Reversing the Warburg Effect: YW3-56 Induces Leukemia Differentiation via AKT-Mediated Glucose Metabolic Reprogramming
Abstract
1. Introduction
2. Results
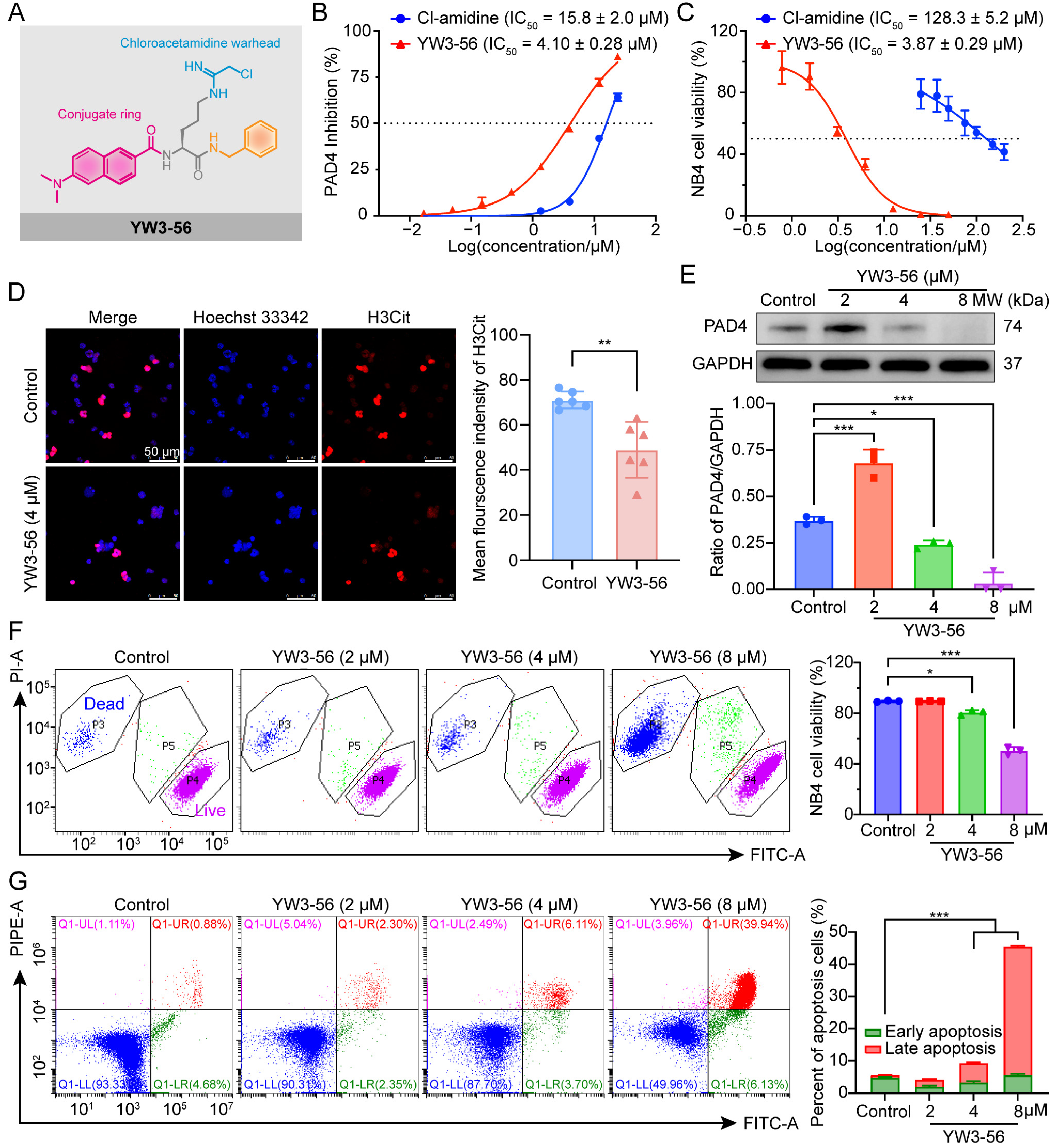
2.1. The PAD4 Inhibitor YW3-56 Suppresses Cell Viability in APL Cells
2.2. YW3-56 Induces Dose-Dependent Apoptosis in APL Cells
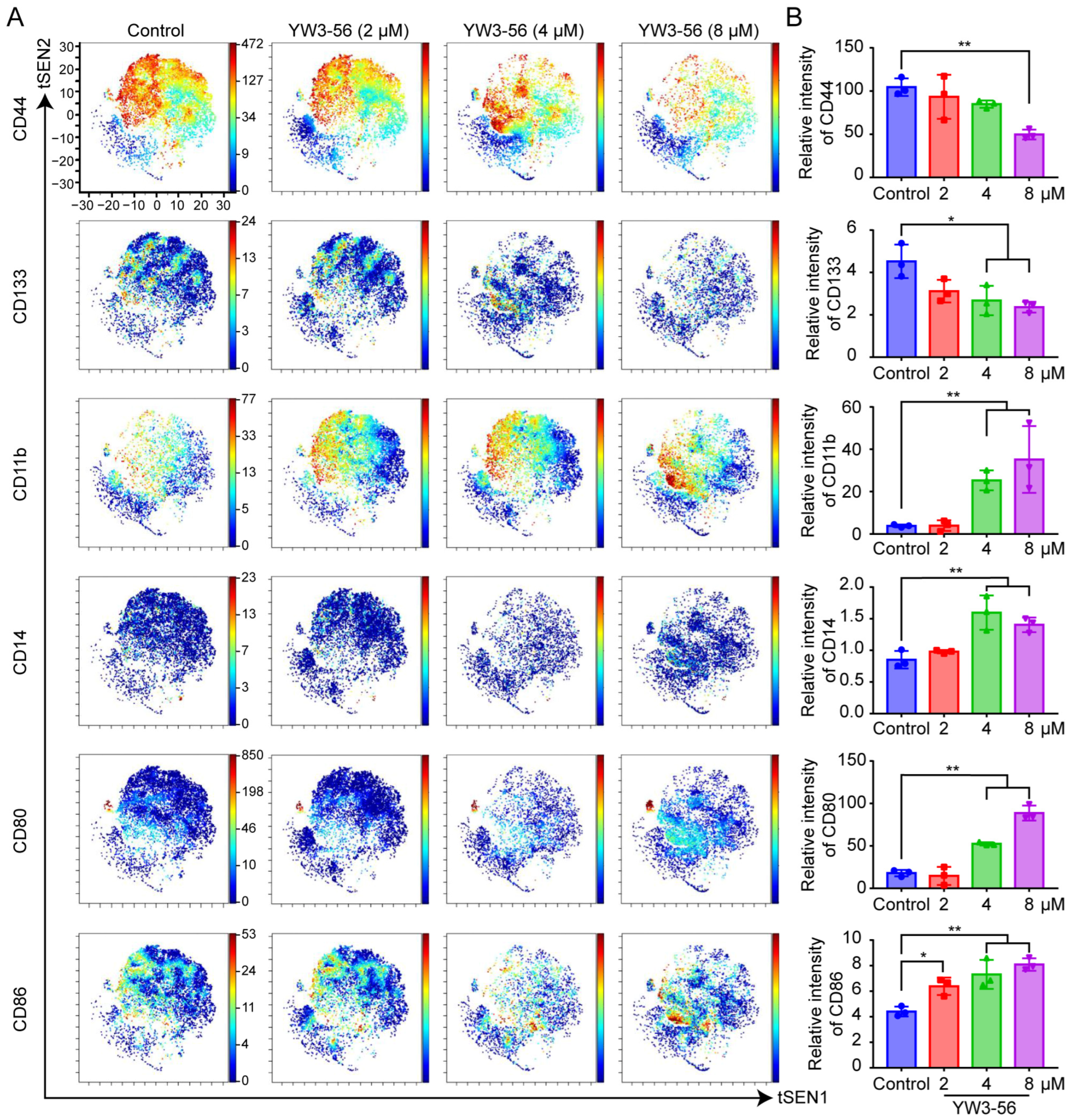
2.3. Mechanistic Insights into PAD4 Inhibitor-Induced Differentiation and Immune Activation
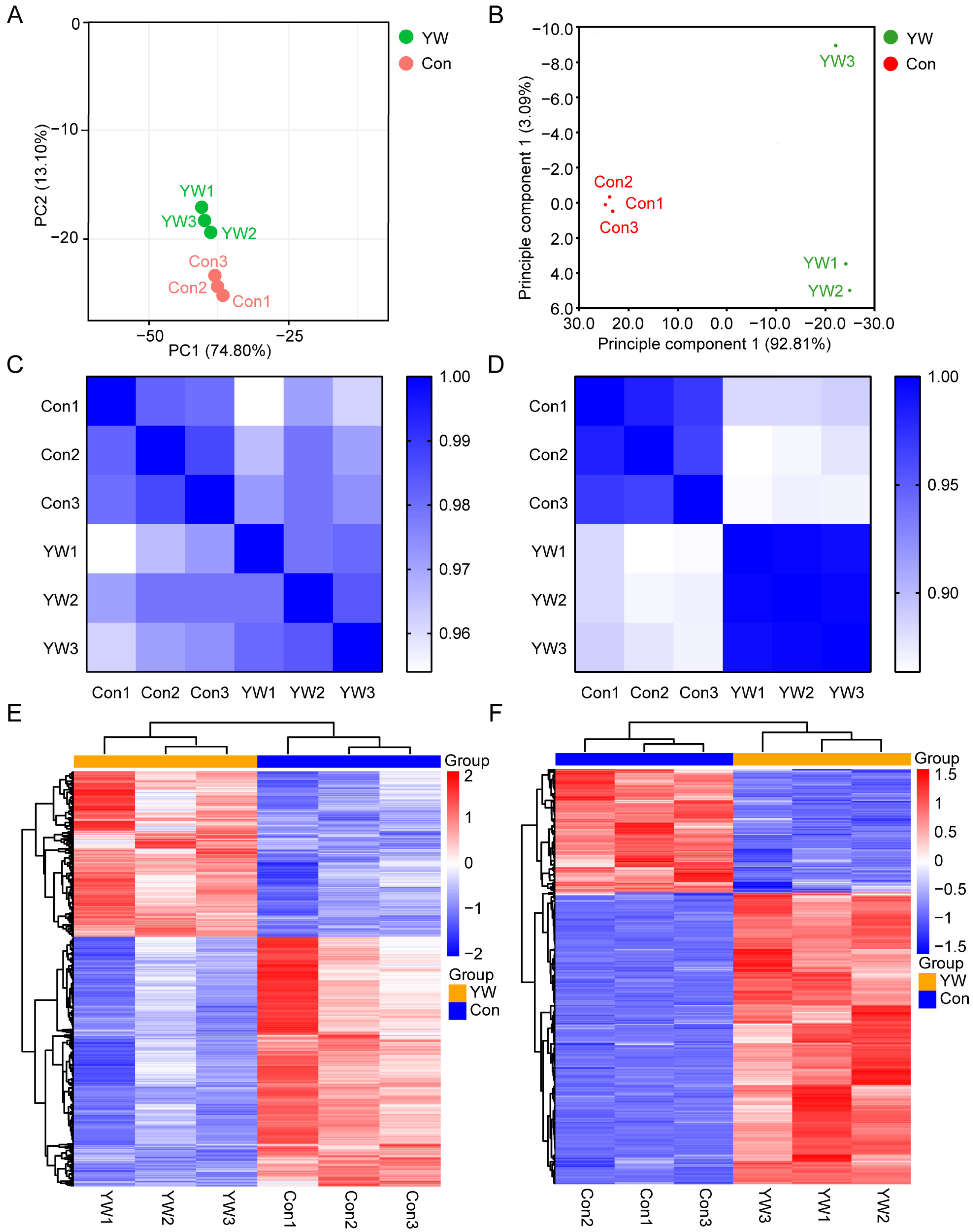
2.4. Quality Control and Correlation Analysis of Transcriptomes and Proteomes
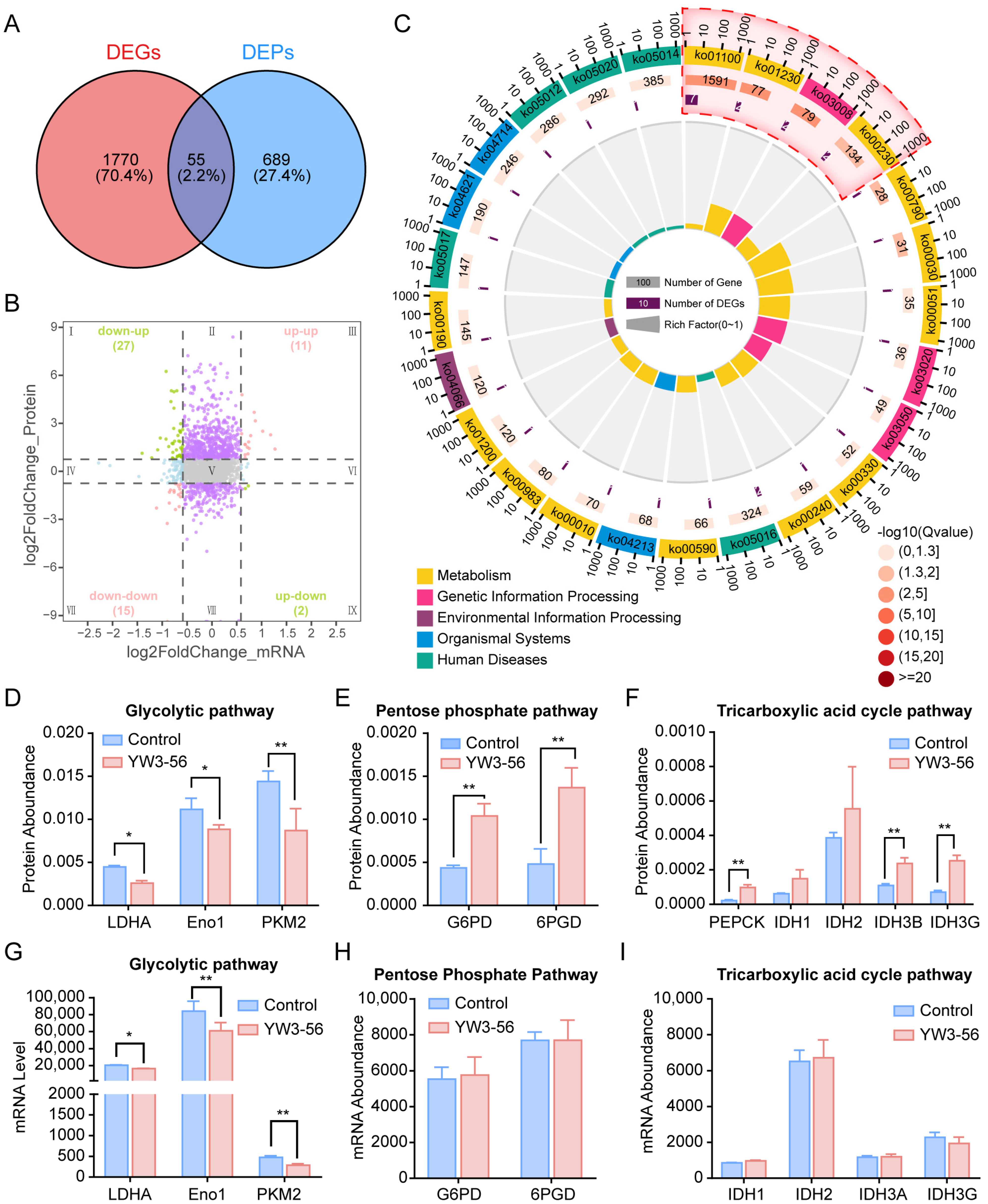
2.5. Integrated Multiomics Analysis Revealed That YW3-56 Reprograms Glucose Metabolism
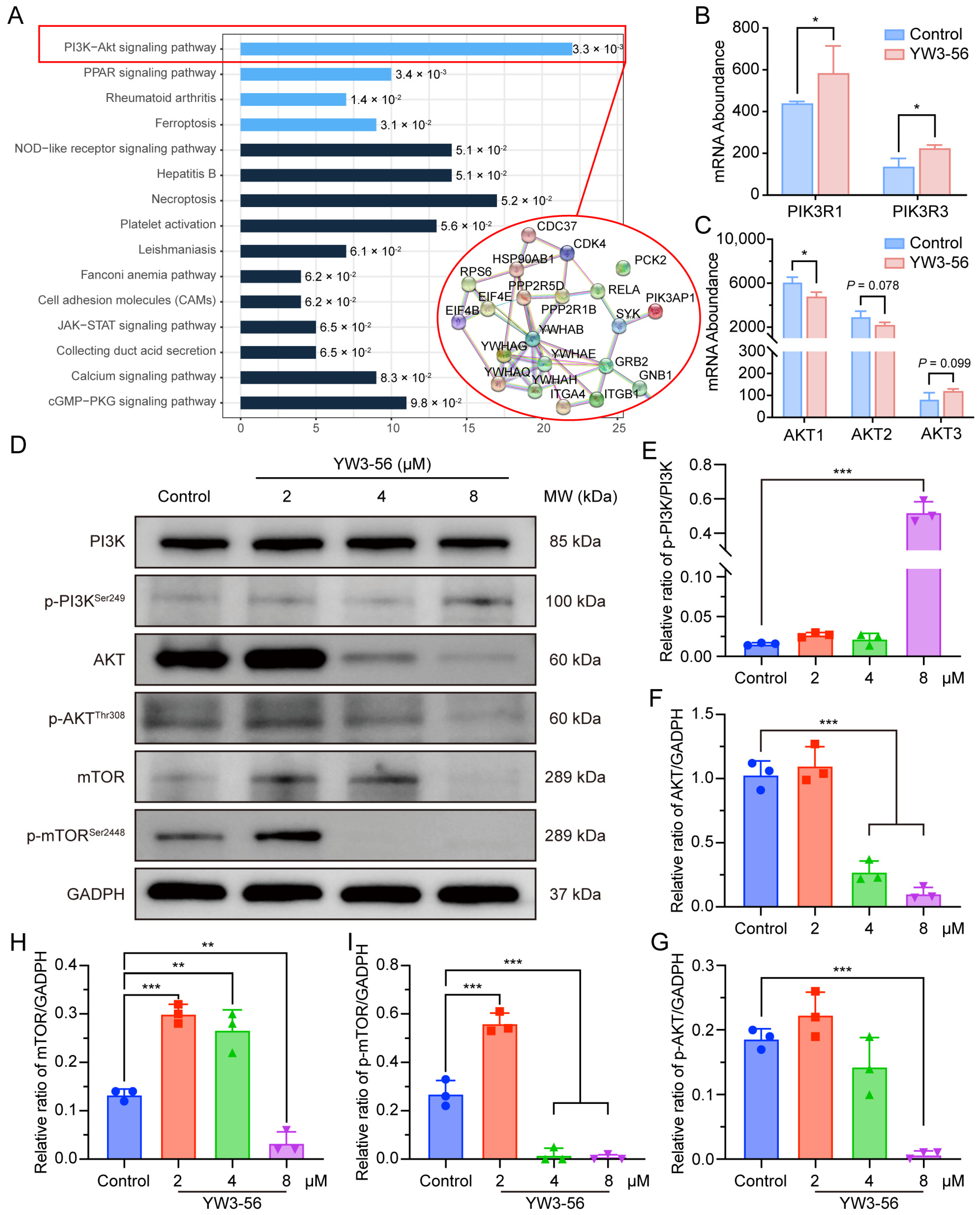
2.6. YW3-56 Orchestrates Differentiation and Glucose Metabolism Through PI3K-AKT-mTOR Axis Modulation
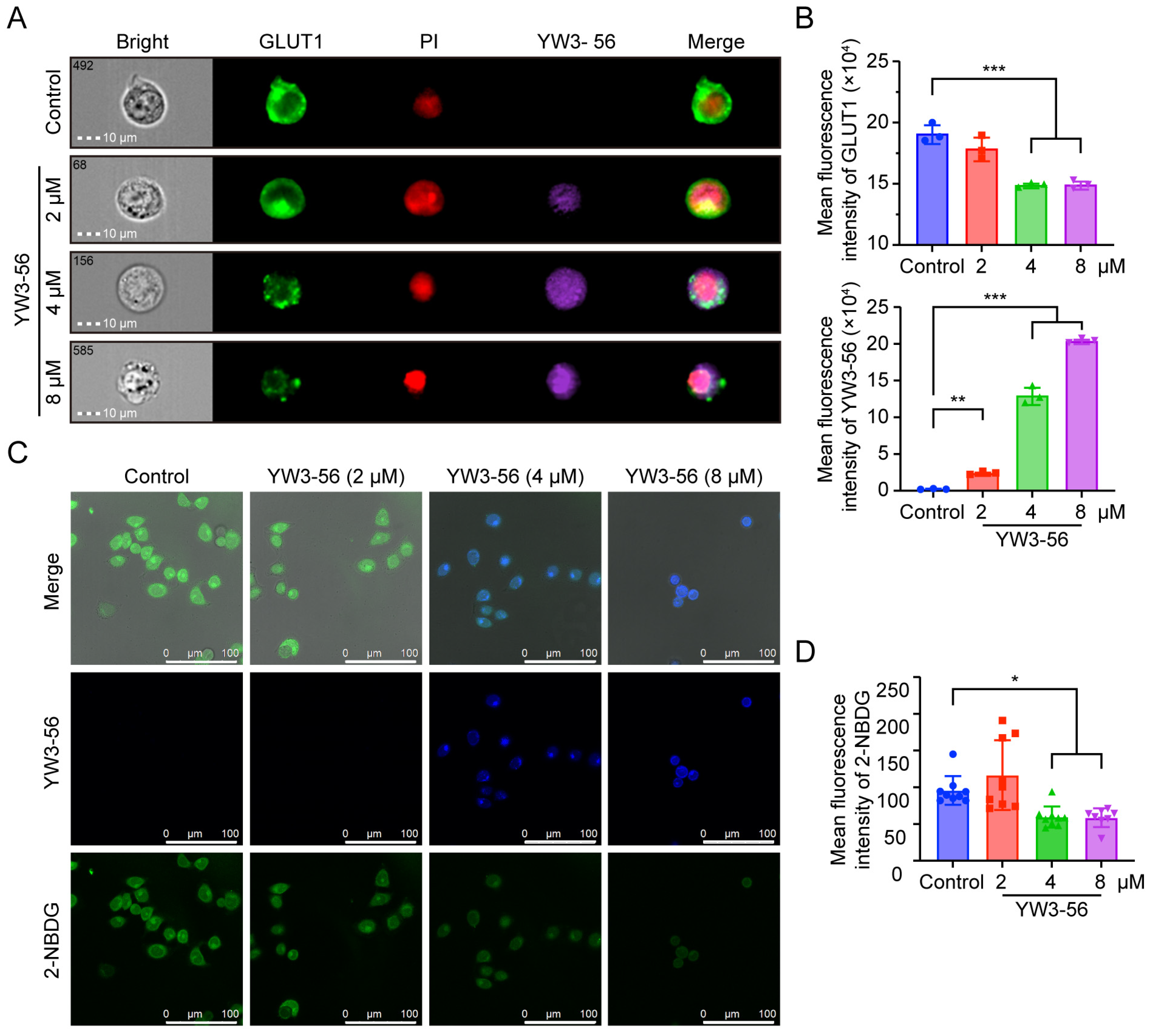
2.7. YW3-56 Disrupts Glucose Uptake via GLUT1 Downregulation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. PAD4 Inhibition
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Live/Dead Cell Assay
4.6. Cell Apoptosis Assay
4.7. Cellular H3Cit Immunofluorescence Assay
4.8. Western Blot Analysis
4.9. Mass Cytometry
4.10. Maturation-Related Cell Surface Differentiation Antigen Expression Assay
4.11. RNA and Protein Preparation
4.12. GLUT1 Expression Assay
4.13. 2-NBDG Glucose Uptake Assay
4.14. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-NBDG | 2-NBD-Glucose |
| 6PGD | 6-phosphogluconate dehydrogenase |
| AKT | Alpha serine/threonine-protein kinase |
| APL | Acute promyelocytic leukemia |
| ATRA | All-trans-retinoic acid |
| ATO | Arsenic trioxide |
| DEGs | differentially expressed genes |
| DEPs | differentially expressed proteins |
| DMSO | Dimethyl sulfoxide |
| Eno1 | Enolase 1 |
| G6PD | Glucose-6-phosphate 1-dehydrogenase |
| GLUT1 | Glucose transporter 1 |
| H3Cit | Histone 3 citrullination |
| IDH3B | Isocitrate dehydrogenase [NAD] subunit beta |
| IDH3G | Isocitrate dehydrogenase [NAD] subunit gamma |
| LDHA | Lactate Dehydrogenase A |
| LSCs | Leukemia stem cells |
| mTOR | Mechanistic target of rapamycin |
| NET | Neutrophil extracellular trap |
| PAD4 | Protein arginine deiminase 4 |
| PCA | Principal component analysis |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PI3K | Phosphatidylinositol 3-kinase |
| PKM2 | Pyruvate kinase M2 |
| TCA | Tricarboxylic acid |
References
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Chale, R.S.; Abad, A.C.; Schally, A.V. Acute promyelocytic leukemia (APL): A review of the literature. Oncotarget 2020, 11, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.D.; Evangelista, F.C.G.; Sabino, A.d.P. Acute Promyelocytic Leukemia (APL): A Review of the Classic and Emerging Target Therapies towards Molecular Heterogeneity. Future Pharmacol. 2023, 3, 162–179. [Google Scholar] [CrossRef]
- Molinaro, A.; Zanta, D.; Moleti, M.L.; Giona, F.; Conter, V.; Rizzari, C.; Biondi, A.; Testi, A.M. Challenging Management of Severe Differentiation Syndrome in Pediatric Acute Promyelocytic Leukemia Treated with ATRA/ATO. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022027. [Google Scholar] [CrossRef]
- Estey, E.H. Acute myeloid leukemia: 2025 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Wang, S.; Hu, J.; Chen, X.A.; Wu, J.; Fisher, M.; Oshaben, K.; Zhao, N.; Gu, Y.; et al. Anticancer peptidylarginine deiminase (PAD) inhibitors regulate the autophagy flux and the mammalian target of rapamycin complex 1 activity. J. Biol. Chem. 2012, 287, 25941–25953. [Google Scholar] [CrossRef]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef]
- Laridan, E.; Martinod, K.; De Meyer, S. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef]
- Liu, X.; Arfman, T.; Wichapong, K.; Reutelingsperger, C.P.M.; Voorberg, J.; Nicolaes, G.A.F. PAD4 takes charge during neutrophil activation: Impact of PAD4 mediated NET formation on immune-mediated disease. J. Thromb. Haemost. 2021, 19, 1607–1617. [Google Scholar] [CrossRef]
- Jung, H.S.; Gu, J.; Kim, J.E.; Nam, Y.; Song, J.W.; Kim, H.K. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE 2019, 14, e216055. [Google Scholar] [CrossRef]
- Zhu, D.; Lu, Y.; Wang, Y.; Wang, Y. PAD4 and Its Inhibitors in Cancer Progression and Prognosis. Pharmaceutics 2022, 14, 2414. [Google Scholar] [CrossRef]
- Song, G.; Shi, L.; Guo, Y.; Yu, L.; Wang, L.; Zhang, X.; Li, L.; Han, Y.; Ren, X.; Guo, Q.; et al. A novel PAD4/SOX4/PU.1 signaling pathway is involved in the committed differentiation of acute promyelocytic leukemia cells into granulocytic cells. Oncotarget 2016, 7, 3144–3157. [Google Scholar]
- Zhu, D.; Zhang, Y.; Wang, S. Histone citrullination: A new target for tumors. Mol. Cancer 2021, 20, 17–90. [Google Scholar] [CrossRef]
- Slack, J.L.; Causey, C.P.; Thompson, P.R. Protein arginine deiminase 4: A target for an epigenetic cancer therapy. Cell Mol. Life Sci. 2011, 68, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Ghanavat, M.; Shahrouzian, M.; Deris Zayeri, Z.; Banihashemi, S.; Kazemi, S.M.; Saki, N. Digging deeper through glucose metabolism and its regulators in cancer and metastasis. Life Sci. 2021, 264, 118603. [Google Scholar] [CrossRef]
- Nong, Q.W.; Zhang, C.; Liu, Q.H.; Xie, R.; Dong, M. Effect of daunorubicin on acute promyelocytic leukemia cells using nuclear magnetic resonance spectroscopy-based metabolomics. Environ. Toxicol. Pharmacol. 2020, 78, 103382. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Wang, J.H.; Zhao, A.H.; Xu, X.; Wang, Y.H.; Chen, T.L.; Li, J.M.; Mi, J.Q.; Zhu, Y.M.; Liu, Y.F.; et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 2014, 124, 1645–1654. [Google Scholar] [CrossRef]
- Mishra, S.K.; Millman, S.E.; Zhang, L. Metabolism in acute myeloid leukemia: Mechanistic insights and therapeutic targets. Blood 2023, 141, 1119–1135. [Google Scholar] [CrossRef]
- Kreitz, J.; Schönfeld, C.; Seibert, M.; Stolp, V.; Alshamleh, I.; Oellerich, T.; Steffen, B.; Schwalbe, H.; Schnütgen, F.; Kurrle, N.; et al. Metabolic Plasticity of Acute Myeloid Leukemia. Cells 2019, 8, 805. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Wen, G.M.; Xu, X.Y.; Xia, P. Metabolism in Cancer Stem Cells: Targets for Clinical Treatment. Cells 2022, 11, 3790. [Google Scholar] [CrossRef]
- Sebastian, C. Tracking down the origin of cancer: Metabolic reprogramming as a driver of stemness and tumorigenesis. Crit. Rev. Oncog. 2014, 19, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Lu, Y.; Yan, Z.; Deng, Q.; Hu, B.; Wang, Y.; Wang, W.; Wang, Y.; Wang, Y. A β-Carboline Derivate PAD4 Inhibitor Reshapes Neutrophil Phenotype and Improves the Tumor Immune Microenvironment against Triple-Negative Breast Cancer. J. Med. Chem. 2024, 67, 7973–7994. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; Sultan, M.; Vidovic, D.; Dean, C.A.; Cruickshank, B.M.; Lee, K.; Loung, C.Y.; Holloway, R.W.; Hoskin, D.W.; Waisman, D.M.; et al. Retinoic acid and arsenic trioxide induce lasting differentiation and demethylation of target genes in APL cells. Sci. Rep. 2019, 9, 9414. [Google Scholar] [CrossRef]
- Chen, H.; Wei, L.; Luo, M.; Wang, X.; Zhu, C.; Huang, H.; Liu, X.; Lu, H.; Zhong, Y. LINC00324 suppresses apoptosis and autophagy in nasopharyngeal carcinoma through upregulation of PAD4 and activation of the PI3K/AKT signaling pathway. Cell Biol. Toxicol. 2022, 38, 995–1011. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, H. Biomarkers and targeted therapy for cancer stem cells. Trends Pharmacol. Sci. 2024, 45, 56–66. [Google Scholar]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar]
- Trzupek, D.; Dunstan, M.; Cutler, A.J.; Lee, M.; Godfrey, L.; Jarvis, L.; Rainbow, D.B.; Aschenbrenner, D.; Jones, J.L.; Uhlig, H.H.; et al. Discovery of CD80 and CD86 as recent activation markers on regulatory T cells by protein-RNA single-cell analysis. Genome Med. 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Waters, E.; Rowshanravan, B.; Hinze, C.; Williams, C.; Janman, D.; Fox, T.A.; Booth, C.; Pesenacker, A.M.; Halliday, N.; et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat. Immunol. 2022, 23, 1365–1378. [Google Scholar] [CrossRef]
- Sefah, K.; Tang, Z.W.; Shangguan, D.H.; Chen, H.; Lopez-Colon, D.; Li, Y.; Parekh, P.; Martin, J.; Meng, L.; Phillips, J.A.; et al. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia 2009, 23, 235–244. [Google Scholar] [CrossRef]
- Liu, W.; Li, T.; Wang, P.; Liu, W.; Liu, F.; Mo, X.; Liu, Z.; Song, Q.; Lv, P.; Ruan, G.; et al. LRRC25 plays a key role in all-trans retinoic acid-induced granulocytic differentiation as a novel potential leukocyte differentiation antigen. Protein Cell 2018, 9, 785–798. [Google Scholar] [CrossRef]
- Murai, T.; Matsuda, S. Targeting the PI3K-AKT-mTOR signaling pathway involved in vasculogenic mimicry promoted by cancer stem cells. Am. J. Cancer Res. 2023, 13, 5039–5046. [Google Scholar] [PubMed]
- Karami Fath, M.; Ebrahimi, M.; Nourbakhsh, E.; Zia Hazara, A.; Mirzaei, A.; Shafieyari, S.; Salehi, A.; Hoseinzadeh, M.; Payandeh, Z.; Barati, G. PI3K/AKT/mTOR signaling pathway in cancer stem cells. Pathol. Res. Pract. 2022, 237, 154010. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/AKT/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, A.; Houde, V.P.; Bellmann, K.; Marette, A. Chronic inhibition of the mTORC1/S6K1 pathway increases insulin-induced PI3K activity but inhibits Akt2 and glucose transport stimulation in 3T3-L1 adipocytes. Mol. Endocrinol. 2010, 24, 766–778. [Google Scholar] [CrossRef]
- Um, S.H.; D’Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006, 3, 393–402. [Google Scholar] [CrossRef]
- Saito, Y.; Chapple, R.H.; Lin, A.; Kitano, A.; Nakada, D. AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell 2015, 17, 585–596. [Google Scholar] [CrossRef]
- Bukkuri, A.; Gatenby, R.A.; Brown, J.S. GLUT1 production in cancer cells: A tragedy of the commons. npj Syst. Biol. Appl. 2022, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, X.; Luan, H.; Li, L.; Dai, W.; Li, Z.; Bian, J. The progress and development of GLUT1 inhibitors targeting cancer energy metabolism. Future Med. Chem. 2019, 11, 2333–2352. [Google Scholar] [CrossRef]
- Beg, M.; Abdullah, N.; Thowfeik, F.S.; Altorki, N.K.; McGraw, T.E. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. eLife 2017, 6, e26896. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpoor, M.; Hedayati, M. Regulators of glucose uptake in thyroid cancer cell lines. Cell Commun. Signal. 2020, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.S.; Wilkinson, S.; James, J.; Ryan, K.M.; Vousden, K.H. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009, 16, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thomas, H.R.; Li, Z.; Yeo, N.C.F.; Scott, H.E.; Dang, N.; Hossain, M.I.; Andrabi, S.A.; Parant, J.M. Puma, noxa, p53, and p63 differentially mediate stress pathway induced apoptosis. Cell Death Dis. 2021, 12, 659. [Google Scholar] [CrossRef]
- Koo, K.Y.; Moon, K.; Song, H.S.; Lee, M.S. Metabolic regulation by p53: Implications for cancer therapy. Mol. Cells 2025, 48, 100198. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Zabala, M.; Ramakrishnan, R.; Reinbach, K.; Ghosh, S.; Oburoglu, L.; Falqués-Costa, A.; Bellamkonda, K.; Ehinger, M.; Peña-Martínez, P.; Puente-Moncada, N.; et al. Combined GLUT1 and OXPHOS inhibition eliminates acute myeloid leukemia cells by restraining their metabolic plasticity. Blood Adv. 2023, 7, 5382–5395. [Google Scholar] [CrossRef]
- Åbacka, H.; Hansen, J.S.; Huang, P.; Venskutonytė, R.; Hyrenius-Wittsten, A.; Poli, G.; Tuccinardi, T.; Granchi, C.; Minutolo, F.; Hagström-Andersson, A.K.; et al. Targeting GLUT1 in acute myeloid leukemia to overcome cytarabine resistance. Haematologica 2021, 106, 1163–1166. [Google Scholar]
- Ciesielski, O.; Pirola, L.; Balcerczyk, A. Peptidylarginine Deiminases Inhibitors Decrease Endothelial Cells Angiogenic Potential by Affecting Akt Signaling and the Expression and Secretion of Angiogenic Factors. Cell Physiol. Biochem. 2024, 58, 63–82. [Google Scholar] [CrossRef]
- Schaefer, T.; Steiner, R.; Lengerke, C. SOX2 and p53 Expression Control Converges in PI3K/AKT Signaling with Versatile Implications for Stemness and Cancer. Int. J. Mol. Sci. 2020, 21, 4902. [Google Scholar] [CrossRef]
- Singh, B.; Reddy, P.G.; Goberdhan, A.; Walsh, C.; Dao, S.; Ngai, I.; Chou, T.C.; O-Charoenrat, P.; Levine, A.J.; Rao, P.H.; et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002, 16, 984–993. [Google Scholar] [CrossRef] [PubMed]
| Sample | Raw Reads | Clean Reads | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|
| control_1 | 40,956,314 | 39,955,562 | 0.03 | 97.27 | 92.33 | 53.04 |
| control_2 | 47,137,690 | 45,968,034 | 0.03 | 97.37 | 92.53 | 52.69 |
| control_3 | 46,282,384 | 44,900,684 | 0.03 | 97.35 | 92.46 | 52.34 |
| YW3-56_1 | 45,422,734 | 44,242,556 | 0.03 | 97.44 | 92.66 | 51.56 |
| YW3-56_2 | 46,369,202 | 45,278,048 | 0.03 | 97.46 | 92.74 | 52.08 |
| YW3-56_3 | 45,600,868 | 44,619,054 | 0.03 | 97.23 | 92.27 | 51.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Gao, D.; Lu, Y.; Chen, N.; Zhang, L.; Zhang, L.; Wang, Y. Reversing the Warburg Effect: YW3-56 Induces Leukemia Differentiation via AKT-Mediated Glucose Metabolic Reprogramming. Pharmaceuticals 2025, 18, 1646. https://doi.org/10.3390/ph18111646
Zhu D, Gao D, Lu Y, Chen N, Zhang L, Zhang L, Wang Y. Reversing the Warburg Effect: YW3-56 Induces Leukemia Differentiation via AKT-Mediated Glucose Metabolic Reprogramming. Pharmaceuticals. 2025; 18(11):1646. https://doi.org/10.3390/ph18111646
Chicago/Turabian StyleZhu, Di, Dan Gao, Yu Lu, Na Chen, Li Zhang, Lan Zhang, and Yuji Wang. 2025. "Reversing the Warburg Effect: YW3-56 Induces Leukemia Differentiation via AKT-Mediated Glucose Metabolic Reprogramming" Pharmaceuticals 18, no. 11: 1646. https://doi.org/10.3390/ph18111646
APA StyleZhu, D., Gao, D., Lu, Y., Chen, N., Zhang, L., Zhang, L., & Wang, Y. (2025). Reversing the Warburg Effect: YW3-56 Induces Leukemia Differentiation via AKT-Mediated Glucose Metabolic Reprogramming. Pharmaceuticals, 18(11), 1646. https://doi.org/10.3390/ph18111646







