Abstract
Protein kinase inhibitors (PKIs) are important therapeutic agents. As of 31 May 2021, the United States Food and Drug Administration (USFDA) has approved 70 PKIs. Most of the PKIs are employed to treat cancer and inflammatory diseases. Imatinib was the first PKI approved by USFDA in 2001. This review summarizes the compound patents and the essential polymorph patents of the PKIs approved by the USFDA from 2001 to 31 May 2021. The dates on the generic drug availability of the PKIs in the USA market have also been forecasted. It is expected that 19 and 48 PKIs will be genericized by 2025 and 2030, respectively, due to their compound patent expiry. This may reduce the financial toxicity associated with the existing PKIs. There are nearly 535 reported PKs. However, the USFDA approved PKIs target only about 10–15% of the total said PKs. As a result, there are still a large number of unexplored PKs. As the field advances during the next 20 years, one can anticipate that PKIs with many scaffolds, chemotypes, and pharmacophores will be developed.
1. Introduction
Protein kinases (PKs) are ubiquitous intracellular and cell surface enzymatic proteins that selectively catalyzes phosphate group’s relocation from ATP, GTP, and other phosphate donors to protein substrates [1]. The PKs mainly catalyze the relocation of a γ-phosphatase group of ATP to the oxygen atom of the -OH group of threonine, serine, and tyrosine residues in peptides/polypeptides, thereby making a conformational variation from an inactive to an active form [1,2]. They constitute an extensive family of structurally related enzymes that are known to be implicated in almost all the signal transduction activities, frequently with cascades of phosphorylation proceedings taking place within the cell [3]. The signal transduction involves the reversible phosphorylation of proteins that helps to regulate mature proteins by altering their structure and function [4,5]. To date, nearly 535 human PKs have been identified [6], wherein more than 478 belong to a superfamily whose catalytic domains are sequentially interrelated. These PKs are additionally categorized into groups, families, and subfamilies established on their biochemical activities. The main two classifications are Serine/threonine PKs and Tyrosine-specific PKs [5]. The seven significant groups with the description of families, subfamilies, and functions are listed in Table 1.
TKs form a distinct group, which phosphorylates proteins on tyrosine, whereas others phosphorylate serine and threonine residues. In addition to this category, there are atypical kinases, which are not related to any sequence resemblance to characteristic kinases but are well recognized for their enzymatic activity similar to specific kinases. Some kinases are believed to lack the catalytic domain for effective phosphorylation and are called pseudokinases. Still, they are distributed across all kinase families, indicating that an absence of catalysis is not a formal barricade to the evolution of unique or irreplaceable biological functions [7].

Table 1.
Families and subfamilies of PKs.
Table 1.
Families and subfamilies of PKs.
| S. No. | Kinase | Families | Subfamilies | Functions |
|---|---|---|---|---|
| Serine/Threonine-Specific Protein Kinases | ||||
| 1 | AGC | PKA, PKG, PKC, DMPK, NDR, AKT, SGK, RSK, PKN, GRK, PDK1, RSKR, RSKL, MAST | DMPK: GEK, ROCK, CRIK PKC: Alpha, Delta, Gamma, Epsilon RSK: MSK, P70 RSKL: RSKL1, RSKL2 MAST: MAST1, MAST 2, MAST3, MAST4, MASTL | They are implicated in various cellular activities and are prospective targets to treat cancer, inflammation, viral infections, obesity, diabetes, and neurological disorders [8] |
| 2 | CAMK | Calcium/calmodulin-dependent protein kinase-CAMK1, Unique VACAMKL, PSK, DAPK, MLCK, TRIO, CASK, CAMK2, PHK, DCAMKL, MAPKAPK, CAMKL, TSK, PIM, TRB1, Unique STK33, PKD, RAD53 | MAPKAPK: MNK, MAPKAPK1, MAPKAPK2, MAPKAPK3, JNK CAMKL: AMPK, BRSK, MELK, MARK, QIK, NUAK, NIMI, SNRK, PASK, CHK1, LKB1, HUNK | They are implicated in the phosphorylation of transcription factors and the control of gene expression. They also control the life cycle of the cell [9] |
| 3 | CK1 | Casein kinase 1, TTBK, VRK | - | They are involved in the phosphorylation of significant governing molecules in cellular translation/transcription, cell–cell adhesion, and receptor coupled signal transduction. They control main signaling trails, particularly in cancer evolution [10] |
| 4 | CMGC | CDK, MAPK, GSK3, CLK families, CDKL, CLK, RCK, DYRK | - | Critical role in cell cycle regulation and intracellular signal transduction [11] |
| 5 | STE | Homologs of yeast Sterile 7/MAP3K, Sterile 11/MAP2K, Sterile 20/MAP4K | MAP4K: FRAY, STLK, PAKA, PAKB, MST, YSK, TAO, MSN, NINAC, KHS, SLK | Crucial role in MAP kinase pathways, which require a sequential PK reaction to activate the next kinase in the pathway, especially in cascade process [12] |
| Tyrosine-Specific Protein Kinases | ||||
| 6 | TK | Tyrosine kinase | Receptor Tyrosine Kinases (RTKs): EGFR, EPH, SEV, ALK, TRK, INSR, CCK4, AXL, VEGFR, FGFR, MUSK, LMR, DDR, ROR, TIE, SEF, PDGFR, RET, MET, RYK | They play a vital role in controlling cellular differentiation, cell division, and morphogenesis. They primarily act as growth factor receptors and in downstream signaling [13] |
| Non-Receptor Tyrosine Kinases (nRTKs): CSK, JAK, SRC (SFKs, BCR), BTK, ACK, SYK, FER, TEC, ABL, FAK | They are involved in signaling cascades, particularly those implicated in growth hormone and cytokine signaling. Some of them are involved in synaptic transmission, myelination, axon guidance, and oligodendrocyte formation [13] | |||
| 7 | TKL | Tyrosine kinase-like | IRAK, MLKL, LIMK, TESK, LRRK, ALK, ACTR, TGFR, MISR, BMPR, RAF, KSR, TAK, ILK, DLK, LZK, MLK, ZAK, RIPK, ANKRP, SGK, RIPK | They control apoptosis, cell differentiation/growth, angiogenesis, vascular development, and the protective response against pathogens [5,14] |
PKs perform a significant function in signal transduction and control of most cellular processes, including cell growth, differentiation, proliferation, angiogenesis, apoptosis, cytoskeletal arrangement, regulation of metabolic reactions, membrane transport, and motility, etc. [6]. Non-catalytic functions of PKs are also essential and include the allosteric effect, subcellular targeting, the scaffolding of protein complexes, competition for protein interactions, and DNA binding [15]. Because PKs regulate most fundamental biological processes, any dysregulation, genetic alteration, and abrupt change in kinase function are typically linked with pathological conditions such as cancer, immunologic, neurological, cardiovascular, and metabolic disorders [3,5]. Hence, manipulation of PKs signaling pathway, regulation, and inhibition constitutes important clinical targets for pharmacological intervention and thus for the identification and development of Protein Kinase Inhibitors (PKIs) to manage and treat several chronic diseases [4,6,16]. Over the past two decades, approximately 1/5th-1/3rd drug discovery programs worldwide have targeted PKs for the drug development of various illnesses.
Kinase mutation frequency is much less, and thus targeting kinases could be helpful in life-saving therapies especially for cancer. A well-known example is receptor tyrosine kinase ALK where gene fusion between EML4 and ALK occurs only in 5% of NSCLC patients and therefore many patients responded to the kinome therapy effectively. Identification of additional effective kinome targets will therefore represent an Achilles heel in a subset of cancer. The use of bioinformatics tools in predicting the likelihood that a given mutation will alter the function of a kinase will be essential in pinpointing cancer-associated kinases [17].
There are about 175 kinase drugs under clinical trials and newer targets are also under evaluation including AKT, Aurora kinases, CHEK1, and CDK1. However, most of the drugs under investigation are well known for targeting EGFR, VEGFR, PI3K, and mTOR [18]. Even though CAMK, CK1, or AGC kinases groups are well-known and evidenced as the primary targets for cancer, there are no investigational drugs that target these kinases are enrolled. So far only 8% of the entire kinome has been effectively “drugged” and a quarter of human kinases are vastly understudied [19]. A wide-ranging scoring system to rank and prioritize clinically relevant kinase targets of different solid tumor cancers from The Cancer Genome Atlas (TCGA) has been developed [19].
Successful applications and deep insights into the ever-diversifying therapeutic space occupied by kinase targets are also explored. For effective target validation and to avoid complicating off-target mediated response it is essential to achieve the desired selectivity while targeting kinases, though it is still an ongoing challenge. The application of large-scale omics data has been modernized to combine multiple parameters to evaluate the protein’s potential as a drug target or biomarker [19].
In recent years, intricately selective kinase chemical probes have been generated by the exploitation of unique pockets using molecular modeling and bioinformatics, prioritizing the ligand-efficient leads and novel chemotypes and the extensive use of kinome-wide profiling [20].
Chemical proteomics and broad kinome profiling of compound libraries have been implemented as an efficient method to lead to discovery, analyzing targets, and optimization [21]. Results revealed that unknown targets for established drugs presented a viewpoint on the "druggable" kinome, emphasized non-kinase off-targets, and recommended for potential therapeutic applications. A database of the cellular targets of 243 clinical kinase inhibitors has been made available using kinobead technology [21].
The ongoing research will undoubtedly pave the way for a better understanding of molecular pathways that will further unravel the role of PKs in pathogenesis. As of now, the majority of the USFDA-approved PKIs are Protein Tyrosine Kinase inhibitors (PTKIs) followed by protein-serine/threonine PKIs. Most of these drugs are clinically used to treat solid (breast, lung, colon) and non-solid tumors (leukemia). Some PKIs are also effective in treating non-malignant diseases, including myelofibrosis, rheumatoid arthritis, glaucoma, ulcerative colitis, pulmonary fibrosis, etc. [22,23].
2. USFDA Approved Protein Kinase Inhibitors
In 2001, the USFDA approved the marketing of the first clinical PKI, imatinib. Since then, the USFDA has approved about 70 PKIs for clinical use (Table 2) (Figure 1). The data provided in Table 2 have been obtained from USFDA’s Orange Book website (https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm?resetfields=1 (accessed on 31 May 2021) using the drug’s name.

Table 2.
The Orange Book data of the USFDA approved PKIs.
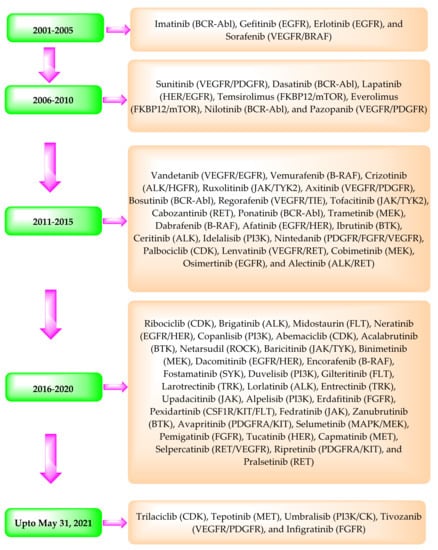
Figure 1.
Timeline depicting the approval of the PKIs by the USFDA and their primary targets in brackets.
3. Patent Searching
The patent searching was performed using the Sci-finder database (CAS Number search, and the exact structure search of each TKI), USFDA’s Orange Book website (mentioned above), and the Drugbank’s website (https://go.drugbank.com/ (accessed on 31 May 2021)) using the drug’s name. The patents disclosing the specific TKI, its marketed active pharmaceutical ingredient, and important polymorphs from the innovative company for the first time were identified and included in this review. The patents of each TKI that claim its treatment methods, dosage forms, formulations, drug combinations, particle size, impurity, preparation process, intermediates, etc., have been excluded from this review. The expiry dates of the selected patents were calculated (20 years from the patent application filing date comprising patent term extension, if any). Sometimes, the drug’s patent term is extended up to five years based on the USPTO’s laws. Accordingly, the expiry dates of the selected patients were also verified from the USPTO’s website. It was also observed that some TKIs were disclosed in different patents of the same patent family and had other expiry dates. In such cases, the patent that had a more extended expiry date was selected for this review because the generic launch of the drug is based on the expiry date of the drug’s patent. The legal status of the patents cited herein was obtained from the website of USPTO (https://portal.uspto.gov/pair/PublicPair (accessed on 31 May 2021)).
4. Summary of the Patents
The proprietary name, approved dosage form, approval date, and marketing status of each marketed PKIs are mentioned in Table 2. The patent number, applicant/assignee, expiry date, and legal status of the cited patents of each PKI are provided in Table 3. A brief description of the PKIs and their important patents are provided below.

Table 3.
Patent number, applicant/assignee, expiry date, and legal status of the cited patents.
4.1. Imatinib Mesylate
Imatinib mesylate (Figure 2) is a pyridine-pyrimidine based piperazine derivative (MF: C29H31N7O·CH4SO3; MW: 589.7; CAS Number: 220127-57-1) [24]. US5521184A claims N-phenyl-2-pyrimidine-amine compounds, including imatinib and its pharmaceutically acceptable salts, as antitumor drugs [25]. USRE43932E (Re-issue of US7544799B2) claims the β-crystal form of imatinib mesylate as having favorable thermodynamic stability, flow properties, and low hygroscopicity that makes it a suitable active pharmaceutical ingredient (API) to be used in the tablet/capsule dosage forms [26].
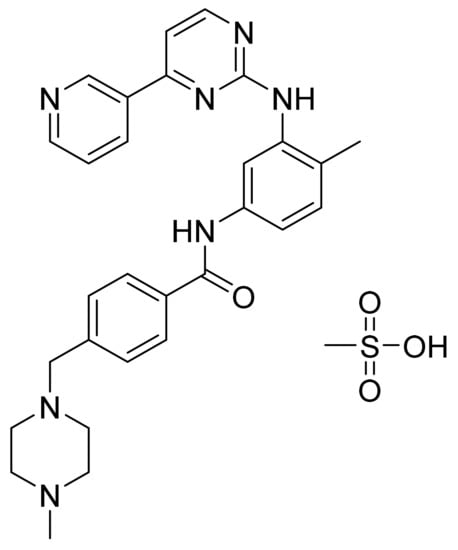
Figure 2.
Imatinib mesylate (4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide methanesulfonate).
4.2. Gefitinib
Gefitinib (Figure 3) is a morpholine based quinazolinamine derivative (MF: C22H24ClFN4O3; MW: 446.9; CAS Number: 184475-35-2) [27]. US5457105A unveils quinazoline derivatives and their salts to treat neoplastic disease. This patent claims gefitinib generically [28]. US5770599A also covers quinazoline derivatives as anticancer agents. This patent claims gefitinib specifically, along with its pharmaceutically acceptable acid-addition salts [29].
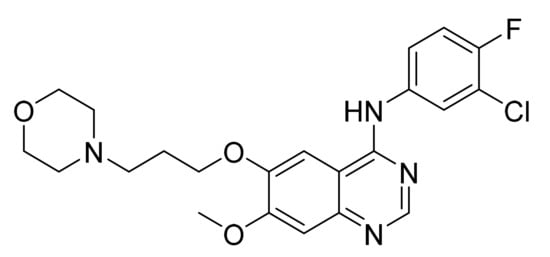
Figure 3.
Gefitinib (N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine).
4.3. Erlotinib Hydrochloride
Erlotinib hydrochloride (Figure 4) is a quinazolinamine derivative (MF: C22H23N3O4·HCl; MW: 429.90; CAS Number: 183319-69-9) [30]. USRE41065E (Reissue patent of US5747498) discloses 4-(substituted phenylamino)quinazoline derivatives, which are useful in treating cancers. It also claims erlotinib hydrochloride specifically [31]. US6900221B1 provides polymorphs of erlotinib hydrochloride and processes for their selective production. It claims homogeneous thermodynamically stable crystalline polymorph of erlotinib hydrochloride (Form B), suitable for making tablet dosage forms [32].
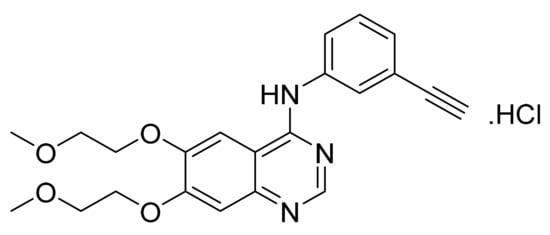
Figure 4.
Erlotinib hydrochloride (N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine hydrochloride).
4.4. Sorafenib Tosylate
Sorafenib tosylate (Figure 5) is a urea-pyridine based diaryl ether derivative (MF: C21H16ClF3N4O3·C7H8O3S; MW: 637.0; CAS Number: 475207-59-1) [33]. US7235576B1 provides aryl urea derivatives for treating RAF-mediated diseases like cancer and their pharmaceutical compositions. It claims sorafenib tosylate specifically [34]. US8877933B2 discloses novel polymorphs of sorafenib tosylate, processes for its synthesis, and compositions comprising it. It claims thermodynamically stable polymorph (Form I) of sorafenib tosylate, which can provide quality dosage form concerning bioavailability and patient safety [35].
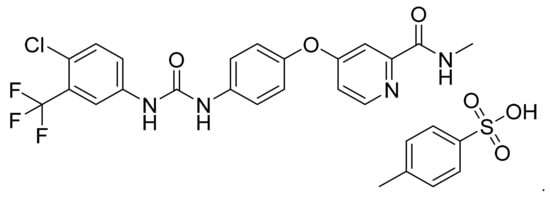
Figure 5.
Sorafenib tosylate (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methylpyridine-2-carboxamide 4-methylbenzenesulfonate).
4.5. Sunitinib Malate
Sunitinib malate (Figure 6) is an indole based pyrrole-3-carboxamide derivative (MF: C22H27FN4O2·C4H6O5; MW: 532.6; CAS Number: 341031-54-7) [36]. US7125905B2 covers 3-pyrrole substituted 2-indolinone compounds as PK activity modulators for treating disorders related to abnormal PK activity. It claims sunitinib malate specifically [37]. The sunitinib malate is also claimed in US6573293B2 [38].
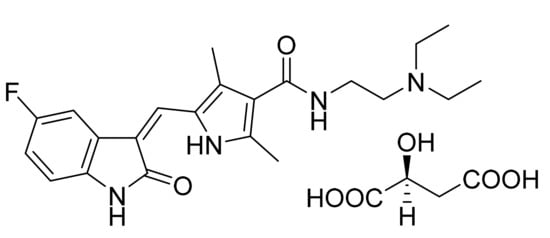
Figure 6.
Sunitinib malate (N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (2S)-2-hydroxybutanedioic acid).
4.6. Dasatinib Monohydrate
Dasatinib monohydrate (Figure 7) is a piperazine-pyrimidine-thiazole based anilide (MF: C22H26ClN7O2S·H2O; MW: 506.02; CAS Number: 863127-77-9) [39]. US6596746B1 provides cyclic compounds for use as PKIs to treat cancer. It claims dasatinib specifically [40]. US7491725B2 claims crystalline monohydrate of dasatinib and process for its preparation [41].
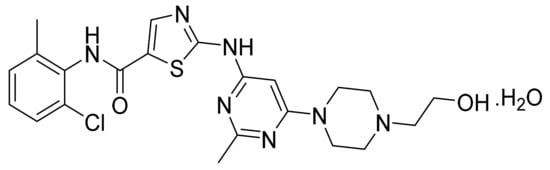
Figure 7.
Dasatinib monohydrate (N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazole carboxamide monohydrate).
4.7. Lapatinib Ditosylate Monohydrate
Lapatinib ditosylate monohydrate (Figure 8) is a furan based quinazolinamine derivative (MF: C29H26ClFN4O4S·(C7H8O3S)2.H2O; MW: 943.5; CAS Number: 388082-78-8) [42]. US8513262B2 discloses substituted heteroaromatic compounds, their synthesis, compositions, and their use in medicine as PTKIs. It claims lapatinib specifically [43]. US7157466B2 relates to quinazoline compounds, anhydrate and hydrate ditosylate salts thereof, and the process for their preparation. It claims lapatinib ditosylate monohydrate specifically. The claimed lapatinib ditosylate possesses physical stability and moisture sorption properties superior to di-HCl salt, making it suitable for developing tablet formulations [44].
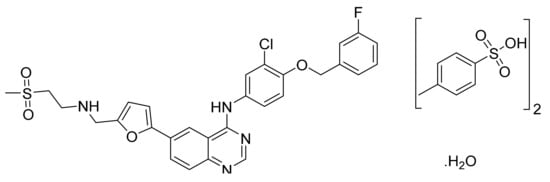
Figure 8.
Lapatinib ditosylate monohydrate (N-(3-chloro-4-{[(3-fluorophenyl) methyl]oxy}phenyl)-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furanyl]-4-quinazolinamine bis(4-methylbenzenesulfonate) monohydrate).
4.8. Temsirolimus
Temsirolimus (Figure 9) is a piperidine-tetrahydropyran based macrolide lactams (MF: C56H87NO16; MW: 1030.30; CAS Number: 162635-04-3) [45]. USRE44768E (Reissue of US5362718) relates to hydroxy esters of rapamycin for treating T-cell leukemia/lymphoma, solid tumors, and hyperproliferative vascular disorders. It claims temsirolimus specifically [46].
Figure 9.
Temsirolimus ((3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26- hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone 4′-[2,2-bis(hydroxymethyl)propionate]).
4.9. Everolimus
Everolimus (Figure 10) is a piperidine-tetrahydropyran based macrolide lactam (MF: C53H83NO14; MW: 958.25; CAS Number: 159351-69-6) [47]. US5665772A provides alkylated derivatives of rapamycin as immunosuppressants. It claims everolimus specifically [48].
Figure 10.
Everolimus ((1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone).
4.10. Nilotinib Hydrochloride Monohydrate
Nilotinib hydrochloride monohydrate (Figure 11) is a pyridine-pyrimidine-imidazole-based benzanilide derivative (MF: C28H22F3N7O·HCl·H2O; MW: 584; CAS Number: 923288-90-8) [49]. US7169791B2 covers substituted pyrimidinyl aminobenzamides, methods of synthesis, and their compositions to treat neoplastic diseases like leukemia. It claims nilotinib and its salts [50]. US8163904B2 claims nilotinib hydrochloride monohydrate as having physicochemical properties required to develop a good dosage form [51]. US8415363B2 claims crystalline form B of nilotinib hydrochloride monohydrate having superior crystallinity and physical stability over other polymorphs [52].
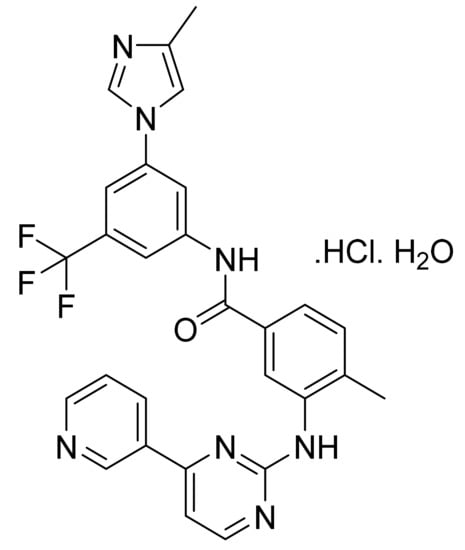
Figure 11.
Nilotinib hydrochloride monohydrate (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-benzamide monohydrochloride monohydrate).
4.11. Pazopanib Hydrochloride
Pazopanib hydrochloride (Figure 12) is a benzenesulfonamide bearing benzimidazole-pyrimidinyl compound (MF: C21H23N7O2S·HCl; MW: 473.99; CAS Number: 635702-64-6) [53]. US7105530B2 reports pyrimidine derivatives as inhibitors of VEGFR-2 to treat disorders, including cancer, associated with inappropriate angiogenesis. It claims pazopanib and its salts [54]. US8114885B2 claims pazopanib hydrochloride precisely [55]. The claimed hydrochloride salt possesses advantageous properties like stability and solubility to develop quality dosage forms.
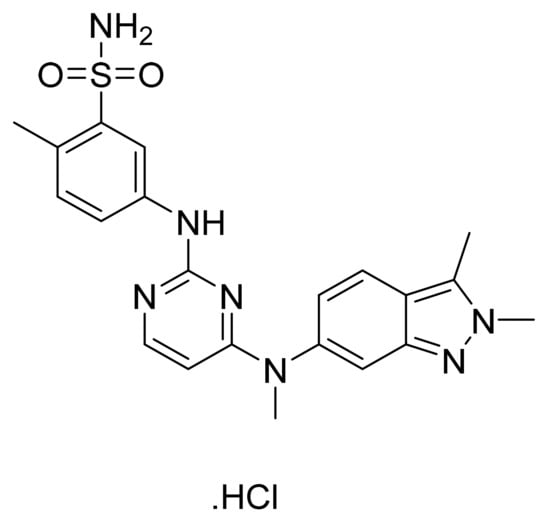
Figure 12.
Pazopanib hydrochloride (5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide monohydrochloride).
4.12. Vandetanib
Vandetanib (Figure 13) is a piperidine based 4-aminoquinazolinamine derivative (MF: C22H24BrFN4O2; MW: 475.36; CAS Number: 443913-73-3) [56]. USRE42353E (Reissue of US6414148B1) provides quinazoline derivatives, synthesis, and compositions to treat illness linked with angiogenesis and amplified vascular permeability. It claims vandetanib precisely [57].
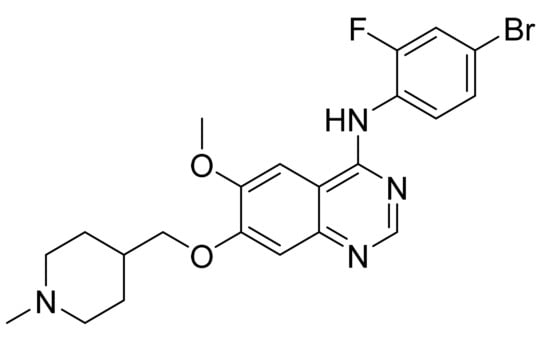
Figure 13.
Vandetanib (N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)methoxy]quinazolin-4-amine).
4.13. Vemurafenib
Vemurafenib (Figure 14) is a phenylketone based pyrrolopyridine (MF: C23H18ClF2N3O3S; MW: 489.9; CAS Number: 918504-65-1) [58]. US8143271B2 describes pyrrolopyridine based compounds as PTKIs to treat diseases and conditions associated with aberrant activity of PTKs. It claims vemurafenib specifically [59].
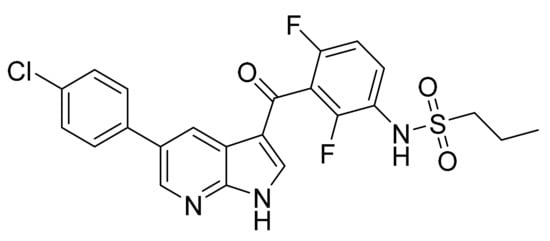
Figure 14.
Vemurafenib (Propane-1-sulfonic acid {3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide).
4.14. Crizotinib
Crizotinib (Figure 15) is a piperidine based pyrazolylpyridine derivative (MF: C21H22Cl2FN5O; MW: 450.34; CAS Number: 877399-52-5) [60]. US7858643B2 describes aminopyridines and aminopyrazines having PTKI activity, methods of synthesizing and using these compounds as anticancer agents. It claims crizotinib and its salts [61]. US8217057B2 claims a crystalline form of a free base of crizotinib with improved solubility, stability, and physicochemical properties to develop solid dosage forms, such as capsules [62].
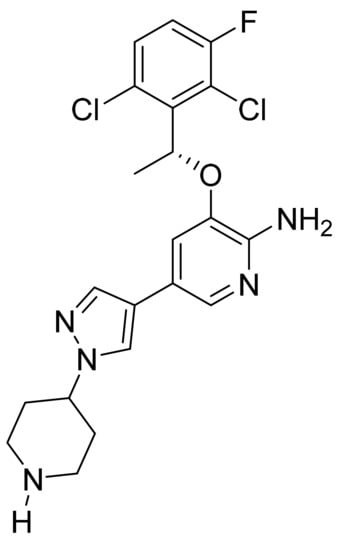
Figure 15.
Crizotinib ((R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine).
4.15. Ruxolitinib Phosphate
Ruxolitinib phosphate (Figure 16) is a pyrrolo[2,3-d]pyrimidine based pyrazole derivative (MF: C17H21N6O4P; MW: 404.36; CAS Number: 1092939-17-7) [63]. US7598257B2 provides pyrrolo[2,3-b]pyridines as JAK modulators, which are beneficial to treat immune-related disorders, skin diseases, myeloid proliferative ailments, and cancer. It claims ruxolitinib and its salts [64]. US8722693B2 claims ruxolitinib phosphate, which has improved water solubility, dissolution rate, chemical stability, long shelf life, excipients, and reproducibility compared to the free base [65].
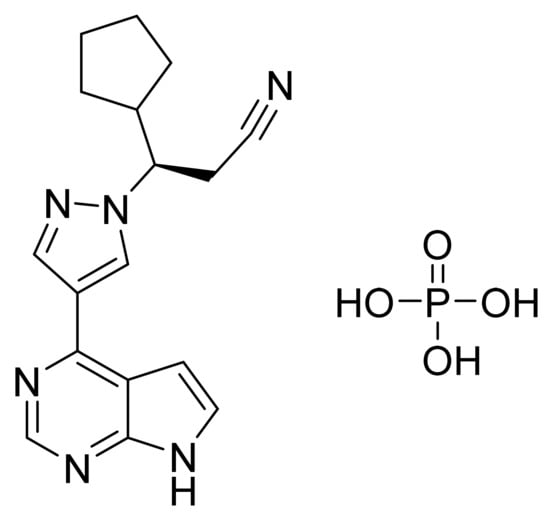
Figure 16.
Ruxolitinib phosphate ((R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate).
4.16. Axitinib
Axitinib (Figure 17) is a pyridine based indazolylphenyl thioether (MF: C22H18N4OS; MW: 386.47; CAS Number: 319460-85-0) [66]. US6534524B1 relates to indazole compounds as PTKIs and their pharmaceutical compositions to treat diseases linked with undesirable angiogenesis and cellular proliferation. It claims axitinib specifically [67]. US8791140B2 claims crystalline forms of axitinib that have advantages in bioavailability, stability, manufacture ability, and suitability for bulk preparation [68].
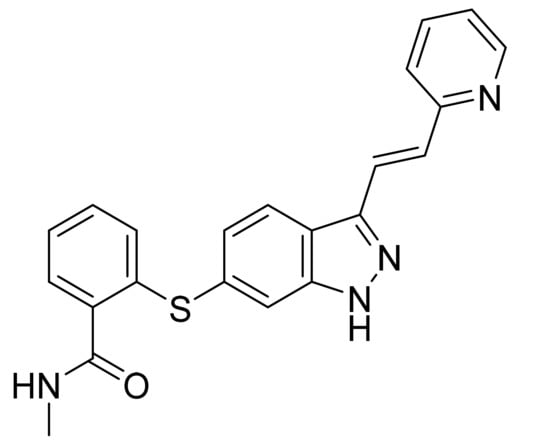
Figure 17.
Axitinib (N-methyl-2-[3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide).
4.17. Bosutinib Monohydrate
Bosutinib monohydrate (Figure 18) is a piperazine based 3-quinolinecarbonitrile derivative (MF: C26H29Cl2N5O3·H2O; MW: 548.46; CAS Number: 918639-08-4) [69]. USRE42376E (Reissue of US6297258B1) describes substituted 3-cyano quinoline compounds as PTKIs to treat diseases resulting from deregulation of PTKs, for example, cancer and polycystic kidney disease. It claims bosutinib [70]. US7767678B2 claims non-hygroscopic and stable crystalline bosutinib monohydrate (Form I) having good solubility that can be used to prepare different solid dosage forms [71].
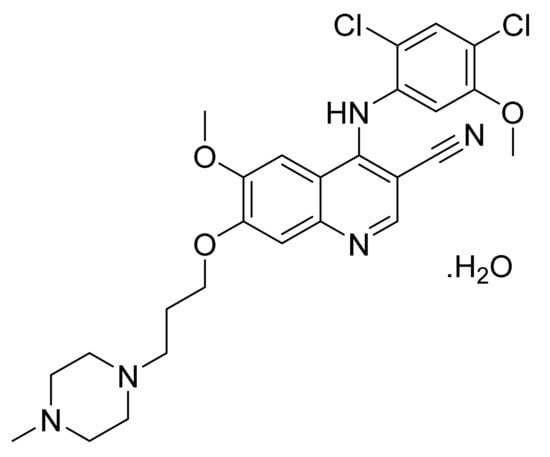
Figure 18.
Bosutinib monohydrate (4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile monohydrate).
4.18. Regorafenib Monohydrate
Regorafenib monohydrate (Figure 19) is pyridinylphenyl urea derivative (MF: C21H15ClF4N4O3·H2O; MW: 500.83; CAS Number: 1019206-88-2) [72]. US8637553B2 discloses omega-carboxyaryl diphenyl urea derivatives as potent inhibitors of PDGFR, VEGFR, RAF, and p38 kinase to treat cancer, inflammatory diseases, and osteoporosis. It claims regorafenib and its salts [73]. US9957232B2 claims regorafenib monohydrate with high stability and good physicochemical features to manufacture pharmaceutical compositions [74].
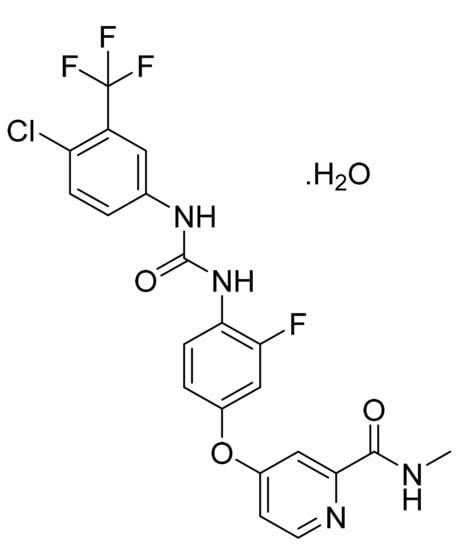
Figure 19.
Regorafenib monohydrate (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl] carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide monohydrate).
4.19. Tofacitinib Citrate
Tofacitinib citrate (Figure 20) is an pyrrolo[2,3-d]pyrimidine based piperidine derivative (MF: C16H20N6O·C6H8O7; MW: 504.5; CAS Number: 540737-29-9) [75]. USRE41783E (Reissue of US6627754B2) provides pyrrolo[2,3-d]pyrimidines as JAK3 inhibitors to treat rheumatoid arthritis, psoriasis, cancer, and leukemia. It claims tofacitinib and its salt [76]. US6965027B2 claims a crystalline form of tofacitinib mono citrate salt with solid-state properties (solubility, stability, compressibility, etc.), which are acceptable to support tablet development [77].
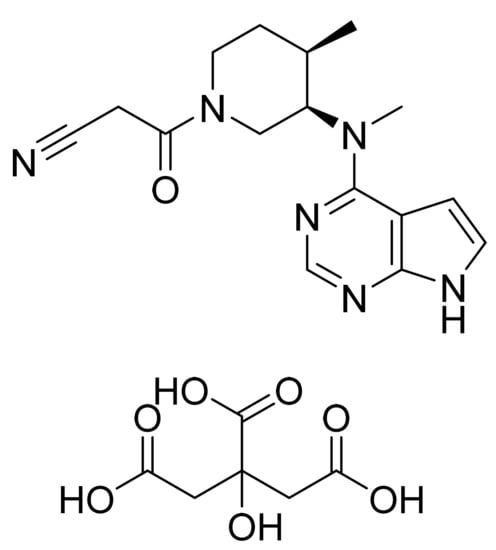
Figure 20.
Tofacitinib citrate ((3R,4R)-4-methyl-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-ß-oxo-1-piperidinepropanenitrile 2-hydroxy-1,2,3-propanetricarboxylate (1:1)).
4.20. Cabozantinib S-Malate
Cabozantinib S-malate (Figure 21) is a quinolinylphenyl ether derivative (MF: C28H24FN3O5·C4H6O5; MW: 635.6; CAS Number: 1140909-48-3) [78]. US7579473B2 relates to quinazolines and quinolines as TKIs, and their pharmaceutical compositions to treat psoriasis, multiple sclerosis, and rheumatoid arthritis. It claims cabozantinib and its salts [79]. US8877776B2 claims cabozantinib (L)-malate salt having desirable solubility and chemical/physical stability to develop a tablet/capsule dosage forms for intended use [80].
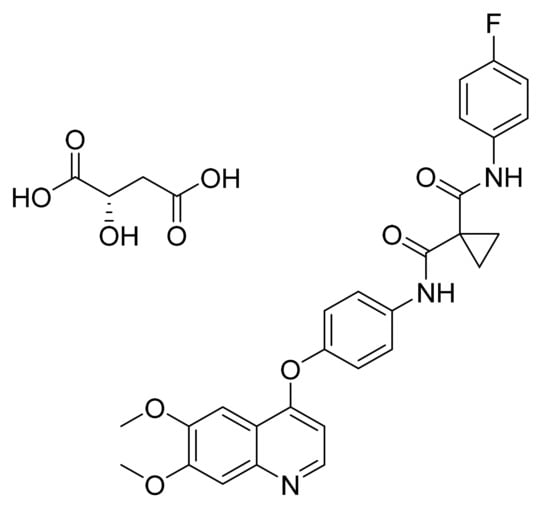
Figure 21.
Cabozantinib (S)-malate (N-(4-(6,7-dimethoxyquinolin-4-yloxy)phenyl)-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamid (2S)-hydroxybutanedioate).
4.21. Ponatinib Hydrochloride
Ponatinib hydrochloride (Figure 22) is animidazo[1,2-b]pyridazine based piperazine derivative (MF: C29H28ClF3N6O; MW: 569.02; CAS Number: 1114544-31-8) [81]. US8114874B2 describes imidazo[1,2-b]pyridazines as PTKIs and their pharmaceutical compositions to treat cancer and other diseases mediated by PTKs. It claims ponatinib hydrochloride specifically [82]. US9493470B2 claims stable crystalline form A of ponatinib hydrochloride that is advantageous for the commercial preparation of solid dosage forms because of its physicochemical stability compared to amorphous ponatinib hydrochloride [83].
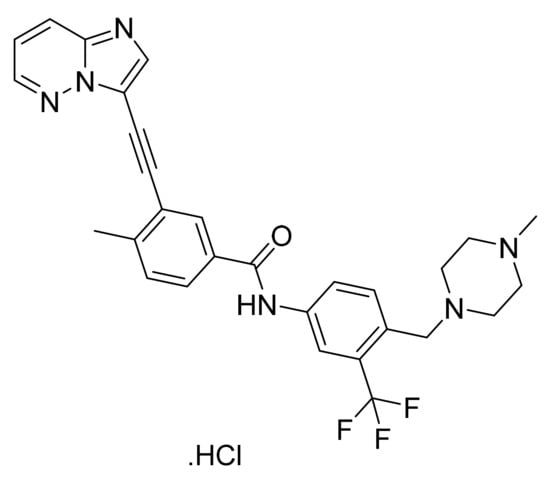
Figure 22.
Ponatinib hydrochloride (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide hydrochloride).
4.22. Trametinib Dimethyl Sulfoxide
Trametinib dimethyl sulfoxide (Figure 23) is a pyridopyrimidine derivative (MF: C26H23FIN5O4.C2H6OS; MW: 693.53; CAS Number: 1187431-43-1) [84]. US7378423B2 unveils pyrimidine compounds, their salts, synthetic procedures, and compositions to treat ailments caused by unwanted cell proliferation, for example, cancer. It claims trametinib dimethyl sulfoxide specifically [85].
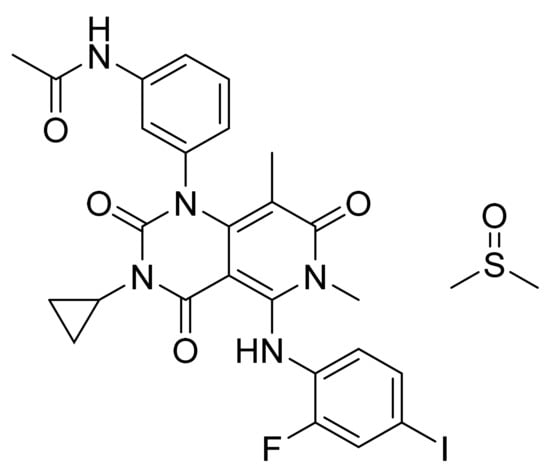
Figure 23.
Trametinib dimethyl sulfoxide (N-(3-{3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-1H,2H,3H,4H,6H,7H-pyrido[4,3-d]pyrimidin-1-yl}phenyl)acetamide dimethyl sulfoxide).
4.23. Dabrafenib Mesylate
Dabrafenib mesylate (Figure 24) is a pyrimidine-thiazole based diphenyl sulfonamide derivative (MF: C23H20F3N5O2S2.CH4O3S; MW: 615.68; CAS Number: 1195768-06-9) [86]. US7994185B2 provides benzene sulfonamide thiazole and oxazole compounds, their pharmaceutical compositions, processes for their preparation, and methods of using these compounds and compositions for treating cancer and melanoma. It claims dabrafenib mesylate specifically [87].
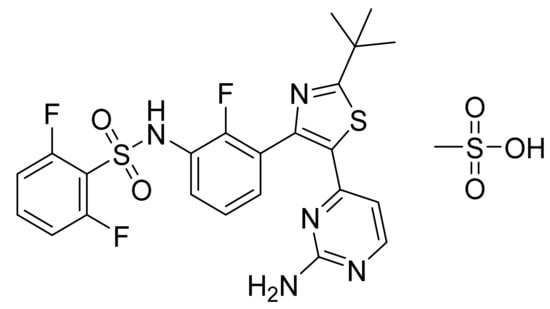
Figure 24.
Dabrafenib mesylate (N-{3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzene sulfonamide mesylate).
4.24. Afatinib Dimaleate
Afatinib dimaleate (Figure 25) is a tetrahydrofuran based quinazolinamine derivative (MF: C32H33ClFN5O11; MW: 718.1; CAS Number: 850140-73-7) [88]. USRE43431E (Reissue of US7019012B2) unveils quinazoline derivatives and their physiologically acceptable salts possessing an inhibitory effect on signal transduction mediated by PTKs to treat tumoral diseases, diseases of the lungs, and respiratory tract. It claims afatinib dimaleate precisely [89]. US8426586B2 claims crystalline afatinib dimaleate, synthesis, and its compositions. The claimed crystalline form is stable and has advantageous properties to develop quality dosage forms [90].
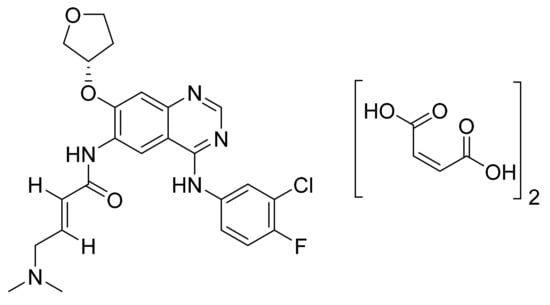
Figure 25.
Afatinib dimaleate (N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)but-2-enamide dimaleate).
4.25. Ibrutinib
Ibrutinib (Figure 26) is a piperidine based pyrazolo[3,4-d]pyrimidine (MF: C25H24N6O2; MW: 440.50; CAS Number: 936563-96-1) [91]. US8735403B2 describes pyrazolo[3,4-d]pyrimidine based inhibitors of BTK, their synthesis, and compositions to treat diseases, wherein inhibition of BTK delivers therapeutic advantage to the diseased person. It claims ibrutinib specifically [92]. US9296753B2 claims stable, water-soluble, and non-hygroscopic crystalline ibrutinib that can be used to manufacture quality dosage forms [93].
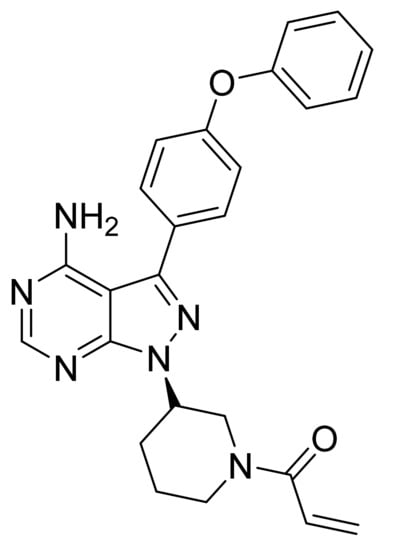
Figure 26.
Ibrutinib (1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one).
4.26. Ceritinib
Ceritinib (Figure 27) is a pyrimidine based phenylpiperidine derivative (MF: C28H36N5O3ClS; MW: 558.14; CAS Number: 1032900-25-6) [94]. US8039479B2 reveals pyrimidine and pyridine derivatives and their pharmaceutical compositions to treat a condition that responds to inhibition of ALK, FAK, ZAP-70, IGF-1R, or a combination thereof. It claims ceritinib specifically [95]. US9309229B2 claims a pure and stable crystalline form of ceritinib with desirable physicochemical properties to provide good dosage forms [96].
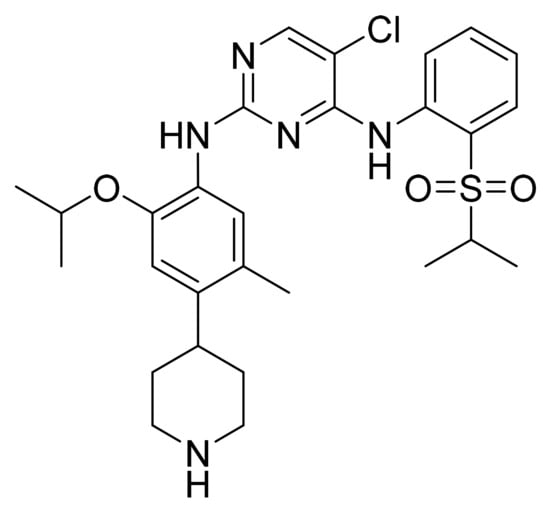
Figure 27.
Ceritinib (5-Chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine).
4.27. Idelalisib
Idelalisib (Figure 28) is a purine based quinazolinone derivative (MF: C22H18FN7O; MW: 415.42; CAS Number: 870281-82-6) [97]. USRE44638E (Reissue of US7932260B2) reports substituted quinazolinone compounds as PI3Kδ inhibitors to treat diseases like bone-resorption disorders, hematopoietic cancers, lymphomas, multiple myelomas, and leukemia. It claims idelalisib and its salts [98]. US9469643B2 claims a water-soluble bioavailable and stable polymorph of idelalisib (Form II) that can be used to provide quality dosage forms [99].
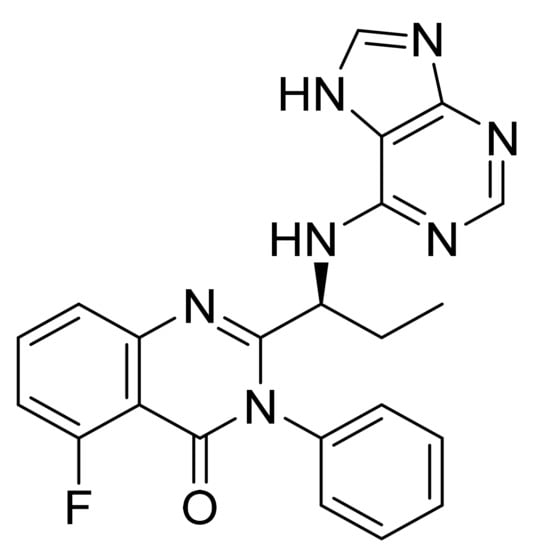
Figure 28.
Idelalisib (5-fluoro-3-phenyl-2-[(1S)-1-(9H-purin-6-ylamino)propyl]quinazolin-4(3H)-one).
4.28. Nintedanib Esylate
Nintedanib esylate (Figure 29) is a piperazine based indole carboxylic acid derivative (MF: C31H33N5O4.C2H6O3S; MW: 649.76; CAS Number: 656247-18-6) [100]. US6762180B1 states indolinone derivatives as PTKIs, synthesis, and compositions to treat proliferative sicknesses. It claims nintedanib and its salts [101]. US7119093B2 claims a stable nintedanib esylate salt specifically characterized by good crystallinity and low amorphization during grinding and compression. This salt is claimed to have good physicochemical characteristics to support quality dosage forms [102].
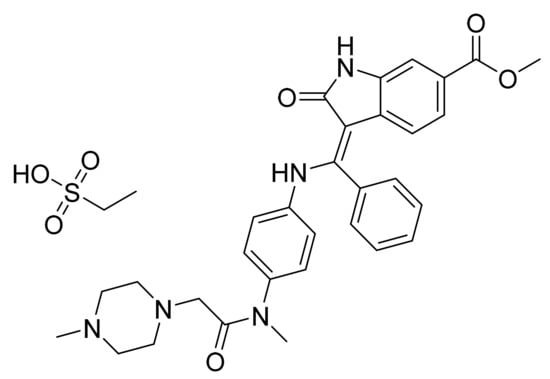
Figure 29.
Nintedanib esylate (methyl (3Z)-3-[({4-[N-methyl-2-(4-methylpiperazin-1-yl)acetamido]phenyl}amino)(phenyl)methylidene]-2-oxo-2,3-dihydro-1H-indole-6-carboxylate esylate).
4.29. Palbociclib
Palbociclib (Figure 30) is a pyrido[2,3-d]pyrimidine based pyridinylpiperazine derivative (MF: C24H29N7O2; MW: 447.54; CAS: 571190-30-2) [103]. USRE47739E (Reissue of US7208489B2) delivers substituted 2-amino pyridines as potent inhibitors of CDK 4, useful for treating inflammation and proliferative cell diseases such as cancer and restenosis. It claims palbociclib and its salts [104]. US10723730B2 claims a stable crystalline free base of palbociclib with larger primary particle size, reduced specific surface area, lower surface energy measurements, and physicochemical properties to formulate a good dosage form [105].
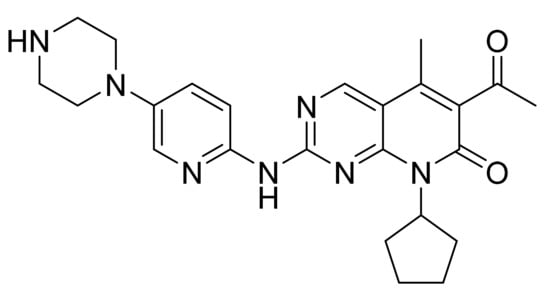
Figure 30.
Palbociclib (6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one).
4.30. Lenvatinib Mesylate
Lenvatinib mesylate (Figure 31) is a quinoline carboxamide derivative (MF: C21H19ClN4O4.CH4O3S; MW: 522.96; CAS Number: 857890-39-2) [106]. US7253286B2 reports nitrogen-containing aromatic derivatives and salts or hydrates thereof to treat various diseases associated with abnormal angiogenesis. It claims lenvatinib and its pharmacologically active salts [107]. US7612208B2 claims a crystalline form of lenvatinib mesylate with improved features (physical/pharmacokinetics) compared to the free-form [108].
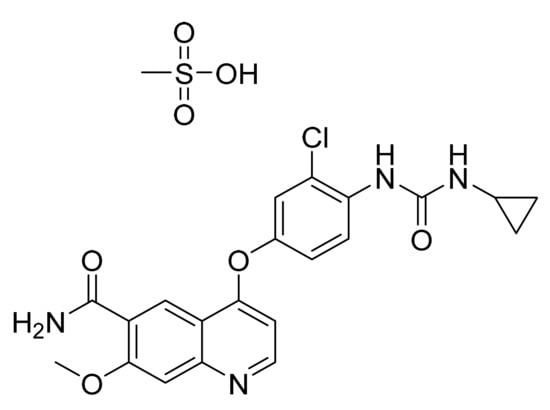
Figure 31.
Lenvatinib mesylate (4-[3-chloro-4-(N′-cyclopropylureido)phenoxy]-7-methoxyquinoline-6-carboxamide methanesulfonate).
4.31. Cobimetinib Fumarate
Cobimetinib fumarate (Figure 32) is a piperidine-azetidine based anthranilamide derivative (MF: C46H46F6I2N6O8 (2C21H21F3IN3O2.C4H4O4); MW: 1178.71; CAS Number: 1369665-02-0) [109]. US7803839B2 provides azetidin-1-yl(2-(2-fluorophenylamino)cyclic)methanone derivatives as inhibitors of MEK that are useful in cancer treatment. It claims cobimetinib and its salts [110]. US10590102B2 claims a thermodynamically stable and non-hygroscopic crystalline fumarate salt (Form A) of cobimetinib with suitable properties for use in a pharmaceutical composition [111].
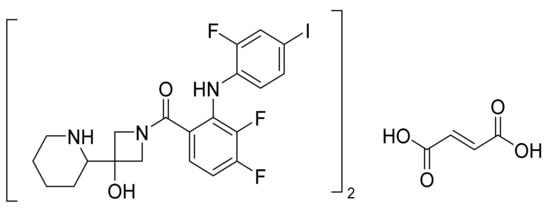
Figure 32.
Cobimetinib fumarate ((S)-[3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl][3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl]methanone hemifumarate).
4.32. Osimertinib Mesylate
Osimertinib mesylate (Figure 33) is a pyrimidine based indole derivative (MF: C28H33N7O2.CH4O3S; MW: 596; CAS Number: 1421373-66-1) [112]. US8946235B2 states 2-(2,4,5-substituted-anilino)pyrimidines, useful in treating a disease mediated by EGFR, for example, cancer. It claims osimertinib mesylate specifically [113].
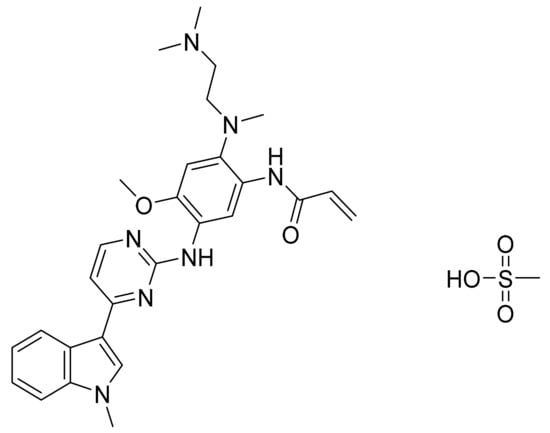
Figure 33.
Osimertinib mesylate (N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide mesylate).
4.33. Alectinib Hydrochloride
Alectinib hydrochloride (Figure 34) is a morpholine-piperidine based carbazole derivatives (MF: C30H34N4O2·HCl; MW: 519.08; CAS Number: 1256589-74-8) [114]. US9126931B2 relates to tetracyclic compounds as ALK inhibitors for treating a disease accompanied by an abnormality in ALK, for example, cancer, depression, and cognitive function disorder. It claims alectinib and its salts [115].
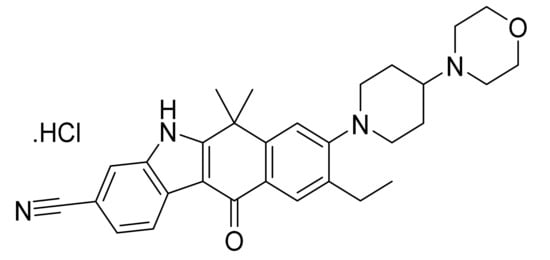
Figure 34.
Alectinib hydrochloride (9-Ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride).
4.34. Ribociclib Succinate
Ribociclib succinate (Figure 35) is a pyridine-piperazine based pyrrolo[2,3-d]pyrimidine derivative (MF: C23H30N8O·C4H6O4; MW: 552.64; CAS Number: 1374639-75-4) [116]. US8415355B2 discloses pyrrolopyrimidine compounds, the process for their preparation, and their pharmaceutical compositions to treat a disease linked with CDK 4 inhibition. It claims ribociclib and its salts [117]. US9193732B2 claims succinate salt of ribociclib that has good stability, non-hygroscopicity, and good solubility. These features make this salt a suitable salt to develop the desired formulation [118].
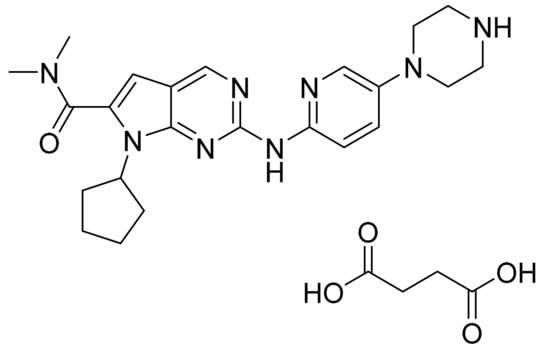
Figure 35.
Ribociclib succinate (7-cyclopentyl-N,N-dimethyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide succinate).
4.35. Brigatinib
Brigatinib (Figure 36) is a piperazine-piperidine based pyrimidine derivative (MF: C29H39ClN7O2P; MW: 584.10; CAS Number: 1197953-54-0) [119]. US9012462B2 narrates phosphorous compounds as PTKIs and their use in treating cancers. It claims brigatinib and its salts [120]. US10385078B2 claims a stable and non-hygroscopic anhydrous crystalline form A of brigatinib suitable for pharmaceutical formulation development [121].
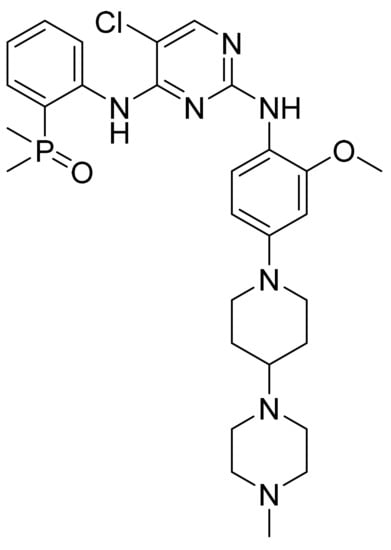
Figure 36.
Brigatinib (5-chloro-N4-[2-(dimethylphosphoryl)phenyl]-N2-{2-methoxy-4[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}pyrimidine-2,4-diamine).
4.36. Midostaurin
Midostaurin (Figure 37) is an indolocarbazole derivative (MF: C35H30N4O4; MW: 570.65; CAS Number: 120685-11-2) [122]. US5093330A relates to staurosporine derivatives, their salts, synthesis, and compositions encompassing them to treat cancer and inflammation. It discloses midostaurin [123]. US7973031B2 claims a method for treating AML using a dosage form (a microemulsion, soft gel, or solid dispersion) of midostaurin, wherein the AML is characterized by deregulated FLT3 receptor tyrosine kinase activity [124].
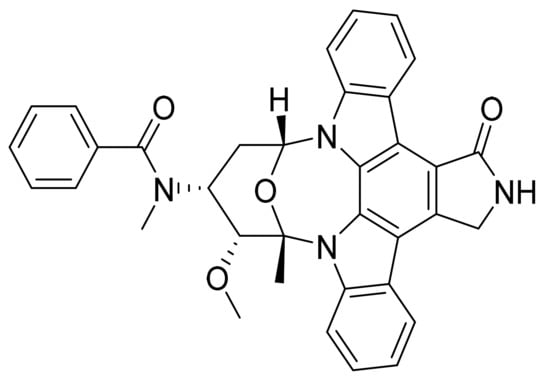
Figure 37.
Midostaurin (N-[(2S,3R,4R,6R)-3-Methoxy-2-methyl-16-oxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-4-yl]-N-methylbenzamide).
4.37. Neratinib Maleate
Neratinib maleate (Figure 38) is a pyridine based 4-aminoquinoline derivative (MF: C30H29ClN6O3·C4H4O4; MW: 673.11; CAS Number: 915942-22-2) [125]. US7399865B2 reports substituted 3-cyanoquinoline compounds and their salts as inhibitors of HER-2 and EGFR to treat cancer. It claims neratinib and its salts [126].
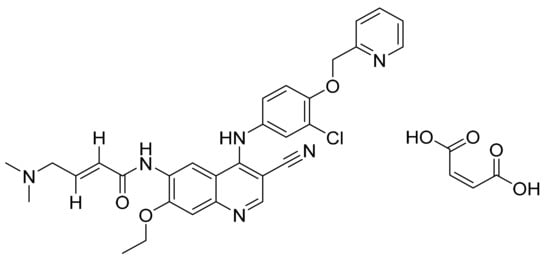
Figure 38.
Neratinib maleate ((E)-N-{4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl}-4-(dimethylamino)but-2-enamide maleate).
4.38. Copanlisib Dihydrochloride
Copanlisib dihydrochloride (Figure 39) is a morpholine-pyrimidine based 2,3-dihydroimidazo[1,2-c]quinazoline derivative (MF: C23H28N8O4·2HCl; MW: 553.45; CAS Number: 1402152-13-9) [127]. USRE46856E (Reissue of US8466283B2) unveils 2,3-dihydroimidazo[1,2-c]quinazoline derivatives, pharmaceutical compositions comprising them, and the use of these compounds for treating hyperproliferative and angiogenesis disorders. It claims copanlisib and its salts [128]. US10383876B2 claims copanlisib dihydrochloride salt that possesses technically advantageous properties (stability, solubility, hygroscopicity, etc.) to develop a quality pharmaceutical composition [129].
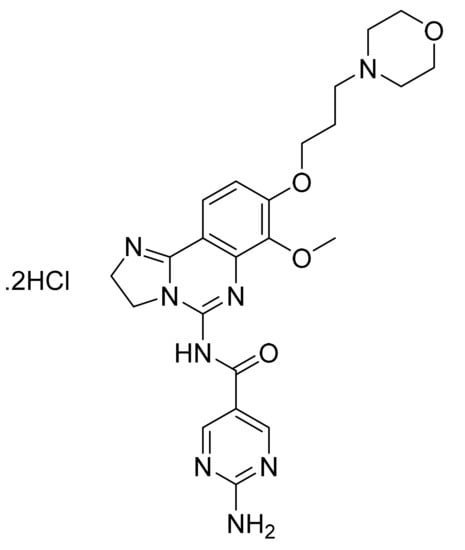
Figure 39.
Copanlisib dihydrochloride (2-amino-N-{7-methoxy-8-[3-(morpholin-4-yl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl}pyrimidine-5-carboxamide dihydrochloride).
4.39. Abemaciclib
Abemaciclib (Figure 40) is a piperazine-pyridine-pyrimidine based benzimidazole derivative (MF: C27H32F2N8; MW: 506.59; CAS Number: 1231929-97-7) [130]. US7855211B2 reports piperazine-pyridine-pyrimidine based benzimidazole derivatives and salts thereof, a pharmaceutical formulation comprising them to treat cancers selected from the group colorectal cancer, breast cancer, NSCLC, prostate cancer, glioblastoma, MCL, CML, and AML. It claims abemaciclib and its salts [131].
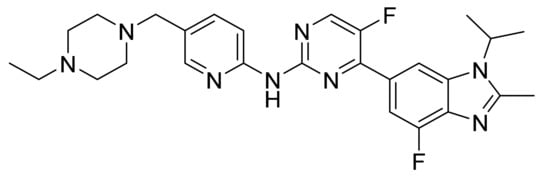
Figure 40.
Abemaciclib (N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]pyrimidin-2-amine).
4.40. Acalabrutinib
Acalabrutinib (Figure 41) is a pyrrolidine-pyridine based imidazo[1,5-a]pyrazine derivative (MF: C26H23N7O2; MW: 465.51; CAS Number: 1420477-60-6) [132]. US9290504B2 provides 4-imidazopyridazin-1-yl-benzamides for the treatment of BTK mediated disorders. It claims acalabrutinib and its salts [133]. US9796721B2 claims a stable and non-hygroscopic anhydrate crystal form of acalabrutinib as having advantageous parameters for making quality pharmaceutical compositions [134].
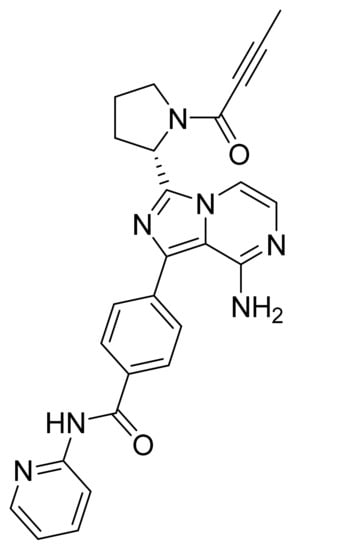
Figure 41.
Acalabrutinib (4-{8-amino-3-[(2S)-1-(but-2-ynoyl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide).
4.41. Netarsudil Dimesylate
Netarsudil dimesylate (Figure 42) is an isoquinoline based beta-amino acid derivative (MF: C30H35N3O9S2; MW: 645.74; CAS Number: 1422144-42-0) [135]. US8394826B2 relates to isoquinoline amide and benzamide based compounds as dual inhibitors of Rho kinase and a monoamine transporter (MAT), useful in treating diseases like glaucoma and cancer. It claims netarsudil [136]. US9415043B2 claims a chemically stable and water-soluble dimesylate salt of netarsudil that can provide a quality ophthalmic solution [137].
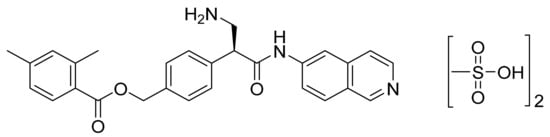
Figure 42.
Netarsudil dimesylate ((S)-4-(3-amino-1-(isoquinolin-6-ylamino)-1-oxopropan-2-yl)benzyl-2,4-dimethylbenzoate dimesylate).
4.42. Baricitinib
Baricitinib (Figure 43) is a pyrazole-azetidine based pyrrolo[2,3-d]pyrimidine derivative (MF: C16H17N7O2S; MW: 371.42; CAS Number: 1187594-09-7) [138]. US8158616B2 provides azetidine derivatives as JAK inhibitors, synthetic methods, and compositions encompassing them to treat inflammatory and autoimmune disorders, along with cancer. It claims baricitinib and its salts [139].
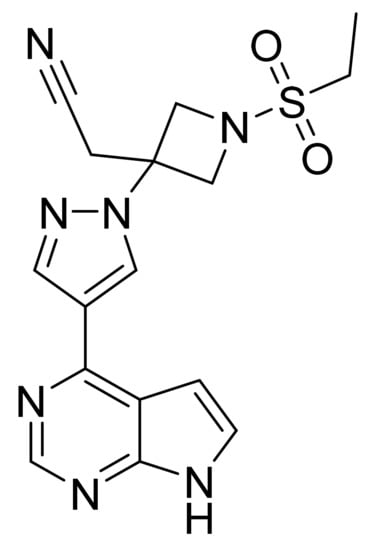
Figure 43.
Baricitinib ({1-(ethylsulfonyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile).
4.43. Binimetinib
Binimetinib (Figure 44) is a benzimidazole derivative (MF: C17H15BrF2N4O3; MW: 441.2; CAS Number: 606143-89-9) [140]. US7777050B2 states alkylated (1H-Benzoimidazol-5-yl)-(4-substituted-phenyl)-amine derivatives, helpful in managing sicknesses like cancer. It claims binimetinib and pharmaceutically acceptable salts thereof [141]. US9562016B2 claims a crystallized form of binimetinib with better purity and an enhanced physical characteristic, beneficial in pharmaceutical dosage form preparation [142].
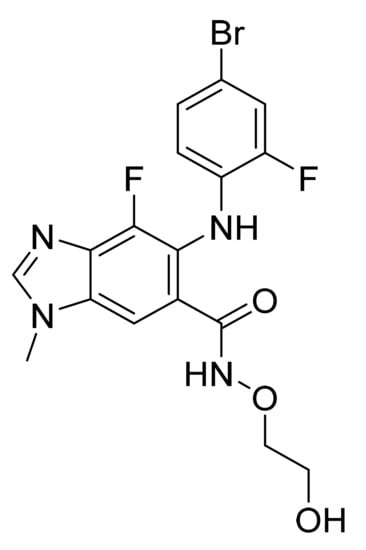
Figure 44.
Binimetinib (5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide).
4.44. Dacomitinib Monohydrate
Dacomitinib monohydrate (Figure 45) is a piperidine based quinazolinamine derivatives (MF: C24H25ClFN5O2·H2O; MW: 487.95; CAS Number: 1042385-75-0) [143]. US7772243B2 unveils 4-anilino-6-substituted alkenoylamino-quinazoline compounds as TKIs to treat proliferative diseases, including cancer and restenosis endometriosis and psoriasis. It claims dacomitinib and its salts [144].
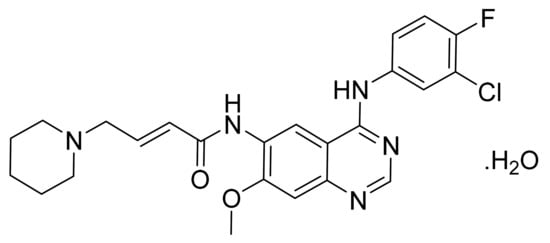
Figure 45.
Dacomitinib monohydrate ((2E)-N-{4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl}-4-(piperidin-1-yl)but-2-enamide monohydrate).
4.45. Encorafenib
Encorafenib (Figure 46) is a pyrazole based pyrimidine derivative (MF: C22H27ClFN7O4S; MW: 540; CAS Number: 1269440-17-6) [145]. US8501758B2 provides pyrazole based pyrimidine and pharmaceutical compositions comprising them to treat disorders associated with the deregulated activity of B-Raf. It claims encorafenib and its salts [146].
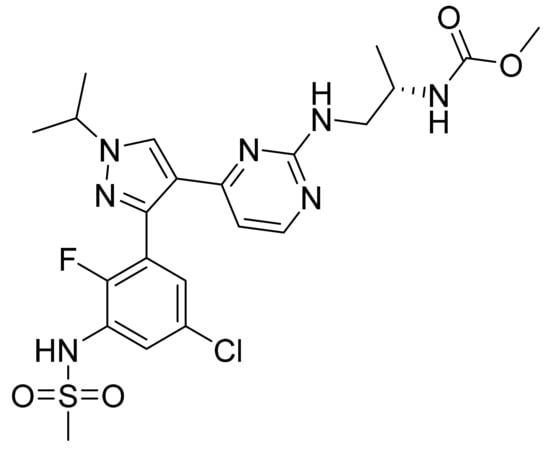
Figure 46.
Encorafenib (N-{(2S)-1-[(4-{3-[5-chloro-2-fluoro-3-(methanesulfonamido)phenyl]-1-(propan-2-yl)-1H-pyrazol-4-yl}pyrimidin-2-yl)amino]propan-2-yl}carbamate).
4.46. Fostamatinib Disodium Hexahydrate
Fostamatinib disodium hexahydrate (Figure 47), a phosphate prodrug of tamatinib, is a pyrimidine based pyrido[3,2-b][1,4]oxazine derivative (MF: C23H24FN6Na2O9P·6H2O; MW: 732.52; CAS Number: 914295-16-2) [147]. US7449458B2 reports prodrugs of pharmacologically active 2,4-pyrimidinediamine derivatives, intermediates thereof, the process of manufacturing them, and pharmaceutical compositions comprising them to treat diseases mediated by the activation of PTKs. It claims fostamatinib disodium hexahydrate, which has increased solubility concerning the parent phosphate prodrug [148]. US8163902B2 claims a thermodynamically stable crystalline form of fostamatinib disodium hexahydrate that is stable over a wide range of relative humidity and requires substantial heating to lose its water molecules. This property makes it a suitable API to develop the desired dosage form [149].
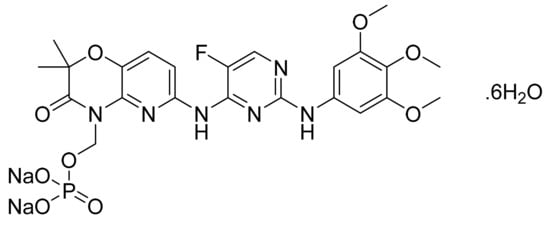
Figure 47.
Fostamatinib disodium hexahydrate (Disodium (6-[[5-fluoro-2-(3,4,5-trimethoxyanilino) pyrimidin-4-yl]amino]-2,2-dimethyl-3-oxo-pyrido[3,2-b][1,4]oxazin-4-yl)methyl phosphate hexahydrate).
4.47. Duvelisib Hydrate
Duvelisib hydrate (Figure 48) is a purine based isoquinolone derivative (MF: C22H17ClN6O·H2O; MW: 434.88; CAS Number: 1201438-56-3) [150]. US8193182B2 provides isoquinolin-1(2H)-one derivatives as modulators of PI3 kinase activity and pharmaceutical compositions comprising them to treat diseases associated with P13 kinase activity. It claims duvelisib and its salts [151]. USRE46621E (Reissue of US8809349B2) claims physically and chemically stable polymorphs of duvelisib, salt, solvate, or hydrate that do not readily decompose or change in chemical makeup or physical state for more than 60 months and are suitable to develop the desired dosage forms of the API [152].
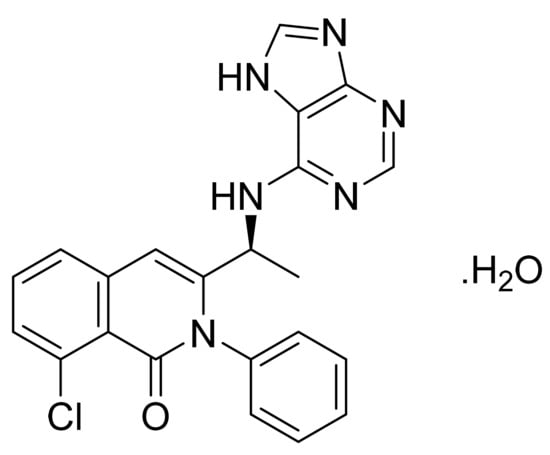
Figure 48.
Duvelisib hydrate ((S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one hydrate).
4.48. Gilteritinib Fumarate
Gilteritinib fumarate (Figure 49) piperazine-piperidine based pyrazine carboxamide derivative (MF: (C29H44N8O3)2·C4H4O4; MW: 1221.50; CAS Number: 1254053-84-3) [153]. US8969336B2 states diamino heterocyclic carboxamide derivatives as having outstanding inhibitory activity against EML4-ALK fusion proteins for use in cancer therapy. It claims gilteritinib and its salts [154]. The gilteritinib fumarate salt is stable in heat, humidity, and storage conditions.
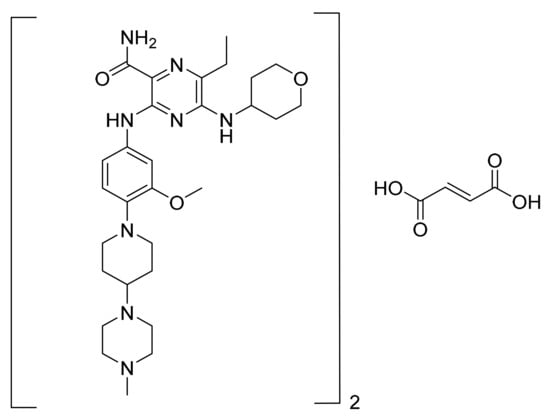
Figure 49.
Gilteritinib fumarate (2-Pyrazinecarboxamide 6-ethyl-3-[[3-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl] phenyl]amino]-5-[(tetrahydro-2H-pyran-4-yl)amino]-, (2E)-2-butenedioate (2:1)).
4.49. Larotrectinib Sulfate
Larotrectinib sulfate (Figure 50) is a pyrrolidine based pyrazolo[1,5-a]pyrimidine derivative (MF: C21H24F2N6O6S; MW: 526.51; CAS Number: 1223405-08-0) [155]. US9127013B2 relates to pyrazolo[1,5-a] pyrimidine derivatives as TRK family PTKIs that are useful to treat cancer, inflammation, and certain infectious diseases. It claims larotrectinib sulfate specifically [156]. US10172861B2 claims crystalline larotrectinib sulfate having stable physicochemical properties, which can be used to develop quality dosage forms [157].
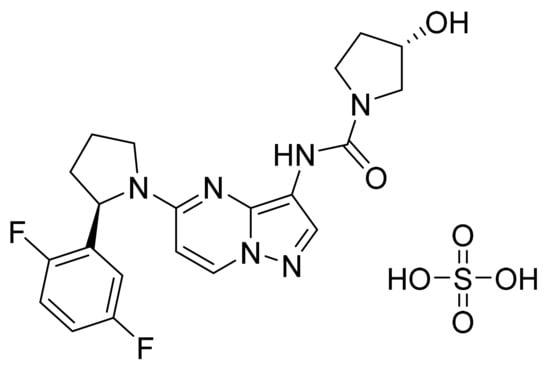
Figure 50.
Larotrectinib sulfate ((3S)-N-{5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-a]pyrimidin-3-yl}-3-hydroxy-1-pyrrolidinecarboxamide sulfate).
4.50. Lorlatinib
Lorlatinib (Figure 51) is a pyrazole-pyridine based benzoxadiazacyclotetradecine derivative (MF: C21H19FN6O2; MW: 406.41; CAS Number: 1223403-58-4) [158]. US8680111B2 discloses macrocyclic compounds as inhibitors of ALK and/or EML4-ALK and their pharmaceutical composition to treat illnesses linked with the deregulation of ALK and EML4-ALK. It claims lorlatinib and its salts [159]. US10420749B2 claims crystalline polymorphs of lorlatinib having high crystallinity and purity, low hygroscopicity, and favorable dissolution and mechanical properties to develop quality pharmaceutical formulations [160].
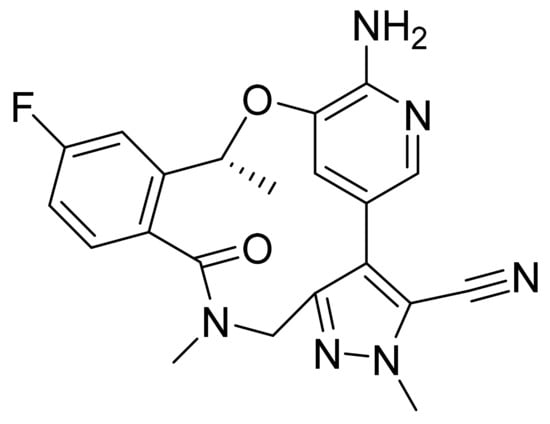
Figure 51.
Lorlatinib ((10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-4,8-methenopyrazolo[4,3-H][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile).
4.51. Entrectinib
Entrectinib (Figure 52) is a tetrahydropyran-piperazine based indazole derivative (MF: C31H34F2N6O2; MW: 560.64; CAS Number: 1108743-60-7) [161]. US8299057B2 discloses indazole derivatives as potent PKIs that are useful in anticancer therapy. It claims entrectinib and its salts [162]. US10738037B2 claims a crystalline Form 4 of entrectinib that exhibits greater thermodynamic stability at a temperature of about 40 °C than other known polymorphs and offers advantages in preparing dosage forms [163].
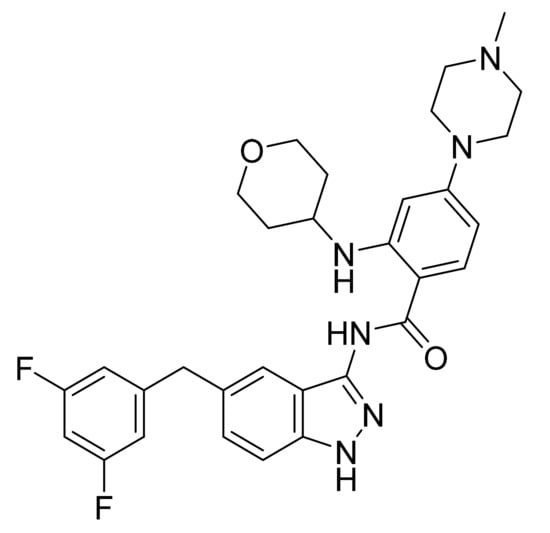
Figure 52.
Entrectinib (N-[5-(3,5-difluorobenzyl)-1H-indazol-3-yl]-4-(4-methylpiperazin-1-yl)-2-(tetrahydro-2H-pyran-4-ylamino)benzamide).
4.52. Upadacitinib Hemihydrate
Upadacitinib hemihydrate (Figure 53) is an imidazo[1,2-a]pyrrolo[2,3-e]pyrazine based pyrrolidine derivative (MF: C17H19F3N6O·½H2O; MW: 389.38; CAS Number: 1310726-60-3) [164]. USRE47221E (Reissue of US8426411B2) describes tricyclic compounds that inhibit JAK family kinase activity for treating diseases, including rheumatoid arthritis, multiple sclerosis, and psoriasis. It claims upadacitinib [165]. US9951080B2 claims physicochemically stable crystalline hemihydrate of upadacitinib having solid-state properties to develop quality pharmaceutical dosage forms [166].
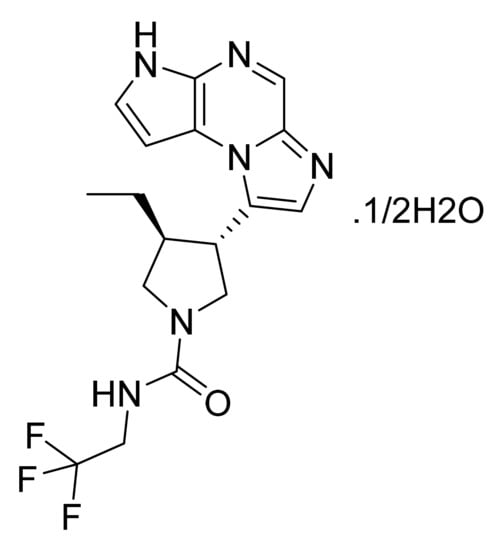
Figure 53.
Upadacitinib hemihydrate ((3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide hydrate (2:1)).
4.53. Alpelisib
Alpelisib (Figure 54) is a pyridine-thiazole based pyrrolidine derivative (MF: C19H22F3N5O2S; MW: 441.47; CAS Number: 1217486-61-7) [167]. US8227462B2 unveils pyrrolidine-1,2-dicarboxamide derivatives for the treatment of illnesses ameliorated by inhibition of PI3Ks. It claims alpelisib in a free form and its salts [168].
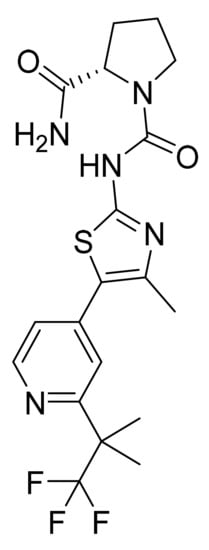
Figure 54.
Alpelisib ((2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidine dicarboxamide).
4.54. Erdafitinib
Erdafitinib (Figure 55) is a pyrazole based quinoxaline derivative (MF: C25H30N6O2; MW: 446.56; CAS Number: 1346242-81-6) [169]. US8895601B2 relates to pyrazole based quinoxaline derivatives and their pharmaceutical compositions to treat diseases like cancer. It claims erdafitinib and its salts [170].
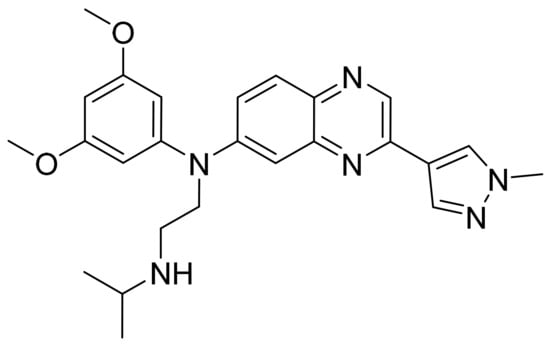
Figure 55.
Erdafitinib (N-(3,5-dimethoxyphenyl)-3-(1-methyl-1H-pyrazol-4-yl)-N-{2-[(propan-2-yl)amino]ethyl}quinoxalin-6-amine).
4.55. Pexidartinib Hydrochloride
Pexidartinib hydrochloride (Figure 56) is a pyrrolo[2,3-b]pyridine based pyridine derivative (MF: C20H15ClF3N5·HCl; MW: 454.28; CAS Number: 1029044-16-3) [171]. US9169250B2 provides fused azacyclic compounds as dual inhibitors of c-FMS and c-KIT to treat diseases that arise due to deregulation of c-FMS and c-KIT. It claims pexidartinib hydrochloride [172]. US9802932B2 claims a stable crystalline form of pexidartinib hydrochloride having attributes for developing a quality pharmaceutical composition [173].
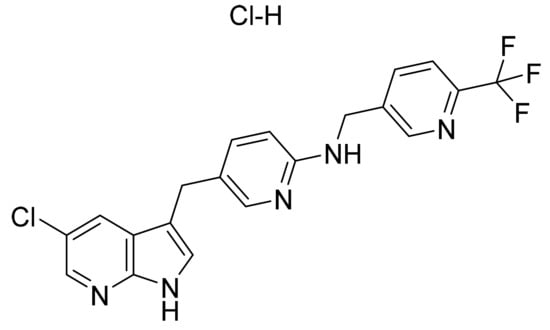
Figure 56.
Pexidartinib hydrochloride (5-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-N-{[6-(trifluoromethyl)pyridin-3-yl]methyl}pyridin-2-amine monohydrochloride).
4.56. Fedratinib Dihydrochloride Monohydrate
Fedratinib dihydrochloride monohydrate (Figure 57) is a pyrrolidine-pyrimidine based benzenesulfonamide derivative (MF: C27H36N6O3S·2HCl·H2O; MW: 615.62; CAS Number: 1374744-69-0) [174]. US7528143B2 unveils biaryl m-pyrimidine compounds as an inhibitor of the JAK family and their pharmaceutical compositions to treat diseases mediated by modulation of JAK activity. It claims fedratinib and its salts [175].
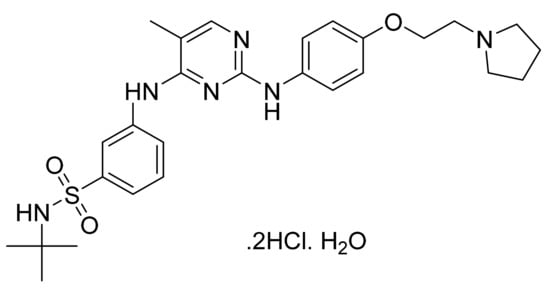
Figure 57.
Fedratinib dihydrochloride monohydrate (N-tert-butyl-3-{[5-methyl-2-({4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}amino)pyrimidin-4-yl]amino}benzene-1-sulfonamide dihydrochloride monohydrate).
4.57. Zanubrutinib
Zanubrutinib (Figure 58) is a piperidine based pyrazolo[1,5-a]pyrimidine derivative (MF: C27H29N5O3; MW: 471.56; CAS Number: 1691249-45-2) [176]. US9447106B2 states substituted pyrazolo[1,5-a]pyrimidines as BTK modulators and used these compounds to treat diseases intervened by BTK. It claims zanubrutinibas and its salts [177].
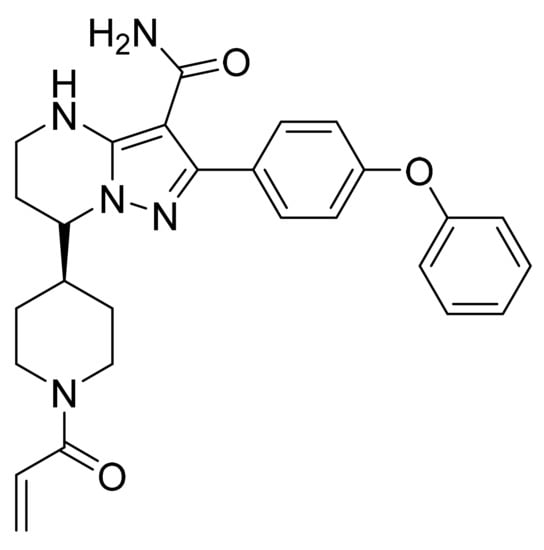
Figure 58.
Zanubrutinib ((S)-7-(1-Acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide).
4.58. Avapritinib
Avapritinib (Figure 59) is a pyrazole-piperazine-pyrimidine based pyrrolo[2,1-f][1,2,4]triazine derivative (MF: C26H27FN10; MW: 498.57; CAS Number: 1703793-34-3) [178]. US9944651B2 refers to piperazine-based pyrrolo[2,1-f][1,2,4]triazine derivatives for treating conditions like mastocytosis and mast cell diseases by modifying the activity of KIT. It claims avapritinib and its salts [179].
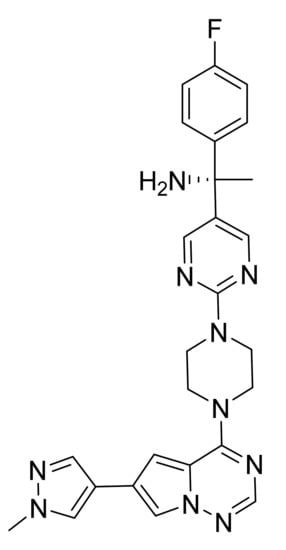
Figure 59.
Avapritinib ((S)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1H-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-yl)pyrimidin-5-yl)ethan-1-amine).
4.59. Selumetinib Sulfate
Selumetinib sulfate (Figure 60) is a benzimidazole derivative (MF: C17H17BrClFN4O7S; MW: 555.76; CAS Number: 943332-08-9) [180]. US7425637B2 reports N3-alkylated benzimidazole compounds that inhibit MEK and are helpful to treat cancer and inflammation. It claims selumetinib and its salts [181]. US9156795B2 claims a stable crystalline hydrogen sulfate salt of selumetinib with enhanced solubility and bioavailability, making it a suitable API to develop desired pharmaceutical dosage forms [182].
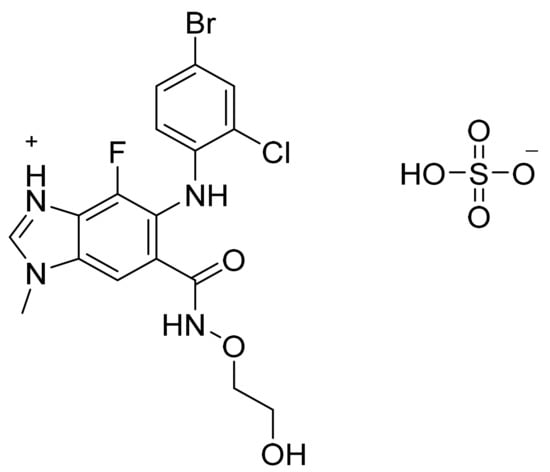
Figure 60.
Selumetinib sulfate (5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-6-[(2-hydroxyethoxy)carbamoyl]-1-methyl-1H-benzimidazol-3-ium hydrogen sulfate).
4.60. Pemigatinib
Pemigatinib (Figure 61) is a morpholine based pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidine derivative (MF: C24H27F2N5O4; MW: 487.5; CAS Number: 1513857-77-6) [183]. US9611267B2 relates to tricyclic compounds as inhibitors of FGFR, useful in ailments facilitated by FGFR malfunctioning like cancer. It claims pemigatinib and its salts [184].
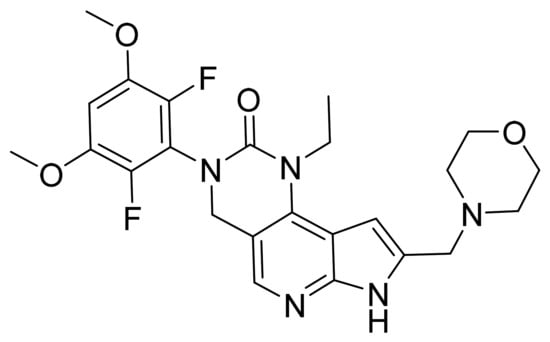
Figure 61.
Pemigatinib (3-(2,6-difluoro-3,5-dimethoxyphenyl)1-ethyl-8-(morpholin-4-ylmethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-2-one).
4.61. Tucatinib
Tucatinib (Figure 62) is a quinazoline-oxazoline based triazolo[1,5-a]pyridine derivative (MF: C26H24N8O2; MW: 480.52; CAS Number: 937263-43-9) [185]. US8648087B2 discloses N4-phenyl-quinazoline-4-amine derivatives as TKIs to treat cancer and inflammation. It claims tucatinib [186].
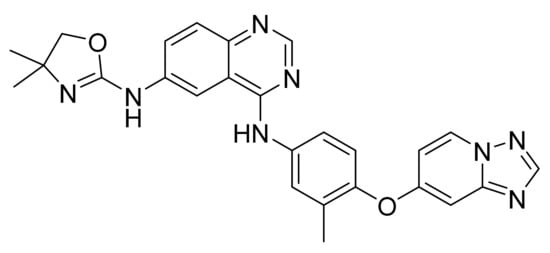
Figure 62.
Tucatinib (N6-(4,4-dimethyl-4,5-dihydro-1,3-oxazol-2-yl)-N4-(3-methyl-4-{[1,2,4]triazolo[1,5-a]pyridin-7-yloxy}phenyl)quinazoline-4,6-diamine).
4.62. Capmatinib Dihydrochloride Monohydrate
Capmatinib dihydrochloride monohydrate (Figure 63) is an imidazo[1,2-b][1,2,4]triazine based quinoline derivative (MF: C23H21Cl2FN6O2; MW: 503.36; CAS Number: 1865733-40-9) [187]. US7767675B2 reveals imidazotriazines and imidazopyrimidines as MET inhibitors and their pharmaceutical compositions useful in cancer treatment. It claims capmatinib and its salts [188]. US8420645B2 claims a stable capmatinib dihydrochloride monohydrate with pharmaceutical attributes to manufacture quality pharmaceutical formulations [189].
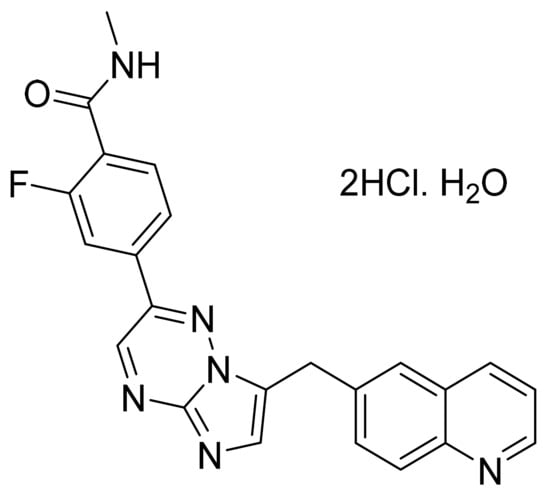
Figure 63.
Capmatinib dihydrochloride monohydrate (2-fluoro-N-methyl-4-{7-[(quinolin-6-yl)methyl]imidazo[1,2-b][1,2,4]triazin-2-yl}benzamide dihydrochloride monohydrate).
4.63. Selpercatinib
Selpercatinib (Figure 64) is a pyridine-diazabicycloheptane based pyrazolo[1,5-a]pyridine derivative (MF: C29H31N7O3; MW: 525.61; CAS Number: 2152628-33-4) [190]. US10112942B2 uncovers pyrazolo[1,5-a]pyridines as RET inhibitors, useful to treat RET-associated diseases. It claims selpercatinib and its salts [191]. US10584124B2 claims a stable crystalline polymorph of selpercatinib that is useful for developing pharmaceutical formulations [192].
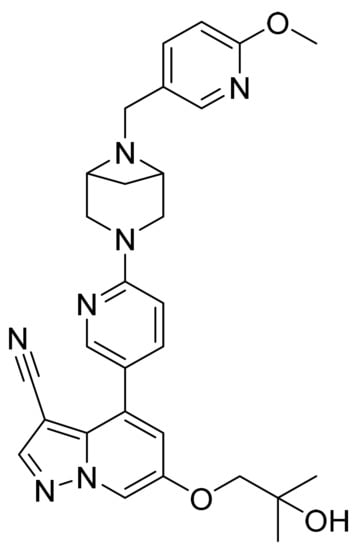
Figure 64.
Selpercatinib (6-(2-hydroxy-2-methylpropoxy)-4-(6-(6-((6-methoxypyridin-3-yl)methyl)-3,6-diazabicyclo[3.1.1]heptan-3-yl)pyridin-3-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile).
4.64. Ripretinib
Ripretinib (Figure 65) is a naphthyridine based phenylurea derivative (MF: C24H21BrFN5O2; MW: 510.36; CAS Number: 1442472-39-0) [193]. US8461179B1 uncovers dihydronaphthyridine derivatives that inhibit c-KIT and that have utility to treat GIST, mast cell leukemia, or mastocytosis. It claims ripretinib and its salts [194].
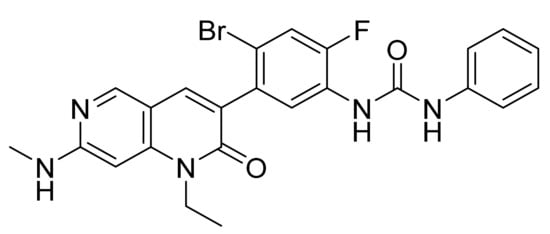
Figure 65.
Ripretinib (1-(4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl]-2-fluorophenyl)-3-phenylurea).
4.65. Pralsetinib
Pralsetinib (Figure 66) is a pyridine-pyrimidine based pyrazole derivative (MF: C27H32FN9O2; MW: 533.61; CAS Number: 2097132-94-8) [195]. US10030005B2 discloses pyrazole based RET inhibitors and their pharmaceutical compositions to treat a condition mediated by aberrant RET activity, e.g., cancer. It claims pralsetinib [196].
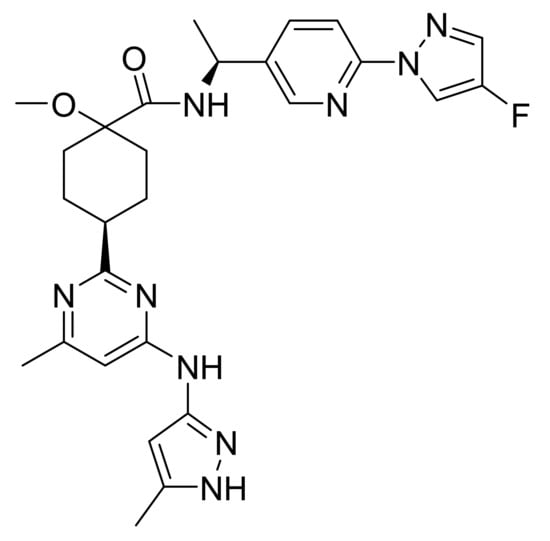
Figure 66.
Pralsetinib ((Cis)-N-((S)-1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-1-methoxy-4-(4-methyl-6-(5-methyl-1H-pyrazol-3-ylamino)pyrimidin-2-yl)cyclohexane carboxamide).
4.66. Trilaciclib Dihydrochloride
Trilaciclib dihydrochloride (Figure 67) is a piperazine-pyridine based pyrazino[1′,2′:1,5]pyrrole derivative (MF: C24H30N8O·2HCl; MW: 519.48; CAS Number: 1977495-97-8) [197]. US8598186B2 reveals tricyclic compounds as CDK inhibitors, which have utility in the treatment of disorders intervened by CDK malfunction like cancer. It claims trilaciclib and its salts [198].
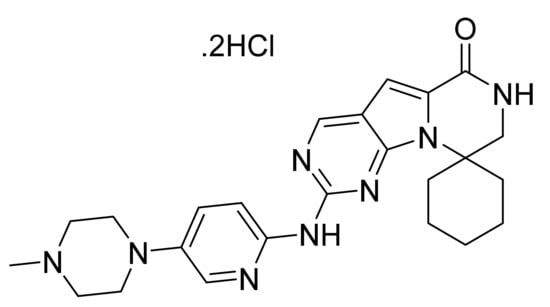
Figure 67.
Trilaciclib dihydrochloride (2′-{[5-(4-methylpiperazin-1-yl)pyridin-2-yl]amino}-7′,8′-dihydro-6′H-spiro[cyclohexane-1,9′-pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyrimidin]-6′-one).
4.67. Tepotinib Hydrochloride Monohydrate
Tepotinib hydrochloride monohydrate (Figure 68) is a piperidine-pyrimidine based dihydropyridazine derivative (MF: C29H28N6O2·HCl·H2O; MW: 547.05; CAS Number: 1946826-82-9) [199]. US8580781B2 reveals certain pyridazinones as MET inhibitors to treat tumors. It claims tepotinibor and its salts [200]. Tepotinib hydrochloride hydrate is claimed explicitly in US8329692B2 [201].
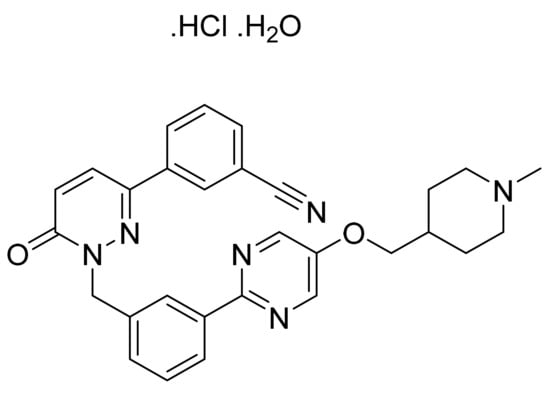
Figure 68.
Tepotinib hydrochloride monohydrate (3-{1-[(3-{5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl}phenyl)methyl]-6-oxo-1,6-dihydropyridazin-3-yl}benzonitrile hydrochloride monohydrate).
4.68. Umbralisib Tosylate
Umbralisibtosylate (Figure 69) is a chromen-4-one based pyrazolo[3,4-d]pyrimidine derivative (MF: C38H32F3N5O6S; 743.75; 1532533-72-4) [202]. US10570142B2 provides pyrazolo[3,4-d]pyrimidines as inhibitors of PI3Kδ and their pharmaceutical compositions to treat PI3Kδ mediated disorders. It claims umbralisib tosylate having at least 95% enantiomeric excess [203]. US10414773B2 unveils a stable crystalline form of umbralisib tosylate possessing specified particle sizes with enhanced solubility and improved pharmacokinetics. This property makes it suitable to prepare a quality oral dosage form [204].
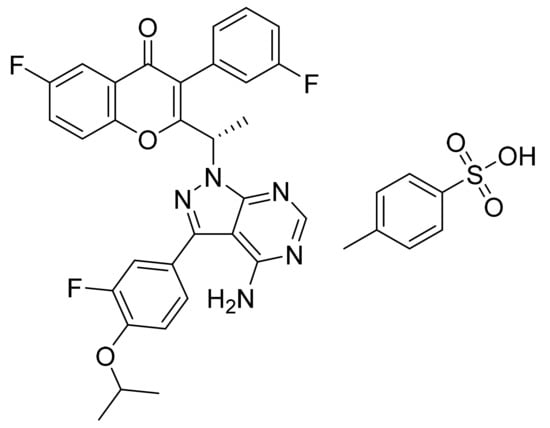
Figure 69.
Umbralisib tosylate ((S)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)-ethyl)-6-fluoro-3-(3-fluorophenyl)-4H-chromen-4-one tosylate)).
4.69. Tivozanib Hydrochloride Monohydrate
Tivozanib hydrochloride monohydrate (Figure 70) is an isoxazole base quinoline derivative (MF: C22H19ClN4O5·HCl·H2O; MW: 509.34; CAS Number: 682745-41-1) [205]. US6821987B2 and US7211587B2 unveil quinoline derivatives having azolyl group, useful for treating tumors, chronic rheumatism, psoriasis, and Kaposi’s sarcoma. These patents claim tivozanib and its salts [206,207]. US7166722B2 claims a physically stable crystalline form of tivozanib hydrochloride monohydrate stable under high temperature and humidity. This form is suitable for developing quality dosage forms [208].
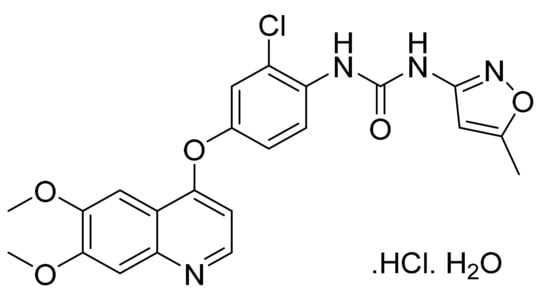
Figure 70.
Tivozanib hydrochloride monohydrate (1-{2-chloro-4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-3-(5-methylisoxazol-3-yl)urea hydrochloride monohydrate).
4.70. Infigratinib Phosphate
Infigratinib (Figure 71) is a piperazine based pyrimidine derivative (MF: C26H31Cl2N7O3. H3PO4; MW: 658.47; CAS Number: 1310746-10-1) [209]. US8552002B2 claims infigratinib and its salts [210]. US9067896B2 claims a monophosphoric acid salt of infigratinib as well as its anhydrous crystalline polymorph (Form A) and amorphous polymorph. The stability and physicochemical parameters of the crystalline Form A were better than other disclosed polymorphs [211].
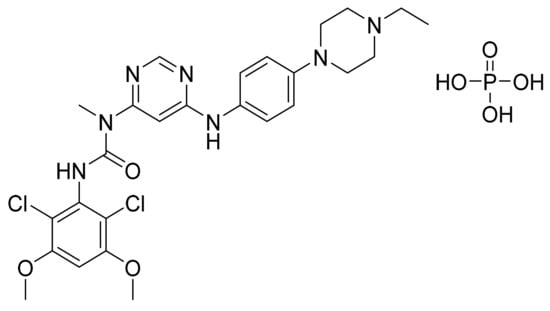
Figure 71.
Infigratinib phosphate (3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-{6-[4-(4-ethylpiperazin-1-yl)phenylamino]pyrimidin-4-yl}-1-methylurea phosphate).
5. Expert Opinion
In 2001, USFDA approved the marketing of the first clinical PKI, imatinib. From 2001 to 31 May 2021, about 70 PKIs have been approved by the USFDA (Table 2). The USFDA has also approved antibodies as PKIs such as trastuzumab and bevacizumab. A few antibodies are also in the clinical trial (amivantamab and patritumab). This review is limited to small molecules as PKIs. Accordingly, USFDA approved antibodies such as PKIs have not been discussed here. The physicochemical properties of about 55 USFDA approved PKIs from 2001 to 2020 have been described in the literature [22,23]. However, these reports are silent about the patent data of the PKIs reported therein.
According to the patent literature, and the data presented in Table 2 and Table 3, the major players that developed the marketed PKIs include Novartis (imatinib, lapatinib, everolimus, nilotinib, pazopanib, trametinib, dabrafenib, ceritinib, ribociclib, midostaurin, alpelisib, capmatinib, and infigratinib), Pfizer (tofacitinib, palbociclib, dacomitinib, and lorlatinib), Astrazeneca (gefitinib, osimertinib, acalabrutinib, and selumetinib), Bayers (sorafenib, regorafenib, copanlisib, and larotrectinib), and PF Prism (temsirolimus, crizotinib, axitinib, and bosutinib). Nearly 535 PKs have been reported [6]. However, the major primary target of the approved PKIs includes ALK, BCR-Abel, B-RAF, BTK, CDK, EGFR, JAK, MEK, PDGFR, PI3K, RET, and VEGFR (Table 2). Accordingly, there remains a large number of unexplored PKs. Some KIs have specificity for multiple kinases and are called multikinase inhibitors (MKIs), such as sunitinib, regorafenib, imatinib, sorafenib, axitinib, lenvatinib, cabozantinib, vandetanib, and pazopanib. The MKIs are supposed to reduce the chances of developing resistance. However, they are also linked to causing adverse effects in patients, for example, hypertension, gastric upset, and dermatological reactions [212]. The development of the covalent PKIs (ibrutinib, dacomitinib, osimertinib, afatinib, and neratinib) had been an unwilling strategy because they can bind to certain proteins and cause toxicity. Furthermore, the allosteric PKIs (trametinib, ascinimib, and selumetinib) are considered better than covalent inhibitors as they are not supposed to bind with other proteins. However, many new kinases have been identified possessing cysteine residues at their active sites. Therefore, the design of potent and selective covalent inhibitors may be useful against such kinases [213,214]. The pharmaceutical industries are trying to develop more potent and safer PKIs that can be used to treat many more PKs associated disorders with fewer adverse events [23]. Some example of PKIs, which are under development and/or waiting for the USFDA approval, include abrocitinib, belumosudil, dovitinib, sitravatinib, abivertinib, enzastaurin, rivoceranib (apatinib), asciminib, ensartinib, mobocertinib, momelotinib, pacritinib, quizartinib, vorolanib, GLPG3970, CA-4948, BAY1834845, BAY1830839, and PF-06650833 [213,214].
The PKIs contain one or more heterocyclic moieties in their structure that can explain the difference in their binding to the target and thus the spectrum of activity. The primary heterocyclic moieties include quinazoline, quinoline, isoquinoline, pyridine, pyrimidine, pyrazole, benzimidazole, indazole, imidazole, indole, carbazole, or their fused structures. This observation suggests that many clinical PKIs have been developed by the chemical modification of a formerly approved drug, and PKs are promiscuous targets. Further, most of the PKIs are marketed as acid-addition salts (hydrochloride, mesylate, tosylate, phosphate, malate, citrate, esylate, fumarate, succinate, and sulfate). This observation indicates the basic nature of the chemical nucleus of the PKIs.
The majority of the PKIs are approved to treat cancer and inflammatory disorders. Some of the PKIs have shown efficacy towards autoimmune diseases, Alzheimer’s disease (neflamapimod, tideglusib, and saracitinib), and Parkinson’s disease (DNL201). It is also expected that PKIs of PKC/WNK that control the activity of ion transporters may be developed to treat hypertension [214].
The malignant cells have genomic instability, which may cause the development of resistance to PKIs. This phenomenon is the reason for developing 2nd, 3rd, and later generations of PKIs targeting the equivalent PKs and their related disorders [212]. To combat resistance development, scientists are exploring different chemical templates and pharmacophores to develop novel PKIs [22]. Besides, inflammatory conditions do not exhibit genomic instability. Therefore, the PKIs, which are approved to treat inflammatory disorders, seldom demonstrate the development of resistance [22,23].
The main marketed dosage form of about 66 USFDA approved PKIs is either a tablet or capsule (Table 2). These are solid dosage forms. The quality of the formulation of a solid dosage form depends upon the solid-state properties (stability, solubility, compressibility, etc.) of the drug [215]. Therefore, many patents related to salts and polymorphs (mostly crystalline forms) of the USFDA approved PKIs have been obtained by the innovator companies. The innovator companies have done this to capture the market for a longer time.
The development of the PKIs is considered a medical breakthrough. However, the prices of these therapeutics cause financial toxicity. The financial burden can make the patients non-compliant with the treatment instructions as they may take lower doses than the prescribed doses. This causes failure of the treatment [216,217]. One way to avoid financial toxicity is to develop the generic version of a drug [218]. Currently, seven PKIs have been genericized (imatinib, erlotinib, sorafenib, dasatinib, lapatinib, temsirolimus, and everolimus) (Table 3). These generic versions must have lower prices than the innovator products. The data given in Table 3 also suggest that twelve more PKIs (gefitinib, sunitinib, pazopanib, vandetanib, axitinib, bosutinib, tofacitinib, idelalisib, nintedanib, lenvatinib, midostaurin, and neratinib) may be genericized by 2025 due to basic/compound/governing patent expiry or expiry of the drug exclusivity. It means by the end of 2025, 19 PKIs will have their generic version in the USA market. Besides, it is also expected that the generic version of about 48 PKIs will be available in the USA market by the end of 2030. Thus, it is hoped that the generic availability of these PKIs will reduce the financial toxicity on a patient.
Although great strides have been made in developing small molecule such as PKIs during the past 20 years, this field is still in its infancy. PKs are ubiquitous, and hence specificity has always been an issue regarding the design of new therapies targeting them. The major disadvantage of the existing PKIs is that they target a minor portion of the kinome, with countless clinically significant kinases missing validated inhibitors [22,23]. There are essential kinases without any inhibitors, and this is a critical area for further research. As the field advances during the next 20 years, one can anticipate that PKIs with many scaffolds, chemotypes, and pharmacophores will be developed. Other innovative strategies are also expected soon. A summary of the PKIs is provided in Figure 72.
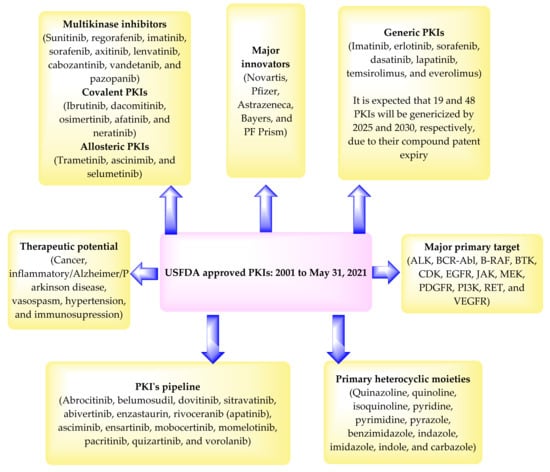
Figure 72.
Summary of the USFDA approved PKIs.
In conclusion, there is a huge scope for discovering PKIs, and it will dominate other cancer discovery strategies for decades. The rate of discovery of better and selective PKIs having less propensity for resistance development will be faster than the last two decades because of the better understanding of the molecular and structural aspects of the human kinases. The development of PKIs to treat hypertension, Alzheimer’s disease, and Parkinson’s disease are foreseeable.
Author Contributions
Conceptualization, M.I. and S.A.K.; methodology, S.M.B.A. and M.A. (Majid Alhomrani); validation, D.U.M. and E.H.A.; formal analysis, A.S.A. (Abdulhakeem S. Alamri), M.T., A., S.I.A., M.A.B. and A.K.A.; resources, W.F.A. and O.A.; data curation, M.A. (Mohammed AlMotairi) and A.S.A. (Ahmed Subeh Alshrari); writing—original draft preparation, M.I.; writing—review and editing, S.A.K., D.U.M., M.T., A.; visualization, S.I.A. and M.A.B.; supervision, M.I. and S.A.K.; project administration, Y.M.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors are thankful to AlMaarefa University, Riyadh for providing support to write this review article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ALK: Anaplastic lymphoma kinase; ALL: Acute lymphoblastic leukemia; AML: Acute myelogenous leukemia; API: Active pharmaceutical ingredient; ARCC: Advanced renal cell carcinoma; ATC: Anaplastic thyroid cancer; ATP: Adenosine triphosphate; BCR-Abl: Breakpoint cluster region/-abl oncogene; BRAF/B-raf: Murine sarcoma viral oncogene homolog; BTK: Bruton’s tyrosine kinase; CDK: Cyclin-dependent protein kinase; CLL: Chronic lymphocytic leukemia; CML: Chronic myelonoid leukemia; CSF1R: Colony stimulating factor 1 receptor; DTC: Differentiated thyroid cancer; EGFR: Epidermal growth factor receptor; FKBP12/mTOR: FK Binding Protein-12/mammalian target of rapamycin; FL: Follicular lymphoma; Flt3: fms-like tyrosine kinase 3; GISTs: Gastrointestinal stromal tumors; GTP: Guanosine triphosphate; HCC: Hepatocellular carcinoma; HER-1/HER-2: Human epidermal growth factor receptor ½; HGFR: Hepatocyte growth factor receptor; ILDs: Interstitial lung disease; IPF: Idiopathic pulmonary fibrosis; ITP: Immune thrombocytopenic purpura; JAK: Janus kinase; MAPK/MEK1/2: Mitogen-activated protein kinase kinase; MAT: Monoamine transporter; MCL: Mantle cell lymphoma; MTC: Medullary thyroid cancer; mUC: Metastatic urothelial carcinoma); MZL: Marginal zone lymphoma; NF1: Neurofibromatosis type 1; NSCLC: Non-small cell lung cancer; PDGFR: Platelet-derived growth factor receptor; Ph+-ALL: Philadelphia chromosome-Positive Acute lymphoblastic leukemia; Ph+-CML: Philadelphia chromosome-positive chronic myeloid leukemia; PI3K: Phosphatidylinositol 3-kinase; PKIs: Protein kinase inhibitors; PKs: Protein kinases; pNET: Primitive neuroectodermal tumor; RCC: Renal cell carcinoma; SLL: Small lymphocytic lymphoma; SSc: Systemic sclerosis; STS: Soft-tissue sarcomas; TKI: Tyrosine Kinase inhibitors; Tyk2: Tyrosine kinase; USFDA: United States Food and Drug Administration; VEGFR: Vascular endothelial growth factor receptor.
References
- Keohane, E.M.; Otto, C.N.; Walenga, J.M. Rodak’s Hematology-E-Book: Clinical Principles and Applications; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Prescott, J.C.; Braisted, A. Identification of Kinase Inhibitors. PCT Patent Application Publication Number WO2005034840A2, 21 April 2005. [Google Scholar]
- Charrier, J.D.; Durrant, S. Protein Kinase Inhibitors. U.S. Patent Application Publication Number US20120028966A1, 2 February 2012. [Google Scholar]
- Liu, C.; Ke, P.; Zhang, J.; Zhang, X.; Chen, X. Protein kinase inhibitor peptide as a tool to specifically inhibit protein kinase A. Front. Physiol. 2020, 11, 574030. [Google Scholar] [CrossRef] [PubMed]
- Plowman, G.; Whyte, D.; Manning, G.; Sudarsanam, S.; Martinez, R. Novel human protein kinases and protein kinase-like enzymes. U.S. Patent Application Publication Number US20040048310A1, 11 March 2004. [Google Scholar]
- Buljan, M.; Ciuffa, R.; van Drogen, A.; Vichalkovski, A.; Mehnert, M.; Rosenberger, G.; Lee, S.; Varjosalo, M.; Pernas, L.E.; Spegg, V.; et al. Kinase interaction network expands functional and disease roles of human kinases. Mol. Cell 2020, 79, 504–520.e9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Linley, A.; Hammond, D.E.; Hood, F.E.; Coulson, J.M.; MacEwan, D.J.; Ross, S.J.; Slupsky, J.R.; Smith, P.D.; Eyers, P.A.; et al. New perspectives, opportunities, and challenges in exploring the human protein kinome. Cancer Res. 2018, 78, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, J.M.; Pastor-Flores, D.; Bauer, A.F.; Schulze, J.O.; Biondi, R.M. AGC protein kinases: From structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim. Biophys. Acta 2013, 1834, 1302–1321. [Google Scholar] [CrossRef]
- Junho, C.V.C.; Caio-Silva, W.; Trentin-Sonoda, M.; Carneiro-Ramos, M.S. An overview of the role of calcium/calmodulin-dependent protein kinase in cardiorenal syndrome. Front. Physiol. 2020, 11, 735. [Google Scholar] [CrossRef]
- Schittek, B.; Sinnberg, T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol. Cancer 2014, 13, 231. [Google Scholar] [CrossRef]
- Strang, B.L. RO0504985 is an inhibitor of CMGC kinase proteins and has anti-human cytomegalovirus activity. Antivir. Res. 2017, 144, 21–26. [Google Scholar] [CrossRef]
- Sawa, M.; Masai, H. Drug design with Cdc7 kinase: A potential novel cancer therapy target. Drug Des. Devel. Ther. 2009, 2, 255–264. [Google Scholar] [CrossRef]
- Matrone, C.; Petrillo, F.; Nasso, R.; Ferretti, G. Fyn tyrosine kinase as harmonizing factor in neuronal functions and dysfunctions. Int. J. Mol. Sci. 2020, 21, 4444. [Google Scholar] [CrossRef]
- Petrie, E.J.; Hildebrand, J.M.; Murphy, J.M. Insane in the membrane: A structural perspective of MLKL function in necroptosis. Immunol. Cell Biol. 2017, 95, 152–159. [Google Scholar] [CrossRef]
- Rauch, J.; Volinsky, N.; Romano, D.; Kolch, W. The secret life of kinases: Functions beyond catalysis. Cell Commun. Signal. 2011, 9, 23. [Google Scholar] [CrossRef]
- Ochoa, D.; Bradley, D.; Beltrao, P. Evolution, dynamics and dysregulation of kinase signalling. Curr. Opin. Struct. Biol. 2018, 48, 133–140. [Google Scholar] [CrossRef]
- Brognard, J.; Hunter, T. Protein kinase signaling networks in cancer. Curr. Opin. Genet. Dev. 2011, 21, 4–11. [Google Scholar] [CrossRef]
- Oprea, T.I.; Bologa, C.G.; Brunak, S.; Campbell, A.; Gan, G.N.; Gaulton, A.; Gomez, S.M.; Guha, R.; Hersey, A.; Holmes, J.; et al. Unexplored therapeutic opportunities in the human genome. Nat. Rev. Drug Discov. 2018, 17, 317–332. [Google Scholar] [CrossRef]
- Essegian, D.; Khurana, R.; Stathias, V.; Schürer, S.C. The clinical kinase index A method to prioritize understudied kinases as drug targets for the treatment of cancer. Cell Rep. Med. 2020, 1, 100128. [Google Scholar] [CrossRef]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar] [CrossRef]
- Klaeger, S.; Heinzlmeir, S.; Wilhelm, M.; Polzer, H.; Vick, B.; Koenig, P.A.; Reinecke, M.; Ruprecht, B.; Petzoldt, S.; Meng, C.; et al. The target landscape of clinical kinase drugs. Science 2017, 358, eaan4368. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef]
- Zimmermann, J. Pyrimidine Derivatives and Processes for the Preparation Thereof. U.S. Patent Number US5521184A, 28 May 1996. [Google Scholar]
- Zimmermann, J.; Sutter, B.; Buerger, H.M. Crystal Modification of a N-Phenyl-2-Pyrimidineamine Derivative, Processes for its Manufacture and Its Use. U.S. Patent Number USRE43932E1, 15 January 2013. [Google Scholar]
- Sim, E.H.; Yang, I.A.; Wood-Baker, R.; Bowman, R.V.; Fong, K.M. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2018, 1, CD006847. [Google Scholar] [CrossRef]
- Barker, A.J. Quinazoline Derivatives Useful for Treatment of Neoplastic Disease. U.S. Patent Number US5457105A, 10 October 1995. [Google Scholar]
- Gibson, K.H. Quinazoline Derivatives. U.S. Patent Number US5770599A, 23 June 1998. [Google Scholar]
- Steins, M.; Thomas, M.; Geibler, M. Erlotinib. Recent Results Cancer Res. 2018, 211, 1–17. [Google Scholar] [CrossRef]
- Schnur, R.C.; Arnold, L.D. Alkynl and Azido-Substituted 4-Anilinoquinazolines. U.S. Patent Number USRE41065E1, 29 December 2009. [Google Scholar]
- Norris, T.; Raggon, J.W.; Connell, R.D.; Moyer, J.D.; Morin, M.J.; Kajiji, S.M.; Foster, B.A.; Ferrante, K.J.; Silberman, S.L. Stable Polymorph on N-(3-ethynylphenyl)-6,7-Bis (2methoxyethoxy)-4-Quinazolinamine Hydrochloride, Methods of Production, and Pharmaceutical Uses Thereof. U.S. Patent Number US6900221B1, 31 May 2005. [Google Scholar]
- Chen, F.; Fang, Y.; Zhao, R.; Le, J.; Zhang, B.; Huang, R.; Chen, Z.; Shao, J. Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 179, 916–935. [Google Scholar] [CrossRef]
- Riedl, B.; Dumas, J.; Khire, U.; Lowinger, T.B.; Scott, W.J.; Smith, R.A.; Wood, J.E.; Monahan, M.K.; Natero, R.; Renick, J.; et al. Omega-Carboxyaryl Substituted Diphenyl Ureas as Raf Kinase Inhibitors. U.S. Patent Number US7235576B1, 26 June 2007. [Google Scholar]
- Alfons, T.G.; Jana, L. Hermodynamically Stable Form of a Tosylate Salt. U.S. Patent Number US8877933B2, 27 August 2009. [Google Scholar]
- Carlisle, B.; Demko, N.; Freeman, G.; Hakala, A.; MacKinnon, N.; Ramsay, T.; Hey, S.; London, A.J.; Kimmelman, J. Benefit, Risk, and Outcomes in Drug Development: A Systematic Review of Sunitinib. J. Natl. Cancer Inst. 2015, 108, djv292. [Google Scholar] [CrossRef]
- Tang, P.C.; Miller, T.A.; Li, X.; Sun, L.; Wei, C.C.; Shirazian, S.; Liang, C.; Vojkovsky, T.; Nematalla, A.S.; Hawley, M. Pyrrole Substituted 2-Indolinone Protein Kinase Inhibitors. U.S. Patent Number US7125905B2, 11 August 2005. [Google Scholar]
- Tang, P.C.; Miller, T.A.; Li, X.; Sun, L.; Wei, C.C.; Shirazian, S.; Liang, C.; Vojkovsky, T.; Nematalla, A.S.; Hawley, M. Pyrrole Substituted 2-Indolinone Protein Kinase Inhibitors. U.S. Patent Number US6573293B2, 24 October 2002. [Google Scholar]
- Korashy, H.M.; Rahman, A.F.; Kassem, M.G. Dasatinib. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 205–237. [Google Scholar] [CrossRef]
- Das, J.; Padmanabha, R.; Chen, P.; Norris, D.J.; Doweyko, A.M.P.; Barrish, J.C.; Wityak, J. Cyclic Protein Tyrosine Kinase Inhibitors. U.S. Patent Number US6596746B1, 22 July 2003. [Google Scholar]
- Lajeunesse, J.; Dimarco, J.D.; Galella, M.; Chidambaram, R. Process for Preparing 2-Aminothiazole-5-Aromatic Carboxamides as Kinase Inhibitors. U.S. Patent Number US7491725B2, 17 February 2009. [Google Scholar]
- Gross-Goupil, M.; Bernhard, J.C.; Ravaud, A. Lapatinib and renal cell carcinoma. Expert Opin. Investig. Drugs 2012, 21, 1727–1732. [Google Scholar] [CrossRef]
- Carter, M.C.; Cockerill, G.S.; Lackey, K.E. Bicyclic Heteroaromatic Compounds as Protein Tyrosine Kinase Inhibitors. U.S. Patent Number US8513262B2, 20 September 2013. [Google Scholar]
- Mcclure, M.S.; Osterhout, M.H.; Roschangar, F.; Sacchetti, M.J. Quinazoline Ditosylate Salt Compounds. U.S. Patent Number US7157466B2, 2 January 2007. [Google Scholar]
- Bukowski, R.M. Temsirolimus: A safety and efficacy review. Expert Opin. Drug Saf. 2012, 11, 861–879. [Google Scholar] [CrossRef]
- Skotnicki, J.S.; Leone, C.L.; Schiehser, G.A. Rapamycin Hydroxyesters. U.S. Patent Number USRE44768E1, 18 February 2014. [Google Scholar]
- Guarini, A.; Minoia, C.; Giannoccaro, M.; Rana, A.; Iacobazzi, A.; Lapietra, A.; Raimondi, A.; Silvestris, N.; Gadaleta, C.D.; Ranieri, G. mTOR as a target of everolimus in refractory/relapsed Hodgkin lymphoma. Curr. Med. Chem. 2012, 19, 945–954. [Google Scholar] [CrossRef]
- Cottens, S.; Sedrani, R. O-alkylated Rapamycin Derivatives and Their Use, Particularly as Immunosuppressants. U.S. Patent Number US5665772A, 9 September 1997. [Google Scholar]
- Vaid, A. Nilotinib as first-line therapy for chronic myeloid leukemia. Indian J. Cancer 2011, 48, 438–445. [Google Scholar] [CrossRef]
- Breitenstein, W.; Furet, P.; Jacob, S.; Manley, P.W. Inhibitors of Tyrosine Kinases. U.S. Patent Number US7169791B2, 30 January 2007. [Google Scholar]
- Manley, P.W.; Shieh, W.C.; Sutton, P.A.; Karpinski, P.P.H.; Wu, R.R.; Monnier, S.M.; Brozio, J. Salts of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide. U.S. Patent Number US8163904B2, 24 April 2012. [Google Scholar]
- Manley, P.W.; Shieh, W.C.; Sutton, P.A.; Karpinski, P.P.H.; Wu, R.R.; Monnier, S.M.; Brozio, J. Crystalline Forms of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide. U.S. Patent Number US8415363B2, 9 April 2013. [Google Scholar]
- Schutz, F.A.; Choueiri, T.K.; Sternberg, C.N. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit. Rev. Oncol. Hematol. 2011, 77, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Boloor, A.; Cheung, M.; Davis, R.; Harris, P.A.; Hinkle, K.; Mook, R.A., Jr.; Stafford, J.A.; Veal, J.M. Pyrimidineamines as Angiogenesis Modulators. U.S. Patent Number US7105530B2, 12 September 2006. [Google Scholar]
- Boloor, A.; Cheung, M.; Hinkle, K.; Hinkle, K.; Veal, J.M.; Harris, P.A.; Mook, R.A., Jr.; Stafford, J.A. Chemical Compounds. U.S. Patent Number US8114885B2, 12 September 2012. [Google Scholar]
- Sim, M.W.; Cohen, M.S. The discovery and development of vandetanib for the treatment of thyroid cancer. Expert Opin. Drug Discov. 2014, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Johnstone, C.; Clayton, E.; Stokes, E.S.E.; Lohmann, J.J.M.; Hennequin, L.F.A. Quinazoline Derivatives and Pharmaceutical Compositions Containing Them. U.S. Patent Number USRE42353E1, 10 May 2011. [Google Scholar]
- Kim, A.; Cohen, M.S. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin. Drug Discov. 2016, 11, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.R.; Bollag, G.; Bremer, R.; Cho, H.; Hirth, K.P.; Ibrahim, P.N.; Tsai, J.; Zhang, C.; Zhang, J. Compounds and Methods for Kinase Modulation, and Indications Therefor. U.S. Patent Number US8143271B2, 27 March 2012. [Google Scholar]
- Roskoski, R., Jr. The preclinical profile of crizotinib for the treatment of non-small-cell lung cancer and other neoplastic disorders. Expert Opin. Drug Discov. 2013, 8, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Ia, L.; Kung, P.P.; Shen, H.; Tran, D.M.; Cui, J.J.; Funk, L.A.; Meng, J.J.; Nambu, M.D.; Pairish, M.A. Enantiomerically Pure Aminoheteroaryl Compounds as Protein Kinase Inhibitors. U.S. Patent Number US7858643B2, 28 December 2010. [Google Scholar]
- Cui, J.J.; Tran, D.M.B. Polymorphs of a c-MET/HGFR Inhibitor. U.S. Patent Number US8217057B2, 10 July 2012. [Google Scholar]
- Naqvi, K.; Verstovsek, S.; Kantarjian, H.; Ravandi, F. A potential role of ruxolitinib in leukemia. Expert Opin. Investig. Drugs 2011, 20, 1159–1166. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Shepard, S. Heteroaryl Substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus Kinase Inhibitors. U.S. Patent Number US7598257B2, 6 October 2009. [Google Scholar]
- Li, H.Y.; Rodgers, J.D. Salts of the Janus kinase Inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile. U.S. Patent Number US8722693B2, 13 May 2014. [Google Scholar]
- Zakharia, Y.; Zakharia, K.; Rixe, O. Axitinib: From preclinical development to future clinical perspectives in renal cell carcinoma. Expert Opin. Drug Discov. 2015, 10, 925–935. [Google Scholar] [CrossRef]
- Bender, S.L.; Borchardt, A.J.; Collins, M.R.; Hua, Y.; Johnson, M.D.; Johnson, T.O., Jr.; Luu, H.T.; Palmer, C.L.; Reich, S.H.; Tempczyk-Russell, A.M.; et al. Indazole Compounds and Pharmaceutical Compositions for Inhibiting Protein Kinases, and Methods for Their Use. U.S. Patent Number US6534524B1, 18 March 2003. [Google Scholar]
- Campeta, A.M.; Chekal, B.P.; Singer, R.A. Crystalline Forms of 6-[2-(methylcarbamoyl) phenylsulfanyl]-3-E-[2-(pyridin-2-yl)ethenyondazole Suitable for the Treatment of Abnormal Cell Growth in Mammals. U.S. Patent Number US8791140B2, 29 July 2014. [Google Scholar]
- Quintás-Cardama, A.; Kantarjian, H.; Cortes, J. Bosutinib for the treatment of chronic myeloid leukemia in chronic phase. Drugs Today (Barc). 2012, 48, 177–188. [Google Scholar] [CrossRef]
- Berger, D.M.; Floyd, M.B.; Frost, P.; Hamann, P.R.; Tsou, H.R.; Wissner, A.; Zhang, N. Substituted 3-cyanoquinolines. U.S. Patent Number USRE42376E, 17 May 2011. [Google Scholar]
- Feigelson, G.; Strong, H.; Wen, H.; Tesconi, M.S. Crystalline Forms of 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3 Quinolinecarbonitrile and Methods of Preparing the Same. U.S. Patent Number US7767678B2, 3 August 2010. [Google Scholar]
- Miura, K.; Satoh, M.; Kinouchi, M.; Yamamoto, K.; Hasegawa, Y.; Philchenkov, A.; Kakugawa, Y.; Fujiya, T. The preclinical development of regorafenib for the treatment of colorectal cancer. Expert Opin. Drug Discov. 2014, 9, 1087–1101. [Google Scholar] [CrossRef]
- Boyer, S.; Dumas, J.; Riedl, B.; Wilhelm, S. Fluoro Substituted omega-carboxyaryl Diphenyl Urea for the Treatment and Prevention of Diseases and Conditions. U.S. Patent Number US8637553B2, 28 January 2014. [Google Scholar]
- Grunenberg, A.; Keil, B.; Stiehl, J.; Tenbieg, K. 4-[4-({[4-chloro-3-(trifluoromethyl) phenyl] carbamoyl} amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide Monohydrate. U.S. Patent Number US9957232B2, 1 May 2018. [Google Scholar]
- Kaur, K.; Kalra, S.; Kaushal, S. Systematic review of tofacitinib: A new drug for the management of rheumatoid arthritis. Clin. Ther. 2014, 36, 1074–1086. [Google Scholar] [CrossRef]
- Blumenkopf, T.A.; Flanagan, M.E.; Munchhof, M.J. Pyrrolo [2,3-d]pyrimidine Compounds. U.S. Patent Number USRE41783E, 28 September 2010. [Google Scholar]
- Flanagan, M.E.; Li, Z.J. Crystalline 3-{4-methyl-3-[methyl-(7H- pyrrolo [2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile Citrate. U.S. Patent Number US6965027B2, 15 November 2005. [Google Scholar]
- Grassi, P.; Verzoni, E.; Ratta, R.; Mennitto, A.; de Braud, F.; Procopio, G. Cabozantinib in the treatment of advanced renal cell carcinoma: Design, development, and potential place in the therapy. Drug Des. Devel. Ther. 2016, 10, 2167–2172. [Google Scholar] [CrossRef][Green Version]
- Chan, D.S.M.; Forsyth, T.P.; Khoury, R.G.; Leahy, J.W.; Mann, L.W.; Nuss, J.M.; Parks, J.J.; Wang, Y.; Xu, W.; Bannen, L.C.; et al. c-Met Modulators and Methods of Use. U.S. Patent Number US7579473B2, 28 May 2009. [Google Scholar]
- Brown, A.S.C.; Gallagher, W.P.; Lamb, P. (L)-malate Salt of N-(4-{[6,7-bis (methyloxy) quinolin-4-yl] oxy} phenyl)-N′-(4-fluorophenyl) cyclopropane-1, 1-dicarboxamide. U.S. Patent Number US8877776B2, 4 November 2014. [Google Scholar]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. Onco. Targets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef]
- Dalgarno, D.C.; Huang, W.S.; Qi, J.; Sawyer, T.K.; Shakespeare, W.C.; Sundaramoorthi, R.; Wang, Y.; Zhu, X.; Zou, D.; Metcalf, C.A., III; et al. Substituted Acetylenic imidazo[1,2-b]pyridazine Compounds as Kinase Inhibitors. U.S. Patent Number US8114874B2, 14 February 2012. [Google Scholar]
- Chaber, J.J.; Murray, C.K.; Rozamus, L.W.; Sharma, P. Crystalline Forms of 3-(imidazo[1,2-b] pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl) methyl]-3-(trifluoromethyl)phenyl}benzamide and Its Mono Hydrochloride Salt. U.S. Patent Number US9493470B2, 15 November 2016. [Google Scholar]
- Jeanson, A.; Boyer, A.; Greillier, L.; Tomasini, P.; Barlesi, F. Therapeutic potential of trametinib to inhibit the mutagenesis by inactivating the protein kinase pathway in non-small cell lung cancer. Expert Rev. Anticancer Ther. 2019, 19, 11–17. [Google Scholar] [CrossRef]
- Abe, H.; Hayakawa, K.; Hori, Y.; Iida, T.; Kawasaki, H.; Kikuchi, S.; Kurachi, H.; Nanayama, T.; Sakai, T.; Takahashi, M.; et al. Pyrimidine Compound and Medical Use Thereof. U.S. Patent Number US7378423B2, 17 May 2008. [Google Scholar]
- Knispel, S.; Zimmer, L.; Kanaki, T.; Ugurel, S.; Schadendorf, D.; Livingstone, E. The safety and efficacy of dabrafenib and trametinib for the treatment of melanoma. Expert Opin. Drug Saf. 2018, 17, 73–87. [Google Scholar] [CrossRef]
- Rheault, T.R. Benzene Sulfonamide Thiazole and Oxazole Compounds. U.S. Patent Number US7994185B2, 9 August 2011. [Google Scholar]
- Brückl, W.; Tufman, A.; Huber, R.M. Advanced non-small cell lung cancer (NSCLC) with activating EGFR mutations: First-line treatment with afatinib and other EGFR TKIs. Expert Rev. Anticancer Ther. 2017, 17, 143–155. [Google Scholar] [CrossRef]
- Himmelsbach, F.; Blech, S.; Langkopf, E.; Jung, B.; Baum, A.; Solca, F. Quinazoline Derivatives and Pharmaceutical Compositions Containing Them. U.S. Patent Number USRE43431E1, 29 May 2012. [Google Scholar]
- Kulinna, C.; Rall, W.; Schnaubelt, J.; Sieger, P.; Soyka, R. Process for Preparing Amino Crotonyl Compounds. U.S. Patent Number US8426586B2, 23 April 2013. [Google Scholar]
- Liu, L.; Shi, B.; Wang, X.; Xiang, H. Strategies to overcome resistance mutations of Bruton’s tyrosine kinase inhibitor ibrutinib. Future Med. Chem. 2018, 10, 343–356. [Google Scholar] [CrossRef]
- Honigberg, L.; Pan, Z.; Verner, E. Inhibitors of Bruton’s Tyrosine Kinase. U.S. Patent Number US8735403B2, 27 May 2014. [Google Scholar]
- Goldman, E.; Purro, N.; Smyth, M.; Wirth, D.D. Crystalline Forms of a Bruton’s Tyrosine Kinase Inhibitor. U.S. Patent Number US9296753B2, 29 March 2016. [Google Scholar]
- De Pas, T.; Pala, L.; Catania, C.; Conforti, F. Molecular and clinical features of second-generation anaplastic lymphoma kinase inhibitors: Ceritinib. Future Oncol. 2017, 13, 2629–2644. [Google Scholar] [CrossRef]
- Michellys, P.Y.; Pei, W.; Marsilje, T.H.; Chen, B.; Uno, T. Compounds and Compositions as Protein Kinase Inhibitors. U.S. Patent Number US8039479B2, 18 October 2011. [Google Scholar]
- Feng, L.; Gong, B.; Karpinski, P.H.; Waykole, L.M. Crystalline Forms of 5-chloro-N2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2, 4-diamine. U.S. Patent Number US9309229B2, 12 April 2016. [Google Scholar]
- Zirlik, K.; Veelken, H. Idelalisib. Recent Results Cancer Res. 2018, 212, 243–264. [Google Scholar] [CrossRef]
- Fowler, K.W.; Huang, D.; Kesicki, E.A.; Oliver, A.; Ooi, H.C.; Puri, K.D.; Ruan, F.; Treiberg, J. Quinazolinones as Inhibitors of Human Phosphatidylinositol 3-kinase Delta. U.S. Patent Number USRE44638E, 10 December 2013. [Google Scholar]
- Carra, E.; Evarts, J.B.; Gerber, M.; Shi, B.; Sujino, K.; Tran, D.; Wang, F. Polymorphic Forms of (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one. U.S. Patent Number US9469643B2, 18 October 2016. [Google Scholar]
- Khalique, S.; Banerjee, S. Nintedanib in ovarian cancer. Expert Opin. Investig. Drugs 2017, 26, 1073–1081. [Google Scholar] [CrossRef]
- Heckel, A.; Hilberg, F.; Redemann, N.; Roth, G.J.; Spevak, W.; Tontsch, G.U.; Van, M.J.; Walter, R. Substituted Indolines which Inhibit Receptor Tyrosine Kinases. U.S. Patent Number US6762180B1, 13 July 2004. [Google Scholar]
- Bock, T.; Hilberg, F.; Linz, G.; Rall, W.; Roth, G.J.; Sieger, P. 3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the Use Thereof as a Pharmaceutical Composition. U.S. Patent Number US7119093B2, 10 October 2006. [Google Scholar]
- de Dueñas, E.M.; Gavila-Gregori, J.; Olmos-Antón, S.; Santaballa-Bertrán, A.; Lluch-Hernández, A.; Espinal-Domínguez, E.J.; Rivero-Silva, M.; Llombart-Cussac, A. Preclinical and clinical development of palbociclib and future perspectives. Clin. Transl. Oncol. 2018, 20, 1136–1144. [Google Scholar] [CrossRef]
- Barvian, M.; Booth, R.J.; Quinn, J., III; Repine, J.T.; Sheehan, D.J.; Toogood, P.L.; Vanderwel, S.N.; Zhou, H. 2-(pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones. U.S. Patent Number USRE47739E, 26 November 2019. [Google Scholar]
- Chekal, B.P.; Ide, N.D. Solid Forms of a Selective CDK4/6 Inhibitor. U.S. Patent Number US10723730B2, 28 July 2020. [Google Scholar]
- Capozzi, M.; De Divitiis, C.; Ottaiano, A.; von Arx, C.; Scala, S.; Tatangelo, F.; Delrio, P.; Tafuto, S. Lenvatinib, a molecule with versatile application: From preclinical evidence to future development in anti-cancer treatment. Cancer Manag. Res. 2019, 11, 3847–3860. [Google Scholar] [CrossRef]
- Arimoto, I.; Fukuda, Y.; Funahashi, Y.; Haneda, T.; Kamat, J.; Matsui, J.; Matsui, K.; Matsukura, M.; Matsushima, T.; Mimura, F.; et al. Nitrogen-Containing Aromatic Derivatives. U.S. Patent Number US7253286B2, 7 August 2007. [Google Scholar]
- Matsushima, T.; Arimoto, I.; Ayata, Y.; Gotoda, M.; Kamada, A.; Nakamura, T.; Sakaguchi, T.; Suzuki, N.; Yoshizawa, K. Crystalline Form of the Salt of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide or the Solvate of the Salt and a Process for Preparing the Same. U.S. Patent Number US7612208B2, 3 November 2009. [Google Scholar]
- Eagles, J.R.; Jimeno, A. Cobimetinib: Inhibiting MEK1/2 in BRAF V600-mutant melanoma. Drugs Today (Barc) 2016, 52, 593–605. [Google Scholar] [CrossRef]
- Aay, N.; Anand, N.K.; Blazey, C.M.; Bowles, O.J.; Bussenius, J.; Costanzo, S.; Curtis, J.K.; Defina, S.C.; Dubenko, L.; Joshi, A.A.; et al. Azetidines as MEK Inhibitors for the Treatment of Proliferative Diseases. U.S. Patent Number US7803839B2, 28 September 2010. [Google Scholar]
- Brown, A.C. Crystalline Fumarate Salt of (S)-[3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl] [3-hydroxy-3-(piperidin-2-yl) azetidin-1-yl]methanone. U.S. Patent Number US10590102B2, 17 March 2020. [Google Scholar]
- Santarpia, M.; Liguori, A.; Karachaliou, N.; Gonzalez-Cao, M.; Daffinà, M.G.; D’Aveni, A.; Marabello, G.; Altavilla, G.; Rosell, R. Osimertinib in the treatment of non-small-cell lung cancer: Design, development and place in therapy. Lung Cancer (Auckl) 2017, 8, 109–125. [Google Scholar] [CrossRef]
- Butterworth, S.; Finlay, M.R.V.; Redfearn, H.M.; Ward, R.A. 2-(2,4,5-substituted-anilino) pyrimidine Compounds. U.S. Patent Number US8946235B2, 3 February 2015. [Google Scholar]
- Srinivasamaharaj, S.; Salame, B.K.; Rios-Perez, J.; Kloecker, G.; Perez, C.A. The role of alectinib in the treatment of advanced ALK-rearranged non-small-cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 1227–1233. [Google Scholar] [CrossRef]
- Asoh, K.; Emura, T.; Furuichi, N.; Hong, W.; Ishii, N.; Ito, T.; Kawada, H.; Kinoshita, K.; Morikami, K.; Oikawa, N.; et al. Tetracyclic Compound. U.S. Patent Number US9126931B2, 8 September 2015. [Google Scholar]
- Curigliano, G.; Criscitiello, C.; Esposito, A.; Intra, M.; Minucci, S. Pharmacokinetic drug evaluation of ribociclib for the treatment of metastatic, hormone-positive breast cancer. Expert Opin. Drug Metab. Toxicol. 2017, 13, 575–581. [Google Scholar] [CrossRef]
- Brain, C.T.; Sung, M.J.E.; Lagu, B. Pyrrolopyrimidine Compounds and Their Uses. U.S. Patent Number US8415355B2, 9 April 2013. [Google Scholar]
- Calienni, J.V.; Chen, G.P.; Gong, B.; Kapa, P.K.; Saxena, V. Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic Acid Dimethylamide and Processes of Making Thereof. U.S. Patent Number US9193732B2, 24 November 2015. [Google Scholar]
- Ali, R.; Arshad, J.; Palacio, S.; Mudad, R. Brigatinib for ALK-positive metastatic non-small-cell lung cancer: Design, development and place in therapy. Drug Des. Devel. Ther. 2019, 13, 569–580. [Google Scholar] [CrossRef]
- Dalgarno, D.C.; Huang, W.S.; Li, F.; Liu, S.; Qi, J.; Romero, J.A.C.; Shakespeare, W.C.; Thomas, R.M.; Wang, Y.; Zhu, X.; et al. Phosphorous Derivatives as Kinase Inhibitors. U.S. Patent Number US9012462B2, 21 April 2015. [Google Scholar]
- Rozamus, L.W.; Sharma, P. Crystalline Forms of 5-chloro-N4-[-2 (dimethylphosphoryl)phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl]phenyl}pyrimidine-2,4-diamine. U.S. Patent Number US10385078B2, 20 August 2019. [Google Scholar]
- Kim, E.S. Midostaurin: First Global Approval. Drugs 2017, 77, 1251–1259. [Google Scholar] [CrossRef]
- Caravatti, G.; Fredenhagen, A. Staurosporine Derivatives Substituted at Methylamino Nitrogen. U.S. Patent Number US5093330A, 3 March 1992. [Google Scholar]
- Griffin, J.D.; Manley, P.W. Staurosporine Derivatives as Inhibitors of FLT3 Receptor Tyrosine Kinase Activity. U.S. Patent Number US7973031B2, 5 July 2011. [Google Scholar]
- Deeks, E.D. Neratinib: First Global Approval. Drugs 2017, 77, 1695–1704. [Google Scholar] [CrossRef]
- Rabindran, S.K.; Tsou, H.R.; Wissner, A. Protein Tyrosine Kinase Enzyme Inhibitors. U.S. Patent Number US7399865B2, 15 July 2008. [Google Scholar]
- Markham, A. Copanlisib: First Global Approval. Drugs 2017, 77, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Bullion, A.M.; Campbell, A.M.; Hentemann, M.; Michels, M.; Redman, A.; Rowley, B.R.; Scott, W.; Wood, J. Substituted 2,3-dihydroimidazo[1,2-c]quinazoline Derivatives Useful for Treating Hyper-Proliferative Disorders and Diseases Associated with Angiogenesis. U.S. Patent Number USRE46856E, 22 May 2018. [Google Scholar]
- Militzer, H.C.; Müller, H.; Peters, J.G. Substituted 2,3-dihydroimidazo[1,2-c]quinazoline Salts. U.S. Patent Number US10383876B2, 20 August 2019. [Google Scholar]
- Kim, E.S. Abemaciclib: First Global Approval. Drugs 2017, 77, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- De Dios, M.A.; De Prado, G.A.; Filadelfa, D.P.C.M.; Garcia, P.M.C.; Gelbert, L.M.; Knobeloch, J.M.; Martin, D.L.N.E.M.; Martin, O.F.M.D.; Martinez, P.J.A. Protein Kinase Inhibitors. U.S. Patent Number US7855211B2, 21 December 2010. [Google Scholar]
- Markham, A.; Dhillon, S. Acalabrutinib: First Global Approval. Drugs 2018, 78, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Barf, T.A.; Man, P.A.D.A.; Oubrie, A.A.; Rewinkel, J.B.M.; Sterrenburg, J.G.; Jans, C.G.J.M.; Raaijmakers, H. 4-imidazopyridazin-1-yl-benzamides and 4-imidazotriazin-1-yl-benzamides as Btk Inhibitors. U.S. Patent Number US9290504B2, 22 March 2016. [Google Scholar]
- Aret, E.; Barf, T.; Blatter, F.; Evarts, J.; Ingallinera, T.; Krejsa, C. Crystal forms of (S)-4-(8-amino-3-(1-(but-2-ynoyl) pyrrolidin-2-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(pyridin-2-yl)benzamide. U.S. Patent Number US9796721B2, 24 October 2017. [Google Scholar]
- Lin, C.W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.W.; Pattabiraman, P.P.; Rao, P.V.; deLong, M.A.; Kopczynski, C.C. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Elong, M.A.; Royalty, S.M.; Sturdivant, J.M. Dual Mechanism Inhibitors for the Treatment of Disease. U.S. Patent Number US8394826B2, 12 March 2013. [Google Scholar]
- Kopczynski, C.; Lin, C.W.; Sturdivant, J.M.; deLong, M.A. Combination Therapy. U.S. Patent Number US9415043B2, 16 August 2016. [Google Scholar]
- Markham, A. Baricitinib: First Global Approval. Drugs 2017, 77, 697–704. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Shepard, S. Azetidine and Cyclobutane Derivatives as JAK Inhibitors. U.S. Patent Number US8158616B2, 17 April 2012. [Google Scholar]
- Tran, B.; Cohen, M.S. The discovery and development of binimetinib for the treatment of melanoma. Expert Opin. Drug Discov. 2020, 15, 745–754. [Google Scholar] [CrossRef]
- Wallace, E.M.; Lyssikatos, J.P.; Marlow, A.L.; Hurley, T.B. N3 Alkylated Benzimidazole Derivatives as MEK Inhibitors. U.S. Patent Number US7777050B2, 17 August 2010. [Google Scholar]
- Rell, C.M.; Liu, W.; Misun, M.; Nichols, P.; Niederer, D.A.; Pachinger, W.H.; Stengel, P.J.; Wolf, M.C.; Zimmermann, D. Preparation of and Formulation Comprising a MEK Inhibitor. U.S. Patent Number US9562016B2, 7 February 2017. [Google Scholar]
- Shirley, M. Dacomitinib: First Global Approval. Drugs 2018, 78, 1947–1953. [Google Scholar] [CrossRef]
- Fakhoury, S.A.; Lee, H.T.; Reed, J.E.; Schlosser, K.M.; Sexton, K.E.; Tecle, H.; Winters, R.T. 4-phenylamino-quinazolin-6-yl-amides. U.S. Patent Number US7772243B2, 10 August 2010. [Google Scholar]
- Koelblinger, P.; Thuerigen, O.; Dummer, R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr. Opin. Oncol. 2018, 30, 125–133. [Google Scholar] [CrossRef]
- Huang, S.; Jin, X.; Liu, Z.; Poon, D.; Tellew, J.; Wan, Y.; Wang, X.; Xie, Y. Compounds and Compositions as Protein Kinase Inhibitors. U.S. Patent Number US8501758B2, 6 August 2013. [Google Scholar]
- Markham, A. Fostamatinib: First Global Approval. Drugs 2018, 78, 959–963. [Google Scholar] [CrossRef]
- Bhamidipati, S.; Singh, R.; Stella, V.J.; Sun, T. Prodrugs of 2,4-pyrimidinediamine Compounds and Their Uses. U.S. Patent Number US7449458B2, 11 November 2008. [Google Scholar]
- Bhamidipati, S.; Masuda, E.; Singh, R.; Sun, T. Prodrugs of 2,4-pyrimidinediamine Compounds and Their Uses. U.S. Patent Number US8163902B2, 24 April 2012. [Google Scholar]
- Blair, H.A. Duvelisib: First Global Approval. Drugs 2018, 78, 1847–1853. [Google Scholar] [CrossRef]
- Chan, K.; Li, L.; Liu, Y.; Ren, P.; Rommel, C.; Wilson, T.E. Substituted Isoquinolin-1(2H)-ones, and Methods of Use Thereof. U.S. Patent Number US8193182B2, 5 June 2012. [Google Scholar]
- Isbester, P.; Kropp, J.; Lane, B.S.; Michael, M.; Pingda, R. Processes for Preparing Isoquinolinones and Solid Forms of Isoquinolinones. U.S. Patent Number USRE46621E, 5 December 2017. [Google Scholar]
- Dhillon, S. Gilteritinib: First Global Approval. Drugs 2019, 79, 331–339. [Google Scholar] [CrossRef]
- Kazuhiko, I.; Yoshinori, I.; Akio, K.; Yutaka, K.; Kazuo, K.; Takahiro, M.; Itsuro, S.; Hiroshi, T. Diamino Heterocyclic Carboxamide Compound. U.S. Patent Number US8969336B2, 3 March 2015. [Google Scholar]
- Scott, L.J. Larotrectinib: First Global Approval. Drugs 2019, 79, 201–206. [Google Scholar] [CrossRef]
- Andrews, S.W.; Haas, J.; Jiang, Y.; Zhang, G. Method of treatment using substituted pyrazolo[1,5-a] pyrimidine compounds. U.S. Patent Number US9127013B2, 8 September 2015. [Google Scholar]
- Alisha, B.; Juengst, D.; Shah, K. Crystalline Form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide Hydrogen Sulfate. U.S. Patent Number US10172861B2, 8 January 2019. [Google Scholar]
- Syed, Y.Y. Lorlatinib: First Global Approval. Drugs 2019, 79, 93–98. [Google Scholar] [CrossRef]
- Bailey, S.; Burke, B.J.; Collins, M.R.; Cui, J.J.; Deal, J.G.; Hoffman, R.L.; Huang, Q.; Johnson, T.W.; Kania, R.S.; Kath, J.C. Macrocyclic Derivatives for the Treatment of Diseases. U.S. Patent Number US8680111B2, 25 March 2014. [Google Scholar]
- Birch, M.J.; Pencheva, K.D. Crystalline Form of Lorlatinib Free Base. U.S. Patent Number US10420749B2, 24 September 2019. [Google Scholar]
- Al-Salama, Z.T.; Keam, S.J. Entrectinib: First Global Approval. Drugs 2019, 79, 1477–1483. [Google Scholar] [CrossRef]
- Lombardi, B.A.; Marchionni, C.; Menichincheri, M.; Nesi, M.; Orsini, P.; Panzeri, A.; Perrone, E.; Vanotti, E. Substituted Indazole Derivatives Active as Kinase Inhibitors. U.S. Patent Number US8299057B2, 30 October 2012. [Google Scholar]
- Candiani, I.; Ottaiano, G.; Tomasi, A. Crystalline Form of N-[5-(3,5-difluoro-benzyl)-1H-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2-(tetrahydro-pyran-4-ylamino)-benzamide. U.S. Patent Number US10738037B2, 11 August 2020. [Google Scholar]
- Duggan, S.; Keam, S.J. Upadacitinib: First Approval. Drugs 2019, 79, 1819–1828. [Google Scholar] [CrossRef]
- Frank, K.E.; Friedman, M.; George, D.M.; Stewart, K.D.; Wallace, G.A.; Wishart, N. Tricyclic Compounds. U.S. Patent Number USRE47221E, 5 February 2019. [Google Scholar]
- Allian, A. Processes for the Preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-alpha]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and Solid State Forms Thereof. U.S. Patent Number US9951080B2, 24 April 2018. [Google Scholar]
- Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef]
- Caravatti, G.; Fairhurst, R.A.; Furet, P.; Guagnano, V.; Imbach, P. Pyrrolidine-1,2-dicarboxamide Derivatives. U.S. Patent Number US8227462B2, 24 July 2012. [Google Scholar]
- Hanna, K.S. Erdafitinib to treat urothelial carcinoma. Drugs Today (Barc.) 2019, 55, 495–501. [Google Scholar] [CrossRef]
- Akkari, R.; Berdini, V.; Besong, G.E.; Embrechts, W.C.J.; Freyne, E.J.E.; Gilissen, R.A.H.J.; Hamlett, C.C.F.; Johnson, C.N.; Lacrampe, J.F.A.; Meerpoel, L.; et al. Pyrazolyl Quinoxaline Kinase Inhibitors. U.S. Patent Number US8895601B2, 25 November 2014. [Google Scholar]
- Monestime, S.; Lazaridis, D. Pexidartinib (TURALIO™): The first FDA-indicated systemic treatment for Tenosynovial Giant Cell Tumor. Drugs R D 2020, 20, 189–195. [Google Scholar] [CrossRef]
- Bremer, R.; Ibrahim, P.N.; Zhang, J. Compounds Modulating c-fms and/or c-kit Activity and Uses Therefor. U.S. Patent Number US9169250B2, 27 October 2015. [Google Scholar]
- Ibrahim, P.N.; Visor, G.C. Solid Forms of a Compound Modulating Kinases. U.S. Patent 2017. [Google Scholar]
- Blair, H.A. Fedratinib: First Approval. Drugs 2019, 79, 1719–1725. [Google Scholar] [CrossRef]
- Cao, J.; Hood, J.D.; Lohse, D.L.; Mcpherson, A.; Noronha, G.; Pathak, V.P.; Renick, J.; Soll, R.M.; Zeng, B.; Mak, C.C. Bi-aryl meta-pyrimidine Inhibitors of Kinases. U.S. Patent Number US7528143B2, 5 May 2009. [Google Scholar]
- Syed, Y.Y. Zanubrutinib: First Approval. Drugs 2020, 80, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z. Substituted pyrazolo[1,5-a]pyrimidines as Bruton’s Tyrosine Kinase Modulators. U.S. Patent Number US9447106B2, 20 September 2016. [Google Scholar]
- Dhillon, S. Avapritinib: First Approval. Drugs 2020, 80, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Hodous, B.L.; Kim, J.L.; Wilson, D.; Wilson, K.J.; Zhang, Y. Compositions Useful for Treating Disorders Related to Kit. U.S. Patent Number US9944651B2, 17 April 2018. [Google Scholar]
- Markham, A.; Keam, S.J. Selumetinib: First Approval. Drugs 2020, 80, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.M.; Lyssikatos, J.P.; Marlow, A.L.; Hurley, T.B. N3 Alkylated Benzimidazole Derivatives as MEK Inhibitors. U.S. Patent Number US7425637B2, 16 September 2008. [Google Scholar]
- Chuang, T.H.; Demattei, J.; Dickinson, P.A.; Ford, J.G.; Pervez, M.; Roberts, R.J.; Sharma, S.G.; Squire, C.J.; Storey, R.A. Hydrogen Sulfate Salt. U.S. Patent Number US9156795B2, 13 October 2015. [Google Scholar]
- Hoy, S.M. Pemigatinib: First Approval. Drugs 2020, 80, 923–929. [Google Scholar] [CrossRef]
- He, C.; Lu, L.; Wu, L.; Yao, W.; Zhang, C. Substituted Tricyclic Compounds as FGFR Inhibitors. U.S. Patent Number US9611267B2, 4 April 2017. [Google Scholar]
- Lee, A. Tucatinib: First Approval. Drugs 2020, 80, 1033–1038. [Google Scholar] [CrossRef]
- Greschuk, J.M.; Hennings, D.D.; Liu, W.; Lyssikatos, J.P.; Marmsaeter, F.P.; Zhao, Q. N4-phenyl-quinazoline-4-amine Derivatives and Related Compounds as ErbB type I Receptor Tyrosine Kinase Inhibitors for the Treatment of Hyperproliferative Diseases. U.S. Patent Number US8648087B2, 11 February 2014. [Google Scholar]
- Dhillon, S. Capmatinib: First Approval. Drugs 2020, 80, 1125–1131. [Google Scholar] [CrossRef]
- He, C.; Metcalf, B.; Qian, D.Q.; Xu, M.; Yao, W.; Zhang, C.; Zhuo, J. Imidazotriazines and Imidazopyrimidines as Kinase Inhibitors. U.S. Patent Number US7767675B2, 3 August 2010. [Google Scholar]
- Liu, P.; Pan, Y.; Qiao, L.; Weng, L.; Zhou, J. Salts of 2-fluoro-N-methyl-4-[7-(quinolin-6-yl-methyl)-imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide and Processes Related to Preparing the Same. U.S. Patent Number US8420645B2, 16 April 2013. [Google Scholar]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Andrews, S.W.; Aronow, S.; Blake, J.F.; Brandhuber, B.J.; Cook, A.; Haas, J.; Jiang, Y.; Kolakowski, G.R.; McFaddin, E.A.; McKenney, M.L.; et al. Substituted Pyrazolo[1,5-a]pyridine Compounds as RET Kinase Inhibitors. U.S. Patent Number US10112942B2, 30 October 2018. [Google Scholar]
- Metcalf, A.T.; Fry, D.; McFaddin, E.A.; Kolakowski, G.R.; Haas, J.; Tang, T.P.; Jiang, Y. Crystalline Forms. U.S. Patent Number US10584124B2, 10 March 2020. [Google Scholar]
- Dhillon, S. Ripretinib: First Approval. Drugs 2020, 80, 1133–1138. [Google Scholar] [CrossRef]
- Flynn, D.L.; Kaufman, M.D.; Petillo, P.A. Dihydronaphthyridines and Related Compounds Useful as Kinase Inhibitors for the Treatment of Proliferative Diseases. U.S. Patent Number US8461179B1, 11 June 2013. [Google Scholar]
- Markham, A. Pralsetinib: First Approval. Drugs 2020, 80, 1865–1870. [Google Scholar] [CrossRef]
- Brubaker, J.D.; DiPietro, L.V.; Kim, J.L.; Wilson, D.W.; Wilson, K.J. Inhibitors of RET. U.S. Patent Number US10030005B2, 24 July 2018. [Google Scholar]
- Dhillon, S. Trilaciclib: First Approval. Drugs 2021, 81, 867–874. [Google Scholar] [CrossRef]
- Strum, J.C.; Tavares, F.X. CDK Inhibitors. U.S. Patent Number US8598186B2, 3 December 2013. [Google Scholar]
- Markham, A. Tepotinib: First Approval. Drugs 2020, 80, 829–833. [Google Scholar] [CrossRef]
- Dorsch, D.; Steiber, F.; Schadt, O.; Blaukat, A. Pyridazinone Derivatives. U.S. Patent Number US8580781B2, 12 November 2013. [Google Scholar]
- Schadt, O.; Dorsch, D.; Steiber, F.; Blaukat, A. Pyrimidinyl Pyridazinone Derivatives. U.S. Patent Number US8329692B2, 11 December 2012. [Google Scholar]
- Dhillon, S.; Keam, S.J. Umbralisib: First Approval. Drugs 2021, 81, 857–866. [Google Scholar] [CrossRef]
- Muthuppalaniappan, M.; Nagarathnam, D.; Vakkalanka, S.K. Selective PI3K Delta Inhibitors. U.S. Patent 2020. [Google Scholar]
- Vakkalanka, S.K. Forms of a PI3K Delta Selective Inhibitor for Use in Pharmaceutical Formulations. U.S. Patent 2019. [Google Scholar]
- Tivozanib Hydrochloride. Am. J. Health Syst. Pharm. 2021, zxab199. [CrossRef]
- Kubo, K.; Sakai, T.; Nagao, R.; Fujiwara, Y.; Isoe, T.; Hasegawa, K. Quinoline Derivatives and Quinazoline Derivatives Having Azolyl Group. U.S. Patent Number US6821987B2, 23 November 2004. [Google Scholar]
- Kubo, K.; Sakai, T.; Nagao, R.; Fujiwara, Y.; Isoe, T.; Hasegawa, K. Quinoline Derivatives and Quinazoline Derivatives Having Azolyl Group. U.S. Patent Number US7211587B2, 1 May 2007. [Google Scholar]
- Matsunaga, N.; Yoshida, S.; Yoshino, A.; Nakajima, T. N-{2-chloro-4-[(6,7-dimethoxy-4-quinolyl)oxy]phenyl}-N′-(5-methyl-3-isoxazolyl)urea Salt in Crystalline Form. U.S. Patent Number US7166722B2, 23 January 2007. [Google Scholar]
- Botrus, G.; Raman, P.; Oliver, T.; Bekaii-Saab, T. Infigratinib (BGJ398): An investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 309–316. [Google Scholar] [CrossRef]
- Ding, Q.; Gray, N.S.; Li, B.; Liu, Y.; Sim, T.; Uno, T.; Zhang, G.; Soldermann, C.P.; Breitenstein, W.; Bold, G.; et al. Compounds and Compositions as Protein Kinase Inhibitors. U.S. Patent Number US8552002B2, 8 October 2013. [Google Scholar]
- Berghausen, J.; Kapa, P.K.; McKenna, J.; Slade, J.; Wu, R.; Du, Z.; Stowasswer, F. Crystalline Forms of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl) -phenylamino]-pyrimidin-4-yl}-1-methyl-urea and Salts Thereof. U.S. Patent Number US9067896B2, 30 June 2015. [Google Scholar]
- Kanev, G.K.; de Graaf, C.; de Esch, I.J.P.; Leurs, R.; Würdinger, T.; Westerman, B.A.; Kooistra, A.J. The Landscape of Atypical and Eukaryotic Protein Kinases. Trends Pharmacol. Sci. 2019, 40, 818–832. [Google Scholar] [CrossRef]
- 2020 Medicines in Development–Cancer. Available online: https://www.phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/MID-Reports/MID-Cancer2020_Product-List_FINAL.pdf (accessed on 31 May 2021).
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 1–19. [Google Scholar] [CrossRef]
- Couillaud, B.M.; Espeau, P.; Mignet, N.; Corvis, Y. State of the art of pharmaceutical solid forms: From crystal property issues to nanocrystals formulation. Chem. Med. Chem. 2019, 14, 8–23. [Google Scholar] [CrossRef]
- Smalley, K.S.M. Pharmacological research and cancer: A call to arms. Pharmacol. Res. 2019, 146, 104291. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Fojo, T.; Mathisen, M.; Zwelling, L.A. Cancer drugs in the United States: Justum Pretium--the just price. J. Clin. Oncol. 2013, 31, 3600–3604. [Google Scholar] [CrossRef]
- Mishuk, A.U.; Fasina, I.; Qian, J. Impact of U.S. federal and state generic drug policies on drug use, spending, and patient outcomes: A systematic review. Res. Soc. Adm. Pharm. 2020, 16, 736–745. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).