Solvent-Free Microwave Extraction of Thymus mastichina Essential Oil: Influence on Their Chemical Composition and on the Antioxidant and Antimicrobial Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield and Quality of T. mastichina Essential Oil
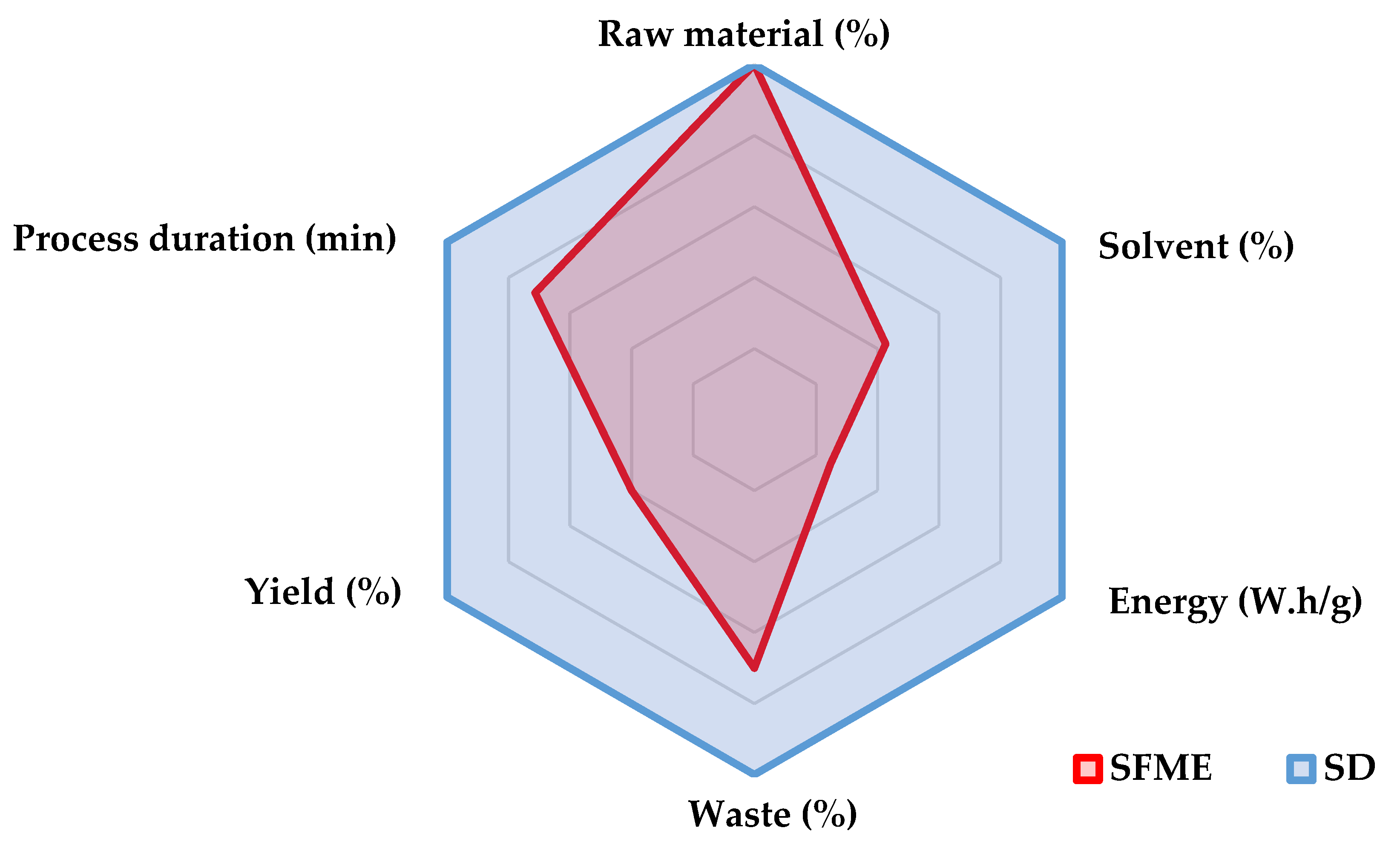
2.2. Large Scale, Cost and Environmental Impact of T. mastichina Extraction
3. Materials and Methods
3.1. T. mastichina Production and Harvest
3.2. Extraction Procedures
3.2.1. Conventional Techniques: Hydrodistillation (HD) and Steam Distillation (SD)
3.2.2. Solvent-Free Microwave Extraction (SFME): Laboratory and Pilot Scale
3.3. Chemical Analysis of Essential Oils Compounds by Gas Chromatography with Flame Ionization Detection (GC-FID) and Mass Spectrometry (GC-MS)
3.3.1. Chromatographic Method (Independent Laboratory 1)
3.3.2. Chromatographic Method (Independent Laboratory 2)
3.4. Antioxidant Assays
3.4.1. Free Radical Scavenging Activity
3.4.2. Total Polyphenol Analysis
3.5. Antimicrobial Activity
3.5.1. Microbial Strains
3.5.2. Determination of Minimum Inhibitory Concentration (MIC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Da Silva, D.V.; Duarte, J.M.; Miguel, M.G.; Leitão, J.M. AFLP assessment of the genetic relationships among 12 Thymus taxa occurring in Portugal. Plant Genet. Resour. 2017, 15, 89–92. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. From famine plants to tasty and fragrant spices: Three Lamiaceae of general dietary relevance in traditional cuisine of Trás-os-Montes (Portugal). LWT-Food Sci. Technol. 2011, 44, 543–548. [Google Scholar] [CrossRef][Green Version]
- Miguel, G.; Guerrero, C.; Rodrigues, H.; Brito, J.; Venâncio, F.; Tavares, R.; Duarte, F. Effect of substrate on the essential oils composition of Thymus mastichina (L.) L. subsp. mastichina collected in Sesimbra region (Portugal). In Natural Products in the New Millennium: Prospects and Industrial Application; Springer: Dordrecht, The Netherlands, 2002; pp. 143–148. [Google Scholar]
- Miguel, M.G.; Guerrero, C.; Rodrigues, H.; Brito, J.C.; Duarte, F.; Venâncio, F.; Tavares, R. Main components of the essential oils from wild portuguese Thymus mastichina (L.) L. ssp. mastichina in different developmental stages or under culture conditions. J. Essent. Oil Res. 2004, 16, 111–114. [Google Scholar] [CrossRef]
- Cutillas, A.-B.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thymus mastichina L. essential oils from Murcia (Spain): Composition and antioxidant, antienzymatic and antimicrobial bioactivities. PLoS ONE 2018, 13, e0190790. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venâncio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Arantes, S.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; Martins da Costa, P.; Belo, A.D.F. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus Labill. and seven mediterranean aromatic plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [PubMed]
- Pina-Vaz, C.; Rodrigues, A.G.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Aazza, S.; El-Guendouz, S.; Miguel, M.G.; Dulce Antunes, M.; Leonor Faleiro, M.; Isabel Correia, A.; Cristina Figueiredo, A. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of essential oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Nat. Prod. Commun. 2016, 11, 1029–1038. [Google Scholar] [PubMed]
- Arantes, S.; Piçarra, A.; Candeias, F.; Caldeira, A.T.; Martins, M.R.; Teixeira, D. Antioxidant activity and cholinesterase inhibition studies of four flavouring herbs from Alentejo. Nat. Prod. Res. 2017, 31, 2183–2187. [Google Scholar] [CrossRef]
- Albano, S.M.; Sofia Lima, A.; Graça Miguel, M.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C. Antioxidant, anti-5-lipoxygenase and antiacetylcholinesterase activities of essential oils and decoction waters of some aromatic plants. Rec. Nat. Prod. 2012, 6, 35–48. [Google Scholar]
- Méndez-Tovar, I.; Sponza, S.; Asensio-S-Manzanera, C.; Schmiderer, C.; Novak, J. Volatile fraction differences for Lamiaceae species using different extraction methodologies. J. Essent. Oil Res. 2015, 27, 497–505. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Mohamadi, M.; Shamspur, T.; Mostafavi, A. Comparison of microwave-assisted distillation and conventional hydrodistillation in the essential oil extraction of flowers Rosa damascena Mill. J. Essent. Oil Res. 2013, 25, 55–61. [Google Scholar] [CrossRef]
- Chemat, F.; Lucchesi, M.E.; Smadia, J. Solvent-Free Microwave Extraction of Volatile Natural Substances. U.S. Patent Application No 10/751,988, 30 September 2004. [Google Scholar]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction: An innovative tool for rapid extraction of essential oil from aromatic herbs and spices. J. Microw. Power Electromagn. Energy 2004, 39, 135–139. [Google Scholar] [CrossRef]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent-free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy: Experimental and theoretical study. J. Chromatogr. A 2009, 1216, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Chenni, M.; EI Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Qi, X.L.; Li, T.T.; Wei, Z.F.; Guo, N.; Luo, M.; Wang, W.; Zu, Y.G.; Fu, Y.J.; Peng, X. Solvent-free microwave extraction of essential oil from pigeon pea leaves [Cajanus cajan (L.) Millsp.] and evaluation of its antimicrobial activity. Ind. Crops Prod. 2014, 58, 322–328. [Google Scholar] [CrossRef]
- Vernès, L.; Vian, M.; Chemat, F. Ultrasound and microwave as green tools for solid-liquid extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–374. [Google Scholar]
- Miguel, M.G.; Guerrero, C.; Rodrigues, H.; Brito, J.; Duarte, F.; Venâncio, F.; Tavares, R. Essential oils of Portuguese Thymus mastichina (L.) L. subsp. mastichina grown on different substrates and harvested on different dates. J. Hortic. Sci. Biotechnol. 2003, 78, 355–358. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomàs, X.; Cañigueral, S.; Da Cunha, A.P.; Adzet, T. Composition and variability of the essential oils of Thymus species from section mastichina from Portugal. Biochem. Syst. Ecol. 1997, 25, 659–672. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017, 28, 1168–1180. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds; Chemat, F., Cravotto, G., Eds.; Food Engineering Series; Springer US: Boston, MA, USA, 2013; ISBN 978-1-4614-4829-7. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation Carol Stream: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Boelens Aroma Chemical Information Service. The Complete Database of Essential Oils. ESO 2000; BACIS: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Kondjoyan, N.; Berdagué, J.L. A Compilation of Relative Retention Indices for the Analysis of Aromatic Compounds; Édition du Laboratoire Flaveur; INRA: Theix, France, 1996; ISBN 9782951062207. [Google Scholar]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.S.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What is the best ethanol-water ratio for the extraction of antioxidants from rosemary? Impact of the solvent on yield, composition, and activity of the extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
| Identification | Molecular Formula | Retention Indice (Lab 1) | Retention Indice (Lab 2) | Literature Retention Index | %SD Flower (Lab 2) | %ETHOS X Flower (Lab 2) | %SD Fresh Aerial Parts (Lab 1) | %SD Fresh Aerial Parts (Lab 2) | %ETHOS X Fresh Aerial Parts (Lab 1) | %ETHOS X Fresh Aerial Parts (Lab 2) | %HD Dry Aerial Parts Rehydrated (Lab 1) | %HD Dry Aerial Parts Rehydrated (Lab 2) | %SD Dry Aerial Parts Rehydrated (Lab 1) | %SD Dry Aerial Parts Rehydrated (Lab 2) | %ETHOS X Dry Aerial Parts Rehydrated (Lab 1) | %ETHOS X Dry Aerial Parts Rehydrated (Lab 2) | %Mac 75 Dry Aerial Parts Rehydrated (Lab 1) | %Mac 75 Dry Aerial Parts Rehydrated (Lab 2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpenes | ||||||||||||||||||
| Tricyclene | C10H16 | 914 | 914 | 920 | - | - | t | 0.02 | t | 0.02 | t | - | t | 0.02 | t | 0.01 | t | 0.02 |
| α-Thujene | C10H16 | 920 | 924 | 924 | 0.20 | 0.21 | 0.12 | 0.15 | 0.14 | 0.18 | 0.19 | 0.20 | 0.25 | 0.29 | 0.20 | 0.24 | 0.26 | 0.29 |
| α-Pinene | C10H16 | 927 | 927 | 932 | 3.21 | 3.41 | 3.27 | 3.71 | 3.63 | 4.35 | 3.32 | 3.31 | 4.15 | 4.47 | 3.31 | 3.71 | 4.15 | 4.39 |
| Camphene | C10H16 | 938 | 938 | 946 | 0.21 | 0.23 | 0.26 | 0.30 | 0.28 | 0.32 | 0.28 | 0.27 | 0.34 | 0.35 | 0.27 | 0.29 | 0.30 | 0.31 |
| Sabinene | C10H16 | 968 | 968 | 968 | 3.17 | 3.61 | 4.45 | 4.39 | 4.64 | 4.98 | 4.62 | 3.37 | 5.21 | 4.44 | 4.39 | 3.90 | 5.09 | 4.27 |
| β-Pinene | C10H16 | 974 | 974 | 4.55 | 4.87 | - | 5.23 | - | 5.83 | - | 4.82 | - | 6.20 | - | 5.22 | - | 6.02 | |
| 3-Octanone | C8H16O | 979 | 979 | - | - | - | - | - | 0.01 | - | - | - | - | - | 0.01 | - | 0.02 | |
| 2,3-Dehydro-1,8-cineole | C10H16O | 976 | 978 | - | - | t | - | t | t | - | t | - | t | - | t | - | ||
| Myrcene | C10H16 | 983 | 983 | 983 | 1.40 | 1.57 | 1.94 | 1.72 | 2.03 | 2.00 | 1.72 | 1.46 | 2.26 | 2.04 | 1.83 | 1.76 | 2.35 | 2.10 |
| 3-Octanol | C8H18O | 988 | 988 | - | 0.02 | - | - | - | 0.02 | - | - | - | 0.02 | - | 0.02 | - | 0.02 | |
| α-Phellandrene | C10H16 | 997 | 997 | 997 | 0.05 | 0.04 | t | 0.05 | t | 0.05 | t | 0.04 | t | 0.06 | t | 0.05 | t | 0.05 |
| α-Terpinene | C10H16 | 1008 | 1008 | 1009 | 0.20 | 0.17 | t | 0.17 | t | - | t | 0.22 | 0.11 | 0.31 | t | 0.21 | t | 0.33 |
| Cymene * | C10H14 | 1015 | 1015 | 1014 | 1.12 | 0.85 | - | 0.79 | t | 0.85 | t | 0.95 | - | 0.89 | t | 0.95 | t | 0.78 |
| 1,8- cineole (eucalyptol) | C10H18O | 1028 | 1028 | 1022 | 62.53 | 59.79 | 67.89 | 63.40 | 66.28 | 52.01 | 68.46 | 60.92 | 70.60 | 63.63 | 67.41 | 55.68 | 64.99 | 56.30 |
| (Z)-β-Ocimene | C10H16 | 1032 | 1032 | 0.05 | 0.04 | 0.25 | 0.04 | 0.26 | 0.04 | 0.20 | 0.04 | 0.26 | 0.05 | 0.20 | 0.03 | 0.25 | 0.05 | |
| (E)-β-Ocimene | C10H16 | 1039 | 1039 | 1037 | 0.16 | 0.20 | - | 0.30 | - | 0.36 | - | 0.21 | - | 0.28 | - | 0.23 | - | 0.28 |
| γ-Terpinene | C10H16 | 1049 | 1049 | 1051 | 0.51 | 0.65 | 0.52 | 0.83 | 0.58 | 0.99 | 0.65 | 0.77 | 0.82 | 1.04 | 0.59 | 0.73 | 0.82 | 1.10 |
| (E)-Sabinene hydrate | C12H20O2 | 1055 | 1055 | 1058 | 0.43 | 0.66 | 0.55 | 0.54 | 0.43 | 0.60 | 0.48 | 0.49 | 0.35 | 0.38 | 0.62 | 0.72 | 0.30 | 0.33 |
| Linalool oxide * | C10H18O2 | 1058 | 1058 | 1076 | - | 0.04 | t | 0.03 | t | 0.03 | t | 0.03 | t | 0.02 | t | 0.04 | t | 0.02 |
| Terpinolene | C10H16 | 1077 | 1077 | 1080 | 0.10 | 0.12 | t | 0.11 | t | 0.14 | t | 0.12 | 0.11 | 0.15 | t | 0.13 | 0.12 | 0.18 |
| (Z)-Sabinene Hydrate | C10H18O | 1085 | 1055 | 1080 | - | - | t | - | t | - | 0.15 | - | t | - | 0.15 | t | - | |
| Linalool | C10H18O | 1091 | 1091 | 1086 | 3.19 | 4.15 | 3.64 | 3.61 | 3.51 | 4.24 | 3.56 | 3.97 | 3.19 | 3.70 | 3.73 | 4.39 | 3.98 | 4.44 |
| Hotrienol | C10H16O | 1101 | 1101 | - | 0.06 | - | 0.04 | - | 0.05 | - | 0.05 | - | 0.04 | - | 0.04 | - | 0.06 | |
| p-Menth-2-en-1-ol | C10H18O | 1105 | 1105 | 1108 | 0.05 | 0.05 | t | 0.04 | t | 0.05 | - | 0.06 | t | 0.03 | - | 0.05 | - | 0.05 |
| α-Campholene aldehyde | C10H16O | 1111 | 1111 | - | 0.02 | - | 0.02 | - | 0.03 | - | - | - | 0.02 | - | 0.03 | - | 0.02 | |
| Pinocarveol | C10H16O | 1123 | 1123 | 1133 | 0.10 | 0.08 | t | 0.07 | t | 0.10 | t | 0.12 | t | 0.07 | t | 0.10 | t | - |
| Camphor | C10H16O | 1126 | 1126 | 1140 | 0.07 | 0.11 | t | 0.05 | t | 0.06 | t | 0.09 | t | 0.04 | t | 0.06 | t | 0.10 |
| Pinocarvone | C10H14O | 1138 | 1138 | 1140 | 0.04 | - | t | 0.04 | t | 0.05 | t | 0.05 | t | 0.03 | t | 0.05 | t | 0.02 |
| Borneol | C10H18O | 1144 | 1154 | - | - | t | - | t | - | t | - | t | - | t | - | t | - | |
| Terpineol * | C10H18O | 1152 | 1152 | 1160 | 2.45 | 2.40 | 2.00 | 2.27 | 1.82 | 2.64 | 2.13 | 2.69 | 1.09 | 1.51 | 2.03 | 2.70 | 1.60 | 2.06 |
| Terpinen-4-ol | C10H18O | 1173 | 1173 | 1165 | 0.63 | 0.52 | t | 0.46 | t | 0.58 | t | 0.77 | t | 0.46 | t | 0.55 | t | 0.70 |
| α-Terpineol | C10H18O | 1179 | 1179 | 1175 | 6.33 | 5.98 | 4.50 | 5.31 | 4.07 | 6.07 | 4.60 | 6.20 | 2.31 | 3.36 | 4.51 | 6.20 | 3.68 | 4.97 |
| (Z)-Dihydrocarvone | C10H16O | 1183 | 1183 | 1181 | 0.29 | 0.25 | t | 0.21 | t | 0.25 | t | 0.29 | t | 0.15 | t | 0.27 | t | 0.20 |
| (E)-Dihydrocarvone | C10H16O | 1217 | 1217 | 1190 | 0.20 | 0.18 | t | 0.18 | t | 0.22 | t | 0.18 | t | 0.09 | t | 0.18 | t | 0.13 |
| Isobornyl formate | C11H18O2 | 1218 | 1228 | - | - | t | - | t | - | t | - | - | - | t | - | - | - | |
| Piperitol | C10H18O | 1207 | 1207 | - | - | - | - | - | - | - | - | - | - | - | 0.02 | - | 0.01 | |
| trans-Carveol | C10H16O | 1215 | 1215 | - | 0.02 | - | - | - | 0.02 | - | 0.02 | - | - | - | 0.03 | - | - | |
| Bornyl or isobornyl acetate | C12H20O2 | 1268 | 1268 | 1275 | 0.04 | 0.03 | t | 0.02 | t | 0.03 | t | 0.04 | t | 0.03 | t | 0.03 | t | 0.02 |
| Carvacrol methyl ether | C11H16O | 1241 | 1241 | 0.56 | 0.53 | - | 0.49 | - | 0.62 | - | 0.60 | - | 0.47 | - | 0.61 | - | 0.60 | |
| Linalool acetate | C12H20O2 | 1254 | 1254 | - | 0.02 | - | 0.03 | - | 0.04 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | |
| Endobornyl acetate | C12H20O2 | 1272 | 1284 | 0.08 | 0.02 | - | 0.02 | - | 0.01 | - | 0.04 | - | 0.02 | - | 0.02 | - | 0.02 | |
| Carvacrol | C10H14O | 1298 | 1298 | 1.30 | 1.19 | - | - | - | 1.16 | - | 1.26 | - | 0.57 | - | 1.31 | - | 0.91 | |
| Thymol | C10H14O | 1293 | 1293 | 1287 | - | 0.05 | 0.76 | 0.61 | 0.04 | 0.82 | - | 0.37 | - | 0.78 | 0.05 | 0.59 | 0.02 | |
| Sesquiterpenes | ||||||||||||||||||
| Bicycloelemene | C15H24 | 1330 | 1330 | - | 0.03 | - | 0.02 | - | 0.04 | - | 0.03 | - | 0.02 | - | 0.02 | - | 0.02 | |
| α-Copaene | C15H24 | 1371 | 1347 | 1347 | - | 0.02 | t | - | t | 0.02 | t | - | t | 0.02 | t | 0.02 | t | 0.02 |
| Bourbonene * | C15H24 | 1379 | 1379 | 1375 | 0.21 | 0.19 | t | 0.11 | 0.15 | 0.23 | 0.10 | 0.13 | 0.11 | 0.15 | 0.15 | 0.21 | 0.15 | 0.21 |
| β-Elemene | C15H24 | 1385 | 1385 | 1402 | 0.08 | 0.09 | t | 0.06 | t | 0.13 | t | 0.06 | t | 0.07 | t | 0.10 | t | 0.12 |
| α-Gurjunene | C15H24 | 1404 | 1404 | 1420 | 0.03 | 0.03 | t | - | t | 0.04 | t | 0.02 | t | 0.03 | t | 0.04 | t | 0.06 |
| β-Caryophyllene | C15H24 | 1413 | 1413 | 1417 | 0.33 | 0.33 | 0.20 | 0.20 | 0.35 | 0.49 | 0.16 | 0.18 | 0.19 | 0.25 | 0.29 | 0.39 | 0.36 | 0.45 |
| Germacrene D | C15H24 | 1484 | 0.04 | 0.04 | - | 0.02 | - | 0.05 | - | 0.02 | - | 0.03 | - | 0.05 | - | 0.05 | ||
| Aromadendrene | C15H24 | 1448 | 1448 | 1446 | - | - | t | - | t | 0.02 | t | 0.02 | t | 0.02 | t | 0.04 | t | 0.05 |
| β-Farnesene | C15H24 | 1440 | 1440 | 0.04 | 0.04 | - | 0.02 | - | 0.04 | - | 0.02 | - | 0.02 | - | 0.04 | - | 0.05 | |
| α-Bisabolene | C15H24 | 1505 | 1501 | 1505 | 0.06 | 0.07 | - | 0.03 | - | 0.08 | - | 0.04 | - | 0.04 | - | 0.08 | - | 0.09 |
| 4,5-Dehydro-isolongifolene | C15H22 | 1544 | 1544 | - | 0.03 | - | - | - | - | - | 0.02 | - | - | - | 0.03 | - | - | |
| allo-Aromadendrene | C15H24 | 1454 | 1464 | 1460 | 0.10 | 0.10 | t | 0.06 | t | 0.16 | t | 0.06 | t | 0.07 | t | 0.12 | t | 0.05 |
| Aromadendrene isomer | C15H24 | 1458 | 0.06 | 0.06 | - | 0.02 | - | 0.07 | - | 0.03 | - | 0.04 | - | 0.09 | - | - | ||
| β-cubebene | C15H24 | 1387 | 1387 | 0.82 | 0.93 | - | 0.64 | - | 1.54 | - | 0.49 | - | 0.63 | - | 1.08 | - | 1.24 | |
| β-selinene | C15H24 | 1489 | 1489 | - | - | - | - | - | 0.03 | - | 0.02 | - | - | - | 0.04 | - | 0.04 | |
| Eremophilene | C15H24 | 1486 | 1486 | 0.07 | - | - | - | - | 0.09 | - | - | - | - | - | 0.08 | - | 0.09 | |
| Bicyclogermacrene | C15H24 | 1500 | 1500 | 1.90 | 2.15 | - | 1.92 | - | 3.81 | - | 1.35 | - | - | - | 2.47 | - | 2.88 | |
| Valencene | C15H24 | 1489 | 1485 | - | - | 1.45 | - | 2.29 | - | 0.85 | - | 0.84 | - | 1.62 | - | 1.96 | - | |
| α-Bisabolene | C15H24 | 1501 | 1496 | - | - | 0.36 | - | 0.71 | 0.08 | 0.28 | - | 0.36 | - | 0.64 | - | 0.80 | - | |
| β -Bisabolene | C15H24 | 1505 | 1501 | 1505 | 1.32 | 1.31 | - | 0.56 | - | 1.50 | - | 0.61 | - | 0.78 | - | 1.46 | - | 1.56 |
| γ -Cadinene | C15H24 | 1513 | 1513 | 0.05 | 0.05 | - | 0.02 | - | 0.04 | - | 0.03 | - | 0.03 | - | 0.06 | - | 0.07 | |
| δ-Cadinene | C15H24 | 1513 | 1513 | 1516 | 0.12 | 0.11 | t | 0.05 | t | 0.12 | t | 0.06 | t | 0.07 | t | 0.15 | t | 0.17 |
| Spathulenol | C15H24O | 1569 | 1569 | 1567 | 0.35 | 0.38 | t | 0.09 | t | 0.22 | t | 0.57 | t | 0.19 | t | 0.46 | t | 0.24 |
| Caryophyllene oxide | C15H24O | 1582 | 1582 | 0.10 | 0.11 | - | 0.05 | - | 0.10 | - | 0.18 | - | 0.05 | - | 0.13 | - | 0.07 | |
| Viridiflorol | C15H26O | 1592 | 1592 | 0.44 | 0.52 | - | 0.32 | - | 0.59 | - | 0.88 | - | 0.29 | - | 0.69 | - | 0.35 | |
| 10-Epi-γ-eudesmol | C15H26O | 1622 | 1622 | - | 0.02 | - | - | - | 0.02 | - | 0.04 | - | - | - | 0.03 | - | - | |
| Isoespathulenol | C15H24O | 1640 | 0.04 | 0.07 | - | - | - | 0.06 | - | - | - | 0.03 | - | 0.09 | - | - | ||
| τ-Muurolol | C15H26O | 1641 | 1641 | - | - | - | - | - | 0.05 | - | 0.08 | - | - | - | 0.07 | - | - | |
| Juniper camphor | C15H26O | 1691 | 1691 | 0.40 | 0.58 | - | 0.23 | - | 0.53 | - | 0.86 | - | 0.29 | - | 0.70 | - | 0.32 | |
| % of identified compounds | 99.78 | 99.4 | 92.16 | 99.06 | 91.78 | 99.19 | 92.57 | 99.37 | 92.92 | 98.37 | 92.72 | 99.38 | 91.75 | 98.65 | ||||
| % of monoterpenes among the identified compounds | 14.93 | 15.97 | 10.81 | 17.81 | 11.56 | 20.11 | 10.98 | 15.78 | 13.51 | 20.59 | 10.79 | 17.46 | 13.34 | 19.41 | ||||
| % of oxygenated monoterpenes among the identified compounds | 78.29 | 76.17 | 79.34 | 76.83 | 76.72 | 68.93 | 80.2 | 77.79 | 77.91 | 74.66 | 79.23 | 73.18 | 75.14 | 71.04 | ||||
| % of sesquiterpenes among the identified compounds | 6.56 | 7.26 | 2.01 | 4.42 | 3.5 | 10.15 | 1.39 | 5.8 | 1.5 | 3.12 | 2.7 | 8.74 | 3.27 | 8.2 |
| Microorganism | Minimum Inhibitory Concentration (MIC) (% (v/v)) | ||
|---|---|---|---|
| SD | ETHOS X | Mac 75 | |
| Staphylococcus aureus (ATCC 25923) * | 0.031 | 0.031 | 0.031 |
| Staphylococcus aureus (CIP 106760) ** | 0.031 | 0.031 | 0.031 |
| Enterococcus faecalis (ATCC 29212) | 0.031 | 0.031 | 0.031 |
| Escherichia coli (ATCC 25922) | 0.156 | 0.156 | 0.156 |
| Pseudomonas aeruginosa (ATCC 27853) | 0.015 | 0.015 | 0.015 |
| Candida albicans (ATCC 10231) | 0.078 | 0.078 | 0.078 |
| Identification | %SD Flower | %ETHOS X Flower | %SD Fresh Aerial Parts | %ETHOS X Fresh Aerial Parts | %HD Dry Aerial Parts Rehydrated | %SD Dry Aerial Parts Rehydrated | %ETHOS X Dry Aerial Parts Rehydrated | %Mac 75 Dry Aerial Parts Rehydrated |
|---|---|---|---|---|---|---|---|---|
| Extraction time (min) | 60 | 30 | 30 | 30 | 120 | 60 | 30 | 60 |
| Yield (%) | 0.91 | 3.1 | 1 | 1.4 | 3.16 | 2.04 | 2.4 | 2.81 |
| Energy consumption (W.h/g of essential oil) | 395.6 | 58.1 | 75.0 | 53.6 | 227.8 | 176.5 | 75.0 | 53.4 |
| Environmental impact (g CO2/g essential oil) | 316.5 | 46.5 | 60.0 | 42.9 | 182.3 | 141.2 | 60.0 | 42.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, A.R.T.S.; Périno, S.; Fernandez, X.; Cunha, C.; Rodrigues, M.; Ribeiro, M.P.; Jordao, L.; Silva, L.A.; Rodilla, J.; Coutinho, P.; et al. Solvent-Free Microwave Extraction of Thymus mastichina Essential Oil: Influence on Their Chemical Composition and on the Antioxidant and Antimicrobial Activities. Pharmaceuticals 2021, 14, 709. https://doi.org/10.3390/ph14080709
Araujo ARTS, Périno S, Fernandez X, Cunha C, Rodrigues M, Ribeiro MP, Jordao L, Silva LA, Rodilla J, Coutinho P, et al. Solvent-Free Microwave Extraction of Thymus mastichina Essential Oil: Influence on Their Chemical Composition and on the Antioxidant and Antimicrobial Activities. Pharmaceuticals. 2021; 14(8):709. https://doi.org/10.3390/ph14080709
Chicago/Turabian StyleAraujo, André R. T. S., Sandrine Périno, Xavier Fernandez, Cassandra Cunha, Márcio Rodrigues, Maximiano P. Ribeiro, Luisa Jordao, Lúcia A. Silva, Jesus Rodilla, Paula Coutinho, and et al. 2021. "Solvent-Free Microwave Extraction of Thymus mastichina Essential Oil: Influence on Their Chemical Composition and on the Antioxidant and Antimicrobial Activities" Pharmaceuticals 14, no. 8: 709. https://doi.org/10.3390/ph14080709
APA StyleAraujo, A. R. T. S., Périno, S., Fernandez, X., Cunha, C., Rodrigues, M., Ribeiro, M. P., Jordao, L., Silva, L. A., Rodilla, J., Coutinho, P., & Chemat, F. (2021). Solvent-Free Microwave Extraction of Thymus mastichina Essential Oil: Influence on Their Chemical Composition and on the Antioxidant and Antimicrobial Activities. Pharmaceuticals, 14(8), 709. https://doi.org/10.3390/ph14080709












