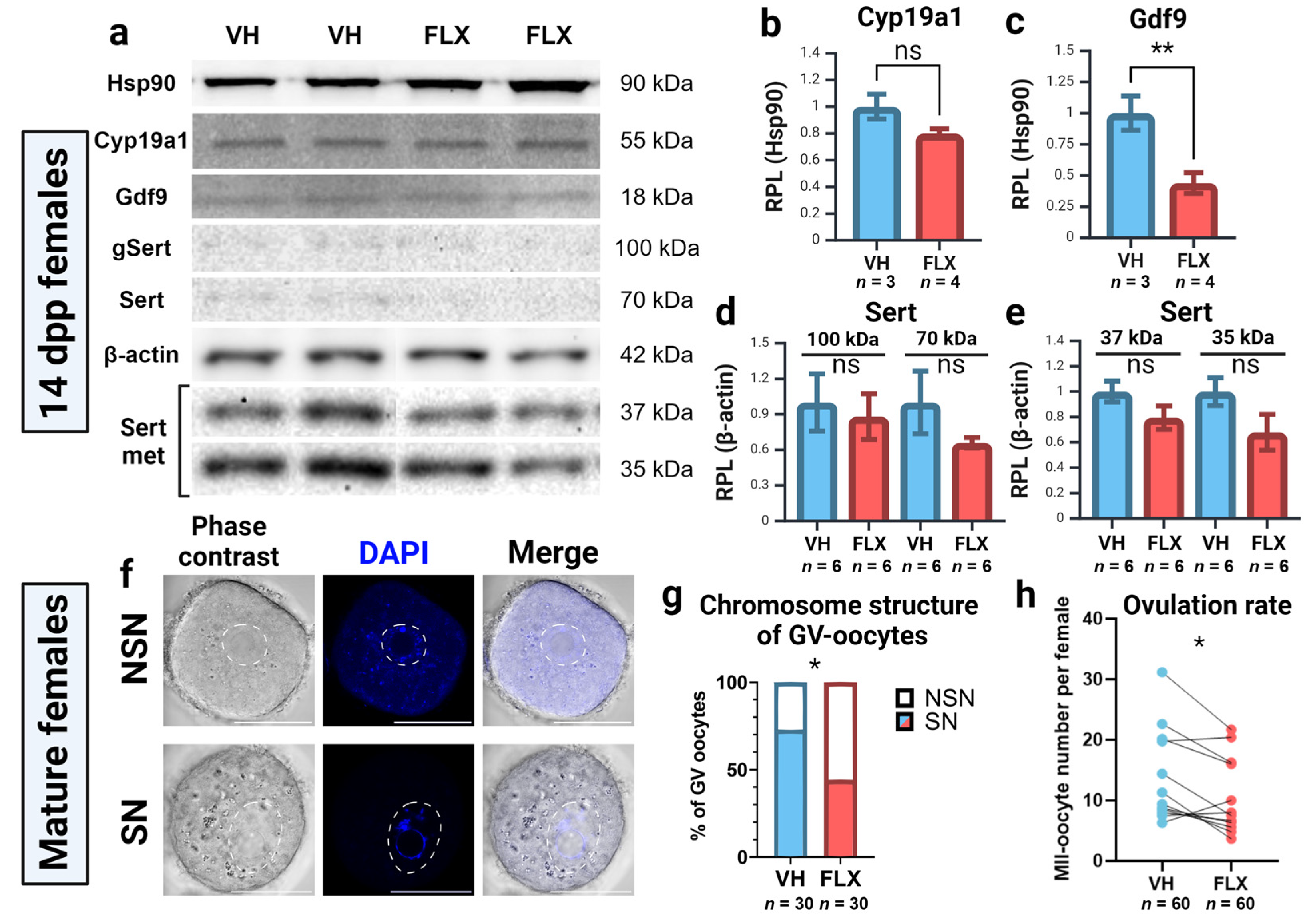
2.5. Fluoxetine Reduces Ovarian GDF9 Protein Levels
2.6. Fluoxetine Impairs Oocyte Competence and Reduces Ovulation Rate
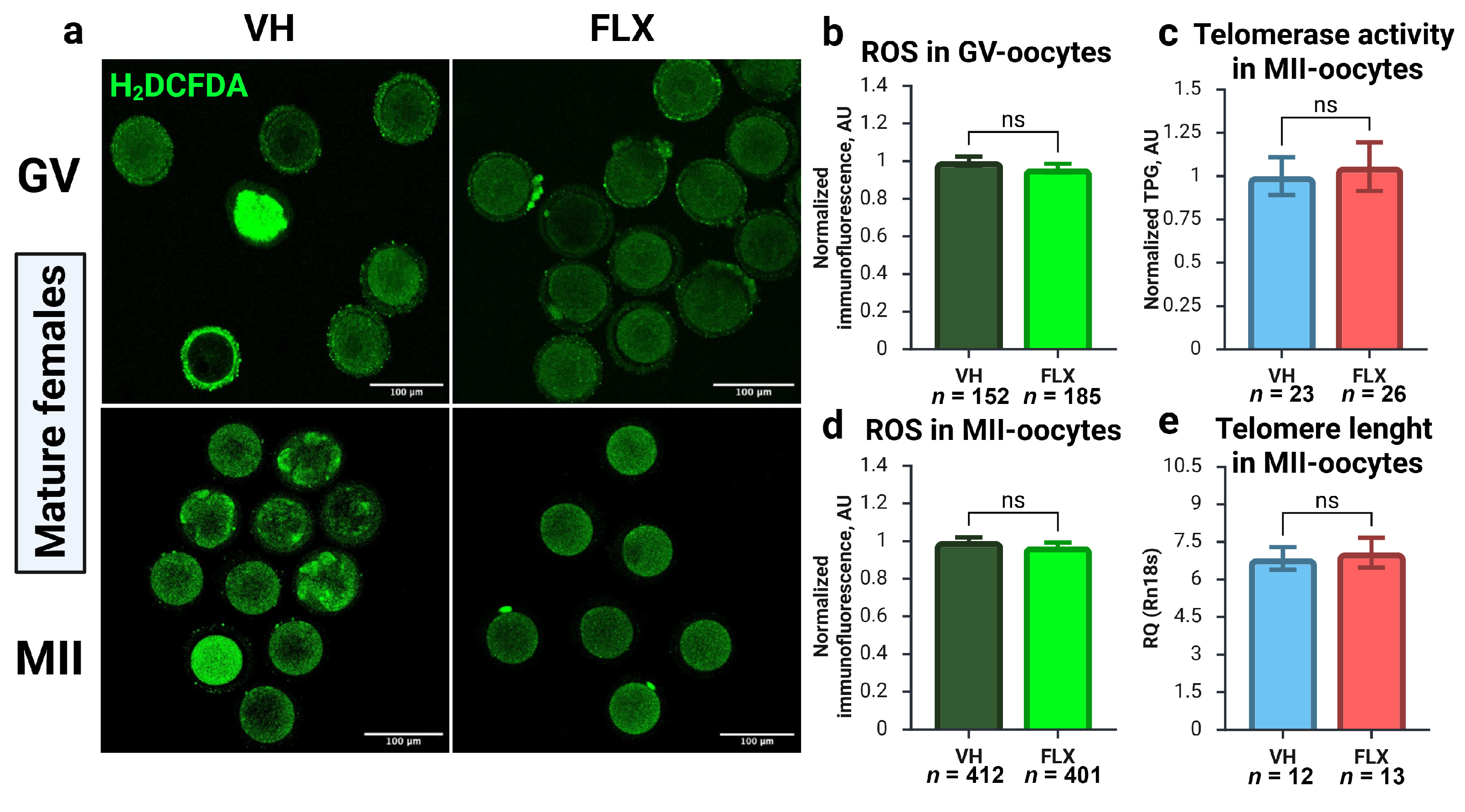
2.7. Fluoxetine-Induced Oocyte Damage Is Independent of Oxidative Stress and Telomere Maintenance Pathways
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Approval
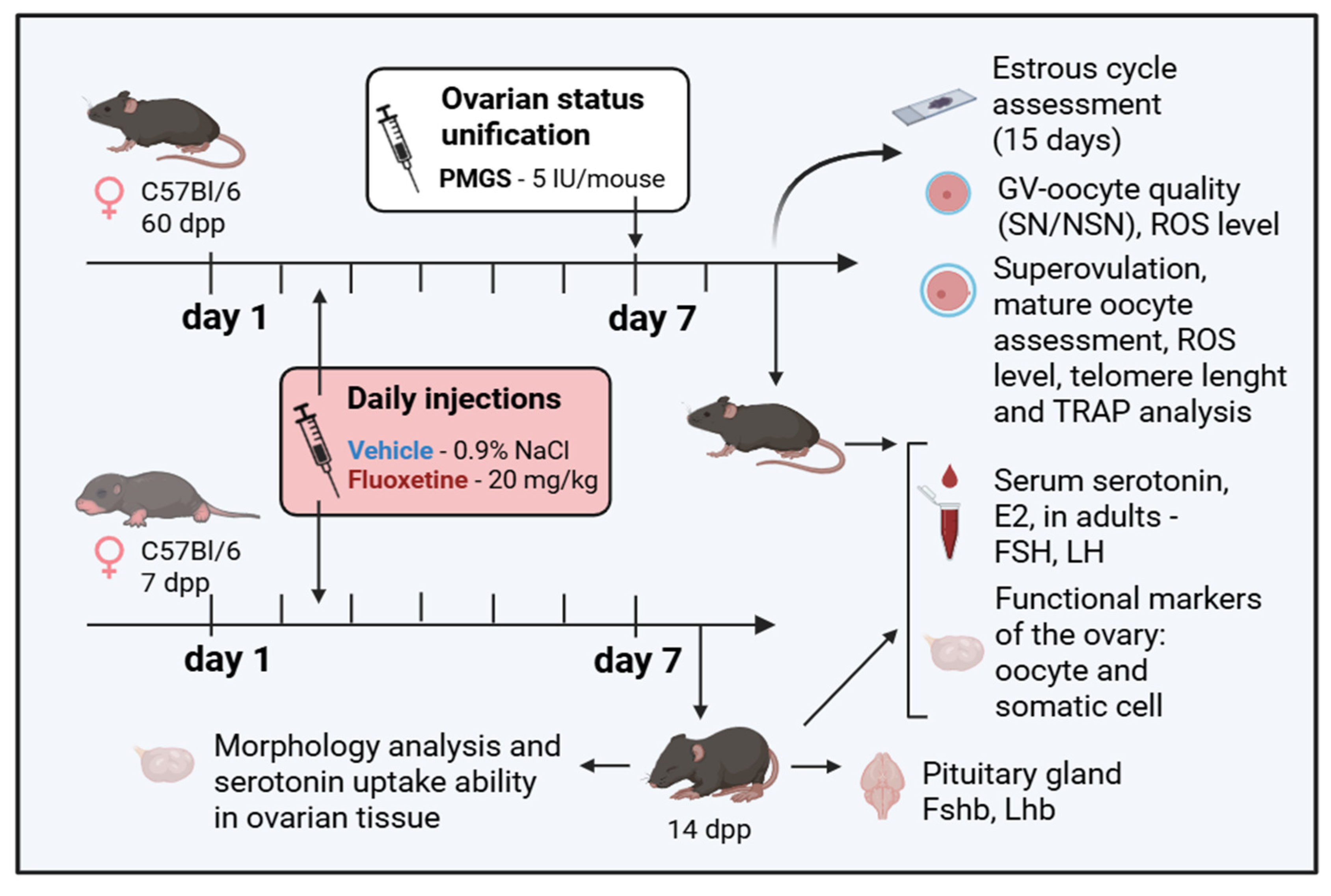
4.2. Experimental Design, Drug Administration, and Sample Collection
4.3. Serum Analysis: HPLC and ELISA
4.4. Oocyte Collection, Staining, and Analysis
4.5. Immunohistochemistry (IHC) and Image Analysis
4.6. Gene Expression Analysis
4.7. Telomerase Activity (TRAP) and Telomere Length Analysis
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine, serotonin |
| dpp | days postpartum |
| E2 | 17β-estradiol |
| FLX | fluoxetine |
| FSH | follicle-stimulating hormone |
| GV | germinal vesicle |
| hCG | human chorionic gonadotropin |
| LH | luteinizing hormone |
| MII | metaphase II |
| NSN | non-surrounded nucleolus-like bodies |
| PMSG | pregnant mare serum gonadotropin |
| RPL | relative protein levels |
| RQ | relative gene expression |
| SN | surrounded nucleolus-like bodies |
| SSRI | selective serotonin reuptake inhibitor |
| VH | vehicle |
References
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Kaihola, H.; Yaldir, F.G.; Hreinsson, J.; Hörnaeus, K.; Bergquist, J.; Olivier, J.D.A.; Åkerud, H.; Sundström-Poromaa, I. Effects of Fluoxetine on Human Embryo Development. Front. Cell. Neurosci. 2016, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-W.; Choe, C.; Kim, E.-J.; Lee, J.-I.; Yoon, S.-Y.; Cho, Y.-W.; Han, S.; Tak, H.-M.; Han, J.; Kang, D. Dual Effects of Fluoxetine on Mouse Early Embryonic Development. Toxicol. Appl. Pharmacol. 2012, 265, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, J.; Cárdenas, M.; Damián-Matsumura, P.; Domínguez, R.; Ayala, M.E. Inhibition of Serotonin Reuptake in the Prepubertal Rat Ovary by Fluoxetine and Effects on Ovarian Functions. Reprod. Toxicol. 2016, 59, 80–88. [Google Scholar] [CrossRef]
- Reefhuis, J.; Devine, O.; Friedman, J.M.; Louik, C.; Honein, M.A. Specific SSRIs and Birth Defects: Bayesian Analysis to Interpret New Data in the Context of Previous Reports. BMJ 2015, 351, h3190. [Google Scholar] [CrossRef]
- Ruiz-Santiago, C.; Rodríguez-Pinacho, C.V.; Pérez-Sánchez, G.; Acosta-Cruz, E. Effects of Selective Serotonin Reuptake Inhibitors on Endocrine System (Review). Biomed. Rep. 2024, 21, 1–13. [Google Scholar] [CrossRef]
- Gök, S.; Gök, B.C.; Alataş, E.; Senol, H.; Topak, O.Z. Effects of Selective Serotonin Reuptake Inhibitor Treatment on Ovarian Reserves in Patients with Depression. Medicina 2023, 59, 517. [Google Scholar] [CrossRef]
- Feng, Y.; Qu, X.; Hao, H. Progress in the Study of the Effects of Selective Serotonin Reuptake Inhibitors (SSRIs) on the Reproductive System. Front. Pharmacol. 2025, 16, 1567863. [Google Scholar] [CrossRef]
- Schloss, P.; Williams, D.C. The Serotonin Transporter: A Primary Target for Antidepressant Drugs. J. Psychopharmacol. 1998, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The Roles of Peripheral Serotonin in Metabolic Homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef]
- Dubé, F.; Amireault, P. Local Serotonergic Signaling in Mammalian Follicles, Oocytes and Early Embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, L.; Liu, X.S.J.S.; Montplaisir, V.; Tiberi, M.; Baltz, J.M.; Liu, X.S.J.S. A Serotonin Receptor Antagonist Induces Oocyte Maturation in Both Frogs and Mice: Evidence That the Same G Protein Ptor Is Responsible for Maintaining Meiosis Arrest in Both Species. J. Cell. Physiol. 2005, 202, 777–786. [Google Scholar] [CrossRef]
- Bódis, J.; Sulyok, E.; Kőszegi, T.; Prémusz, V.; Várnagy, Á.; Koppán, M. Serum and Follicular Fluid Levels of Serotonin, Kisspeptin, and Brain-Derived Neurotrophic Factor in Patients Undergoing in Vitro Fertilization: An Observational Study. J. Int. Med. Res. 2020, 48, 030006051987933. [Google Scholar] [CrossRef] [PubMed]
- Il’ková, G.; Rehák, P.; Veselá, J.; Cikos, S.; Fabian, D.; Czikková, S.; Koppel, J. Serotonin Localization and Its Functional Significance during Mouse Preimplantation Embryo Development. Zygote 2004, 12, 205–213. [Google Scholar] [CrossRef]
- Basu, B.; Desai, R.; Balaji, J.; Chaerkady, R.; Sriram, V.; Maiti, S.; Panicker, M.M. Serotonin in Pre-Implantation Mouse Embryos Is Localized to the Mitochondria and Can Modulate Mitochondrial Potential. Reproduction 2008, 135, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Nikishin, D.A.; Alyoshina, N.M.; Semenova, M.L.; Shmukler, Y.B. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. Int. J. Mol. Sci. 2019, 20, 3070. [Google Scholar] [CrossRef] [PubMed]
- Alyoshina, N.M.; Tkachenko, M.D.; Malchenko, L.A.; Shmukler, Y.B.; Nikishin, D.A. Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary. Int. J. Mol. Sci. 2022, 23, 14828. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Khramova, Y.V.; Alyoshina, N.M.; Malchenko, L.A.; Shmukler, Y.B. Oocyte-Mediated Effect of Serotonin on the Functional Status of Granulosa Cells. Russ. J. Dev. Biol. 2021, 52, 97–104. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Alyoshina, N.M.; Semenova, M.L.; Shmukler, Y.B. Expression Dynamics of the Serotonergic System Components in Granulosa Cells of the Developing Ovarian Follicle and after Luteinization. Genes Cells 2017, XII, 33–38. [Google Scholar] [CrossRef]
- Frolova, V.S.; Ivanova, A.D.; Konorova, M.S.; Shmukler, Y.B.; Nikishin, D.A. Spatial Organization of the Components of the Serotonergic System in the Early Mouse Development. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2023, 17, S59–S64. [Google Scholar] [CrossRef]
- Zha, W.; Ho, H.T.B.; Hu, T.; Hebert, M.F.; Wang, J. Serotonin Transporter Deficiency Drives Estrogen-Dependent Obesity and Glucose Intolerance. Sci. Rep. 2017, 7, 1137. [Google Scholar] [CrossRef]
- Zha, W.; Hu, T.; Hebert, M.F.; Wang, J. Effect of Pregnancy on Paroxetine-Induced Adiposity and Glucose Intolerance in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hudon Thibeault, A.A.; Laurent, L.; Vo Duy, S.; Sauvé, S.; Caron, P.; Guillemette, C.; Sanderson, J.T.; Vaillancourt, C. Fluoxetine and Its Active Metabolite Norfluoxetine Disrupt Estrogen Synthesis in a Co-Culture Model of the Feto-Placental Unit. Mol. Cell. Endocrinol. 2017, 442, 32–39. [Google Scholar] [CrossRef]
- Terranova, P.F.; Uilenbroek, J.T.; Saville, L.; Horst, D.; Nakamura, Y. Serotonin Enhances Oestradiol Production by Hamster Preovulatory Follicles in Vitro: Effects of Experimentally Induced Atresia. J. Endocrinol. 1990, 125, 433–438. [Google Scholar] [CrossRef]
- Tanaka, E.; Baba, N.; Toshida, K.; Suzuki, K. Serotonin Stimulates Steroidogenesis in Rat Preovulatory Follicles: Involvement of 5-HT2 Receptor. Life Sci. 1993, 53, 563–570. [Google Scholar] [CrossRef]
- Koppan, M.; Bodis, J.; Verzar, Z.; Tinneberg, H.-R.; Torok, A. Serotonin May Alter the Pattern of Gonadotropin-Induced Progesterone Release of Human Granulosa Cells in Superfusion System. Endocrine 2004, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Holck, A.; Wolkowitz, O.M.; Mellon, S.H.; Reus, V.I.; Nelson, J.C.; Westrin, Å.; Lindqvist, D. Plasma Serotonin Levels Are Associated with Antidepressant Response to SSRIs. J. Affect. Disord. 2019, 250, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.C.; Gluck, N.; Arnulf, I.; Quintin, P.; Leboyer, M.; Pecquery, R.; Launay, J.M.; Perez-Diaz, F.; Spreux-Varoquaux, O. Decreased Platelet Serotonin Transporter Sites and Increased Platelet Inositol Triphosphate Levels in Patients with Unipolar Depression: Effects of Clomipramine and Fluoxetine. Clin. Pharmacol. Ther. 1999, 66, 617–624. [Google Scholar] [CrossRef]
- Alyoshina, N.M.; Tkachenko, M.D.; Nikishina, Y.O.; Nikishin, D.A.; Koltzov, N.K. Serotonin Transporter Activity in Mouse Oocytes Is a Positive Indicator of Follicular Growth and Oocyte Maturity. Int. J. Mol. Sci. 2023, 24, 11247. [Google Scholar] [CrossRef]
- Tkachenko, M.D.; Alyoshina, N.M.; Nikishina, Y.O.; Frolova, V.S.; Nikishin, D.A. Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model. Int. J. Mol. Sci. 2025, 26, 4858. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Liu, R.; Yang, C.; Wang, X.; Ran, Z.; Zhou, S.; Li, X.; He, C. A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles. Int. J. Mol. Sci. 2021, 22, 5130. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Anderson, N.J.; Zhou, Y.; Pankhurst, M.W. Mouse Primary Follicles Experience Slow Growth Rates after Activation and Progressive Increases That Influence the Duration of the Primary Follicle Phase. Biol. Reprod. 2023, 109, 684–692. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Liu, K. The Two Classes of Primordial Follicles in the Mouse Ovary: Their Development, Physiological Functions and Implications for Future Research. Mol. Hum. Reprod. 2014, 20, 286–292. [Google Scholar] [CrossRef]
- Richard, S.; Zhou, Y.; Jasoni, C.L.; Pankhurst, M.W. Ovarian Follicle Size or Growth Rate Can Both Be Determinants of Ovulatory Follicle Selection in Mice. Biol. Reprod. 2024, 110, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of Chronic Fluoxetine in Animal Models of Anxiety and Depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.A.; van Faassen, M.; de Jong, W.H.; Bouma, G.; Meijer, C.; Walenkamp, A.M.; de Vries, E.G.; Oosting, S.F.; Ruhé, H.G.; Kema, I.P. Use of Selective Serotonin Reuptake Inhibitors Is Associated with Very Low Plasma-Free Serotonin Concentrations in Humans. Ann. Clin. Biochem. Int. J. Lab. Med. 2020, 57, 59–63. [Google Scholar] [CrossRef]
- Urbina, M.; Pineda, S.; Piñango, L.; Carreira, I.; Lima, L. [3H]Paroxetine Binding to Human Peripheral Lymphocyte Membranes of Patients with Major Depression before and after Treatment with Fluoxetine. Int. J. Immunopharmacol. 1999, 21, 631–646. [Google Scholar] [CrossRef]
- Oh, J.; Zupan, B.; Gross, S.; Toth, M. Paradoxical Anxiogenic Response of Juvenile Mice to Fluoxetine. Neuropsychopharmacology 2009, 34, 2197–2207. [Google Scholar] [CrossRef]
- Tiffin, P.A.; Mediavilla, J.L.; Close, H.; Kasim, A.S.; Welsh, P.; Paton, L.W.; Mason, J.M. What Were the Impacts of the Committee on Safety of Medicines Warning and Publication of the NICE Guidelines on Trends in Child and Adolescent Antidepressant Prescribing in Primary Care? A Population Based Study. BMJ Open 2019, 9, e028201. [Google Scholar] [CrossRef]
- Dmitriev, A.D.; Factor, M.I.; Segal, O.L.; Pavlova, E.V.; Massino, Y.S.; Smirnova, M.B.; Yakovleva, D.A.; Dmitriev, D.A.; Kizim, E.A.; Kolyaskina, G.I.; et al. Western Blot Analysis of Human and Rat Serotonin Transporter in Platelets and Brain Using Site-Specific Antibodies: Evidence That Transporter Undergoes Endoproteolytic Cleavage. Clin. Chim. Acta 2005, 356, 76–94. [Google Scholar] [CrossRef]
- Descarries, L.; Riad, M. Effects of the Antidepressant Fluoxetine on the Subcellular Localization of 5-HT 1A Receptors and SERT. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2416–2425. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Blakely, R.D. Phosphorylation and Sequestration of Serotonin Transporters Differentially Modulated by Psychostimulants. Science 1999, 285, 763–766. [Google Scholar] [CrossRef]
- Tavoulari, S.; Forrest, L.R.; Rudnick, G. Fluoxetine (Prozac) Binding to Serotonin Transporter Is Modulated by Chloride and Conformational Changes. J. Neurosci. 2009, 29, 9635–9643. [Google Scholar] [CrossRef]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 Direct the Follicle Symphony. J. Assist. Reprod. Genet. 2018, 35, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Reader, K.L.; Mottershead, D.G.; Martin, G.A.; Gilchrist, R.B.; Heath, D.A.; McNatty, K.P.; Juengel, J.L. Signalling Pathways Involved in the Synergistic Effects of Human Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15. Reprod. Fertil. Dev. 2016, 28, 491–498. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral Role of GDF-9 and BMP-15 in Ovarian Function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Orisaka, S.; Jiang, J.-Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K. Growth Differentiation Factor 9 Is Antiapoptotic during Follicular Development from Preantral to Early Antral Stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.-M.; Joyce, I.M. RNA Interference Evidence That Growth Differentiation Factor-9 Mediates Oocyte Regulation of Cumulus Expansion in Mice. Biol. Reprod. 2005, 72, 195–199. [Google Scholar] [CrossRef]
- Otsuka, F.; Moore, R.K.; Shimasaki, S. Biological Function and Cellular Mechanism of Bone Morphogenetic Protein-6 in the Ovary. J. Biol. Chem. 2001, 276, 32889–32895. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chang, H.M.; Taylor, E.L.; Liu, R.Z.; Leung, P.C.K. BMP6 Downregulates GDNF Expression through SMAD1/5 and ERK1/2 Signaling Pathways in Human Granulosa-Lutein Cells. Endocrinology 2018, 159, 2926–2938. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, Q.; Gao, X.; Du, H. Increased Bone Morphogenetic Protein-6 in Follicular Fluidand Granulosa Cells May Correlate with Fertilization and Embryo Quality in Humans. Exp. Ther. Med. 2017, 14, 1171–1176. [Google Scholar] [CrossRef]
- Choi, Y.; Rajkovic, A. Characterization of NOBOX DNA Binding Specificity and Its Regulation of Gdf9 and Pou5f1 Promoters. J. Biol. Chem. 2006, 281, 35747–35756. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C. Tissue Physiology and Pathology of Aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef]
- Pavlidi, P.; Kokras, N.; Dalla, C. Antidepressants’ Effects on Testosterone and Estrogens: What Do We Know? Eur. J. Pharmacol. 2021, 899, 173998. [Google Scholar] [CrossRef]
- Morton, A.J.; Candelaria, J.I.; McDonnell, S.P.; Zgodzay, D.P.; Denicol, A.C. Review: Roles of Follicle-Stimulating Hormone in Preantral Folliculogenesis of Domestic Animals: What Can We Learn from Model Species and Where Do We Go from Here? Animal 2023, 17 (Suppl. 1), 100743. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Patel, H. An Overview of FSH-FSHR Biology and Explaining the Existing Conundrums. J. Ovarian Res. 2021, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.-G.; Ushizawa, K.; Hosoe, M.; Takahashi, T. Differential Genome-Wide Gene Expression Profiling of Bovine Largest and Second-Largest Follicles: Identification of Genes Associated with Growth of Dominant Follicles. Reprod. Biol. Endocrinol. 2010, 8, 11. [Google Scholar] [CrossRef]
- Russell, D.L.; Robker, R.L. Molecular Mechanisms of Ovulation: Co-Ordination through the Cumulus Complex. Hum. Reprod. Update 2007, 13, 289–312. [Google Scholar] [CrossRef]
- Diaz, F.; O’BRien, M.; Wigglesworth, K.; Eppig, J. The Preantral Granulosa Cell to Cumulus Cell Transition in the Mouse Ovary: Development of Competence to Undergo Expansion. Dev. Biol. 2006, 299, 91–104. [Google Scholar] [CrossRef]
- Anderson, R.A.; Sciorio, R.; Kinnell, H.; Bayne, R.A.L.; Thong, K.J.; de Sousa, P.A.; Pickering, S. Cumulus Gene Expression as a Predictor of Human Oocyte Fertilisation, Embryo Development and Competence to Establish a Pregnancy. Reproduction 2009, 138, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Elvin, J.A.; Clark, A.T.; Wang, P.; Wolfman, N.M.; Matzuk, M.M. Paracrine Actions Of Growth Differentiation Factor-9 in the Mammalian Ovary. Mol. Endocrinol. 1999, 13, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, M.; Ponce, R.H.; Boiani, M.; Guizzardi, S.; Govoni, P.; Scandroglio, R.; Garagna, S.; Redi, C.A. The Analysis of Chromatin Organisation Allows Selection of Mouse Antral Oocytes Competent for Development to Blastocyst. Zygote 2002, 10, 73–78. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Li, M.; Luo, Y.-B.; Song, S.; Tian, D.; Yang, J.; Zhang, B.; Hou, Y.; Schatten, H.; Liu, Z.; et al. Maternal Factors Required for Oocyte Developmental Competence in Mice: Transcriptome Analysis of Non-Surrounded Nucleolus (NSN) and Surrounded Nucleolus (SN) Oocytes. Cell Cycle 2013, 12, 1928–1938. [Google Scholar] [CrossRef]
- Safrany, S.T.; Brimson, J.M. Are Fluoxetine’s Effects Due to Sigma-1 Receptor Agonism? Pharmacol. Res. 2016, 113, 707–708. [Google Scholar] [CrossRef]
- McLean, A.C.; Valenzuela, N.; Fai, S.; Bennett, S.A.L. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J. Vis. Exp. 2012, 67, e4389. [Google Scholar] [CrossRef]
- Kim, A.R.; Nodel, M.R.; Pavlenko, T.A.; Chesnokova, N.B.; Yakhno, N.N.; Ugrumov, M.V. Tear Fluid Catecholamines as Biomarkers of the Parkinson’s Disease: A Clinical and Experimental Study. Acta Naturae 2019, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Alyoshina, N.M.; Rousanova, V.R.; Malchenko, L.A.; Khramova, Y.V.; Nikishina, Y.O.; Konduktorova, V.V.; Evstifeeva, A.Y.; Nikishin, D.A. Analysis of the Ovarian Marker Genes Expression Revealed the Antagonistic Effects of Serotonin and Androstenedione on the Functional State of Mouse Granulosa Cells in Primary Culture. Russ. J. Dev. Biol. 2023, 54, 165–176. [Google Scholar] [CrossRef]
- Filatov, M.A.; Nikishin, D.A.; Khramova, Y.V.; Semenova, M.L. Reference Genes Selection for Real-Time Quantitative PCR Analysis in Mouse Germinal Vesicle Oocytes. Zygote 2019, 27, 392–397. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Filatov, M.A.; Kiseleva, M.V.; Bagaeva, T.S.; Konduktorova, V.V.; Khramova, Y.V.; Malinova, I.V.; Komarova, E.V.; Semenova, M.L. Selection of Stable Expressed Reference Genes in Native and Vitrified/Thawed Human Ovarian Tissue for Analysis by QRT-PCR and Western Blot. J. Assist. Reprod. Genet. 2018, 35, 1851–1860. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Dhillon, V.S.; Thomas, P.; Fenech, M. A Quantitative Real-Time PCR Method for Absolute Telomere Length. Biotechniques 2008, 44, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, Y.E.; Rose, R.D.; Sobinoff, A.P.; Wu, L.L.; Adhikari, D.; Zhang, Q.-H.; Wells, J.K.; Wong, L.H.; Szeto, H.H.; Piltz, S.G.; et al. Telomere Length in Offspring Is Determined by Mitochondrial-Nuclear Communication at Fertilization. Nat. Commun. 2025, 16, 2527. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | NCBI Gene ID | Forward Primer | Tann, °C | Reverse Primer | Tann, °C |
|---|---|---|---|---|---|
| Bmp6 | 12161 | ACCGTACTTTGTGGCAGAGC | 68.3 | GAAAAGGCAAAGAGCAGAGTTAG | 66.4 |
| Bmp15 | 12155 | GAATCTGATGCCTCTTGTCCTT | 69.5 | ATGGCATGGTTGGGTGAAT | 63.2 |
| Cyp11a1 | 13070 | GCCTGGAGCCATCAAGAACT | 70.3 | GAAAAGCGGAATAGGTCATCACT | 66.4 |
| Cyp17a1 | 13074 | CGGTGGCCCCCTTGCTCA | 81.6 | GGCTGGTCCCATTCATTTTTATCGTG | 69.7 |
| Cyp19a1 | 13075 | TCTCCTCATCAAACCAAACATCTTCT | 69.1 | CAGTTGCAAAATCCATACAGTCTTCC | 69.7 |
| Fshb | 14308 | TCGCCCACCCTTGTCCT | 74.4 | CTGGCCCTGGCACTCCTA | 66.8 |
| Fshr | 14309 | TGCTACACCCACATCTACCTCACA | 70.3 | GGATCTTGGCCTTGGACACAGT | 69.5 |
| Gdf9 | 14566 | GCCTCCCCGACCTTTAGA | 71.5 | TGCCTCAGACTCCACATTTTC | 65.0 |
| Has2 | 15117 | GCGGAAGAAGGGACAACA | 69.3 | TGCGGTGCCACAATACTG | 64.5 |
| Igf1 | 16000 | GACCGAGGGGCTTTTACTTCAACA | 73.9 | GGCGCTGGGCACGGATAG | 71.3 |
| Lhb | 16866 | TGGCCGCAGAGAATGAGTT | 68.9 | TGAGGGCTACAGGAAAGGAGAC | 73.2 |
| Lhgr | 16867 | CTCTCACCTATCTCCCTGTCAAAGTAA | 69.4 | TGTAAAAGCACCGGGTTCAATGT | 68.2 |
| Ptgs2 | 19225 | CCCTCCGGTGTTTGTCCTT | 67.5 | CCTGCAGCATTTTTCATCTTGTA | 64.6 |
| Rps18 | 20084 | AAGAAAATTCGAGCCCATAGAGG | 67.8 | TAACAGCAAAGGCCCAGAGACT | 69.5 |
| Rn18s | 19791 | GACTCAACACGGGAAACCTCA | 71.8 | CAAATCGCTCCACCAACTAAGA | 67.6 |
| Sert (Slc6a4) | 15567 | GGGAGACCTGGGGCAAGAAG | 74.8 | CAGGGCGAGCTCCATGTAGAAGA | 71.8 |
| Star | 20845 | GCCCACTTTTCTGTCCCTTAT | 68.9 | CTGCCCTCGCTCACCTTA | 64.5 |
| Tbp | 21374 | GTAGCGGTGGCGGGTATCT | 71.3 | CGTCTTCAATGTTCTGGGTTATCT | 67.0 |
| Tel | - | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT | 76.2 | GGCTTGCCTTACCCTTACCCTT ACCCTTACCCTTACCCT | 80.0 |
| Antibody | Manufacturer | Catalogue Number | Dilution |
|---|---|---|---|
| Primary antibodies | |||
| Anti-β-actin, mouse | Sigma-Aldrich, St. Louis, MO, USA | A5441 | 1:10,000 |
| Anti-Cyp19a1, rabbit | Abcam, Cambridge, Cambridgeshire, UK | ab18995 | 1:1000 |
| Anti-Gdf9, goat | Thermo Fisher Scientific, Waltham, MA, USA | PA5-47924 | 1:200 |
| Anti-Hsp90, rabbit | Sigma-Aldrich, St. Louis, MO, USA | SAB4300541 | 1:5000 |
| Anti-Sert, goat | Abcam, Cambridge, Cambridgeshire, UK | ab130130 | 1:5000 |
| Secondary antibodies | |||
| Horseradish peroxidase, IgG donkey, against goat | Sigma-Aldrich, St. Louis, MO, USA | SAB3700284 | 1:50,000 |
| Horseradish peroxidase, IgG goat, against mouse | Jackson ImmunoResearch Labs, West Grove, PA, USA | 115-035-003 | 1:50,000 |
| Horseradish peroxidase, IgG goat, against rabbit | Jackson ImmunoResearch Labs, West Grove, PA, USA | 111-035-003 | 1:50,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyoshina, N.M.; Beketova, M.V.; Tkachenko, M.D.; Nikishina, Y.O.; Frolova, V.S.; Malchenko, L.A.; Semenova, M.L.; Rubtsova, M.P.; Nikishin, D.A. Fluoxetine Disrupts Ovarian Serotonin Signaling and Oocyte Competence in Mice. Pharmaceuticals 2025, 18, 1647. https://doi.org/10.3390/ph18111647
Alyoshina NM, Beketova MV, Tkachenko MD, Nikishina YO, Frolova VS, Malchenko LA, Semenova ML, Rubtsova MP, Nikishin DA. Fluoxetine Disrupts Ovarian Serotonin Signaling and Oocyte Competence in Mice. Pharmaceuticals. 2025; 18(11):1647. https://doi.org/10.3390/ph18111647
Chicago/Turabian StyleAlyoshina, Nina M., Maria V. Beketova, Maria D. Tkachenko, Yulia O. Nikishina, Veronika S. Frolova, Lyudmila A. Malchenko, Maria L. Semenova, Maria P. Rubtsova, and Denis A. Nikishin. 2025. "Fluoxetine Disrupts Ovarian Serotonin Signaling and Oocyte Competence in Mice" Pharmaceuticals 18, no. 11: 1647. https://doi.org/10.3390/ph18111647
APA StyleAlyoshina, N. M., Beketova, M. V., Tkachenko, M. D., Nikishina, Y. O., Frolova, V. S., Malchenko, L. A., Semenova, M. L., Rubtsova, M. P., & Nikishin, D. A. (2025). Fluoxetine Disrupts Ovarian Serotonin Signaling and Oocyte Competence in Mice. Pharmaceuticals, 18(11), 1647. https://doi.org/10.3390/ph18111647





