Structures of Mammeasins P and Q, Coumarin-Related Polysubstituted Benzofurans, from the Thai Medicinal Plant Mammea siamensis (Miq.) T. Anders.: Anti-Proliferative Activity of Coumarin Constituents against Human Prostate Carcinoma Cell Line LNCaP
Abstract
1. Introduction
2. Results and Discussion
2.1. Anti-Proliferative Effects of the Methanol Extract and Its Fractions against Human Prostate Carcinoma LNCaP Cells
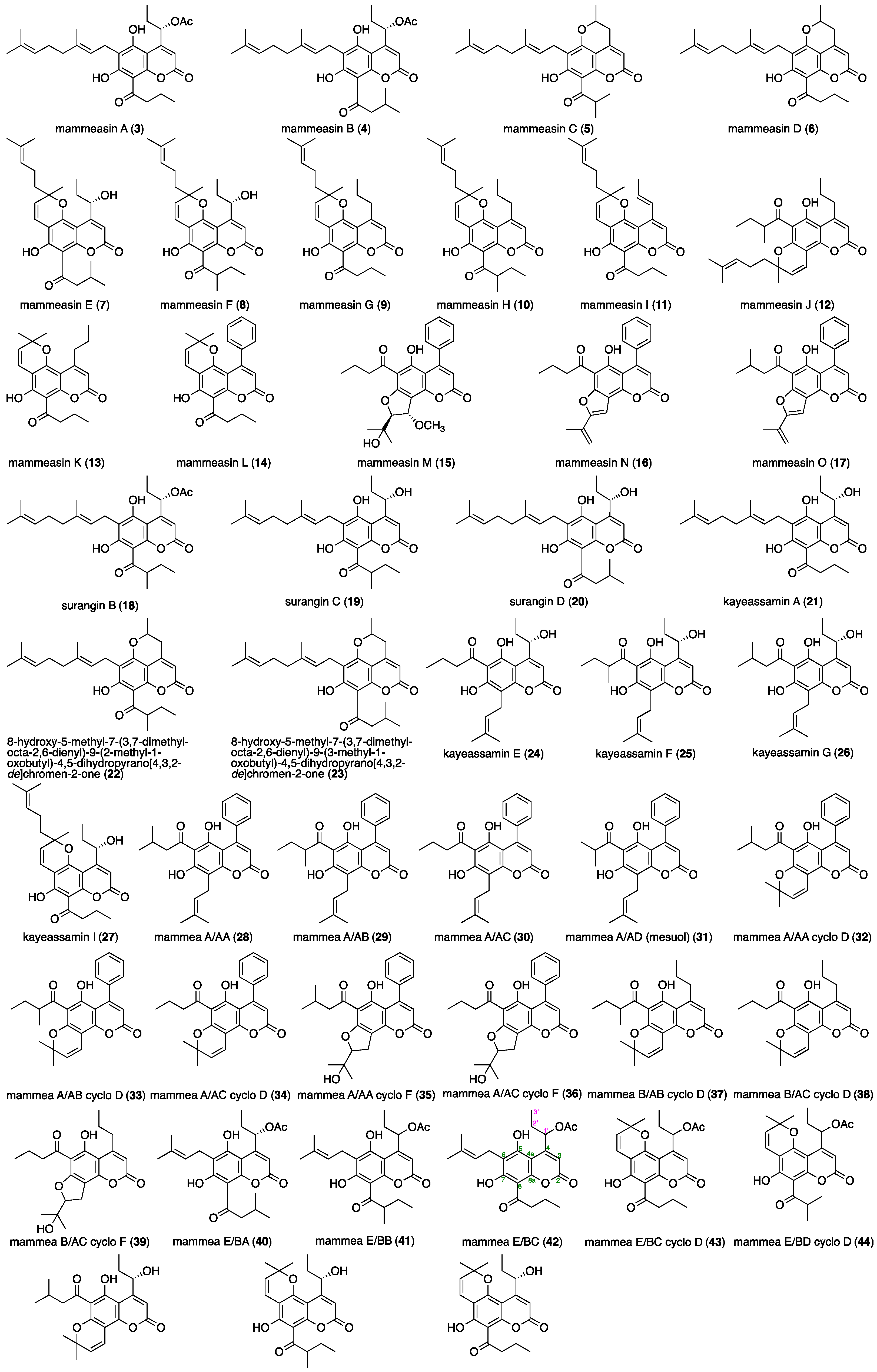
2.2. Isolation
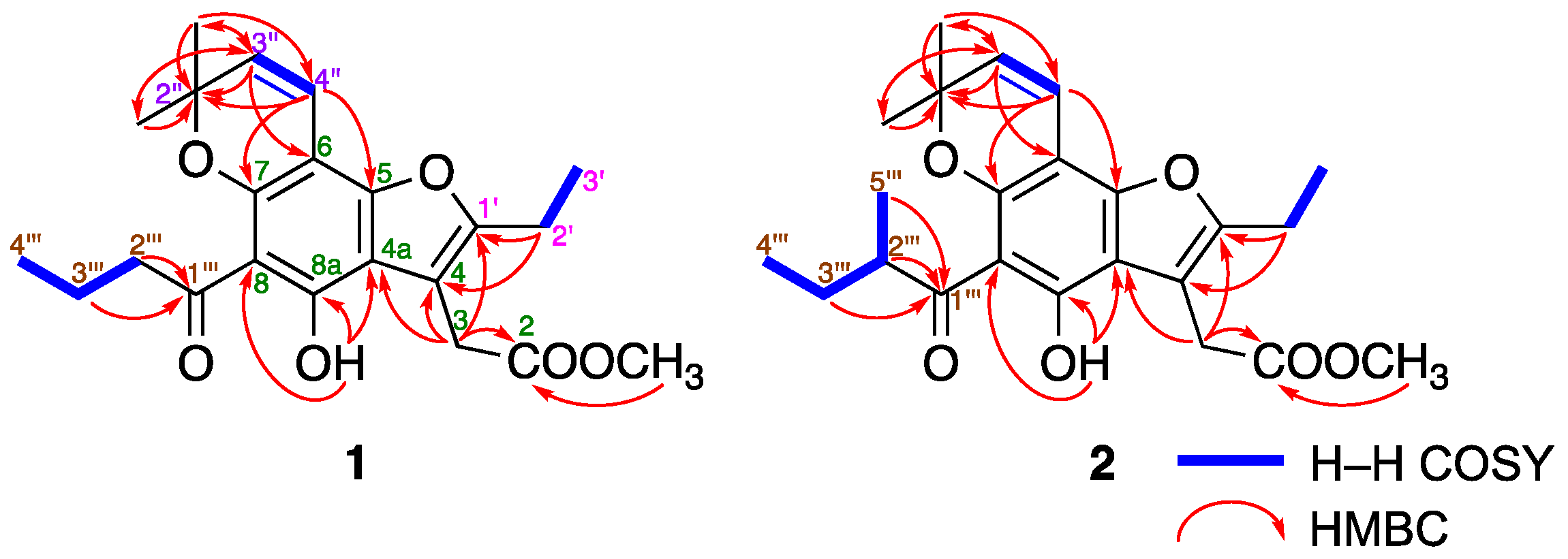
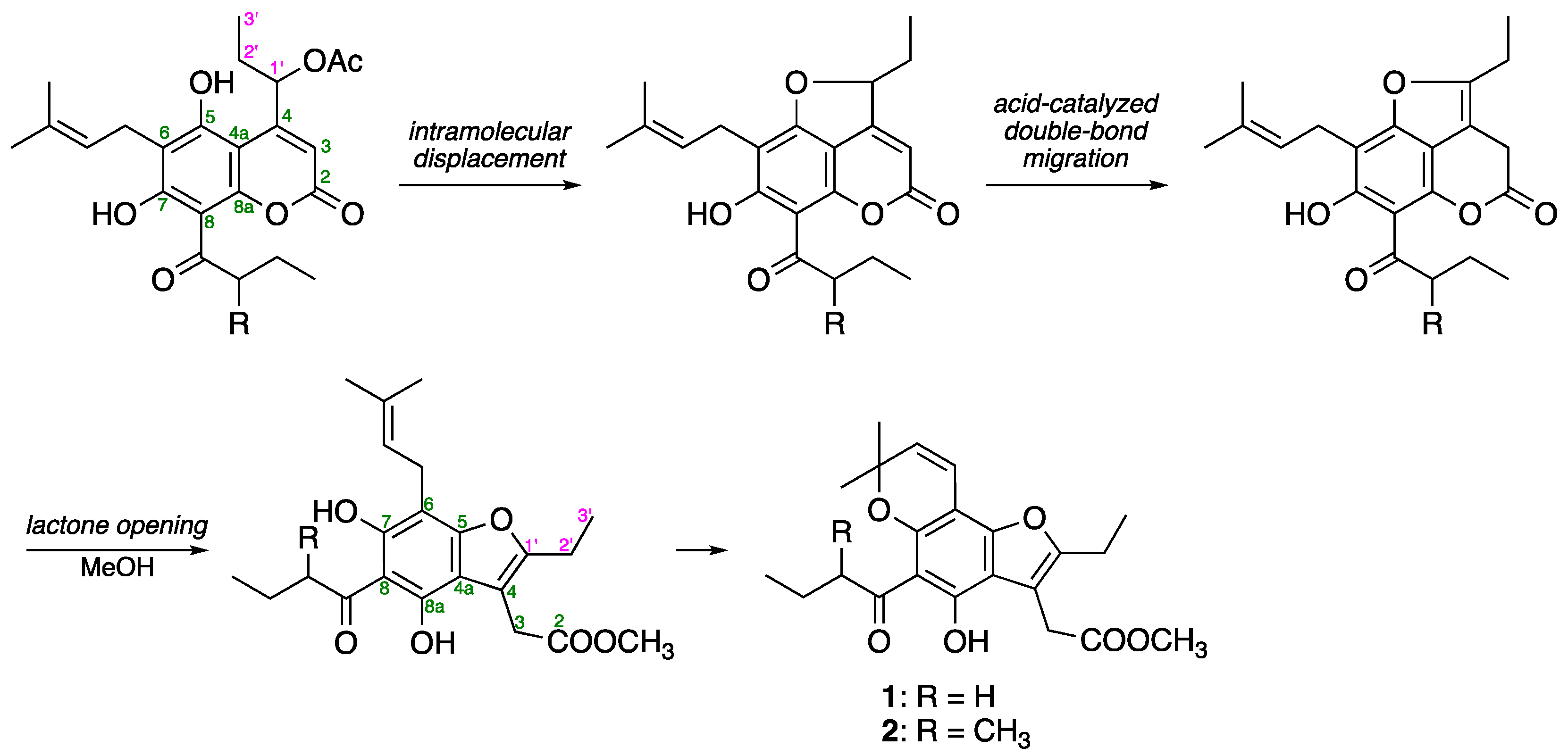
2.3. Structure Determination for Mammeasins P (1) and Q (2)
2.4. Anti-Proliferative Effects of the Coumarin Constituents against Human Prostate Carcinoma LNCaP Cells
3. Materials and Methods
3.1. Spectroscopy and Column Chromatography
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Mammeasin P (1)
3.3.2. Mammeasin Q (2)
3.4. Bioassay
3.4.1. Reagents
3.4.2. Cell Culture Assay
3.4.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agoulnik, I.U.; Weigel, N.L. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J. Cell. Biochem. 2006, 99, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef]
- Sawada, N. Risk and preventive factors for prostate cancer in Japan: The Japan public health center-based prospective (JPHC) study. J. Epidemiol. 2017, 27, 2–7. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Erdogan, S.; Dognalar, O.; Dogaanlar, Z.B.; Turkekul, K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- The World Flora Online. Mammea siamensis (Miq.) T. Anderson. Available online: http://www.worldfloraonline.org/taxon/wfo-0000376586 (accessed on 29 January 2023).
- Manneesri, J.; Masniyom, P.; Pongpiriyadacha, Y. Bacterial cellulose film containing flavonoids from “Sarapee” (Mammea siamensis) flower extract against Salmonella typhimurium TISTR 292. J. Agric. Sci. Technol. A 2012, 2, 86–89. [Google Scholar]
- Poobrasert, O.; Constant, H.L.; Beecher, C.W.; Farnsworth, N.R.; Kinghorn, A.D.; Pezzuto, J.M.; Cordell, G.A.; Santisuk, T.; Reutrakul, V. Xanthones from the twigs of Mammea siamensis. Phytochemistry 1998, 47, 1661–1663. [Google Scholar] [CrossRef]
- Uto, T.; Tung, N.H.; Thongjankaew, P.; Lhieochaiphant, S.; Shoyama, Y. Kayeassamine A isolated from the flower of Mammea siamensis triggers apoptosis by activating caspase-3/-8 in HL-60 human leukemia cells. Pharcogn. Res. 2016, 8, 244–248. [Google Scholar] [CrossRef]
- Chaniad, P.; Chukaew, A.; Payaka, A.; Phuwajaroanpong, A.; Techarang, T.; Plirat, W.; Punsawad, C. Antimalarial potential of compounds isolated from Mammea siamensis T. Anders. flowers.: In vitro and molecular docking studies. BMC Comlement. Med. Ther. 2022, 22, 226. [Google Scholar] [CrossRef]
- Fujii, K.; Hara, Y.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Natural compounds with BMI1 promoter inhibitory activity from Mammea siamensis and Andrographis paniculate. Chem. Pharm. Bull. 2022, 70, 885–891. [Google Scholar] [CrossRef]
- Subhadirasakul, S.; Pechpongs, P.A. A terpenoid and two steroids from the flowers of Mammea siamensis. Songklanakarin J. Sci. Technol. 2005, 27, 555–561. [Google Scholar]
- Sangkaruk, R.; Rungrojsakul, M.; Tima, S.; Anuchapreede, S. Effect of Thai saraphi flower extracts on WT1 and Bcr/Abl protein expression in leukemic cell lines. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 16–24. [Google Scholar] [CrossRef]
- Kaweetripob, W.; Mahidol, C.; Prawat, H.; Puchirawat, S. Chemical investigation of Mammea siamensis. Pharm. Biol. 2000, 38, 55–57. [Google Scholar] [CrossRef]
- Prachyawarakorn, V.; Mahidol, C.; Ruchirawat, S. NMR study of seven coumarins from Mammea siamansis. Pharm. Biol. 2000, 38, 58–62. [Google Scholar] [CrossRef]
- Mahidol, C.; Kaweetripob, W.; Prawat, H.; Ruchirawat, S. Mammea coumarins from the flowers of Mammea siamensis. J. Nat. Prod. 2002, 65, 757–760. [Google Scholar] [CrossRef]
- Prachyawarakorn, V.; Mahidol, C.; Ruchirawai, S. Siamenols A–D, four new coumarins from Mammea siamensis. Chem. Pharm. Bull. 2006, 54, 884–886. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Maneerat, W.; Kiattansakul, R. Phenolic compounds from Mammea siamensis seeds. Can. J. Chem. 2006, 84, 1546–1549. [Google Scholar] [CrossRef]
- Prachyawarakorn, V.; Mahidol, C.; Ruchirawat, S. Pyranocoumarins from the twigs of Mammea siamensis. Phytochemistry 2006, 67, 924–928. [Google Scholar] [CrossRef]
- Mahidol, C.; Prawat, H.; Kaweetripob, W.; Ruchirawat, S. Regioisomers of acylcoumarins from the flowers of Mammea siamensis. Nat. Prod. Commun. 2007, 2, 557–564. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Promnart, P.; Syers, J.K.; Kanjana-Opas, A.; Ponglimanont, C.; Karalai, C. Coumarins and xanthones from the seeds of Mammea siamensis. J. Braz. Chem. Soc. 2007, 18, 1077–1080. [Google Scholar] [CrossRef]
- Ngo, N.T.N.; Nguyen, V.T.; Van Vo, H.; Vang, O.; Duus, F.; Ho, T.-D.H.; Pham, H.D.; Nguyen, L.-H.D. Cytotoxic coumarins from the bark of Mammea siamensis. Chem. Pharm. Bull. 2010, 58, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.H.; Uto, T.; Sakamoto, A.; Hayashida, Y.; Hidaka, Y.; Morinaga, O.; Lhieochaiphant, S.; Shoyama, Y. Antiproliferative and apoptotic effects of compounds from the flower of Mammea siamensis (Miq.) T. Anders. on human cancer cell lines. Bioorg. Med. Chem. 2013, 23, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Noysang, C.; Mahringer, A.; Zeino, M.; Saeed, M.; Luanratana, O.; Fricker, G.; Bauer, R.; Efferth, T. Cytotoxicity and inhibition of P-glycoprotein by selected medicinal plants from Thailand. J. Ethnopharmacol. 2014, 155, 633–641. [Google Scholar] [CrossRef]
- Morikawa, T.; Sueyoshi, M.; Chaipech, S.; Matsuda, H.; Nomura, Y.; Yabe, M.; Matsumoto, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; et al. Suppressive effects of coumarins from Mammea siamensis on inducible nitric oxide synthase expression in RAW264.7 cells. Bioorg. Med. Chem. 2012, 20, 4968–4977. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Shibatani, K.; Sueyoshi, M.; Chaipech, S.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Aromatase inhibitory activity of geranylated coumarins, mammeasins C and D, isolated from the flowers of Mammea siamensis. Chem. Pharm. Bull. 2016, 64, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Manse, Y.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Phytochemicals with chemopreventive activity obtained from the Thai medicinal plant Mammea siamensis (Miq.) T. Anders.: Isolation and structure determination of new prenylcoumarins with inhibitory activity against aromatase. Int. J. Mol. Sci. 2022, 23, 11233. [Google Scholar] [CrossRef]
- Morikawa, T.; Luo, F.; Manse, Y.; Sugita, H.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Ninomiya, K. Geranylated coumarins from Thai medicinal plant Memmea siamensis with testosterone 5α-reductase inhibitory activity. Front. Chem. 2020, 8, 199. [Google Scholar] [CrossRef]
- Luo, F.; Sugita, H.; Muraki, K.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Anti-proliferative activities of coumarins from the Thai medicinal plant Mammea siamensis (Miq.) T. Anders. Against human digestive tract carcinoma cell lines. Fitoterapia 2021, 148, 104780. [Google Scholar] [CrossRef]
- Mayers, R.B.; Parker, M.; Grizzle, W.E. The effects of coumarin and suramin on the growth of malignant renal and prostate cell lines. J. Cancer Res. Clin. Oncol. 1994, 120 (Suppl. 1), S11–S13. [Google Scholar] [CrossRef]
- Han, H.-Y.; Wen, P.; Liu, H.-W.; Wang, N.-L.; Yao, X.-S. Coumarins from Campylotropis hirtella (Franch.) Schindl. And their inhibitory activity on prostate specific antigen secreted from LNCaP cells. Chem. Pharm. Bull. 2008, 56, 1338–1341. [Google Scholar] [CrossRef]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The LNCaP cell line–A new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar] [PubMed]
- Chuu, C.-P.; Kokontis, J.M.; Hiipakka, R.A.; Liao, S. Modulation of liver X receptor signalling as novel therapy for prostate cancer. J. Biomed. Sci. 2007, 14, 543–553. [Google Scholar] [CrossRef]
- Chuu, C.-P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.-P.; Lim, C.-Y.; Huo, C.; Su, L.-C. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J. Biomed. Sci. 2011, 18, 63. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of prostate cancer models for preclinical study: Advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef]
- Crombie, L.; Jones, R.C.F.; Palmer, C.J. Synthesis of the insecticidal 1'-acetoxy-mammeins and surangin B. Tetrahedron Lett. 1985, 26, 2933–2936. [Google Scholar] [CrossRef]
- Crombie, L.; Jones, R.C.F.; Palmer, C.J. Synthesis of the Mammea coumarins. Part 2. Experiments in the mammea E series and synthesis of mammea E/AC. J. Chem. Soc. Perkin Trans. I 1987, 333–343. [Google Scholar] [CrossRef]
- Liew, S.Y.; Looi, C.Y.; Paydar, M.; Cheah, F.K.; Leong, K.H.; Wong, W.F.; Mustafa, M.R.; Litaudon, M.; Awang, K. Subditine, a new monoterpenoid indole alkaloid from bark of Nauclea subdita (Korth.) steud. Induces apoptosis in human prostate cancer cells. PLoS ONE 2014, 9, e87286. [Google Scholar] [CrossRef]
- Pandey, S.; Walpole, C.; Shaw, P.N.; Cabot, P.J.; Hewavitharana, A.K.; Batra, J. Bio-guided fractionation of papaya leaf juice for delineation the components responsible for the selective anti-proliferative effects on prostate cancer cells. Front. Pharmacol. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Tanabe, G.; Tsutsui, N.; Shibatani, K.; Marumoto, S.; Ishikawa, F.; Ninomiya, K.; Muraoka, O.; Morikawa, T. Total syntheses of the aromatase inhibitors, mammeasins C and D, from Thai medicinal plant Mammea siamensis. Tetrahedron 2017, 73, 4481–4486. [Google Scholar] [CrossRef]
- Tindall, D.J.; Rittmaster, R.S. The rationale for inhibiting 5α-reductase isoenzymes in the prevention and treatment of prostate cancer. J. Urol. 2008, 179, 1235–1242. [Google Scholar] [CrossRef]
- Chau, C.H.; Figg, W.D. Revisiting 5α-reductase inhibitors and the risk of prostate cancer. Nat. Rev. Urol. 2018, 15, 400–401. [Google Scholar] [CrossRef]
- Wallerstedt, A.; Strom, P.; Gronberg, H.; Nordstrom, T.; Eklund, M. Risk of prostate cancer in men treated with 5α-reductase inhibitors–A large population-based prospective study. J. Natl. Cancer Inst. 2018, 110, 1216–1221. [Google Scholar] [CrossRef]
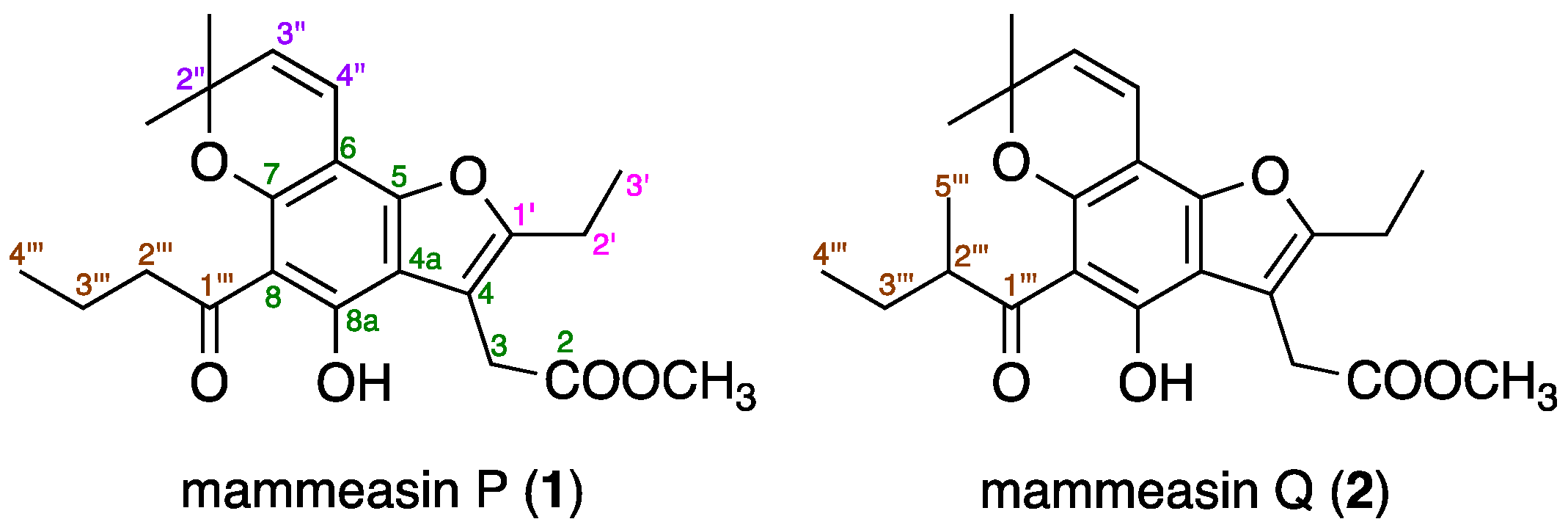
| Treatment | Inhibition (%) | IC50 | ||||
|---|---|---|---|---|---|---|
| 0 μg/mL | 0.3 μg/mL | 1 μg/mL | 3 μg/mL | 10 μg/mL | (μg/mL) | |
| MeOH extract | 100.0 ± 1.7 | 96.2 ± 2.3 | 87.8 ± 2.0 ** | 24.5 ± 1.7 ** | 7.3 ± 0.1 ** | 2.0 |
| EtOAc-soluble fraction | 100.0 ± 1.3 | 97.7 ± 2.0 | 97.0 ± 2.3 | 42.7 ± 1.5 ** | 7.6 ± 0.2 ** | 2.7 |
| 0 μg/mL | 3 μg/mL | 10 μg/mL | 30 μg/mL | 100 μg/mL | ||
| MeOH-eluted fraction | 100.0 ± 2.0 | 99.4 ± 4.6 | 95.8 ± 2.2 | 38.1 ± 1.5 ** | 11.9 ± 0.0 ** | 23.8 |
| H2O-eluted fraction | 100.0 ± 6.1 | 99.7 ± 6.3 | 94.8 ± 4.1 | 86.1 ± 3.1 | 72.4 ± 2.5 ** | >100 |
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 171.9 | 171.9 | ||
| 3 | 3.75 (2H, s) | 29.8 | 3.75 (2H, s) | 29.7 |
| 4 | 107.7 | 107.7 | ||
| 4a | 111.1 | 111.2 | ||
| 5 | * 152.2 | * 151.9 | ||
| 6 | 99.1 | 99.1 | ||
| 7 | * 154.6 | * 154.5 | ||
| 8 | 107.0 | 107.0 | ||
| 8a | 159.8 | 159.8 | ||
| 2-COOCH3 | 3.71 (3H, s) | 52.1 | 3.71 (3H, s) | 52.1 |
| 8a-OH | 14.12 (1H, s) | 14.09 (1H, s) | ||
| 1’ | 155.5 | 155.5 | ||
| 2’ | 2.67 (2H, q, 7.6) | 19.4 | 2.67 (2H, q, 7.5) | 19.5 |
| 3’ | 1.27 (3H, t, 7.6) | 12.8 | 1.27 (3H, t, 7.5) | 12.8 |
| 2’’ | 78.1 | 78.1 | ||
| 3’’ | 5.51 (1H, d, 9.7) | 125.7 | 5.51 (1H, d, 9.8) | 125.7 |
| 4’’ | 6.65 (1H, d, 9.7) | 116.1 | 6.65 (1H, d, 9.8) | 116.1 |
| 2’’-CH3 × 2 | 1.52 (6H, s) | 27.6 | 1.52 (6H, s) | 27.6 |
| 1’’’ | 207.5 | 211.8 | ||
| 2’’’ | 3.07 (2H, t, 7.4) | 128.4 | 3.81 (1H, m) | 46.2 |
| 3’’’ | 1.73 (2H, qt, 7.4, 7.4) | 18.5 | 1.42, 1.87 (each 1H, both m) | 26.8 |
| 4’’’ | 1.01 (3H, t, 7.4) | 14.0 | 0.92 (3H, t, 7.5) | 11.9 |
| 5’’’ | 1.17 (3H, d, 6.9) | 16.9 | ||
| Treatment | IC50 (µM) | Treatment | IC50 (µM) |
|---|---|---|---|
| Mammeasin A (3) | 1.2 | Kayeassamin G (26) | 3.5 |
| Mammeasin B (4) | 0.63 | Kayeassamin I (27) | 16.1 |
| Mammeasin C (5) | 30.5 | Mammea A/AA (28) | 51.9 |
| Mammeasin D (6) | 25.0 | Mammea A/AC (30) | 26.2 |
| Mammeasin E (7) | 5.9 | Mammea A/AB cyclo D (33) | >100 (82.7) (a) |
| Mammeasin F (8) | 16.7 | Mammea A/AC cyclo D (34) | >100 (90.0) (a) |
| Mammeasin G (9) | 83.5 | Mammea A/AA cyclo F (35) | 21.3 |
| Mammeasin H (10) | 69.4 | Mammea A/AC cyclo F (36) | 39.7 |
| Mammeasin I (11) | ca 100 | Mammea B/AB cyclo D (37) | 61.9 |
| Mammeasin J (12) | >100 (86.9) (a) | Mammea B/AC cyclo D (38) | >100 (78.4) (a) |
| Mammeasin K (13) | >100 (79.9) (a) | Mammea E/BA (40) | 0.88 |
| Mammeasin L (14) | 49.4 | Mammea E/BB (41) | 0.52 |
| Mammeasin M (15) | >100 (91.3) (a) | Mammea E/BC (42) | 0.12 |
| Mammeasin N (16) | ca 100 | Mammea E/BC cyclo D (43) | 23.1 |
| Mammeasin O (17) | 35.2 | Mammea E/BD cyclo D (44) | 53.9 |
| Surangin B (18) | 1.5 | Deacetylmammea E/AA cyclo D (45) | 25.9 |
| Surangin C (19) | 11.8 | Deacetylmammea E/BB cyclo D (46) | 34.0 |
| Surangin D (20) | 24.7 | Deacetylmammea E/BC cyclo D (47) | 19.7 |
| Kayeassamin E (24) | 3.0 | IC50 (nM) | |
| Kayeassamin F (25) | 6.2 | Paclitaxel (b) [40,41] | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, F.; Manse, Y.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Structures of Mammeasins P and Q, Coumarin-Related Polysubstituted Benzofurans, from the Thai Medicinal Plant Mammea siamensis (Miq.) T. Anders.: Anti-Proliferative Activity of Coumarin Constituents against Human Prostate Carcinoma Cell Line LNCaP. Pharmaceuticals 2023, 16, 231. https://doi.org/10.3390/ph16020231
Luo F, Manse Y, Chaipech S, Pongpiriyadacha Y, Muraoka O, Morikawa T. Structures of Mammeasins P and Q, Coumarin-Related Polysubstituted Benzofurans, from the Thai Medicinal Plant Mammea siamensis (Miq.) T. Anders.: Anti-Proliferative Activity of Coumarin Constituents against Human Prostate Carcinoma Cell Line LNCaP. Pharmaceuticals. 2023; 16(2):231. https://doi.org/10.3390/ph16020231
Chicago/Turabian StyleLuo, Fenglin, Yoshiaki Manse, Saowanee Chaipech, Yutana Pongpiriyadacha, Osamu Muraoka, and Toshio Morikawa. 2023. "Structures of Mammeasins P and Q, Coumarin-Related Polysubstituted Benzofurans, from the Thai Medicinal Plant Mammea siamensis (Miq.) T. Anders.: Anti-Proliferative Activity of Coumarin Constituents against Human Prostate Carcinoma Cell Line LNCaP" Pharmaceuticals 16, no. 2: 231. https://doi.org/10.3390/ph16020231
APA StyleLuo, F., Manse, Y., Chaipech, S., Pongpiriyadacha, Y., Muraoka, O., & Morikawa, T. (2023). Structures of Mammeasins P and Q, Coumarin-Related Polysubstituted Benzofurans, from the Thai Medicinal Plant Mammea siamensis (Miq.) T. Anders.: Anti-Proliferative Activity of Coumarin Constituents against Human Prostate Carcinoma Cell Line LNCaP. Pharmaceuticals, 16(2), 231. https://doi.org/10.3390/ph16020231







