4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Chiral Separation
2.3. Assignment of the Absolute Configuration
2.4. Cytotoxicity of 4-Isobutylmethcathinone and Its Enantiomers
2.5. Fluorescence Microscopy
2.6. Receptor Binding Studies
3. Materials and Methods
3.1. Chemicals
3.2. NMR and MS Analysis
3.3. Chiral Separation
3.4. Chiroptical Methods and DFT Calculations
3.5. Cell Lines and Cultivation
3.6. Cytotoxicity Tests
3.7. Fluorescence Microscopy and Sample Preparation
3.8. PRESTO-Tango β-Arrestin Recruitment Assay
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- European Drug Report 2019: Trends and Developments; Publications Office of the European Union: Luxembourg, 2019. [CrossRef]
- Kalix, P. Cathinone, an alkaloid from khat leaves with an amphetamine-like releasing effect. Psychopharmacology 1981, 74, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Kalix, P.; Khan, I. Khat: An amphetamine-like plant material. Bull. World Health Organ. 1984, 62, 681–686. [Google Scholar] [PubMed]
- Kelly, J.P. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef]
- Dal Cason, T.A.; Young, R.; Glennon, R.A. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 1997, 58, 1109–1116. [Google Scholar] [CrossRef]
- Calinski, D.M.; Kisor, D.F.; Sprague, J.E. A review of the influence of functional group modifications to the core scaffold of synthetic cathinones on drug pharmacokinetics. Psychopharmacology 2019, 236, 881–890. [Google Scholar] [CrossRef]
- Saha, K.; Partilla, J.S.; Lehner, K.R.; Seddik, A.; Stockner, T.; Holy, M.; Sandtner, W.; Ecker, G.F.; Sitte, H.H.; Baumann, M.H. ‘Second-generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology 2015, 40, 1321–1331. [Google Scholar] [CrossRef]
- Green, A.R.; King, M.V.; Shortall, S.E.; Fone, K.C. The preclinical pharmacology of mephedrone; not just MDMA by another name. Br. J. Pharmacol. 2014, 171, 2251–2268. [Google Scholar] [CrossRef]
- Mayer, F.P.; Wimmer, L.; Dillon-Carter, O.; Partilla, J.S.; Burchardt, N.V.; Mihovilovic, M.D.; Baumann, M.H.; Sitte, H.H. Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters. Br. J. Pharmacol. 2016, 173, 2657–2668. [Google Scholar] [CrossRef]
- Niello, M.; Cintulová, D.; Raithmayr, P.; Holy, M.; Jäntsch, K.; Colas, C.; Ecker, G.F.; Sitte, H.H.; Mihovilovic, M.D. Effects of Hydroxylated Mephedrone Metabolites on Monoamine Transporter Activity in vitro. Front. Pharmacol. 2021, 12, 654061. [Google Scholar] [CrossRef]
- Gregg, R.A.; Baumann, M.H.; Partilla, J.S.; Bonano, J.S.; Vouga, A.; Tallarida, C.S.; Velvadapu, V.; Smith, G.R.; Peet, M.M.; Reitz, A.B.; et al. Stereochemistry of mephedrone neuropharmacology: Enantiomer-specific behavioural and neurochemical effects in rats. Br. J. Pharmacol. 2015, 172, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Philogene-Khalid, H.L.; Hicks, C.; Reitz, A.B.; Liu-Chen, L.-Y.; Rawls, S.M. Synthetic cathinones and stereochemistry: S enantiomer of mephedrone reduces anxiety- and depressant-like effects in cocaine- or MDPV-abstinent rats. Drug Alcohol. Depend. 2017, 178, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.J.; Rice, M.E. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004, 27, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef]
- Niello, M.; Gradisch, R.; Loland, C.J.; Stockner, T.; Sitte, H.H. Allosteric Modulation of Neurotransmitter Transporters as a Therapeutic Strategy. Trends Pharmacol. Sci. 2020, 41, 446–463. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Kirk, K.L. Chirality in Drug Research; Francotte, E., Linder, W., Eds.; Wiley/VCH: Weinheim, Germany, 2006. [Google Scholar]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; de Lourdes Bastos, M.; Carvalho, F.; Capela, J.P. Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells. Neurotoxicology 2019, 75, 158–173. [Google Scholar] [CrossRef]
- Gaspar, H.; Bronze, S.; Oliveira, C.; Victor, B.L.; Machuqueiro, M.; Pacheco, R.; Caldeira, M.J.; Santos, S. Proactive response to tackle the threat of emerging drugs: Synthesis and toxicity evaluation of new cathinones. Forensic Sci. Int. 2018, 290, 146–156. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing…Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Silva, B.; Palmeira, A.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. S-(+)-Pentedrone and R-(+)-methylone as the most oxidative and cytotoxic enantiomers to dopaminergic SH-SY5Y cells: Role of MRP1 and P-gp in cathinones enantioselectivity. Toxicol. Appl. Pharmacol. 2021, 416, 115442. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Rodrigues, J.S.; Almeida, A.S.; Lima, A.R.; Fernandes, C.; de Pinho, P.G.; Miranda, J.P.; Remião, F. Enantioselectivity of Pentedrone and Methylone on Metabolic Profiling in 2D and 3D Human Hepatocyte-like Cells. Pharmaceuticals 2022, 15, 368. [Google Scholar] [CrossRef] [PubMed]
- Kolderová, N.; Jurásek, B.; Kuchař, M.; Lindner, W.; Kohout, M. Gradient supercritical fluid chromatography coupled to mass spectrometry with a gradient flow of make-up solvent for enantioseparation of cathinones. J. Chromatogr. A 2020, 1625, 461286. [Google Scholar] [CrossRef]
- Spálovská, D.; Králík, F.; Kohout, M.; Jurásek, B.; Habartová, L.; Kuchař, M.; Setnička, V. Structure determination of butylone as a new psychoactive substance using chiroptical and vibrational spectroscopies. Chirality 2018, 30, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Spálovská, D.; Maříková, T.; Kohout, M.; Králík, F.; Kuchař, M.; Setnička, V. Methylone and pentylone: Structural analysis of new psychoactive substances. Forensic Toxicol. 2019, 37, 366–377. [Google Scholar] [CrossRef]
- Spálovská, D.; Paškan, M.; Jurásek, B.; Kuchař, M.; Kohout, M.; Setnička, V. Structural spectroscopic study of enantiomerically pure synthetic cathinones and their major metabolites. New J. Chem. 2021, 45, 850–860. [Google Scholar] [CrossRef]
- Wolrab, D.; Frühauf, P.; Moulisová, A.; Kuchař, M.; Gerner, C.; Lindner, W.; Kohout, M. Chiral separation of new designer drugs (Cathinones) on chiral ion-exchange type stationary phases. J. Pharm. Biomed. Anal. 2016, 120, 306–315. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef]
- Luethi, D.; Kolaczynska, K.E.; Docci, L.; Krähenbühl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 2018, 134, 4–12. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef]
- Sulzer, D.; Chen, T.K.; Lau, Y.Y.; Kristensen, H.; Rayport, S.; Ewing, A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995, 15, 4102–4108. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Kolaczynska, K.E.; Thomann, J.; Hoener, M.C.; Liechti, M.E. The Pharmacological Profile of Second Generation Pyrovalerone Cathinones and Related Cathinone Derivative. Int. J. Mol. Sci. 2021, 22, 8277. [Google Scholar] [CrossRef] [PubMed]
- Seaman, R.W.; Doyle, M.R.; Sulima, A.; Rice, K.C.; Collins, G.T. Discriminative stimulus effects of 3,4-methylenedioxypyrovalerone (MDPV) and structurally related synthetic cathinones. Behav. Pharmacol. 2021, 32, 357–367. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Sassano, M.F.; Huang, X.P.; Lansu, K.; McCorvy, J.D.; Giguère, P.M.; Sciaky, N.; Roth, B.L. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 2015, 22, 362–369. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Martel, J.C.; Gatti McArthur, S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Urbanova, M.; Setnicka, V.V.; Volka, K. Measurements of concentration dependence and enantiomeric purity of terpene solutions as a test of a new commercial VCD spectrometer. Chirality 2000, 12, 199–203. [Google Scholar] [CrossRef]
- Bouř, P.; Maloň, P. The MCM Program; Czech Academy of Sciences: Prague, Czech Republic, 1995. [Google Scholar]
- Covington, C.L.; Polavarapu, P.L. Similarity in Dissymmetry Factor Spectra: A Quantitative Measure of Comparison between Experimental and Predicted Vibrational Circular Dichroism. J. Phys. Chem. A 2013, 117, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, P.L.; Covington, C.L. Comparison of Experimental and Calculated Chiroptical Spectra for Chiral Molecular Structure Determination. Chirality 2014, 26, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Okada, S.; Kuroda, A.; Watanabe, S.; Sawada, Y.; Tanaka, H. 4-(Benzoylindolizinyl)butyric Acids; Novel Nonsteroidal Inhibitors of Steroid 5α-Reductase. III. Chem. Pharm. Bull. 2001, 49, 799–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, C.L.; Ostrum, K.G. Selective bromination with copper(II) bromide. J. Am. Chem. Soc. 1964, 29, 3459–3461. [Google Scholar] [CrossRef]
- Malmedy, F.; Wirth, T. Stereoselective Ketone Rearrangements with Hypervalent Iodine Reagents. Chem. Eur. J. 2016, 22, 16072–16077. [Google Scholar] [CrossRef]
- Sonawane, H.R.; Rellur, N.S.; Kulkarni, D.G.; Ayyangar, N.R. Photochemical Rearrangement of α-Chloro-Propiophenones to α-Arylpropanoic Acids: Studies on Chirality Transfer and Synthesis of (S)-(+)-Ibuprofen and (S)-(+)-Ketoprofen. Tetrahedron 1994, 50, 1243–1260. [Google Scholar] [CrossRef]
- Herciková, J.; Spálovská, D.; Frühauf, P.; Izák, P.; Lindner, W.; Kohout, M. Design and synthesis of naphthalene-based strong cation exchangers and their application for chiral separation of basic drugs. J. Sep. Sci. 2021, 44, 3348–3356. [Google Scholar] [CrossRef]



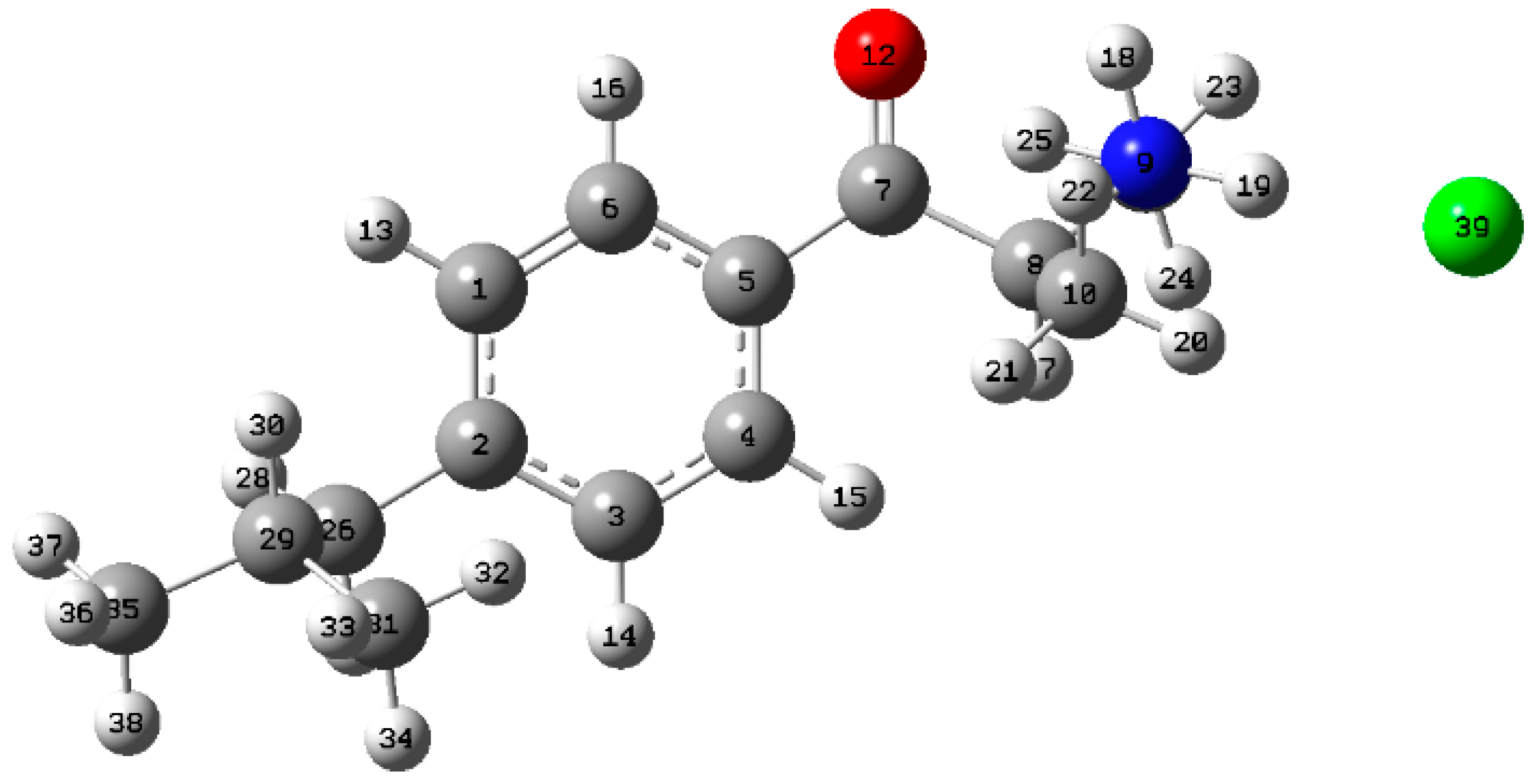





| Dihedral Angles | ||||||
|---|---|---|---|---|---|---|
| Conformer | α1 | α2 | α3 | α4 | ΔG (kJ mol−1) | Relative Abundances (%) |
| 4-isobutylmethcathinone I | 96 | −80 | −75 | −64 | 0 | 38 |
| 4-isobutylmethcathinone II | 97 | −80 | 108 | 173 | 0.23 | 25 |
| 4-isobutylmethcathinone III | 97 | −80 | −105 | −173 | 0.37 | 20 |
| 4-isobutylmethcathinone IV | 97 | −81 | 74 | −173 | 0.54 | 15 |
| 4-isobutylmethcathinone V | 97 | −81 | 89 | 63 | 1.91 | 2 |
| Compound | IBMcat | S-IBMCat | R-IBMCat |
|---|---|---|---|
| Cell Line | |||
| IC50 ± SD [µM] | |||
| HEK 293T | 123 ± 6 | 192 ± 2 | 185 ± 5 |
| Hep G2 | 52 ± 7 | 46 ± 9 | 49 ± 1 |
| HMC-3 | 61 ± 2 | 64 ± 1 | 65 ± 2 |
| SH-SY5Y | 44 ± 5 | 50 ± 1 | 52 ± 4 |
| 5637 | 18 ± 1 | 21 ± 4 | 20 ± 1 |
| L929 | 36 ± 4 | 33 ± 5 | 39 ± 2 |
| CaCo-2 | 389 ± 42 | 390 ± 17 | 331 ± 19 |
| HaCaT | 357 ± 40 | 361 ± 22 | 329 ± 43 |
| MRC-5 | 635 ± 17 | 530 ± 35 | 515 ± 34 |
| BJ | 749 ± 102 | 685 ± 41 | 698 ± 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paškan, M.; Rimpelová, S.; Svobodová Pavlíčková, V.; Spálovská, D.; Setnička, V.; Kuchař, M.; Kohout, M. 4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers. Pharmaceuticals 2022, 15, 1495. https://doi.org/10.3390/ph15121495
Paškan M, Rimpelová S, Svobodová Pavlíčková V, Spálovská D, Setnička V, Kuchař M, Kohout M. 4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers. Pharmaceuticals. 2022; 15(12):1495. https://doi.org/10.3390/ph15121495
Chicago/Turabian StylePaškan, Martin, Silvie Rimpelová, Vladimíra Svobodová Pavlíčková, Dita Spálovská, Vladimír Setnička, Martin Kuchař, and Michal Kohout. 2022. "4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers" Pharmaceuticals 15, no. 12: 1495. https://doi.org/10.3390/ph15121495
APA StylePaškan, M., Rimpelová, S., Svobodová Pavlíčková, V., Spálovská, D., Setnička, V., Kuchař, M., & Kohout, M. (2022). 4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers. Pharmaceuticals, 15(12), 1495. https://doi.org/10.3390/ph15121495








