Abstract
P2Y12 inhibitor monotherapy is a feasible alternative treatment for patients after percutaneous coronary intervention (PCI) in the modern era. Clinical trials have shown that it could lower the risk of bleeding complications without increased ischemic events as compared to standard dual antiplatelet therapy (DAPT). However, the efficacy and safety of this novel approach among patients with acute coronary syndrome (ACS) are controversial because they have a much higher risk for recurrent ischemic events. The purpose of this study is to evaluate the efficacy and safety of this novel approach among patients with ACS. We conducted a meta-analysis of randomized controlled trials that compared P2Y12 inhibitor monotherapy with 12-month DAPT in ACS patients who underwent PCI with stent implantation. PubMed, Embase, the Cochrane library database, ClinicalTrials.gov, and other three websites were searched for data from the earliest report to July 2022. The primary efficacy outcome was major adverse cardiovascular and cerebrovascular events (MACCE), a composite of all-cause mortality, myocardial infarction, stent thrombosis, or stroke. The primary safety outcome was major or minor bleeding events. The secondary endpoint was net adverse clinical events (NACE), defined as a composite of major bleeding and adverse cardiac and cerebrovascular events. Five randomized controlled trials with a total of 21,034 patients were included in our meta-analysis. The quantitative analysis showed a significant reduction in major or minor bleeding events in patients treated with P2Y12 inhibitor monotherapy as compared with standard DAPT(OR: 0.59, 95% CI: 0.46–0.75, p < 0.0001) without increasing the risk of MACCE (OR: 0.98, 95% CI: 0.86–1.13, p = 0.82). The NACE was favorable in the patients treated with P2Y12 inhibitor monotherapy (OR: 0.82, 95% CI: 0.73–0.93, p = 0.002). Of note, the overall clinical benefit of P2Y12 inhibitor monotherapy was quite different between ticagrelor and clopidogrel. The incidence of NACE was significantly lower in ticagrelor monotherapy as compared with DAPT (OR: 0.79, 95% CI: 0.68–0.91), but not in clopidogrel monotherapy (OR: 1.14, 95% CI: 0.79–1.63). Both clopidogrel and ticagrelor monotherapy showed a similar reduction in bleeding complications (OR: 0.46, 95% CI: 0.22–0.94; OR: 0.60, 95% CI: 0.44–0.83, respectively). Although statistically insignificant, the incidence of MACCE was numerically higher in clopidogrel monotherapy as compared with standard DAPT (OR: 1.50, 95% CI: 0.99–2.28, p = 0.06). Based on these findings, P2Y12 inhibitor monotherapy with ticagrelor would be a better choice of medical treatment for ACS patients after PCI with stent implantation in the current era.
1. Introduction
Based on the results of CURE study and concerning stent thrombosis with first-generation drug-eluting stents (DES), 12-months of dual antiplatelet therapy (DAPT) has been the standard care for ACS patients after percutaneous coronary intervention (PCI) and stent implantation for the last few decades [1,2]. Although DAPT could reduce the risk of ischemic events, it also increases the risk of bleeding complications. These bleeding events used to be assumed to be benign, but recent studies had shown that post-PCI bleeding was associated with a substantial risk of recurrent ischemic events and increased mortality [3,4]. With the advent of newer-generation DESs and the advancement of PCI techniques, the risk of stent thrombosis is much lower than before. Some researchers had challenged this standard treatment strategy by trying to shorten the duration of DAPT with the continuation of aspirin monotherapy. Although some studies had shown the safety of this approach, they generally enrolled predominantly low-risk patients and excluded ACS patients. Unlike stable coronary artery disease (CAD) or chronic coronary syndrome (CCS), ACS patients have higher platelet reactivity and risk of recurrent ischemic events in the first year after PCI [5,6,7]. Recent SMART-DATE trial and meta-analysis had tried to shorten the duration of P2Y12 inhibitor in ACS patients, but they all failed [8,9]. The ischemic events were significantly increased once the duration of DAPT was shortened. Therefore, 12-months of DAPT is still strongly recommended for all ACS patients in the current guidelines if there is no specific contraindication for DAPT [10,11,12].
The P2Y12 receptor, a G-protein-coupled receptor (GPCR) coupled to the inhibitory G protein Gαi2, is a platelet ADP-receptor. The activation of platelet P2Y12 receptors by ADP leads to an inhibition of adenylyl cyclase and additional downstream events including the activation of phosphatidylinositol-3-kinase and the inhibition of Ras GTPase-activating protein 3 (RASA3) to promote GTPase Rap1b activity and integrin activation. These reactions eventually cause the amplification and stabilization of platelet aggregation [13]. By blocking P2Y12 receptors and ADP-induced platelet activation, P2Y12 inhibitors demonstrate potent antiplatelet effects. P2Y12 inhibitor monotherapy is a novel treatment strategy that shortens the duration of DAPT to 1–3 months and continues with a P2Y12 inhibitor instead of aspirin. The scientific rationale of this treatment strategy is to use a more potent antiplatelet agent to prevent recurrent ischemic events and avoid potential gastrointestinal side effects caused specifically by aspirin. This novel approach has been tested in several large randomized controlled studies, and nearly all of them had favorable outcomes [14,15,16,17]. Overall, P2Y12 inhibitor monotherapy could lower the risk of bleeding complications without increasing the ischemic events in the general population after PCI and stent implantation [18]. However, whether this novel approach could apply to ACS patients remains under debate,. In particular, the concern of a much higher risk of recurrent ischemic events in ACS patients than in stable CAD patients and the inconsistent antiplatelet effect of clopidogrel concern clinicians [19,20]. The purpose of this study is to evaluate the efficacy and safety of this novel approach among patients with ACS. We conducted a meta-analysis of randomized controlled trials that compared P2Y12 inhibitor monotherapy with 12-month DAPT in ACS patients who underwent PCI with stent implantation.
2. Results
2.1. Search Results and Characteristics of Included Trials
The results of the database search and study selection are shown in Figure 1. A total of 2289 records were identified from the databases and websites mentioned above. Of these, 74 full-text articles were reviewed, and 69 of them were excluded for not meeting the pre-specified inclusion criteria. In the end, five randomized controlled trials with a total of 21,034 patients were included in this meta-analysis. The main characteristics and outcomes of the included trials were summarized in Table 1. There were 10,556 patients who received standard 12-month DAPT, and 10,478 patients received P2Y12 inhibitor monotherapy after PCI and stent implantation. The ischemic and bleeding events of each trial are summarized in Table 2.
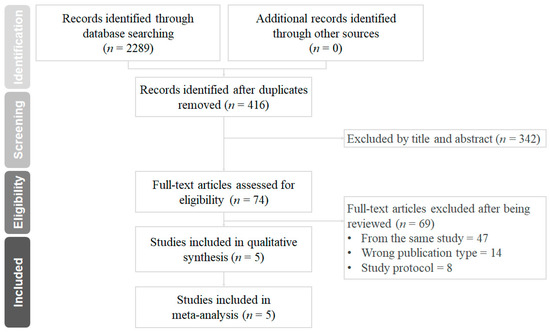
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram for the searching and identification of included studies.

Table 1.
Clinical characteristics and outcomes of included randomized trials.

Table 2.
The efficacy and safety outcomes of P2Y12 inhibitor monotherapy and DAPT in ACS patients of included trials.
2.2. The Primary and Secondary Outcomes
The quantitative analysis of primary and secondary outcomes is shown in Figure 2. In ACS patients, P2Y12 inhibitor monotherapy did not increase the risk of MACCE as compared with standard 12-month DAPT (OR: 0.98, 95% CI: 0.86–1.13, p = 0.82, I2 = 41%, PHeterogeneity = 0.15) (Figure 2A), but the risk of major or minor bleeding events was significantly lower in patients treated with P2Y12 inhibitor monotherapy (OR: 0.59, 95% CI: 0.46–0.75, p < 0.0001, I2 = 58%, PHeterogeneity = 0.05) (Figure 2B). The quantitative analysis of NACE demonstrated a significantly favorable result for P2Y12 inhibitor monotherapy (OR: 0.82, 95% CI: 0.73–0.93, p = 0.002, I2 = 29%, PHeterogeneity = 0.23) in patients with ACS after PCI (Figure 2C).
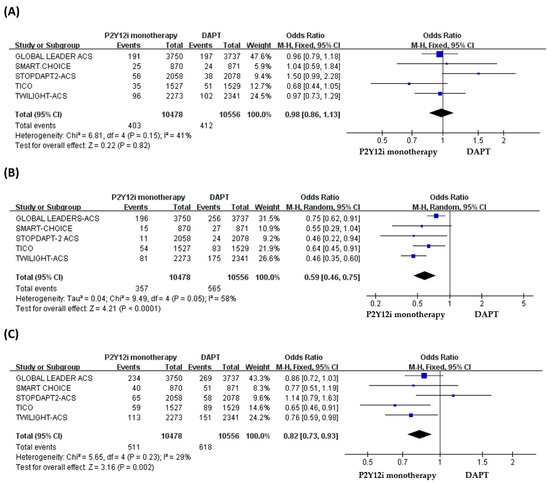
Figure 2.
The efficacy and safety of P2Y12 inhibitor monotherapy in patients with ACS after PCI as compared with 12-month DAPT. (A) MACCE, (B) bleeding events, (C) NACE.
2.3. Subgroup Analysis of Different P2Y12 Inhibitors
The efficacy of P2Y12 inhibitor monotherapy was quite different among patients receiving ticagrelor from those receiving clopidogrel. Although statistically insignificant, patients receiving clopidogrel monotherapy were associated with a trend of higher risk of MACCE as compared with standard DAPT (OR: 1.50, 95% CI: 0.99–2.28, p = 0.06), whereas patients receiving ticagrelor monotherapy were associated with a favorable result (OR: 0.92, 95% CI: 0.78–1.09, p = 0.34) (Figure 3). Both clopidogrel monotherapy and ticagrelor monotherapy showed a similar reduction in the risk of bleeding events (OR: 0.46, 95% CI: 0.22–0.94; OR: 0.60, 95% CI: 0.44–0.83, respectively) (Figure 4). Overall, the NACE was significantly lower in ticagrelor monotherapy (OR: 0.79, 95% CI: 0.69–0.91, p = 0.001) as compared with standard DAPT. However, the NACE was no different between clopidogrel monotherapy and standard DAPT (OR: 1.14, 95% CI: 0.79–1.63, p = 0.49) (Figure 5). The SMART-CHOICE study was not included in the subgroup analysis because there was no available reported data on different P2Y12 inhibitors.
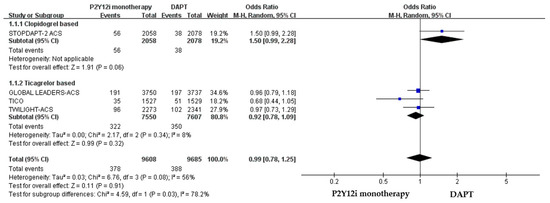
Figure 3.
The efficacy outcomes (MACCE) of different P2Y12 inhibitor monotherapy as compared with 12-month DAPT in patients with ACS after PCI.
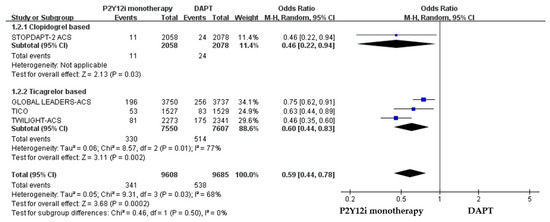
Figure 4.
The safety outcomes (major or minor bleeding events) of different P2Y12 inhibitor monotherapy as compared with 12-month DAPT in patients with ACS after PCI.
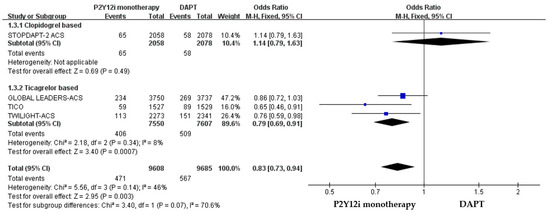
Figure 5.
The NACE of different P2Y12 inhibitor monotherapy as compared with 12-month DAPT in patients with ACS after PCI.
2.4. Extrapolatory Analysis of P2Y12 Inhibitor Monotherapy in Non-ACS Patients as Compared with ACS Patients
The primary efficacy and safety outcomes of P2Y12 inhibitor monotherapy in non-ACS versus non-ACS patients in these included clinical trials are summarized in Table 3. The quantitative analysis of the clinical outcomes in non-ACS patients is demonstrated in Figure 6. P2Y12 inhibitor monotherapy was associated with a favorable result of reducing major bleeding as compared with 12-month DAPT, but statistically insignificant (OR: 0.83, 95% CI: 0.66–1.05, p = 0.13). The 1-year rate of ischemic events was similar between P2Y12 inhibitor monotherapy and DAPT in both non-ACS (OR: 1.03, 95% CI: 0.85–1.25, p = 0.78).

Table 3.
The efficacy and safety outcomes of P2Y12 inhibitor monotherapy in ACS versus non-ACS patients of included trials.
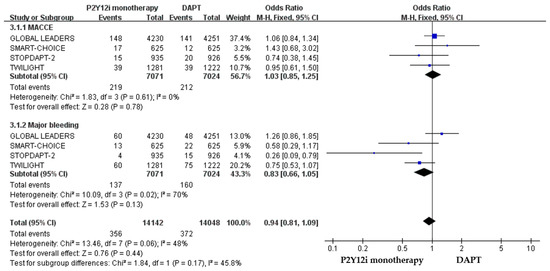
Figure 6.
The efficacy and safety of P2Y12 inhibitor monotherapy in patients without ACS after PCI as compared with 12-month DAPT.
2.5. Extraploartory Analysis of P2Y12 Inhibitor Monotherapy in STE-ACS Patients as Compared with NSTE-ACS Patients
The efficacy and safety outcomes of P2Y12 inhibitor monotherapy in STE-ACS versus NSTE-ACS patients in these included clinical trials were demonstrated in Figure 7 and Figure 8. The incidence of ischemic events was similar between P2Y12 inhibitor monotherapy and DAPT in both STE-ACS (OR: 1.13, 95% CI: 0.86–1.48, p = 0.38) and NSTE-ACS patients (OR: 0.89, 95% CI: 0.76–1.05, p = 0.17). P2Y12 inhibitor monotherapy significantly reduced bleeding events as compared with 12-month DAPT in both STE-ACS (OR: 0.68, 95% CI: 0.49–0.96, p = 0.03) and NSTE-ACS patients (OR: 0.60, 95% CI: 0.51–0.71, p < 0.0001). The SMART-CHOICE study was not included in the subgroup analysis because there was no available reported data on the different diagnoses of ACS.
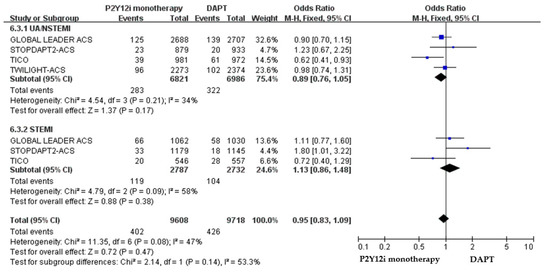
Figure 7.
The efficacy outcomes (MACCE) of P2Y12 inhibitor monotherapy in STE-ACS patients and NSTE-ACS after PCI as compared with 12-month DAPT.
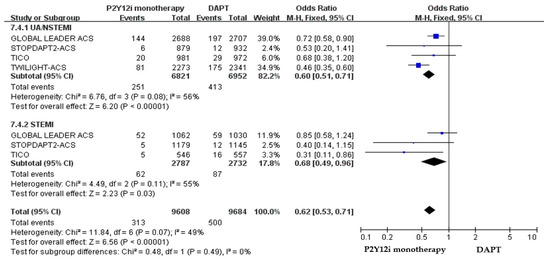
Figure 8.
The safety outcomes (major or minor bleeding events) of P2Y12 inhibitor monotherapy as compared with 12-month DAPT in patients with STE-ACS and NSTE-ACS patients after PCI.
2.6. Quality Assessment and Publication Bias
The overall risk of bias in selection, detection, and reporting bias was low. The detailed quality assessment and risk of bias assessment per study can be found in Supplementary Table S4. All studies in this meta-analysis were randomized controlled trials, but only TWILIGHT-ACS was double-blinded. There was no publication bias in all outcomes. The outcomes of included trials are distributed symmetrically in the funnel plot (Supplementary Figure S1), and heterogeneity was low in all outcomes.
3. Materials and Methods
3.1. Data Sources and Searching
This meta-analysis was conducted following the guidelines of the Preferred Reporting Items for a Systematic review and Meta-analysis (PRISMA) and Cochrane Collaboration. Our protocol was registered on PROSPERO (international prospective register of systematic reviews) and is available online (www.crd.york.ac.uk/prospero, accessed on 29 March 2022, CRD42022312669). We searched PubMed, Embase, the Cochrane library database, ClinicalTrials.gov, and three other websites (www.tctmd.com, www.acc.org/cardiosourceplus, and www.escardio.org) from the earliest record to July 2022. The search terms used included: “P2Y12 inhibitor monotherapy”, “dual antiplatelet therapy”, “randomized trial”, “percutaneous coronary intervention”, “outcome”, and “acute coronary syndrome”. No language restriction was applied.
3.2. Study Selection
The inclusion criteria of the selected study were: (1) ACS patients who underwent PCI with stent implantation, (2) randomized controlled trial (or subgroup analysis of randomized controlled trial), (3) comparing P2Y12 inhibitor monotherapy to standard 12-month dual antiplatelet therapy, (4) follow up patients’ clinical outcomes for at least 12 months, and (5) the study reported the primary efficacy and safety outcomes of interest. The exclusion criteria were: (1) a non-randomized controlled trial, (2) studies not reporting the data of patients with ACS, and (3) ongoing studies or lack of clinical endpoints data, and studies not available in full text were also excluded.
All of the retrieved articles were screened by three reviewers to identify all of the potentially eligible studies.
3.3. Data Extraction and Clinical Outcomes
The baseline characteristics and outcome data of the included studies were independently extracted by multiple reviewers, and the discrepancy was resolved through negotiation. The primary efficacy outcome was major adverse cardiovascular and cerebrovascular events (MACCE), a composite of all-cause mortality, myocardial infarction, stent thrombosis, or stroke. The primary safety outcome was major or minor bleeding events. The secondary endpoint was net adverse clinical event (NACE), defined as a composite of major bleeding and adverse cardiac and cerebrovascular events. A separate subgroup analysis on the type of P2Y12 inhibitor was performed.
3.4. Assessment of Risk of Bias
The quality of each study is independently evaluated by the first and second authors (Wen-Han Feng and Yong-Chieh Chang) by using the Cochrane Collaboration tool. Discrepancies were solved by discussions with the corresponding author.
3.5. Data Synthesis and Analysis
All data were pooled to calculate the odds ratio (OR) and 95% confidence intervals by using a random-effects model. Between-trial heterogeneity was assessed by using an I2 test, and whether the value > 50% was regarded as considerable heterogeneity. Potential publication bias was examined by the visual inspection of funnel plots. Statistical significance is defined as p-value < 0.05. All analyses were performed using Review Manager (RevMan) software, version 5.4. (The Cochrane Collaboration, 2020.)
4. Discussion
The results from this meta-analysis of 21,034 patients from five randomized controlled trials demonstrated that P2Y12 inhibitor monotherapy could significantly lower the risk of bleeding complications without increasing the risk of ischemic events as compared with standard DAPT in ACS patients after PCI and stent implantation. The benefit of this novel approach was clearly demonstrated by the results of NACE. Moreover, this benefit was consistent in both NSTE-ACS and STE-ACS patients. These findings might challenge contemporary practice guideline recommendations of 12-month DAPT as the standard treatment for ACS patients after PCI.
In our exploratory analysis, the benefit of reducing bleeding events in P2Y12 inhibitor monotherapy was more pronounced among ACS patients as compared with non-ACS patients (relative risk reduction was 45% vs. 10%, respectively). This result may reflect the differences in underlying demographic and clinical characteristics between ACS patients and non-ACS patients. In the GLOBAL LEADERS study, non-ACS patients were older and had more co-morbidities (such as diabetes, peripheral vascular disease, chronic kidney disease, and prior MI) than ACS patients. Although ACS patients were younger and had fewer co-morbidities, they were still associated with a more significant reduction of bleeding events by the withdrawal of aspirin [25]. Similar findings were observed both in the TWILIGHT study and the STOPDAPT-2 study [22,26]. These observations also support the preferential benefit of applying P2Y12 inhibitor monotherapy to ACS patients other than non-ACS patients.
How to balance the risk of ischemic and bleeding events in treating ACS patients remains a crucial question. ACS patients have higher platelet reactivity and a higher risk of recurrent ischemic events than non-ACS patients [6]. Therefore, current guidelines recommend at least 12 months of DAPT in ACS patients, even without PCI and stent implantation [27,28]. Notably, the risk of bleeding events was also higher in ACS patients. A previous study had demonstrated that once the patients had post-PCI bleeding, the 2-year mortality risk was about eight times higher than for those without post-PCI bleeding [3].
Aspirin and P2Y12 inhibitors have different pharmacological pathways to inhibit the activation of platelets. Aspirin suppresses the generation of thromboxane A2 by acetylating cyclooxygenase-1. P2Y12 inhibitors block the P2Y12-dependent pathway by either directly blocking adenosine diphosphate (ADP)-induced signal transduction (ticagrelor) or blocking the binding of ADP to P2Y12 receptor (clopidogrel, prasugrel) [29]. DAPT was presumed to have additive inhibitory effects on platelet activation [30]. However, studies found that aspirin only had a slight additional inhibition of platelet aggregation when a P2Y12 inhibitor was used [31]. Ticagrelor monotherapy was demonstrated to have similar levels of inhibition for most platelet activation pathways as compared with DAPT (ticagrelor plus aspirin) in patients that underwent PCI [32]. Experimental studies also showed that the blockade of platelet P2Y12 receptor reduced the generation of thromboxane A2 induced by platelet agonists, and subsequently inhibited the effects of thromboxane A2-induced ADP release [33,34]. Moreover, the aspirin-induced gastrointestinal irritation/bleeding and the potential aspirin-resistance phenotype are the other two concerns. The literature review revealed the prevalence of aspirin resistance to be approximately 20–30% in patients with cardiovascular disease [35,36]. These patients are at a greater risk of clinical adverse cardiovascular events and mortality than those sensitive to aspirin treatment [36].
Different from previous published meta-analyses and reviews on P2Y12 inhibitor monotherapy after PCI [37,38,39,40,41], our meta-analysis for the first time included the latest published STOPDAPT-2 ACS trial and characterized the significant difference between ticagrelor versus clopidogrel monotherapy in ACS patients. This distinction is important given the growing application of P2Y12 inhibitor monotherapy in patients after PCI and stent implantation. There are three oral P2Y12 inhibitors currently available in clinical practice, and they carry very different pharmacological characteristics. Clopidogrel and prasugrel are prodrugs that require the metabolism to cause them to enter their active form and to exert their antiplatelet effects. In contrast, ticagrelor is a direct-acting drug with no effect on P2Y12 genetic polymorphism. Clopidogrel had a relatively slow onset and modest antiplatelet effect as compared with the other two P2Y12 inhibitors [42]. More importantly, the response to clopidogrel is variable, and a substantial portion of patients may have a poor response or even resistance to this drug [19,20]. Current guidelines recommend that ticagrelor and prasugrel are the preferred P2Y12 inhibitor in DAPT for ACS patients by the clinical trial results from PLATO and TRITON-TIMI 38, respectively, unless they are unavailable or cannot be tolerated [43,44]. This recommendation seems to be the same in P2Y12 inhibitor monotherapy. In a STOPDAPT-2 ACS study, clopidogrel monotherapy after 1–2 months of DAPT failed to reach the noninferiority of 12-month DAPT for a composite of cardiovascular and bleeding events (HR: 1.14, 95% CI, 0.80–1.62, p = 0.06 for noninferiority) [24]. Although the major bleeding events were reduced, the incidence of cardiovascular events significantly increased. It is worthy of note that the incidence of myocardial infarction was higher in the clopidogrel monotherapy group than in the 12-month DAPT group (1.59% vs. 0.85%, HR: 1.91, 95% CI: 1.06–3.44), and most of these were spontaneous myocardial infarctions (1.5% vs. 0.8%, HR: 2.03, 95% CI: 1.09–3.78). Furthermore, in the subgroup analysis of STOPDAPT-2 ACS, the incidence of major secondary cardiovascular endpoint (a composite of cardiovascular death, MI, definite stent thrombosis, and stroke) was significantly higher in STEMI patients treated with clopidogrel monotherapy as compared with DAPT (2.84% vs. 1.61%, HR: 1.80, 95% CI: 1.01–3.19, p = 0.04). Based on the above findings, significant attention should be paid to clopidogrel monotherapy in ACS patients, especially in the STEMI patients.
Prasugrel monotherapy was tested in several clinical studies [45,46,47], but none of them involved a randomized controlled trial. Therefore, they were excluded from our meta-analysis. The largest study of prasugrel monotherapy was the PENDULUM mono and registry study [47]. It was a prospective, observational cohort study. The results showed that prasugrel monotherapy could reduce major or minor bleeding (OR: 0.68, 95% CI: 0.47–0.98, p = 0.039) without increasing ischemic events (OR: 0.85, 95% CI: 0.61–1.19, p = 0.348) as compared with DAPT. Prasugrel monotherapy seems to be a potential alternative treatment strategy in patients with high-bleeding risk, but further study is required to prove its efficacy and safety.
The TICO study was the very first randomized study to show that P2Y12 inhibitor monotherapy with ticagrelor could have better outcomes as compared to standard DAPT in ACS patients, even including patients with STEMI. However, the case number was relatively small. Our meta-analysis provided a greater amount of data to evaluate the efficacy and safety of ticagrelor monotherapy in ACS patients, and the findings were very consistent. Our recent real-world observational study also supported the findings of the present meta-analysis by demonstrating ticagrelor monotherapy to be associated with a substantially lower cardiovascular risk as compared with clopidogrel monotherapy in ACS patients that underwent PCI [48]. Therefore, ticagrelor is the preferred choice of the P2Y12 inhibitor when applying P2Y12 inhibitor monotherapy in ACS patients after PCI.
There are several limitations in our study. First, most patients enrolled in these trials were implanted with newer-generation DES. It is unclear whether our findings could apply to first-generation DES or bare-metal stents. Second, baseline characteristics and the indications for PCI were not identical in these included trials. Of note, the TWILIGHT study only enrolled patients who were able to tolerate three months of DAPT without having a major adverse clinical event. Those who had major bleeding or recurrent ischemic events within 90 days after PCI were excluded. Third, only one randomized trial involved the analysis of clopidogrel monotherapy since there was no available data from the SMART-CHOICE study. Fourth, prasugrel monotherapy was not analyzed in our study. This is because prasugrel was used in only 4% of the enrolled patients in the SMART-CHOICE study, and the other four clinical trials were not using prasugrel in P2Y12 inhibitor monotherapy. Fifth, some of our included trials were not global studies. The TICO study and SMART-CHOICE were conducted exclusively in South Korea, whereas the STOPDAPT-2 ACS study was only conducted in Japan. Caution is needed in extrapolating these results outside of East Asian patients. Racial differences are important issues in antiplatelet therapy. Platelet aggregation is indeed a process that may depend upon race [49]. East Asians have a higher frequency of the CYP2C19 loss-of-function alleles, and tend to have a lower incidence of ischemic outcomes and a higher incidence of bleeding outcomes compared to Caucasians. Black individuals have a higher prevalence of CV risk factors, and higher thrombogenic, proinflammatory, and dysfunctional endothelial profiles than Caucasians [50]. Future studies are needed to explore the efficacy and safety of P2Y12 inhibitor monotherapy in ACS in different races.
5. Conclusions
Based on the results of our study, P2Y12 inhibitor monotherapy could significantly decrease bleeding events without increasing the risk of stent thrombosis or myocardial infarction in ACS patients. However, the type of P2Y12 inhibitor did matter. Compared with the standard DAPT, P2Y12 inhibitor monotherapy by ticagrelor, but not clopidogrel, carries a significantly lower NACE. We conclude that P2Y12 inhibitor monotherapy with ticagrelor is a favorable choice for ACS patients after PCI with stent implantation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16020232/s1, Figure S1: The funnel plots of each outcome. (A) MACCE, (B) major or minor bleeding, (C) NACE; Table S1: EMBASE search strategy; Table S2: PubMed search strategy; Table S3: Cochrane Library search strategy; Table S4: Bias risk assessment of the studies.
Author Contributions
Conceptualization, W.-H.F.; data collection, Y.-C.C., Y.-H.L., C.-Y.C., T.-H.L., T.-C.L. and C.-T.C.; data analysis, Y.-C.C., H.-L.C., T.-H.L., T.-C.L. and C.-T.C.; validation, Y.-C.C.; writing—original draft preparation, W.-H.F. and Y.-C.C.; writing—review and editing, Y.-H.L., H.-F.K. and H.-M.C.; supervision, C.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kaohsiung Municipal Ta-Tung Hospital (KMTTH-111-008), Kaohsiung Medical University (grant KMU-TC112A02), and Kaohsiung Medical University Hospital (grant KMUH110-0M07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, E.; Steg, P.G.; Wijns, W. Stent thrombosis late after implantation of first-generation drug-eluting stents: A cause for concern. Circulation 2007, 115, 1440–1455. [Google Scholar] [CrossRef]
- Généreux, P.; Giustino, G.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Rinaldi, M.J.; Neumann, F.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2015, 66, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Bacchi Reggiani, L.; Della Riva, D.; Romanello, M.; Feres, F.; Abizaid, A.; Gilard, M.; Morice, M.C.; Valgimigli, M.; Hong, M.K.; et al. Bleeding-Related Deaths in Relation to the Duration of Dual-Antiplatelet Therapy After Coronary Stenting. J. Am. Coll. Cardiol. 2017, 69, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Neumann, F.J.; Ott, I.; Schiessler, A.; Schömig, A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation 1996, 93, 229–237. [Google Scholar] [CrossRef]
- Giustino, G.; Mehran, R.; Dangas, G.D.; Kirtane, A.J.; Redfors, B.; Généreux, P.; Brener, S.J.; Prats, J.; Pocock, S.J.; Deliargyris, E.N.; et al. Characterization of the Average Daily Ischemic and Bleeding Risk After Primary PCI for STEMI. J. Am. Coll. Cardiol. 2017, 70, 1846–1857. [Google Scholar] [CrossRef]
- Scalone, G.; Coviello, I.; Barone, L.; Battipaglia, I.; Aurigemma, C.; Careri, G.; Pinnacchio, G.; Tarzia, P.; Lanza, G.A.; Crea, F. Evidence of increased platelet reactivity in the first six months after acute ST segment elevation myocardial infarction. Thromb. Res. 2011, 128, 174–178. [Google Scholar] [CrossRef]
- Hahn, J.Y.; Song, Y.B.; Oh, J.H.; Cho, D.K.; Lee, J.B.; Doh, J.H.; Kim, S.H.; Jeong, J.O.; Bae, J.H.; Kim, B.O.; et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): A randomised, open-label, non-inferiority trial. Lancet 2018, 391, 1274–1284. [Google Scholar] [CrossRef]
- Palmerini, T.; Della Riva, D.; Benedetto, U.; Bacchi Reggiani, L.; Feres, F.; Abizaid, A.; Gilard, M.; Morice, M.C.; Valgimigli, M.; Hong, M.K.; et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: An individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur. Heart J. 2017, 38, 1034–1043. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Bittl, J.A.; Baber, U.; Bradley, S.M.; Wijeysundera, D.N. Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016, 134, e156–e178. [Google Scholar] [CrossRef]
- Gimbel, M.E.; Ten Berg, J.M. Safety considerations with the use of platelet inhibitors for elderly patients with non-ST-elevation acute coronary syndrome. Expert Opin. Drug Saf. 2021, 20, 1545–1552. [Google Scholar] [CrossRef]
- von Kügelgen, I. Structure, Pharmacology and Roles in Physiology of the P2Y(12) Receptor. Adv. Exp. Med. Biol. 2017, 1051, 123–138. [Google Scholar] [CrossRef]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef]
- Hahn, J.Y.; Song, Y.B.; Oh, J.H.; Chun, W.J.; Park, Y.H.; Jang, W.J.; Im, E.S.; Jeong, J.O.; Cho, B.R.; Oh, S.K.; et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 2019, 321, 2428–2437. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Jüni, P.; Hamm, C.; Steg, P.G.; Heg, D.; van Es, G.A.; McFadden, E.P.; Onuma, Y.; van Meijeren, C.; et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre, open-label, randomised superiority trial. Lancet 2018, 392, 940–949. [Google Scholar] [CrossRef]
- Feng, W.H.; Hsieh, I.C.; Li, Y.H. P2Y12 Inhibitor Monotherapy after Percutaneous Coronary Intervention: Is It Safe to Abandon Aspirin? Acta Cardiol. Sin. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Steinhubl, S.R.; Berger, P.B.; Malinin, A.I.; Bhatt, D.L.; Topol, E.J. Variability in platelet responsiveness to clopidogrel among 544 individuals. J. Am. Coll. Cardiol. 2005, 45, 246–251. [Google Scholar] [CrossRef]
- Roden, D.M.; Shuldiner, A.R. Responding to the clopidogrel warning by the US food and drug administration: Real life is complicated. Circulation 2010, 122, 445–448. [Google Scholar] [CrossRef]
- Tomaniak, M.; Chichareon, P.; Onuma, Y.; Deliargyris, E.N.; Takahashi, K.; Kogame, N.; Modolo, R.; Chang, C.C.; Rademaker-Havinga, T.; Storey, R.F.; et al. Benefit and Risks of Aspirin in Addition to Ticagrelor in Acute Coronary Syndromes: A Post Hoc Analysis of the Randomized GLOBAL LEADERS Trial. JAMA Cardiol. 2019, 4, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Dangas, G.; Angiolillo, D.J.; Cohen, D.J.; Sharma, S.K.; Nicolas, J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dudek, D.; et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur. Heart J. 2020, 41, 3533–3545. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Hong, S.J.; Cho, Y.H.; Yun, K.H.; Kim, Y.H.; Suh, Y.; Cho, J.Y.; Her, A.Y.; Cho, S.; Jeon, D.W.; et al. Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 2020, 323, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Morimoto, T.; Natsuaki, M.; Yamamoto, K.; Obayashi, Y.; Ogita, M.; Suwa, S.; Isawa, T.; Domei, T.; Yamaji, K.; et al. Comparison of Clopidogrel Monotherapy After 1 to 2 Months of Dual Antiplatelet Therapy With 12 Months of Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome: The STOPDAPT-2 ACS Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Valgimigli, M.; Odutayo, A.; Serruys, P.W.; Hamm, C.; Steg, P.G.; Heg, D.; Mc Fadden, E.P.; Onuma, Y.; Benit, E.; et al. Efficacy and Safety of Ticagrelor Monotherapy by Clinical Presentation: Pre-Specified Analysis of the GLOBAL LEADERS Trial. J. Am. Heart Assoc. 2021, 10, e015560. [Google Scholar] [CrossRef]
- Obayashi, Y.; Watanabe, H.; Morimoto, T.; Yamamoto, K.; Natsuaki, M.; Domei, T.; Yamaji, K.; Suwa, S.; Isawa, T.; Watanabe, H.; et al. Clopidogrel Monotherapy After 1-Month Dual Antiplatelet Therapy in Percutaneous Coronary Intervention: From the STOPDAPT-2 Total Cohort. Circ. Cardiovasc. Interv. 2022, 15, e012004. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 68, 1082–1115. [Google Scholar] [CrossRef]
- Capodanno, D.; Mehran, R.; Valgimigli, M.; Baber, U.; Windecker, S.; Vranckx, P.; Dangas, G.; Rollini, F.; Kimura, T.; Collet, J.P.; et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat. Rev. Cardiol. 2018, 15, 480–496. [Google Scholar] [CrossRef]
- Cadroy, Y.; Bossavy, J.P.; Thalamas, C.; Sagnard, L.; Sakariassen, K.; Boneu, B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation 2000, 101, 2823–2828. [Google Scholar] [CrossRef]
- Armstrong, P.C.; Leadbeater, P.D.; Chan, M.V.; Kirkby, N.S.; Jakubowski, J.A.; Mitchell, J.A.; Warner, T.D. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J. Thromb. Haemost. 2011, 9, 552–561. [Google Scholar] [CrossRef]
- Baos, S.; Underwood, W.; Culliford, L.; Reeves, B.C.; Rogers, C.A.; Bowles, R.; Johnson, T.; Baumbach, A.; Mumford, A. Platelet inhibition during ticagrelor monotherapy versus ticagrelor plus aspirin in patients with coronary artery disease (TEMPLATE study): Study protocol for a randomised controlled trial. Trials 2017, 18, 529. [Google Scholar] [CrossRef]
- Bhavaraju, K.; Georgakis, A.; Jin, J.; Gartner, T.K.; Tomiyama, Y.; Nurden, A.; Nurden, P.; Kunapuli, S.P. Antagonism of P2Y12 reduces physiological thromboxane levels. Platelets 2010, 21, 604–609. [Google Scholar] [CrossRef]
- Armstrong, P.C.; Dhanji, A.R.; Tucker, A.T.; Mitchell, J.A.; Warner, T.D. Reduction of platelet thromboxane A2 production ex vivo and in vivo by clopidogrel therapy. J. Thromb. Haemost. 2010, 8, 613–615. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Farhadi, Z.; Behzadifar, M.; Shabaninejad, H.; Abolghasem Gorji, H.; Taheri Mirghaed, M.; Salemi, M.; Amin, K.; Mohammadibakhsh, R.; Bragazzi, N.L.; et al. Prevalence rate of laboratory defined aspirin resistance in cardiovascular disease patients: A systematic review and meta-analysis. Casp. J. Intern. Med. 2020, 11, 124–134. [Google Scholar] [CrossRef]
- Krasopoulos, G.; Brister, S.J.; Beattie, W.S.; Buchanan, M.R. Aspirin “resistance” and risk of cardiovascular morbidity: Systematic review and meta-analysis. BMJ 2008, 336, 195–198. [Google Scholar] [CrossRef]
- Ho, A.C.; Egolum, U.; Parker, S.; Dimmel, J.; Hawkins, A.; Ling, H. P2Y12 Inhibitor Monotherapy After a Short Dual Antiplatelet Therapy Versus Standard-Term Dual Antiplatelet Therapy in Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Meta-Analysis. Clin. Drug Investig. 2020, 40, 799–808. [Google Scholar] [CrossRef]
- Valgimigli, M.; Gragnano, F.; Branca, M.; Franzone, A.; Baber, U.; Jang, Y.; Kimura, T.; Hahn, J.Y.; Zhao, Q.; Windecker, S.; et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: Individual patient level meta-analysis of randomised controlled trials. BMJ 2021, 373, n1332. [Google Scholar] [CrossRef]
- Giacoppo, D.; Matsuda, Y.; Fovino, L.N.; D’Amico, G.; Gargiulo, G.; Byrne, R.A.; Capodanno, D.; Valgimigli, M.; Mehran, R.; Tarantini, G. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents: A systematic review and meta-analysis of randomized clinical trials. Eur. Heart J. 2021, 42, 308–319. [Google Scholar] [CrossRef]
- Nicolas, J.; Dangas, G.; Chiarito, M.; Pivato, C.A.; Spirit, A.; Cao, D.; Giustino, G.; Beerkens, F.; Camaj, A.; Vogel, B.; et al. Efficacy and Safety of P2Y12 Inhibitor Monotherapy After Complex PCI: A Collaborative Systematic Review and Meta-Analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 1, pvac071. [Google Scholar] [CrossRef]
- Capodanno, D.; Angiolillo, D.J. Timing, Selection, Modulation, and Duration of P2Y12 Inhibitors for Patients With Acute Coronary Syndromes Undergoing PCI. JACC Cardiovasc. Interv. 2023, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Varenhorst, C.; James, S.; Erlinge, D.; Braun, O.O.; Jakubowski, J.A.; Sugidachi, A.; Winters, K.J.; Siegbahn, A. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur. Heart J. 2008, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Hamana, T.; Sawada, T.; Fujimoto, W.; Osue, T.; Tsukiyama, Y.; Uzu, K.; Takaya, T.; Yasaka, Y.; Kawai, H. Comparison of the 9-Month Intrastent Condition and 30-Month Clinical Outcomes After Resolute Zotarolimus-Eluting Stent Implantation Between Standard-Duration and 1-Month Dual Antiplatelet Therapy Followed by Prasugrel Monotherapy. Circ. Rep. 2020, 3, 55–65. [Google Scholar] [CrossRef]
- Nakamura, M.; Morino, Y.; Kakuta, T.; Hata, Y.; Takamisawa, I.; Tanabe, K.; Anzai, H.; Takahashi, A.; Kadota, K.; Suzuki, H.; et al. Monotherapy With Prasugrel After Dual-Antiplatelet Therapy for Japanese Percutaneous Coronary Intervention Patients With High Bleeding Risk—A Prospective Cohort Study (PENDULUM mono Study). Circ. J. Off. J. Jpn. Circ. Soc. 2020, 85, 27–36. [Google Scholar] [CrossRef]
- Nakamura, M.; Kadota, K.; Nakao, K.; Nakagawa, Y.; Shite, J.; Yokoi, H.; Kozuma, K.; Tanabe, K.; Akasaka, T.; Shinke, T.; et al. Single Antiplatelet Therapy With Prasugrel vs. Dual Antiplatelet Therapy in Japanese Percutaneous Coronary Intervention Patients With High Bleeding Risk. Circ. J. Off. J. Jpn. Circ. Soc. 2021, 85, 785–793. [Google Scholar] [CrossRef]
- Chen, P.W.; Feng, W.H.; Ho, M.Y.; Su, C.H.; Huang, S.W.; Cheng, C.W.; Yeh, H.I.; Chen, C.P.; Huang, W.C.; Fang, C.C.; et al. P2Y12 Inhibitor Monotherapy with Clopidogrel Versus Ticagrelor in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2020, 9, 1657. [Google Scholar] [CrossRef]
- Tourdot, B.E.; Conaway, S.; Niisuke, K.; Edelstein, L.C.; Bray, P.F.; Holinstat, M. Mechanism of race-dependent platelet activation through the protease-activated receptor-4 and Gq signaling axis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2644–2650. [Google Scholar] [CrossRef]
- Tamargo, J.; Kaski, J.C.; Kimura, T.; Barton, J.C.; Yamamoto, K.; Komiyama, M.; Drexel, H.; Lewis, B.S.; Agewall, S.; Hasegawa, K. Racial and ethnic differences in pharmacotherapy to prevent coronary artery disease and thrombotic events. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 738–751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).