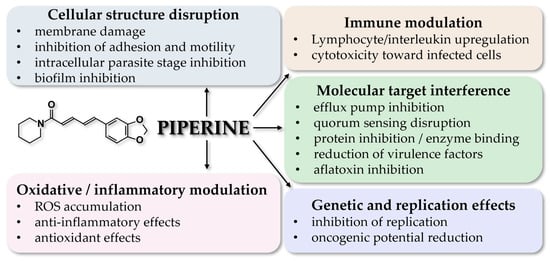
Abstract
Piperine is an alkaloid found in plants of the genus Piper, and particularly in P. nigrum. This compound has been under extensive research lately for its antimicrobial, antiviral, and also anti-inflammatory, anti-oxidant, anticancer, and positive metabolic properties. Regarding its antibacterial applications, current data show that piperine is effective against Bacillus sphaericus, Bacterioides fragilis, Escherichia coli, Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus mutans, Pseudomonas aeruginosa, and Vibrio cholerae; its antifungal potency is exerted against Candida albicans and members of the Aspergillus family; antiviral activity has been documented against MERS-CoV, SARS-CoV2, EBOV, DENV, HCV, ZKV, and HPIV; and antiparasitic activity against Leishmania spp., Plasmodium spp., Trichomonas vaginalis, and Trypanosoma spp. While such applications are promising, more research is required to elucidate the mechanisms of action and to discover new ways of administration.
Keywords:
black pepper; piperine; antibacterial; antifungal; antiparasitic; antiviral; traditional medicine 1. Introduction
The World Health Organization (WHO) reports a continued increase in antimicrobial resistance in recent years [1]. Novel antimicrobial agents are necessary to counter this emerging trend [2]; at the same time, there is also a recognized increasing difficulty in the treatment of certain viral infections [3].
While various applications for phytochemicals have been described, numerous research efforts are currently centered around the effectiveness of plant-derived substances in their antimicrobial and antiviral roles [4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Taking advantage of relevant research in ethnobotany, it is hoped that by examining and verifying reported traditional uses of plants, plant-derived substances can be used to increase the effectiveness of current antimicrobial and antiviral treatments or even become, sometime in the future, frontline treatments of their own.
Piperine, the focus of this review, is predominantly extracted from black pepper (Piper nigrum) of the Piperaceae family, one of the most ancient plant families in tropical regions (Figure 1) [18]. There are other plants of this family like Piper longum [19] which contain piperine, but P. nigrum yields the highest concentration.
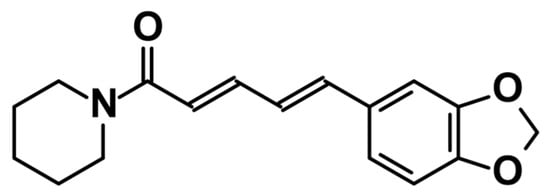
Figure 1.
Chemical structure of piperine.
There are numerous ethnomedical uses of black pepper reported and a lot of research has also been performed, using modern methods, on the bioactivity and properties of its extracts [19]. Piperine, along with some volatile substances, is mostly responsible for the distinctive flavor, spiciness, and pungency of black pepper [20]. Piperine itself, perhaps the most important bioactive compound of black peppercorns, is characterized by anti-inflammatory, anticancer, anti-oxidant, analgesic, antidiabetic, and antilipidemic properties, among others [21]. The essential oil of black pepper has a number of health-related beneficial effects [22,23], while some volatile components exhibit insecticidal properties [24,25].
This review provides an integrative and comparative synthesis of piperine’s antimicrobial, antifungal, antiviral, and antiparasitic properties, bridging data from in vitro, in silico, and limited in vivo studies. The novelty of our paper is the approach of covering all pathogen classes and thoroughly analyzing effective concentrations, mechanisms of action, and translational barriers, including bioavailability and toxicity. The manuscript offers a critical, evidence-based perspective that connects traditional use of Piper nigrum with modern pharmacological validation, highlighting piperine’s potential as a broad-spectrum antimicrobial candidate.
2. Antibacterial Properties of Piperine
There is a recent scientific endeavor looking into the antibacterial potential of plant-derived compounds and metabolites [26]. A number of research efforts have been carried out focusing on piperine (Table 1).

Table 1.
Antimicrobial properties of piperine, based on current research evidence, classified alphabetically by family.
2.1. Antibacterial Activity Against Bacillus spp.
These bacteria are rarely pathogenic, with a few exceptions [39]. Piper-longuminine, a chemical isolate from Piper longum, and piperine were shown to be harmful to this bacteria, exhibiting a minimum inhibitory concentration (MIC) of 25 mg/mL [27].
2.2. Antibacterial Activity Against Bacterioides fragilis
This bacterium is one of the many colonizers of the human gut, but a few strains are implicated in colorectal cancer [40]; on the other hand, it is implicated in potentially severe extraintestinal infections [40]. Piperine was found to have inhibitory action against Bacteroides fragilis at concentrations of 0.10 mg/mL [28].
2.3. Antibacterial Activity Against Escherichia coli
Escherichia coli is a physiological colonizer of the gastrointestinal tract which may become pathogenic in immunocompromised hosts [41]. The most frequent E. coli infections are those of the urogenital and gastrointestinal tract, while in neonates it can cause meningitis [42].
To ascertain the effectiveness of piperine against this pathogen, and its potential interaction with antibiotics, Dusane et al. [29] cultured uropathological species of E. coli. Piperine by itself did not show promising results in inhibiting the growth of bacteria, unless found in relatively high concentrations of 50 μg/mL. In lower concentrations however, piperine was found to decrease the expression of the genes that create the bacterial flagella; it enabled ciprofloxacin and azithromycin to more easily penetrate the created biofilm and inhibit bacterial growth [29].
2.4. Antibacterial Activity Against Helicobacter pylori
Helicobacter pylori is a common bacterium associated with gastric cancer, gastritis, and ulcers, while natural compounds may help reduce the risk of these conditions [30,31]. Another research effort, this time by Tharmalingam et al. [30], found that at an IC50 of 115 μΜ, piperine suppressed H. pylori adhesion to gastric adenocarcinoma cells and the expression of the flagellar flhA and flgE genes, thus reducing motility. It was also observed that treatment of gastric cells with piperine restrained the entry of certain Helicobacter virulence factors into cells, decreased its adhesion potential to cells, and reduced oncogenesis potential via diminished β-catenin translocation into the cell nucleus [31]. While the MIC was higher than the toxicity limit for the given cell type, sub-MICs were effective against the bacterium. Finally, based on the research of Toyoda et al. [32], piperine was found to reduce the expression of interleukin 1β, interferon γ, and interleukin 6, along with that of inducible nitrogen oxide synthase (iNOS).
2.5. Antibacterial Activity Against Mycobacterium tuberculosis
M. tuberculosis is the most well-known pathogen of this genus, being the causative agent of tuberculosis [43]. Despite the existence of a vaccine and individualized antibiotic treatment schemes, there has been an emergence of multi-resistant [44], extremely resistant, and total-resistant strains, which are predicted to be a cause of mortality for millions of people in the next decades [45].
Cell-mediated immunity, where Th1 lymphocytes have a key role, is mostly implicated in defense against M. tuberculosis infections. Based on that, the research team of Sharma et al. [33] revealed that piperine exhibited an important increase in immune response by inducing Th1 lymphocyte production. This effect was exerted at a 1 μg/mL dose of piperine while higher doses showed negative effects on the proliferation of lymphocytes. On the contrary, both 1 and 10 μg/mL of piperine were proven to upregulate interferon-γ and interleukin-2 in a dose-dependent manner. The potential of Piper nigrum as a treatment at least for some symptoms of tuberculosis has also been mentioned by Mohamad et al. [46].
2.6. Antibacterial Activity Against Pseudomonas aeruginosa
P. aeruginosa is an opportunistic human pathogen, which can cause both localized and systemic infections [47] and is a causative agent of nosocomial infections [48]. In certain cases it may persist for decades [49], and may be associated with increased morbidity and mortality [50]. The most worrying aspect concerns reports of emerging resistance to common antibiotic therapies [51].
It must be noted that an initial study by Vázquez-Martínez et al. [52] found that neither piperine on its own nor P. nigrum extract could have an appreciable antibacterial effect against P. aeruginosa. On the other hand, Das et al. [34] demonstrated the potential of piperine to inhibit Pseudomonas-associated biofilm formation, via the accumulation of reactive oxygen species (ROS), to reduce surface hydrophobicity and bacterial motility, and the potential to affect the quorum sensing network, which is associated with the coding of several virulence genes [53]. In the case of carbapenem-resistant P. aeruginosa, piperine was found to reduce the expression of the MexAB-OprM efflux pumps, which was associated with the reported resistance [35].
2.7. Antibacterial Activity Against Staphylococcus aureus
This bacterium is a frequent colonizer of the human body that has raised major health concerns throughout the years, causing a diversity of diseases ranging from skin infections to pneumonia, abscess formation, and even sepsis [54].
Das et al. [36] analyzed the effects of piperine in various concentrations on MRSA. The results showed that the formation of the biofilm was substantially decreased by 36% and 45% in 8 and 16 μg/mL piperine solutions, respectively [36]. Also, when the bacteria were exposed to the higher amount of piperine, they also showed reduced metabolic activity by 33%. Moreover, it was observed that the bacterial expression of the icaA gene was decreased [36]. The aforementioned gene was the gene mostly responsible for the formation of the biofilm that protected the microbe [55]. These conclusions validate earlier experiments which showed piperine to be relatively effective against S. aureus, with an MIC of 12.5 μg/mL [27].
2.8. Antimicrobial Activity Against Streptococcus mutans
This commensal bacterium of the oral cavity can become pathogenic in certain circumstances, and is a prominent cause of dental caries formation [56].
Dwivedi et al. showed piperine to be active against this bacterium, having an MIC of 0.33 ± 0.02 mg/mL and a BIC (biofilm inhibitory concentration) of 0.0407 ± 0.03 mg/mL [37]. The significance of these results is increased when taking into consideration that the tested isolate was SM03, which has a very potent biofilm-formation capacity [37].
2.9. Antimicrobial Activity Against Vibrio cholerae
Cholera is a disease reported since ancient times and the bacterium responsible is a physiological inhabitant of aquatic ecosystems [57,58]. There are numerous pathogenic biotypes which produce different virulence factors [59]; in recent years, resistance to antibiotics is a cause for concern [60,61].
The study of Manjunath et al. [38] on the antibacterial activity of piperine extracted from white pepper against V. cholerae, specifically the O1 El Tor variant, found that it can inhibit, or at least reduce, bacterial growth, although the precise mechanisms require elucidation.
3. Antifungal Properties of Piperine
Even though a limited number of fungi are considered of medical interest compared to bacteria [62], and most of the infections they cause are not life-threatening, there exist cases in which infections with fungal species such as Aspergillus fumigatus and Candida albicans will lead to serious pathological conditions. The current research evidence on the antifungal properties of piperine is presented in Table 2.

Table 2.
Antifungal properties of piperine, based on current research evidence, classified alphabetically by family.
3.1. Antifungal Activity Against Aspergillus spp.
The members of this genus are not generally considered prime suspects for fungal infections in the general population, but can be dangerous under specific circumstances [67]. While piperine had no significant effect on the growth of A. flavus, it was effective in inhibiting aflatoxin production at concentrations ranging from 1000 for aflatoxin G2 to 3000 μg/mL for aflatoxins B1, B2, and G1 [63]. In another study, piperine was used to synthesize a number of derivatives which were effective against A. flavus, A. niger, and A. fumigatus [64].
3.2. Antifungal Activity Against Candida albicans
Candida albicans is one of the most notable commensal microorganisms in the human body, being mainly found lining the mucosa of the gastrointestinal and the genitourinary tracts [68]. If the proper conditions are met, mainly regarding the host’s immune status and the microbiome composition, it may become pathogenic, causing significant morbidity and mortality [68].
Piperine has been shown to damage the membrane of Candida albicans cells, with the ensuing oxidative stress resulting in apoptosis [66]. Moreover, it also enhances the action of the antifungal agent fluconazole [66]. Its MIC ranged from 2.5 to 15 mg/L depending on the strains and isolates [66]. Similarly, the study by Trindale et al. [64] tested a variety of piperine derivatives against the strains ATCC-60193 and LM-92 and their MICs ranged from 256 to 1024 μg/mL−1. The antifungal action of piperine against different candidal albicans strains is also corroborated by the findings of Phuna et al. [65].
5. Antiparasitic Properties of Piperine
While the majority of parasitic infections are mostly dangerous in areas where they are endemic [103,104], they currently show a trend of increasing resistance [105]. There is a notable corpus of research results regarding the antiparasitic potency of piperine (Table 4).

Table 4.
Antiparasitic properties of piperine, based on current research evidence, classified alphabetically by species.
5.1. Antiparasitic Activity Against Leishmania spp.
There is a plethora of Leishmania species, each with their own particularities when it comes to geographical distribution and animal reservoir [115]. The ability of Leishmania parasites to evade the immune system [116] emphasizes that a proper therapeutic approach is of the utmost importance.
The research of Vieira-Araújo et al. [108] showed that a mixture of 50% piperine and 50% meglumine antimoniate resulted in an IC50 of 2.09 ± 0.25 µg/mL against L. infantum promastigotes. Similarly, a combination of 25% piperine and 75% glucantime resulted in an IC50 of 7.25 ± 4.84 µg/mL against the parasite’s amastigote form [108]. It is worth noting that these results are better than those of the pentavalent antimony-based compounds which are commonly used as therapeutic agents [108]. Based on the tests of Chouhan et al. [107], piperine seems to be active against both the promastigotes and the amastigotes of L. donovani too, though the hexane and ethanolic extracts of P. nigrum are superior in this regard.
5.2. Antiparasitic Activity Against Malaria
Malaria is a disease caused by five members of the Plasmodium genus, the rest being rarely pathogenic, and it is one of the most ancient diseases known to humanity [117]. The use of artemisinin or an artemisinin-based combination therapy is the treatment of choice, but it has adverse effects, and malaria parasites have begun developing resistance [118].
When tested on mice, piperine was shown to be effective to a notable extent against P. berghei both prophylactically and therapeutically, particularly in regard to suppressing parasitemia and the clinical manifestations, due to its ability to alter the morphology of infected erythrocytes [111]. The greatest effects on parasitemia, at a maximum of 79.21% suppression, were exerted by doses of 40 mg/kg [111]. Piperine has also exhibited antiparasitic action against Plasmodium falciparum at an IC50 of > 200μM, though some safety concerns were raised regarding its potential risk for reproduction [110], and the concentration at which it was active was quite high. Its cytotoxicity was corroborated by Wansri et al. [77] whose research on Vero cells displayed an IC50 of 61.24 ± 2.83 against P. falciparum 3D7 and an IC50 of 56.67 ± 0.98 against T. brucei rhodesiense. Similar values were also reported by Thiengsusuk et al. [109], who noted that perhaps piperine is more effective during the first 8–12 h of the parasite’s lifecycle.
5.3. Antiparasitic Activity Against Trichomonas vaginalis
Trichomonas vaginalis is the causative agent of trichomoniasis, the most commonly contracted nonviral sexually transmitted disease [119]. This obligate extracellular parasite colonizes the human genitourinary tract, and may be symptomatic [119]. Both the extracts and essential oil derived by P. nigrum exhibited cytolytic effects against the trophozoites of Trichomonas vaginalis, their MLC being up to 100 µg/mL [112]. Notably, the viability of the trophozoites was impaired even in sub-MLC and lower concentrations [112].
5.4. Antiparasitic Activity Against Trypanosoma spp.
Trypanosoma cruzi is the causative agent of Chagas disease [120], a life-threatening condition whose list of endemic areas has been increasing as a result of migration [120]. Trypanosoma brucei rhodesiense on the other hand is implicated in approximately 5% of human African trypanosomiasis cases [121]. In general, trypanosomiasis is severe parasitic disease that is difficult to treat [122].
Piperine has been shown to be active against the epimastigote form of T. cruzi [113,123]. The research results of Cotinguiba et al. [114] suggested that there are piperamides which can be used against the parasite with an IC50 as low as 10.5 μM, better than benznidazole’s IC50 of 42.7 μM, though the effectiveness of piperine itself in that regard was found to be lacking. An important observation was that double bonds conjugated with a carbonyl group are essential for achieving high anti-Trypanosoma cruzi activity [114]. Furthermore, piperine’s cytotoxicity against T. brucei rhodesiense was affirmed by Wansri et al. [77] who calculated its half-maximal effective concentration (EC50) as being 56.7 ± 0.98 μM. Piperine’s potency may be mild, but these results highlight the significance of the methoxy-substituted phenyl amide scaffold of some piperine analogs which were also tested in this study, and whose effectiveness as anti-trypanosomal agents was notably superior [77].
7. Conclusions
Piperine is one of the most active compounds of black pepper (P. nigrum), presents a host of notable antimicrobial and antiviral activities, and is, in some cases, more potent than the agents currently in use. While the mechanisms behind such activity have been under study for quite a while, nevertheless, more research is necessary, exploring both other potential applications and ways of administration.
Author Contributions
Conceptualization, A.P., K.P., C.D.M.D., I.S.-F., and C.S.; methodology, A.P., S.D., C.C., and C.S.; validation, A.C., I.A.B., C.S., and C.C.; formal analysis, A.C., I.S.-F., A.-E.S., and I.A.B.; investigation, A.-T.P., G.-M.A., A.P., L.T., and K.P.; resources, A.-T.P., G.-M.A., A.P., L.T., S.D., and C.C.; data curation, C.D.M.D., A.C., and A.-E.S.; writing—original draft preparation, A.-T.P., G.-M.A., A.P., L.T., K.P., C.D.M.D., A.C., I.S.-F., S.D., A.-E.S., I.A.B., C.S., and C.C.; writing—review and editing, A.P., I.S.-F., S.D., C.S., and C.C.; visualization, A.P.; supervision, A.P., L.T., C.C., and C.S.; project administration, A.P., A.-T.P., L.T., I.A.B., and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila through the institutional program Publish not Perish.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DENV | Dengue Virus |
| EBOV | Ebola Virus |
| EC50 | Half-maximal Effective Concentration |
| HCV | Hepatitis C Virus |
| HPIV | Human Parainfluenza Virus |
| IC50 | Half-maximal Inhibitory Concentration |
| iNOS | Inducible Nitric Oxide Synthase |
| MERS-CoV | Middle East Respiratory Syndrome-related Coronavirus |
| MIC | Minimum Inhibitory Concentration |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| VSV | Indian Vesiculovirus |
| WHO | World Health Organization |
| ZKV | Zika Virus |
References
- Periferakis, A.T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef]
- Brinkac, L.; Voorhies, A.; Gomez, A.; Nelson, K.E. The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 2017, 74, 1001–1008. [Google Scholar] [CrossRef]
- Wang, B.; Mahsoub, H.M.; Li, W.; Heffron, C.L.; Tian, D.; Hassebroek, A.M.; LeRoith, T.; Meng, X.J. Ribavirin Treatment Failure-Associated Mutation, Y1320H, in the RNA-Dependent RNA Polymerase of Genotype 3 Hepatitis E Virus (HEV) Enhances Virus Replication in a Rabbit HEV Infection Model. mBio 2023, 14, e0337222. [Google Scholar] [CrossRef]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-Staphylococcus aureus activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, A.A.; Ayoub, M.T.; Shakya, A.K.; Abu Safieh, K.A.; Mubarak, M.S. Synthesis, characterization, and antimicrobial activity of some new coumarin derivatives. Med. Chem. Res. 2012, 21, 468–476. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.; Campopiano, D.J. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Maloh, J.; Sivamani, R.K. Clinical Efficacy of Topical or Oral Soy Supplementation in Dermatology: A Systematic Review. J. Clin. Med. 2023, 12, 4171. [Google Scholar] [CrossRef]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and Antioxidant Activity of a Hydroalcoholic Extract from Humulus lupulus L. Oxid. Med. Cell Longev. 2018, 2018, 5919237. [Google Scholar] [CrossRef]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.D.; Caruntu, A.; Scheau, A.E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, C.; Horvath, P.; Fischer, I.; Wagner, S.; Thiel, K.; Ladurner, R.; Königsrainer, I.; Königsrainer, A.; Schwab, M.; Beckert, S.; et al. Fluorescence-Guided Laparoscopy after Oral Hypericin Administration for Staging of Locally Advanced Gastric Cancer—A Pilot Study. J. Clin. Med. 2024, 13, 2422. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. Capsaicin: Effects on the Pathogenesis of Hepatocellular Carcinoma. Molecules 2019, 24, 2350. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Savino, R.; Medoro, A.; Ali, S.; Scapagnini, G.; Maes, M.; Davinelli, S. The Emerging Role of Flavonoids in Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2023, 12, 3520. [Google Scholar] [CrossRef]
- Scott, I.M.; Helson, B.V.; Strunz, G.M.; Finlay, H.; Sánchez-Vindas, P.E.; Poveda, L.; Lyons, D.B.; Philogène, B.J.R.; Arnason, J.T. Efficacy of Piper nigrum (Piperaceae) extract for control of insect defoliators of forest and ornamental trees. Can. Entomol. 2007, 139, 513–522. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytosci. 2021, 7, 52. [Google Scholar] [CrossRef]
- Peter, K.V. Futurology of black pepper. In Black Pepper; Ravindran, P.N., Ed.; Harwood Academic: Amsterdam, The Netherlands, 2000; pp. 481–487. [Google Scholar]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CAB International: London, UK, 2008. [Google Scholar]
- Scott, I.; Gagnon, N.; Lesage, L.; Philogene, B.; Arnason, J. Efficacy of botanical insecticides from Piper species (Piperaceae) extracts for control of European chafer (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2005, 98, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.T.; Khan, R.R. Comparison of repellency of essential oils against red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J. Stored Prod. Postharvest Res. 2011, 2, 131–134. [Google Scholar]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Reddy, P.; Jamil, K.; Madhusudhan, P.; Anjani, G.; Das, B. Antibacterial Activity of Isolates from Piper longum and Taxus baccata. Pharm. Biol. 2001, 39, 236–238. [Google Scholar] [CrossRef]
- Lakes, J.E.; Richards, C.I.; Flythe, M.D. Inhibition of Bacteroidetes and Firmicutes by select phytochemicals. Anaerobe 2020, 61, 102145. [Google Scholar] [CrossRef]
- Dusane, D.H.; Hosseinidoust, Z.; Asadishad, B.; Tufenkji, N. Alkaloids modulate motility, biofilm formation and antibiotic susceptibility of uropathogenic Escherichia coli. PLoS ONE 2014, 9, e112093. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Kim, S.H.; Park, M.; Woo, H.J.; Kim, H.W.; Yang, J.Y.; Rhee, K.J.; Kim, J.B. Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect. Agent Cancer 2014, 9, 43. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Park, M.; Lee, M.H.; Woo, H.J.; Kim, H.W.; Yang, J.Y.; Rhee, K.J.; Kim, J.B. Piperine treatment suppresses Helicobacter pylori toxin entry in to gastric epithelium and minimizes β-catenin mediated oncogenesis and IL-8 secretion in vitro. Am. J. Transl. Res. 2016, 8, 885–898. [Google Scholar]
- Toyoda, T.; Shi, L.; Takasu, S.; Cho, Y.M.; Kiriyama, Y.; Nishikawa, A.; Ogawa, K.; Tatematsu, M.; Tsukamoto, T. Anti-Inflammatory Effects of Capsaicin and Piperine on Helicobacter pylori-Induced Chronic Gastritis in Mongolian Gerbils. Helicobacter 2016, 21, 131–142. [Google Scholar] [CrossRef]
- Sharma, S.; Kalia, N.P.; Suden, P.; Chauhan, P.S.; Kumar, M.; Ram, A.B.; Khajuria, A.; Bani, S.; Khan, I.A. Protective efficacy of piperine against Mycobacterium tuberculosis. Tuberculosis 2014, 94, 389–396. [Google Scholar] [CrossRef]
- Das, S.; Paul, P.; Dastidar, D.G.; Chakraborty, P.; Chatterjee, S.; Sarkar, S.; Maiti, D.; Tribedi, P. Piperine Exhibits Potential Antibiofilm Activity Against Pseudomonas aeruginosa by Accumulating Reactive Oxygen Species, Affecting Cell Surface Hydrophobicity and Quorum Sensing. Appl. Biochem. Biotechnol. 2023, 195, 3229–3256. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, R.; Liu, X.; Li, D.; Guo, M.; Fei, B.; Ren, Y.; You, X.; Li, Y. Effect of piperine on the inhibitory potential of MexAB-OprM efflux pump and imipenem resistance in carbapenem-resistant Pseudomonas aeruginosa. Microb. Pathog. 2023, 185, 106397. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Malik, M.; Dastidar, D.G.; Roy, R.; Paul, P.; Sarkar, S.; Chakraborty, P.; Maity, A.; Dasgupta, M.; Gupta, A.D.; et al. Piperine, a phytochemical prevents the biofilm city of methicillin-resistant Staphylococcus aureus: A biochemical approach to understand the underlying mechanism. Microb. Pathog. 2024, 189, 106601. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Singh, V. Effects of the natural compounds embelin and piperine on the biofilm-producing property of Streptococcus mutans. J. Tradit. Complement. Med. 2016, 6, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, G.B.; Awasthi, S.P.; Zahid, M.S.H.; Hatanaka, N.; Hinenoya, A.; Iwaoka, E.; Aoki, S.; Ramamurthy, T.; Yamasaki, S. Piperine, an active ingredient of white pepper, suppresses the growth of multidrug-resistant toxigenic Vibrio cholerae and other pathogenic bacteria. Lett. Appl. Microbiol. 2022, 74, 472–481. [Google Scholar] [CrossRef]
- Tanaka, I.; Kutsuna, S.; Ohkusu, M.; Kato, T.; Miyashita, M.; Moriya, A.; Ohkusu, K. Bacillus subtilis variant natto Bacteremia of Gastrointestinal Origin, Japan. Emerg. Infect. Dis. 2022, 28, 1718–1719. [Google Scholar] [CrossRef]
- Patrick, S. A tale of two habitats: Bacteroides fragilis, a lethal pathogen and resident in the human gastrointestinal microbiome. Microbiology 2022, 168, 001156. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Kim, K.S. Human Meningitis-Associated Escherichia coli. EcoSal Plus 2016, 7, 10.1128/ecosalplus.ESP-0015-2015. [Google Scholar] [CrossRef]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef]
- Mazurek, G.H.; Jereb, J.; LoBue, P.; Iademarco, M.F.; Metchock, B.; Vernon, A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWr Recomm. Rep. 2005, 54, 49–55. [Google Scholar] [PubMed]
- Allué-Guardia, A.; García, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.; Zin, N.M.; Wahab, H.A.; Ibrahim, P.; Sulaiman, S.F.; Zahariluddin, A.S.M.; Noor, S.S.M. Antituberculosis potential of some ethnobotanically selected Malaysian plants. J. Ethnopharmacol. 2011, 133, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, J.; Tümmler, B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research 2017, 6, 1261. [Google Scholar] [CrossRef]
- Buhl, M.; Peter, S.; Willmann, M. Prevalence and risk factors associated with colonization and infection of extensively drug-resistant Pseudomonas aeruginosa: A systematic review. Expert Rev. Anti-Infect. Ther. 2015, 13, 1159–1170. [Google Scholar] [CrossRef]
- Winstanley, C.; O’Brien, S.; Brockhurst, M.A. Pseudomonas aeruginosa Evolutionary Adaptation and Diversification in Cystic Fibrosis Chronic Lung Infections. Trends Microbiol. 2016, 24, 327–337. [Google Scholar] [CrossRef]
- Döring, G.; Parameswaran, I.G.; Murphy, T.F. Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease. FEMS Microbiol. Rev. 2011, 35, 124–146. [Google Scholar] [CrossRef]
- Bassi, G.L.; Ferrer, M.; Marti, J.D.; Comaru, T.; Torres, A. Ventilator-associated pneumonia. Semin. Respir. Crit. Care Med. 2014, 35, 469–481. [Google Scholar] [CrossRef]
- Vázquez-Martínez, J.; Buitemea-Cantúa, G.V.; Gutierrez-Villagomez, J.M.; García-González, J.P.; Ramírez-Chávez, E.; Molina-Torres, J. Bioautography and GC-MS based identification of piperine and trichostachine as the active quorum quenching compounds in black pepper. Heliyon 2020, 6, e03137. [Google Scholar] [CrossRef]
- Miranda, S.W.; Asfahl, K.L.; Dandekar, A.A.; Greenberg, E.P. Pseudomonas aeruginosa Quorum Sensing. Adv. Exp. Med. Biol. 2022, 1386, 95–115. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y.; Choi, K.H. icaA Gene of Staphylococcus aureus Responds to NaCl, Leading to Increased Biofilm Formation. J. Food Prot. 2018, 81, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Correa, C.M.; Rincón Rodríguez, R.J.; Parada-Sanchez, M.T. Genomic and phenotypic diversity of Streptococcus mutans. J. Oral. Biosci. 2019, 61, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Garay, E.; Arnau, A.; Amaro, C. Incidence of Vibrio cholerae and related vibrios in a coastal lagoon and seawater influenced by lake discharges along an annual cycle. Appl. Environ. Microbiol. 1985, 50, 426–430. [Google Scholar] [CrossRef]
- Reidl, J.; Klose, K.E. Vibrio cholerae and cholera: Out of the water and into the host. FEMS Microbiol. Rev. 2002, 26, 125–139. [Google Scholar] [CrossRef]
- Glass, R.I.; Huq, I.; Alim, A.; Yunus, M. Emergence of multiply antibiotic-resistant Vibrio cholerae in Bangladesh. J. Infect. Dis. 1980, 142, 939–942. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Am. Assoc. Adv. Sci. 2012, 336, 647. [Google Scholar] [CrossRef]
- Moon, Y.S.; Choi, W.S.; Park, E.S.; Bae, I.K.; Choi, S.D.; Paek, O.; Kim, S.H.; Chun, H.S.; Lee, S.E. Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds. Toxins 2016, 8, 240. [Google Scholar] [CrossRef]
- Trindade, E.O.; Dutra, T.F.; Brandão, M.C.; Diniz Neto, H.; Lima, E.O.; Lira, B.F.; Athayde-Filho, P.F.d.; Barbosa-Filho, J.M. Synthesis, in silico study and antimicrobial activity of new piperine derivatives containing substituted δ-Esters. J. Braz. Chem. Soc. 2020, 31, 2590–2602. [Google Scholar] [CrossRef]
- Phuna, Z.X.; Yu, J.K.E.; Tee, J.Y.; Chuah, S.Q.; Tan, N.W.H.; Vijayabalan, S.; Abdul Manap, A.S.; Sisinthy, S.P.; Madhavan, P. In vitro evaluation of nanoemulsions of curcumin, piperine, and tualang honey as antifungal agents for Candida species. J. Appl. Biotechnol. Rep. 2020, 7, 189–197. [Google Scholar]
- Thakre, A.; Jadhav, V.; Kazi, R.; Shelar, A.; Patil, R.; Kharat, K.; Zore, G.; Karuppayil, S.M. Oxidative stress induced by piperine leads to apoptosis in Candida albicans. Med. Mycol. 2021, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Xavier, M.O.; Severo, L.C. Differences and similarities amongst pathogenic Aspergillus species. In Aspergillosis: From Diagnosis to Prevention; Springer: Berlin/Heidelberg, Germany, 2010; pp. 7–32. [Google Scholar]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Lavanchy, D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral Hepat. 2004, 11, 97–107. [Google Scholar] [CrossRef]
- Ly, K.N.; Xing, J.; Klevens, R.M.; Jiles, R.B.; Ward, J.W.; Holmberg, S.D. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann. Intern. Med. 2012, 156, 271–278. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef]
- Periferakis, A.; Bolocan, A.; Ion, D. A Review of Innovation in Medicine. Technol. Innov. Life Sci. 2022, 1, 42–48. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Fayad, E.; Althobaiti, F.; Zaki, I.; Abu Almaaty, A.H. Statistical optimization of bile salt deployed nanovesicles as a potential platform for oral delivery of piperine: Accentuated antiviral and anti-inflammatory activity in MERS-CoV challenged mice. Drug Deliv. 2021, 28, 1150–1165. [Google Scholar] [CrossRef]
- Shekunov, E.V.; Efimova, S.S.; Yudintceva, N.M.; Muryleva, A.A.; Zarubaev, V.V.; Slita, A.V.; Ostroumova, O.S. Plant Alkaloids Inhibit Membrane Fusion Mediated by Calcium and Fragments of MERS-CoV and SARS-CoV/SARS-CoV-2 Fusion Peptides. Biomedicines 2021, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Rout, J.; Swain, B.C.; Tripathy, U. In silico investigation of spice molecules as potent inhibitor of SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Wansri, R.; Lin, A.C.K.; Pengon, J.; Kamchonwongpaisan, S.; Srimongkolpithak, N.; Rattanajak, R.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Hengphasatporn, K.; et al. Semi-Synthesis of N-Aryl Amide Analogs of Piperine from Piper nigrum and Evaluation of Their Antitrypanosomal, Antimalarial, and Anti-SARS-CoV-2 Main Protease Activities. Molecules 2022, 27, 2841. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Antunes, B.S.; do Nascimento, G.O.; Kawall, J.C.S.; Oliveira, J.V.B.; Silva, K.; Costa, M.A.T.; Oliveira, C.R. Antiviral activity of medicinal plant-derived products against SARS-CoV-2. Exp. Biol. Med. 2022, 247, 1797–1809. [Google Scholar] [CrossRef]
- Nag, A.; Chowdhury, R.R. Piperine, an alkaloid of black pepper seeds can effectively inhibit the antiviral enzymes of Dengue and Ebola viruses, an in silico molecular docking study. VirusDisease 2020, 31, 308–315. [Google Scholar] [CrossRef]
- Saputro, A.H.; Amelia, T.; Mahardhika, A.B.; Widyawaruyanti, A.; Wahyuni, T.S.; Permanasari, A.A.; Artarini, A.A.; Tjahjono, D.H.; Damayanti, S. Alpha-mangostin, piperine and beta-sitosterol as hepatitis C antivirus (HCV): In silico and in vitro studies. Heliyon 2023, 9, e20141. [Google Scholar] [CrossRef]
- Srinivasan, D.; Venkatesan, M. Activity of phytochemical constituents of black pepper and rosemary against Zika virus: An in silico approach. NanoBioScience 2021, 11, 3393–3404. [Google Scholar] [CrossRef]
- Priya, N.; Kumari, P.S. Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. Int. J. Pharm. Sci. Rev. Res. 2017, 44, 197–202. [Google Scholar]
- Widagdo, W.; Sooksawasdi Na Ayudhya, S.; Hundie, G.B.; Haagmans, B.L. Host Determinants of MERS-CoV Transmission and Pathogenesis. Viruses 2019, 11, 280. [Google Scholar] [CrossRef]
- Chafekar, A.; Fielding, B.C. MERS-CoV: Understanding the Latest Human Coronavirus Threat. Viruses 2018, 10, 93. [Google Scholar] [CrossRef]
- Santos-López, G.; Cortés-Hernández, P.; Vallejo-Ruiz, V.; Reyes-Leyva, J. SARS-CoV-2: Basic concepts, origin and treatment advances. Gac. Med. Mex. 2021, 157, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd; Hewlett, A.; Kraft, C.S.; Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Jadav, S.S.; Kumar, A.; Ahsan, M.J.; Jayaprakash, V. Ebola virus: Current and future perspectives. Infect. Disord. Drug Targets 2015, 15, 20–31. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Bryniarski, K.; Nazimek, K. Ebola haemorrhagic fever virus: Pathogenesis, immune responses, potential prevention. Folia Med. Cracov 2014, 54, 39–48. [Google Scholar]
- Soo, K.M.; Khalid, B.; Ching, S.M.; Chee, H.Y. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. PLoS ONE 2016, 11, e0154760. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, R.; Cheng, G. Progress towards understanding the pathogenesis of dengue hemorrhagic fever. Virol. Sin. 2017, 32, 16–22. [Google Scholar] [CrossRef]
- Oliveira, A.S.d.; Silva, M.L.d.; Oliveira, A.F.; Silva, C.C.d.; Teixeira, R.R.; De Paula, S.O. NS3 and NS5 proteins: Important targets for anti-dengue drug design. J. Braz. Chem. Soc. 2014, 25, 1759–1769. [Google Scholar] [CrossRef]
- González-Grande, R.; Jiménez-Pérez, M.; González Arjona, C.; Mostazo Torres, J. New approaches in the treatment of hepatitis C. World J. Gastroenterol. 2016, 22, 1421–1432. [Google Scholar] [CrossRef]
- Martinez, M.A.; Franco, S. Therapy Implications of Hepatitis C Virus Genetic Diversity. Viruses 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, J.; Qing, J.; Huang, M.; Wu, M.; Gao, F.; Li, D.; Hong, Z.; Kong, L.; Huang, W.; et al. Discovery of Novel Hepatitis C Virus NS5B Polymerase Inhibitors by Combining Random Forest, Multiple e-Pharmacophore Modeling and Docking. PLoS ONE 2016, 11, e0148181. [Google Scholar] [CrossRef] [PubMed]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Pan, P.; Lao, Z.; Xu, J.; Li, Z.; Zhan, S.; Liu, X.; Wu, Y.; Wang, W.; et al. Cinnamic acid inhibits Zika virus by inhibiting RdRp activity. Antivir. Res. 2021, 192, 105117. [Google Scholar] [CrossRef]
- King, C.; Colbourn, T. Global human parainfluenza virus estimates for action on childhood pneumonia. Lancet Glob. Health 2021, 9, e1033–e1034. [Google Scholar] [CrossRef]
- Vilchez, R.A.; Dauber, J.; McCurry, K.; Iacono, A.; Kusne, S. Parainfluenza virus infection in adult lung transplant recipients: An emergent clinical syndrome with implications on allograft function. Am. J. Transpl. 2003, 3, 116–120. [Google Scholar] [CrossRef]
- Tomczyk, T.; Orzechowska, B. Vesicular stomatitis virus (VSV) as a vaccine vector for immunization against viral infections. Postep. Hig. Med. Dosw. (Online) 2013, 67, 1345–1358. [Google Scholar] [CrossRef]
- Barber, G.N. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004, 17, 516–527. [Google Scholar] [CrossRef]
- Barry, M.A.; Weatherhead, J.E.; Hotez, P.J.; Woc-Colburn, L. Childhood parasitic infections endemic to the United States. Pediatr. Clin. N. Am. 2013, 60, 471–485. [Google Scholar] [CrossRef]
- Pisarski, K. The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef]
- Geary, T.G.; Thompson, D.P. Development of antiparasitic drugs in the 21st century. Vet. Parasitol. 2003, 115, 167–184. [Google Scholar] [CrossRef]
- Ferreira, C.; Soares, D.C.; Barreto-Junior, C.B.; Nascimento, M.T.; Freire-de-Lima, L.; Delorenzi, J.C.; Lima, M.E.F.; Atella, G.C.; Folly, E.; Carvalho, T.M.U.; et al. Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry 2011, 72, 2155–2164. [Google Scholar] [CrossRef]
- Chouhan, G.; Islamuddin, M.; Ahmad, F.; Sahal, D.; Afrin, F. Antileishmanial potential of Piper nigrum seed extracts against Leishmania donovani. Open J. Med. Microbiol. 2014, 4, 228–235. [Google Scholar] [CrossRef]
- Vieira-Araújo, F.M.; Macedo Rondon, F.C.; Pinto Vieira, Í.G.; Pereira Mendes, F.N.; Carneiro de Freitas, J.C.; Maia de Morais, S. Sinergism between alkaloids piperine and capsaicin with meglumine antimoniate against Leishmania infantum. Exp. Parasitol. 2018, 188, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Thiengsusuk, A.; Muhamad, P.; Chaijaroenkul, W.; Na-Bangchang, K. Antimalarial Activity of Piperine. J. Trop. Med. 2018, 2018, 9486905. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.; Veloso, M.P.; de Souza Reis, K.; de Matos Passarini, G.; Dos Santos, A.P.A.; do Nascimento Martinez, L.; Fokoue, H.H.; Kato, M.J.; Teles, C.B.G.; Kuehn, C.C. In silico evaluation and in vitro growth inhibition of Plasmodium falciparum by natural amides and synthetic analogs. Parasitol. Res. 2020, 119, 1879–1887. [Google Scholar] [CrossRef]
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Setyowati, E.Y.; Berbudi, A. Oral Administration of Piperine as Curative and Prophylaxis Reduces Parasitaemia in Plasmodium berghei ANKA-Infected Mice. J. Trop. Med. 2022, 2022, 5721449. [Google Scholar] [CrossRef]
- Jamshidi-Zad, A.; Dastan, D.; Fallah, M.; Azizi-Jalilian, F.; Matini, M. Essential Oil Components and Antitrichomonal Effects of Piper nigrum L. J. Kerman Univ. Med. Sci. 2023, 30, 207–212. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Freire-de-Lima, L.; Previato, J.O.; Mendonça-Previato, L.; Heise, N.; de Lima, M.E. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg Med. Chem. Lett. 2004, 14, 3555–3558. [Google Scholar] [CrossRef]
- Cotinguiba, F.; Regasini, L.O.; da Silva Bolzani, V.; Debonsi, H.M.; Duó Passerini, G.; Cicarelli, R.M.B.; Kato, M.J.; Furlan, M. Piperamides and their derivatives as potential anti-trypanosomal agents. Med. Chem. Res. 2009, 18, 703–711. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Stäger, S.; Joshi, T.; Bankoti, R. Immune evasive mechanisms contributing to persistent Leishmania donovani infection. Immunol. Res. 2010, 47, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Menard, D.; Dondorp, A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.; Burke, P.; Smalley, H.; Hobbs, G. Trichomonas vaginalis: Clinical relevance, pathogenicity and diagnosis. Crit. Rev. Microbiol. 2016, 42, 406–417. [Google Scholar] [CrossRef]
- Borgna, E.; Prochetto, E.; Gamba, J.C.; Marcipar, I.; Cabrera, G. Role of myeloid-derived suppressor cells during Trypanosoma cruzi infection. Int. Rev. Cell Mol. Biol. 2023, 375, 117–163. [Google Scholar] [CrossRef]
- Priotto, G.; Franco, J.R.; Lejon, V.; Büscher, P.; Matovu, E.; Ndung’u, J.; Biéler, S.; Mumba, D.; Van Reet, N.; Verlé, P.; et al. Target product profile: Diagnostic test for Trypanosoma brucei rhodesiense. Bull. World Health Organ. 2023, 101, 529–534. [Google Scholar] [CrossRef]
- MacLean, L.; Chisi, J.E.; Odiit, M.; Gibson, W.C.; Ferris, V.; Picozzi, K.; Sternberg, J.M. Severity of human african trypanosomiasis in East Africa is associated with geographic location, parasite genotype, and host inflammatory cytokine response profile. Infect. Immun. 2004, 72, 7040–7044. [Google Scholar] [CrossRef][Green Version]
- Freire-de-Lima, L.; Ribeiro, T.S.; Rocha, G.M.; Brandão, B.A.; Romeiro, A.; Mendonça-Previato, L.; Previato, J.O.; de Lima, M.E.; de Carvalho, T.M.; Heise, N. The toxic effects of piperine against Trypanosoma cruzi: Ultrastructural alterations and reversible blockage of cytokinesis in epimastigote forms. Parasitol. Res. 2008, 102, 1059–1067. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Abass, S.; Parveen, R.; Irfan, M.; Malik, Z.; Husain, S.A.; Ahmad, S. Mechanism of antibacterial phytoconstituents: An updated review. Arch. Microbiol. 2024, 206, 325. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Samsudin, N.; Benbelgacem, F.F.; Hashim, Y.Z.H.-Y.; Salleh, H.M.; Santhanam, J. Phytochemicals with antifungal properties: Cure from nature. Malays. J. Microbiol. 2020, 16, 323. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; López-Ibáñez, S.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Plant phytochemicals in food preservation: Antifungal bioactivity: A review. J. Food Prot. 2020, 83, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D. Phytochemicals from plant foods as potential source of antiviral agents: An overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Zholdybayeva, E.; Kozhakhmetova, S.; Bayanbek, D.; Bekbayeva, A.; Auganova, D.; Kulmambetova, G.; Tarlykov, P. Multi-omics approach for understanding the response of Bacteroides fragilis to carbapenems. Heliyon 2024, 10, e37049. [Google Scholar] [CrossRef]
- Lasko, M.J.; Gethers, M.L.; Tabor-Rennie, J.L.; Nicolau, D.P.; Kuti, J.L. In Vitro Time-Kill Studies of Trimethoprim/Sulfamethoxazole against Stenotrophomonas maltophilia versus Escherichia coli Using Cation-Adjusted Mueller-Hinton Broth and ISO-Sensitest Broth. Antimicrob. Agents Chemother. 2022, 66, e0216721. [Google Scholar] [CrossRef]
- Genestet, C.; Ader, F.; Pichat, C.; Lina, G.; Dumitrescu, O.; Goutelle, S. Assessing the Combined Antibacterial Effect of Isoniazid and Rifampin on Four Mycobacterium tuberculosis Strains Using In Vitro Experiments and Response-Surface Modeling. Antimicrob. Agents Chemother. 2018, 62, e01413-17. [Google Scholar] [CrossRef]
- Shawar, R.M.; MacLeod, D.L.; Garber, R.L.; Burns, J.L.; Stapp, J.R.; Clausen, C.R.; Tanaka, S.K. Activities of tobramycin and six other antibiotics against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 1999, 43, 2877–2880. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhao, H.; Wang, X.; Rao, L.; Ai, W.; Yu, J.; Guo, Y.; Wu, X.; Yu, F.; et al. In Vitro Activity of Vancomycin, Teicoplanin, Linezolid and Daptomycin Against Methicillin-Resistant Staphylococcus aureus Isolates Collected from Chinese Hospitals in 2018–2020. Infect. Drug Resist. 2021, 14, 5449–5456. [Google Scholar] [CrossRef]
- Clark, S.A.; Vinson, L.A.; Eckert, G.; Gregory, R.L. Effect of Commonly Prescribed Liquid Medications on Streptococcus mutans Biofilm. An in vitro study. J. Clin. Pediatr. Dent. 2017, 41, 141–146. [Google Scholar] [CrossRef][Green Version]
- Scrascia, M.; Forcillo, M.; Maimone, F.; Pazzani, C. Susceptibility to rifaximin of Vibrio cholerae strains from different geographical areas. J. Antimicrob. Chemother. 2003, 52, 303–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omran, S.M.; Taghizadeh-Armaki, M.; Zarrinfar, H.; Hedayati, M.T.; Abastabar, M.; Moqarabzadeh, V.; Ansari, S.; Saber, S.; Hoseinnejad, A.; Miri, A.; et al. In-vitro antifungal susceptibility testing of lanoconazole and luliconazole against Aspergillus flavus as an important agent of invasive aspergillosis. J. Infect. Chemother. 2019, 25, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Maenchantrarath, C.; Khumdee, P.; Samosornsuk, S.; Mungkornkaew, N.; Samosornsuk, W. Investigation of fluconazole susceptibility to Candida albicans by MALDI-TOF MS and real-time PCR for CDR1, CDR2, MDR1 and ERG11. BMC Microbiol. 2022, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Pruijssers, A.J.; George, A.S.; Schäfer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H., III; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef]
- Cheng, G.; Tian, Y.; Doehle, B.; Peng, B.; Corsa, A.; Lee, Y.J.; Gong, R.; Yu, M.; Han, B.; Xu, S.; et al. In Vitro Antiviral Activity and Resistance Profile Characterization of the Hepatitis C Virus NS5A Inhibitor Ledipasvir. Antimicrob. Agents Chemother. 2016, 60, 1847–1853. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials. Antibiotics 2019, 8, 55. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Velmathi, S. Synthesis, in vitro and in silico anti-bacterial analysis of piperine and piperic ester analogues. Chem. Biol. Drug Des. 2021, 98, 19–29. [Google Scholar] [CrossRef]
- Petran, E.M.; Periferakis, A.; Troumpata, L.; Periferakis, A.T.; Scheau, A.E.; Badarau, I.A.; Periferakis, K.; Caruntu, A.; Savulescu-Fiedler, I.; Sima, R.M.; et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Curr. Issues Mol. Biol. 2024, 46, 7895–7943. [Google Scholar] [CrossRef]
- Suresh, D.V.; Mahesha, H.G.; Rao, A.G.; Srinivasan, K. Binding of bioactive phytochemical piperine with human serum albumin: A spectrofluorometric study. Biopolymers 2007, 86, 265–275. [Google Scholar] [CrossRef]
- Grace, A.N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Turos, E.; Shim, J.-Y.; Wang, Y.; Greenhalgh, K.; Reddy, G.S.K.; Dickey, S.; Lim, D.V. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg. Med. Chem. Lett. 2007, 17, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.; Santra, C.; Dasgupta, A.K.; Karmakar, P. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Milovanovic, M.; Arsenijevic, A.; Milovanovic, J.; Kanjevac, T.; Arsenijevic, N. Chapter 14—Nanoparticles in antiviral therapy. In Antimicrobial Nanoarchitectonics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–410. [Google Scholar]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current applications and prospects of nanoparticles for antifungal drug delivery. EXCLI J. 2021, 20, 562. [Google Scholar]
- Elmi, T.; Gholami, S.; Fakhar, M.; Azizi, F. A review on the use of nanoparticles in the treatment. J. Maz. Univ. Med. Sci. 2013, 23, 126–133. [Google Scholar]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Bipinchandra, S.; Mouray, E.; Grellier, P.; Satish, P. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol. Int. 2015, 64, 353–356. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, D.; Pan, Y.; Qu, W.; Hao, H.; Wang, X.; Liu, Z.; Xie, S. Nanoparticles for antiparasitic drug delivery. Drug Deliv. 2019, 26, 1206–1221. [Google Scholar] [CrossRef] [PubMed]
- Teli, D.; Satasia, R.; Patel, V.; Nair, R.; Khatri, R.; Gala, D.; Balar, P.C.; Patel, K.; Sharma, A.; Vadodariya, P.; et al. Nature meets technology: Harnessing nanotechnology to unleash the power of phytochemicals. Clin. Tradit. Med. Pharmacol. 2024, 5, 200139. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, Y.; Liang, X.-J. Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr. Med. Chem. 2009, 16, 2924–2942. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Gogoi, N.R.; Vaghela, D.A.; Balar, P.C.; Dawre, S.; Dave, D.J. Parenteral microemulsions for drug delivery: Advances and update. J. Drug Deliv. Sci. Technol. 2023, 89, 104991. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Solouk, A.; Saber-Samandari, S.; Akbari, S.; Ghanbari, H.; Brycki, B.E. Capsaicin-loaded alginate nanoparticles embedded polycaprolactone-chitosan nanofibers as a controlled drug delivery nanoplatform for anticancer activity. J. Colloid Interface Sci. 2023, 638, 616–628. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, Y.; Xiang, M.; Wu, F.; Sun, M.; Du, X.; Chen, L. Biocompatible polyelectrolyte complex nanoparticles for lycopene encapsulation attenuate oxidative stress-induced cell damage. Front. Nutr. 2022, 9, 902208. [Google Scholar] [CrossRef]
- Teskač, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.R.; Philbert, M.; Vu, T.Q.; Huang, Q.; Kokini, J.L.; Saos, E.; Chen, H.; Peterson, C.M.; Friedl, K.E.; McDade-Ngutter, C. Nanotechnology research: Applications in nutritional sciences. J. Nutr. 2010, 140, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Joshi, M.D.; Patravale, V.B. Parasitic diseases: Liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Scheau, C.; Didilescu, A.C.; Caruntu, C. Medical Application of Functional Biomaterials—The Future Is Now. J. Funct. Biomater. 2022, 13, 244. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials adapted to VAT photopolymerization in 3D printing: Characteristics and medical applications. J. Funct. Biomater. 2023, 15, 7. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Garofil, N.D.; Didilescu, A.C.; Caruntu, C.; Scheau, C. 3D Bioprinting in Limb Salvage Surgery. J. Funct. Biomater. 2024, 15, 383. [Google Scholar] [CrossRef]
- Scheau, C.; Didilescu, A.C.; Caruntu, C. Innovative Biomaterials: The Cornerstone of Next-Generation Medical Solutions. J. Funct. Biomater. 2024, 15, 218. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Caruntu, A.; Dragosloveanu, C.D.M.; Scheau, A.-E.; Badarau, I.A.; Periferakis, A.; Dragosloveanu, S.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Head and Neck 3D Bioprinting—A Review on Recent Advancements in Soft Tissue 3D Bioprinting and Medical Applications. J. Funct. Biomater. 2025, 16, 240. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.-A.; Georgatos-Garcia, S.; Scheau, A.-E.; Periferakis, K.; Caruntu, A.; Badarau, I.A. Use of biomaterials in 3D printing as a solution to microbial infections in arthroplasty and osseous reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, A.; Chrcanovic, B. Effects of the local administration of antibiotics on bone formation on implant surface in animal models: A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2020, 56, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; López-Torres, I.I.; Alonso-Berenguel, M.; Torres-Suárez, A.I.; Martín-Sabroso, C. Local antimicrobial delivery systems for prophylaxis and treatment of periprosthetic traumatological infections. Eur. J. Pharm. Sci. 2025, 204, 106940. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef]
- Roca, M.; Villegas, L.; Kortabitarte, M.L.; Althaus, R.L.; Molina, M.P. Effect of heat treatments on stability of β-lactams in milk. J. Dairy Sci. 2011, 94, 1155–1164. [Google Scholar] [CrossRef]
- Stănciuc, N.; Râpeanu, G. 13—Kinetics of Phytochemicals Degradation During Thermal Processing of Fruits Beverages. In Non-Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 407–440. [Google Scholar] [CrossRef]
- Alcorn, J.B. Huastec Mayan Ethnobotany; University of Texas Press: Austin, TX, USA, 1984. [Google Scholar]
- Jain, S.K. Ethnobotany. Interdiscip. Sci. Rev. 1986, 11, 285–292. [Google Scholar] [CrossRef]
- Cotton, C.M. Ethnobotany: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Heinrich, M. Ethnobotany and its role in drug development. Phytother. Res. 2000, 14, 479–488. [Google Scholar] [CrossRef]
- Petran, M.; Dragos, D.; Gilca, M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s–1970s). J. Ethnobiol. Ethnomed. 2020, 16, 15. [Google Scholar] [CrossRef]
- Balick, M.J.; Cox, P.A. Plants, People, and Culture: The Science of Ethnobotany; Garland Science: New York, NY, USA, 2020. [Google Scholar]
- Periferakis, A.; Tsigas, G.; Periferakis, A.-T.; Tone, C.M.; Hemes, D.A.; Periferakis, K.; Troumpata, L.; Badarau, I.A.; Scheau, C.; Caruntu, A.; et al. Agonists, Antagonists and Receptors of Somatostatin: Pathophysiological and Therapeutical Implications in Neoplasias. Curr. Issues Mol. Biol. 2024, 46, 9721–9759. [Google Scholar] [CrossRef]
- Caruntu, C.; Negrei, C.; Ilie Ghita, M.; Caruntu, A.; Bădărău, A.; ioan Buraga, I.B.; Boda, D.; Albu, A.; Brănişteanu, D. Capsaicin, a hot topic in skin pharmacology and physiology. Farmacia 2015, 63, 487–491. [Google Scholar]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.T.; Troumpata, L.; Periferakis, K.; Scheau, A.E.; Savulescu-Fiedler, I.; Caruntu, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. Int. J. Mol. Sci. 2023, 24, 16299. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, Y.; Li, L.; Feng, J.; Lou, B.; Zhou, X.; Wu, H. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar] [CrossRef]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Teow, S.Y.; Ali, S.A. Synergistic antibacterial activity of Curcumin with antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar]
- Yun, D.G.; Lee, D.G. Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, Y.; Yu, Y.; Zeng, Y. Enhanced antibacterial activity of curcumin by combination with metal ions. Colloid. Interface Sci. Commun. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Smyth, T.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 2009, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Georgatos-Garcia, S.; Touriki, G.; Dragosloveanu, C.D.M.; Caruntu, A.; Savulescu-Fiedler, I.; Dragosloveanu, S.; et al. Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential. Curr. Issues Mol. Biol. 2025, 47, 204. [Google Scholar] [CrossRef] [PubMed]
- Molina-Torres, J.; García-Chávez, A.; Ramírez-Chávez, E. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J. Ethnopharmacol. 1999, 64, 241–248. [Google Scholar] [CrossRef]
- Rosas-Piñón, Y.; Mejía, A.; Díaz-Ruiz, G.; Aguilar, M.I.; Sánchez-Nieto, S.; Rivero-Cruz, J.F. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. J. Ethnopharmacol. 2012, 141, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Flores-Vallejo, R.D.C.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; Catalan, C.; deLampasona, M. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. J. Sci. Food Agric. 2004, 84, 1878–1884. [Google Scholar] [CrossRef]
- Leal, S.M.; Pino, N.; Stashenko, E.E.; Martínez, J.R.; Escobar, P. Antiprotozoal activity of essential oils derived from Piper spp. grown in Colombia. J. Essent. Oil Res. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Lee, S.E.; Campbell, B.C. Inhibition of aflatoxin B1 biosynthesis by piperlongumine isolated from Piper longum L. J. Microbiol. Biotechnol. 2002, 12, 679–682. [Google Scholar]
- Krahe, N.-K.; Berger, R.G.; Kahlert, L.; Ersoy, F. Co-Oxidative Transformation of Piperine to Piperonal and 3,4-Methylenedioxycinnamaldehyde by a Lipoxygenase from Pleurotus sapidus. ChemBioChem 2021, 22, 2857–2861. [Google Scholar] [CrossRef]
- Zhao, K.; Wonta, K.B.; Xia, J.; Zhong, F.; Sharma, V. Phytochemical profiling and evaluation of antimicrobial activities of common culinary spices: Syzygium aromaticum (clove) and Piper nigrum (black pepper). Front. Nutr. 2024, 11, 1447144. [Google Scholar] [CrossRef]
- Bukhari, I.A.; Alhumayyd, M.; Mahesar, A.; Gilani, A. The analgesic and anticonvulsant effects of piperine in mice. J. Physiol. Pharmacol. 2013, 64, 789. [Google Scholar]
- Kaleem, M.; Sheema, S.H.; Bano, B. Protective effects of Piper nigrum and Vinca rosea in alloxan induced diabetic rats. Indian. J. Physiol. Pharmacol. 2005, 49, 65–71. [Google Scholar]
- Venkatachalapathi, A.; Sangeeth, T.; Ali, M.A.; Tamilselvi, S.S.; Paulsamy, S.; Al-Hemaidc, F.M.A. Ethnomedicinal assessment of Irula tribes of Walayar valley of Southern Western Ghats, India. Saudi J. Biol. Sci. 2018, 25, 760–775. [Google Scholar] [CrossRef]
- Periferakis, A.; Troumpata, L.; Periferakis, K.; Adalis, G.; Periferakis, A.; Georgatos-Garcia, S.; Maier, C.; Costache, A.; Garofil, D.; Costache, D. Traditional Ethnomedical and Ethnobotanical Applications and Uses of Piper Nigrum. R. J. Mil. Med. 2025, 128, 286–303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).